A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete
Abstract
:1. Introduction
2. Experimental Section
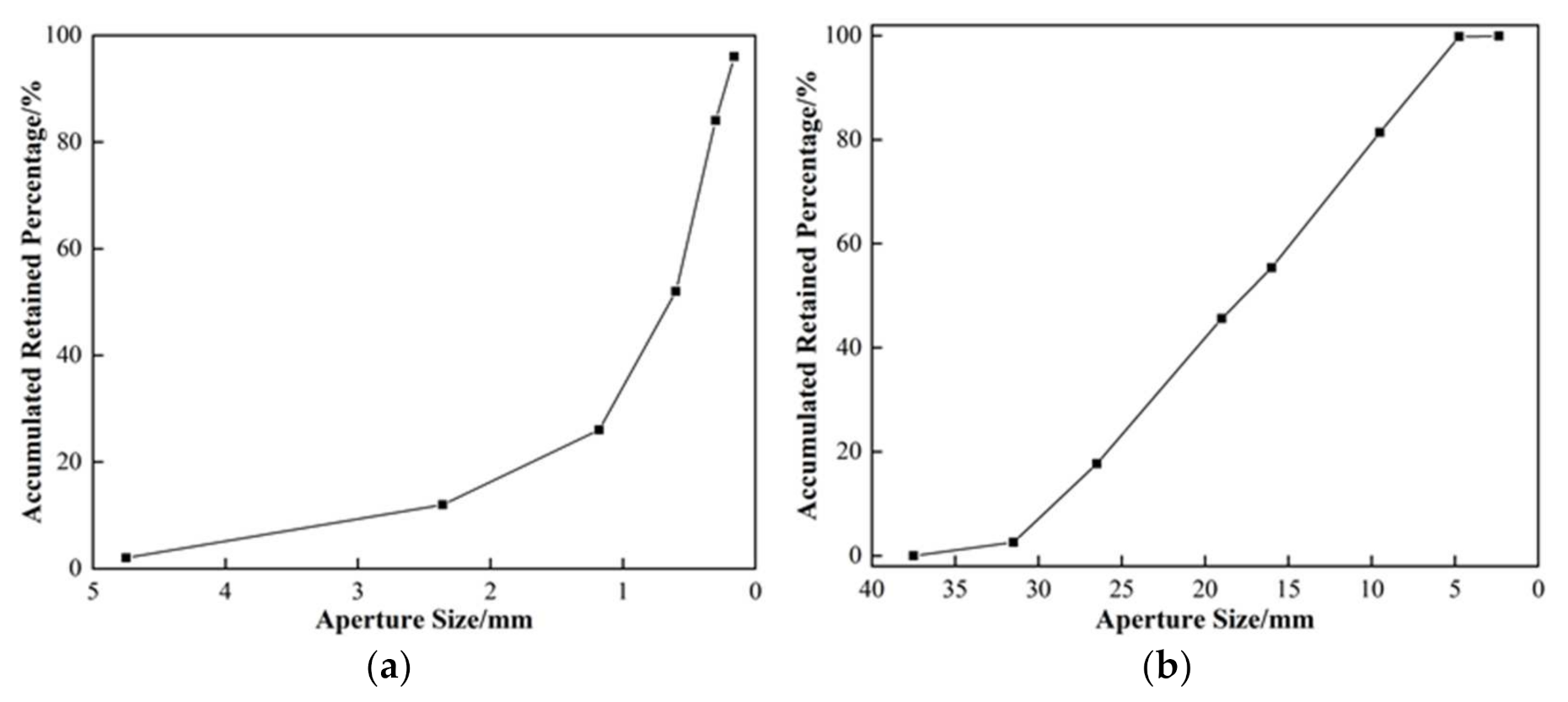
2.1. Materials
2.2. Concrete Mix Ratio
2.3. Preparation of Concrete Specimens
2.4. Concrete Surface Treatment Methods
2.5. Methods
2.5.1. Water Penetration Test
2.5.2. Chloride Penetration Resistance Test
2.5.3. Frost Resistance Test
2.5.4. Sulfate Corrosion Resistance Test
2.5.5. Wear Resistance Test
2.5.6. SEM Test
2.5.7. XRD, FTIR and TGA
3. Results and Discussion
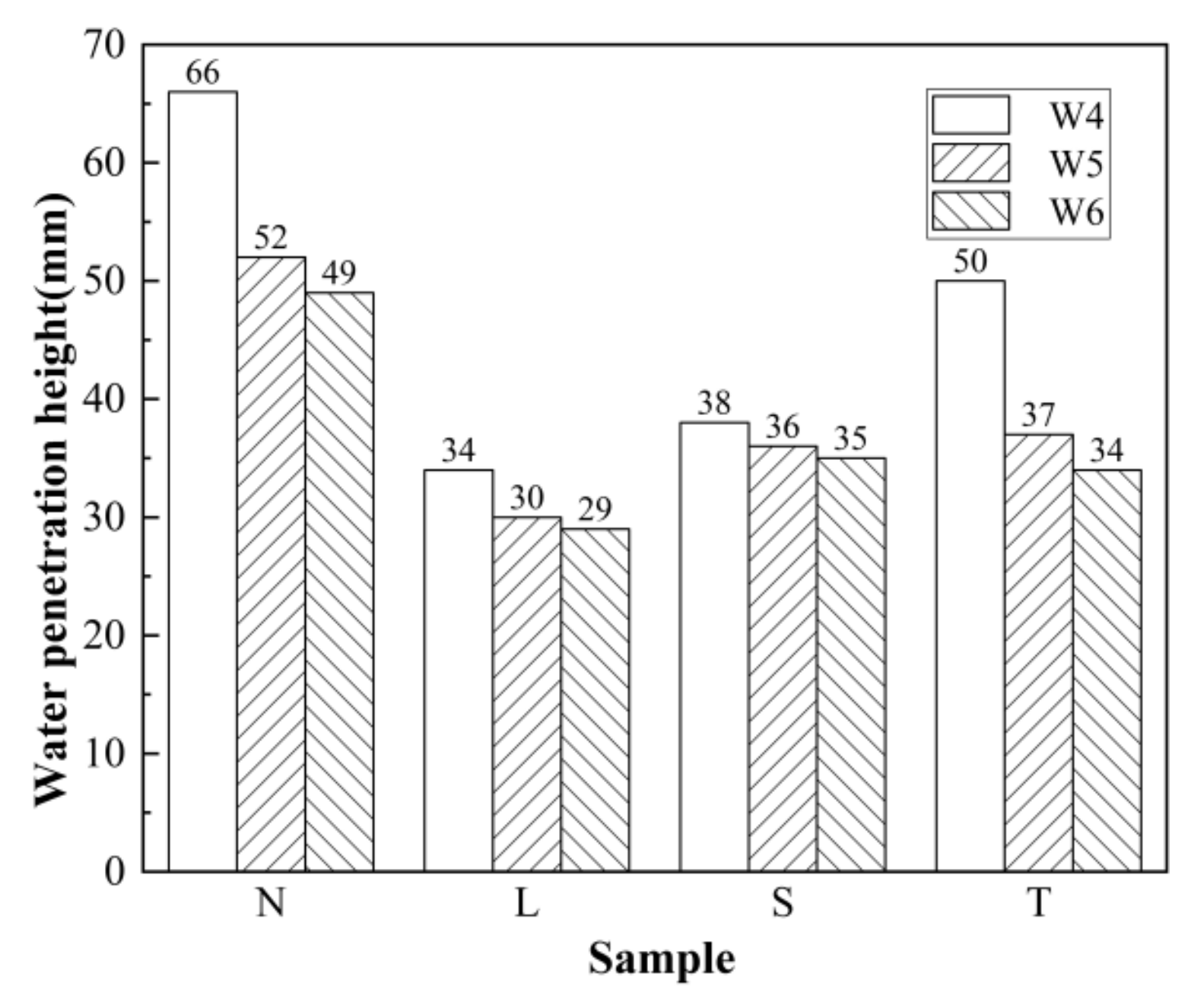
3.1. Water Penetration Test Results and Analysis
3.1.1. Impact of Different Surfacing Agents on the Water Penetration Resistance of Concrete
3.1.2. Water Penetration Resistance of Surfaced Concrete with Different Strengths
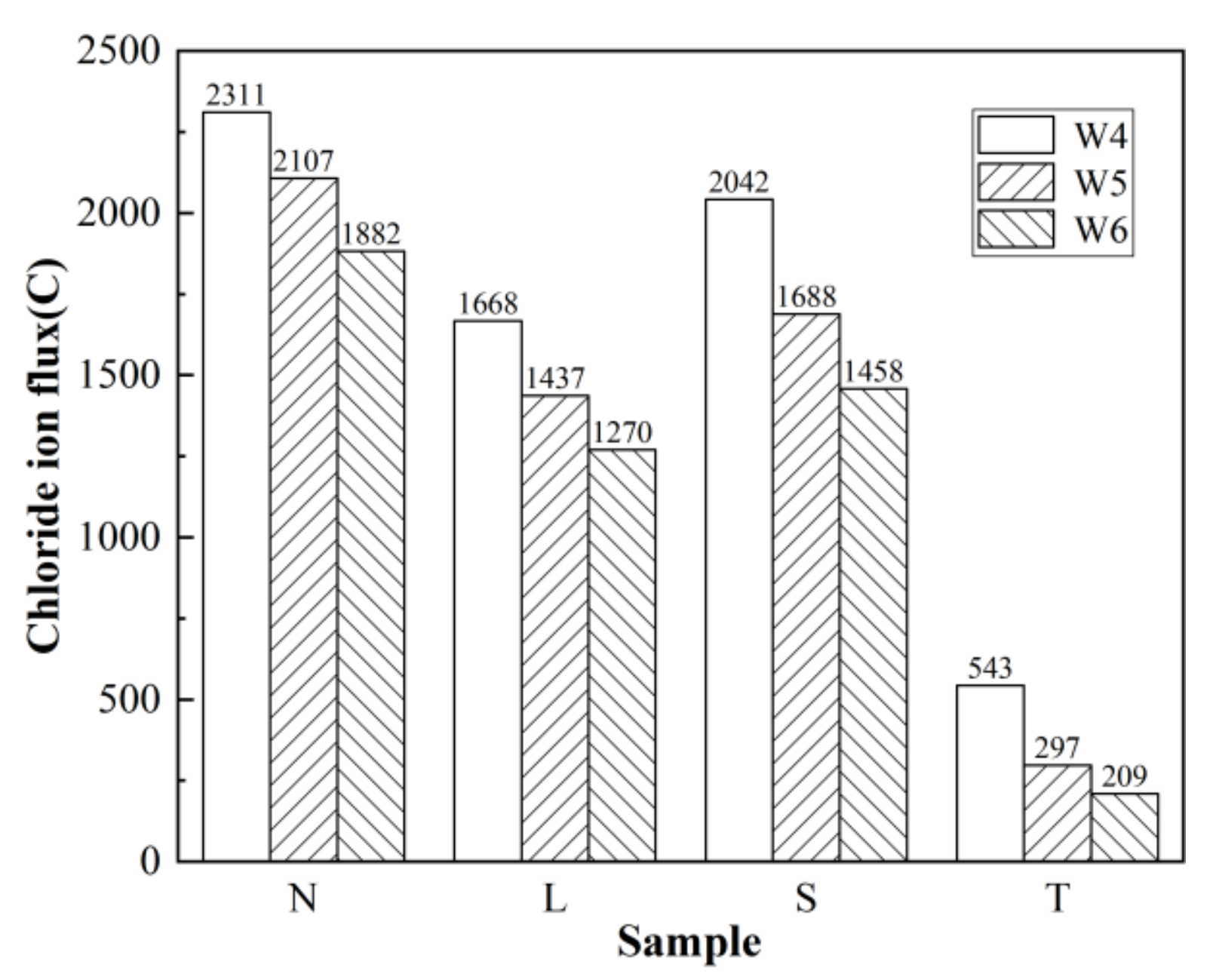
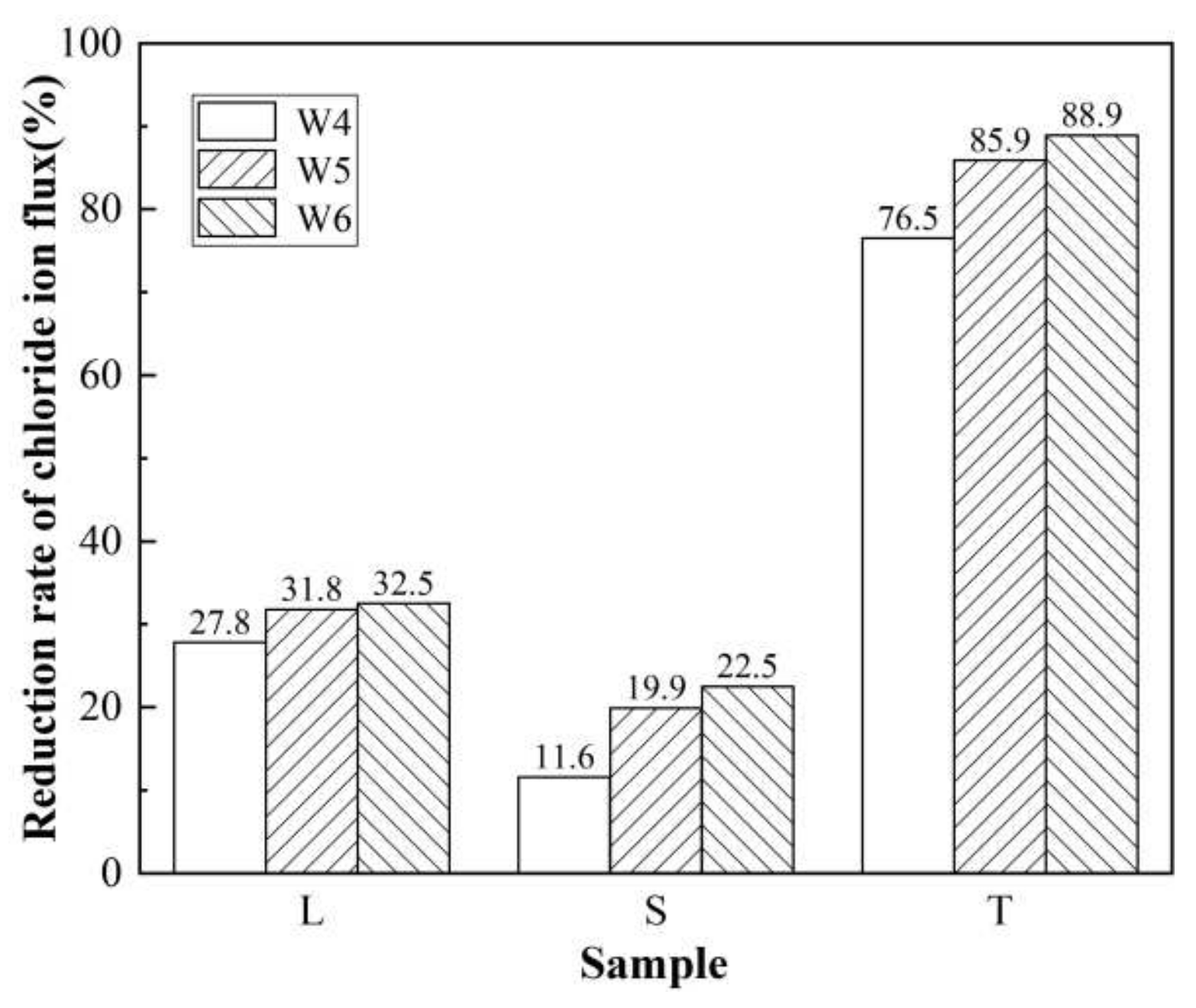
3.2. Rapid Chloride Penetration Test (RCPT) Results and Analysis
3.2.1. Anti-Chloride Ion Permeability of Concrete after Different Surface Treatments
3.2.2. Chloride Ion Permeability of Surfaced Concrete with Different Strengths
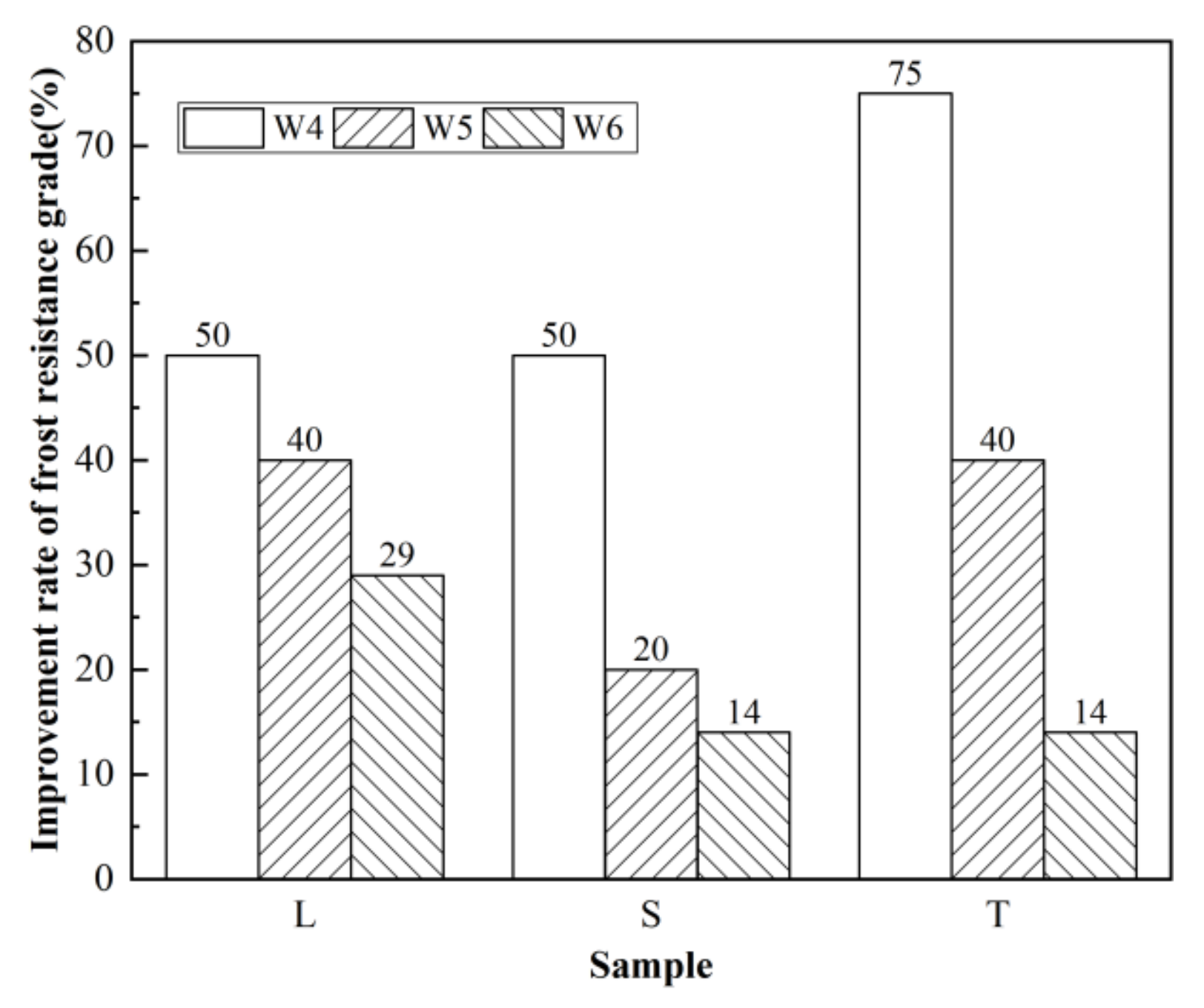
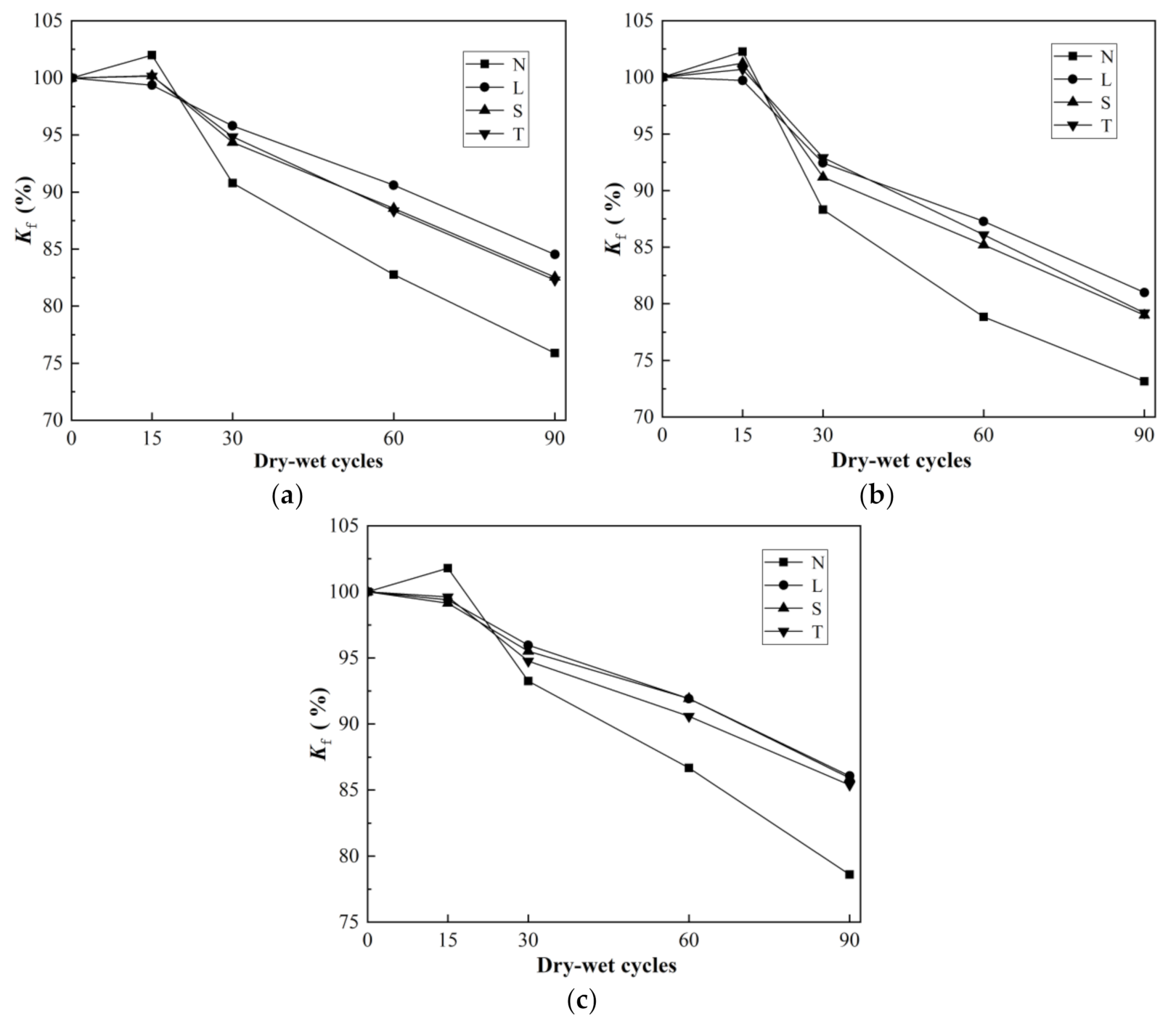
3.3. Frost Resistance Test Results and Analysis
3.3.1. Frost Resistance of Concrete after Different Surface Treatments
3.3.2. Frost Resistance of Surfaced Concrete with Different Strengths
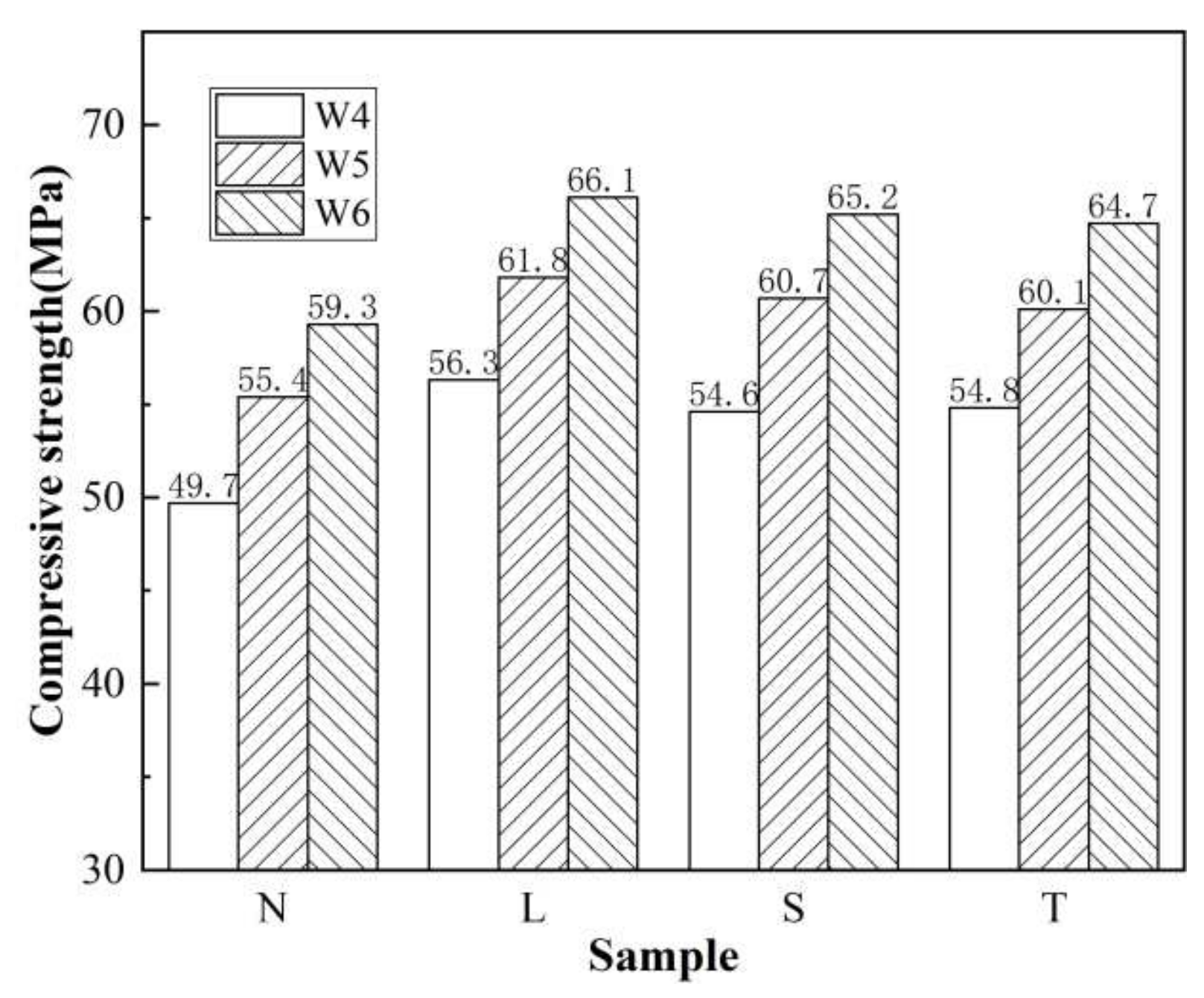
3.4. Sulfate Corrosion Resistance Test Results and Analysis
3.4.1. Sulfate Corrosion Resistance of Concrete after Different Surface Treatments
3.4.2. Sulfate Corrosion Resistance of Surfaced Concrete with Different Strengths
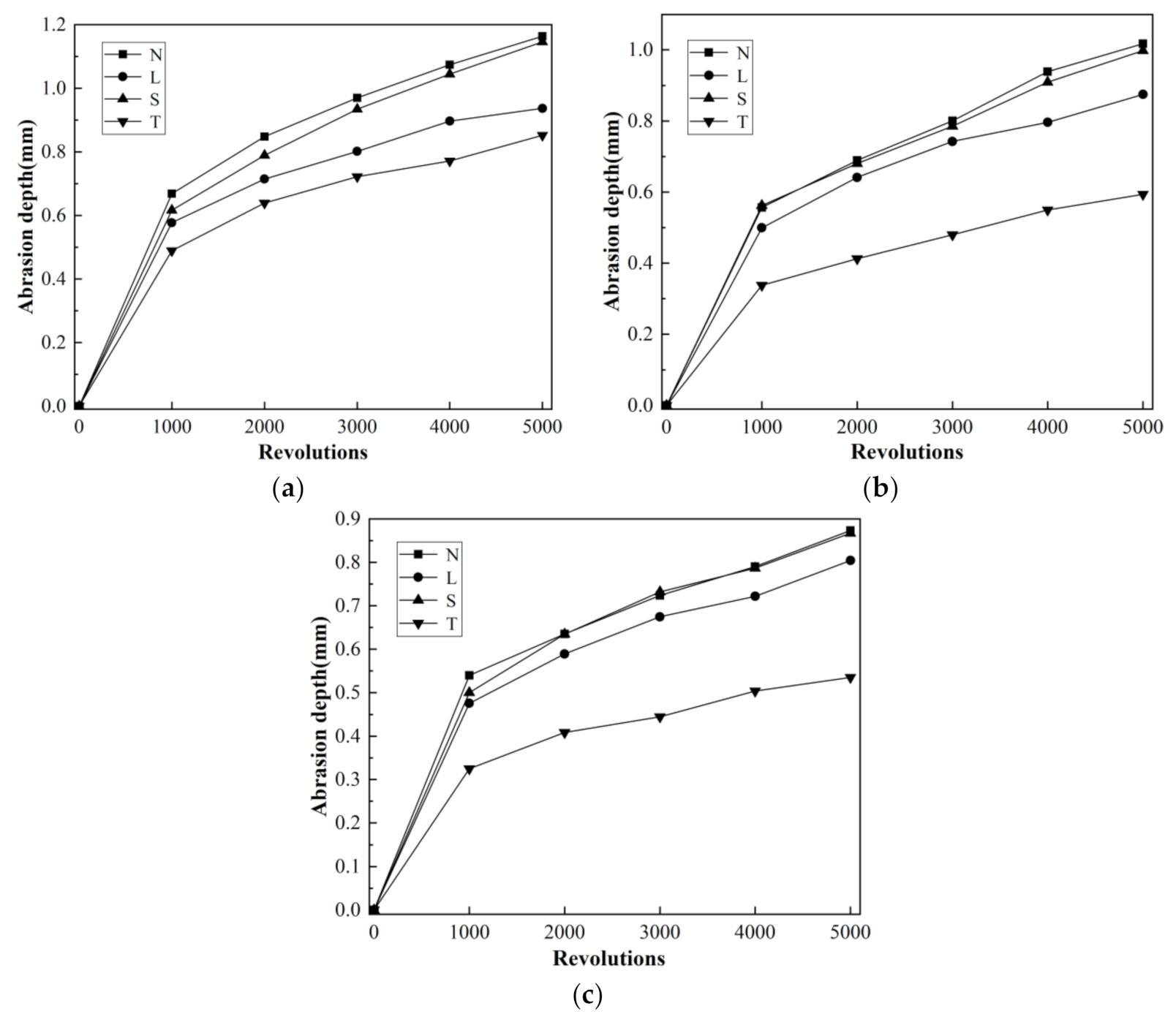
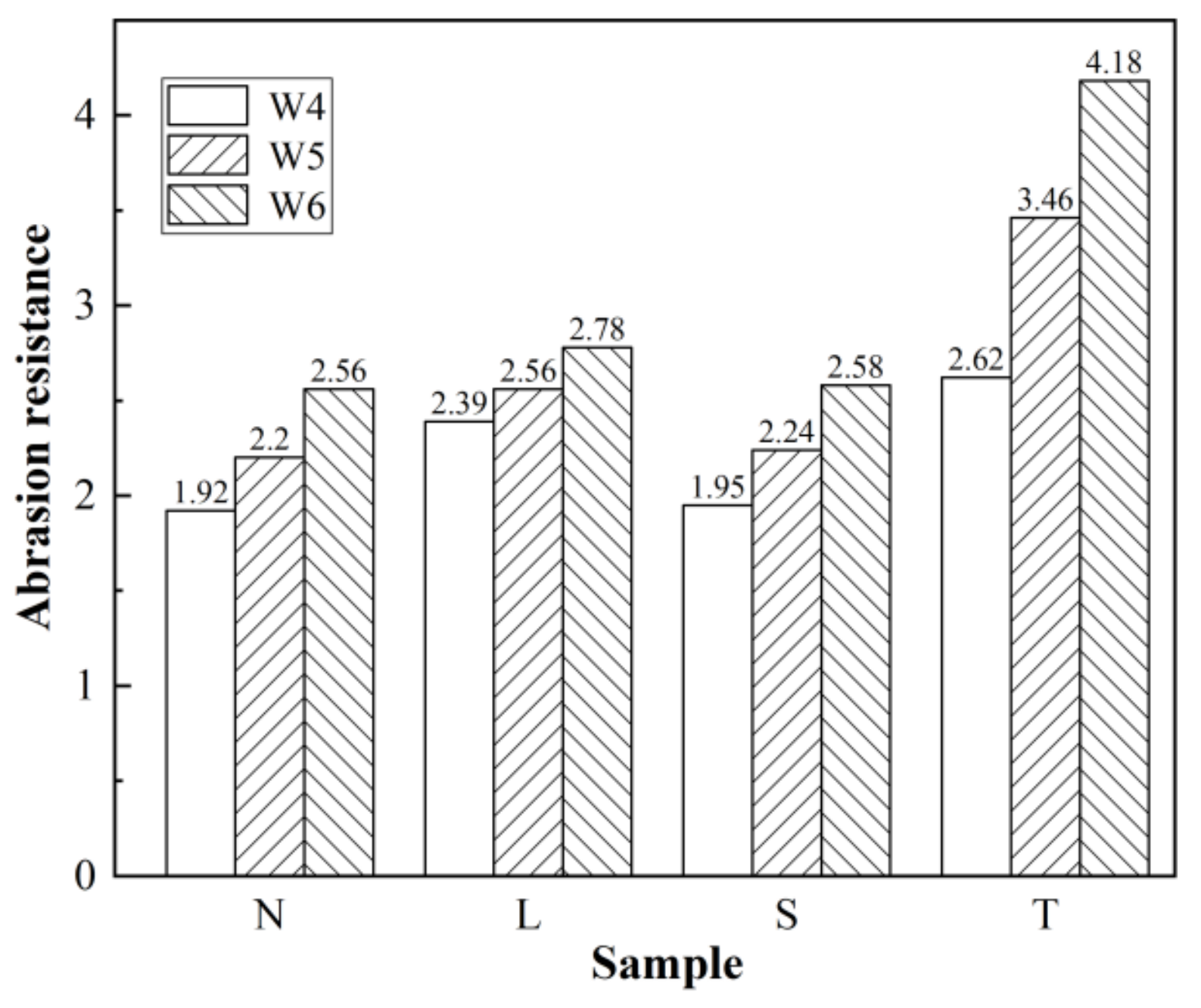
3.5. Wear Resistance Test Results and Analysis
3.5.1. Wear Resistance of Concrete after Different Surface Treatments
3.5.2. Wear Resistance of Surfaced Concrete with Different Strengths
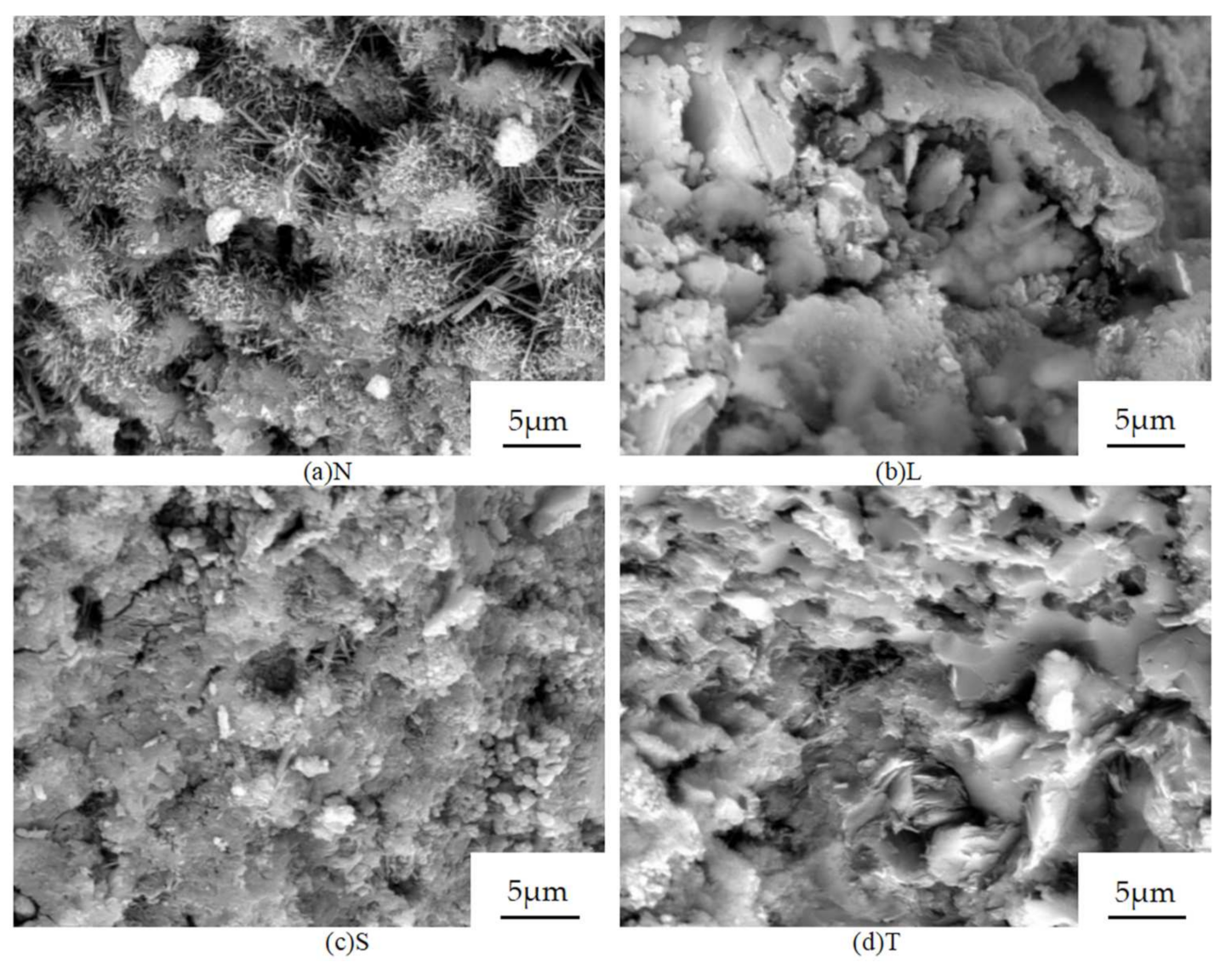
3.6. SEM Test Results and Analysis
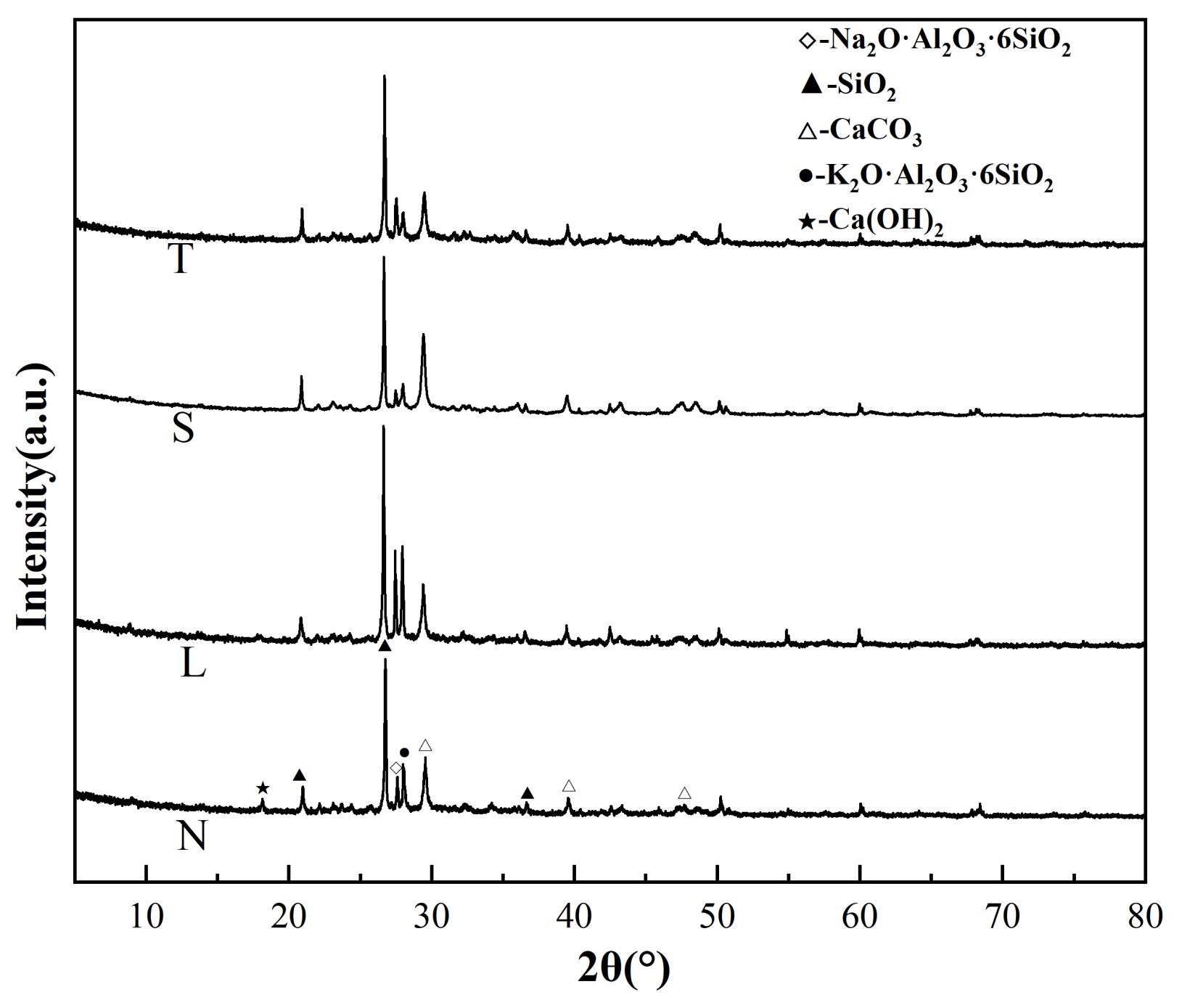
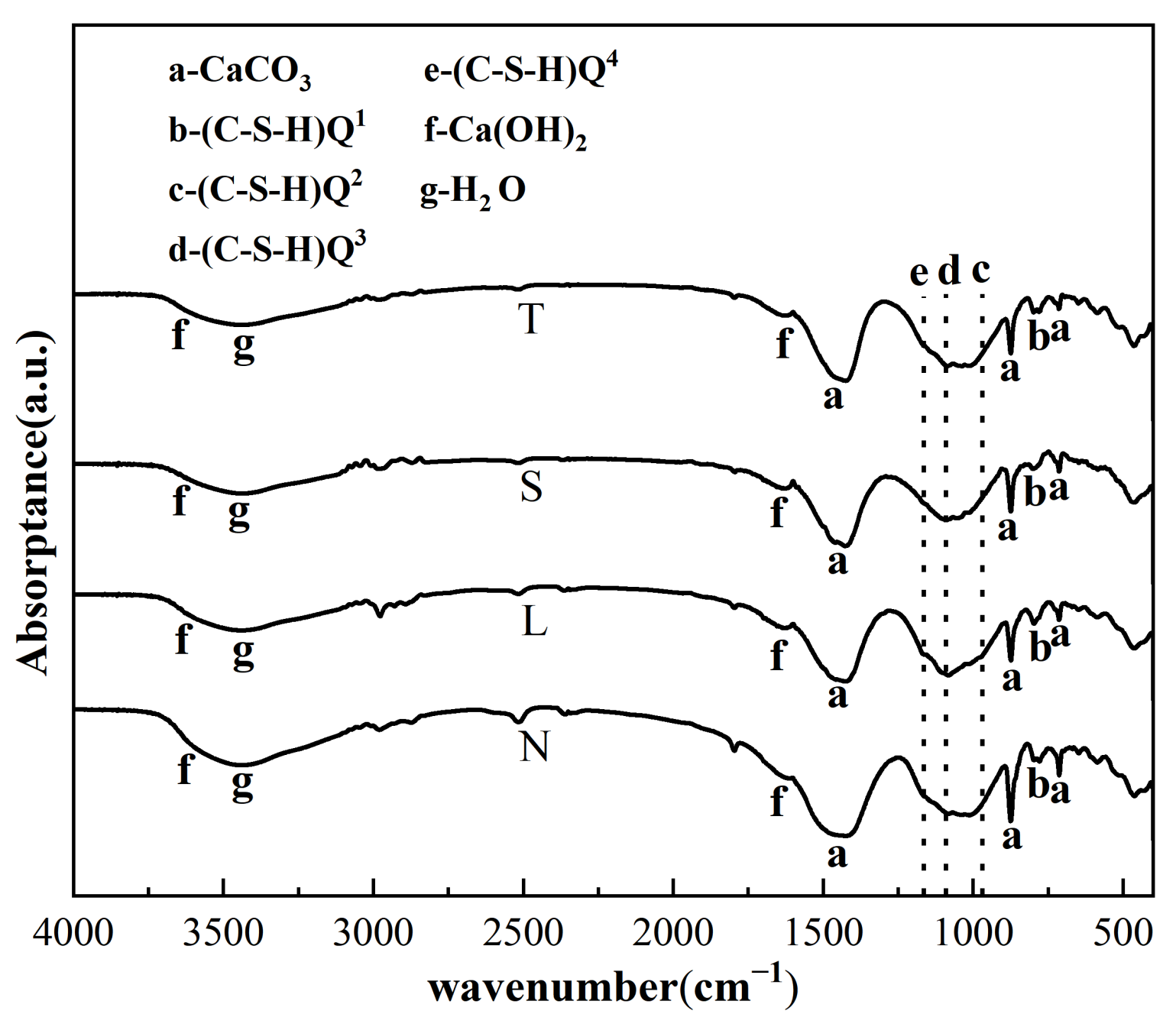
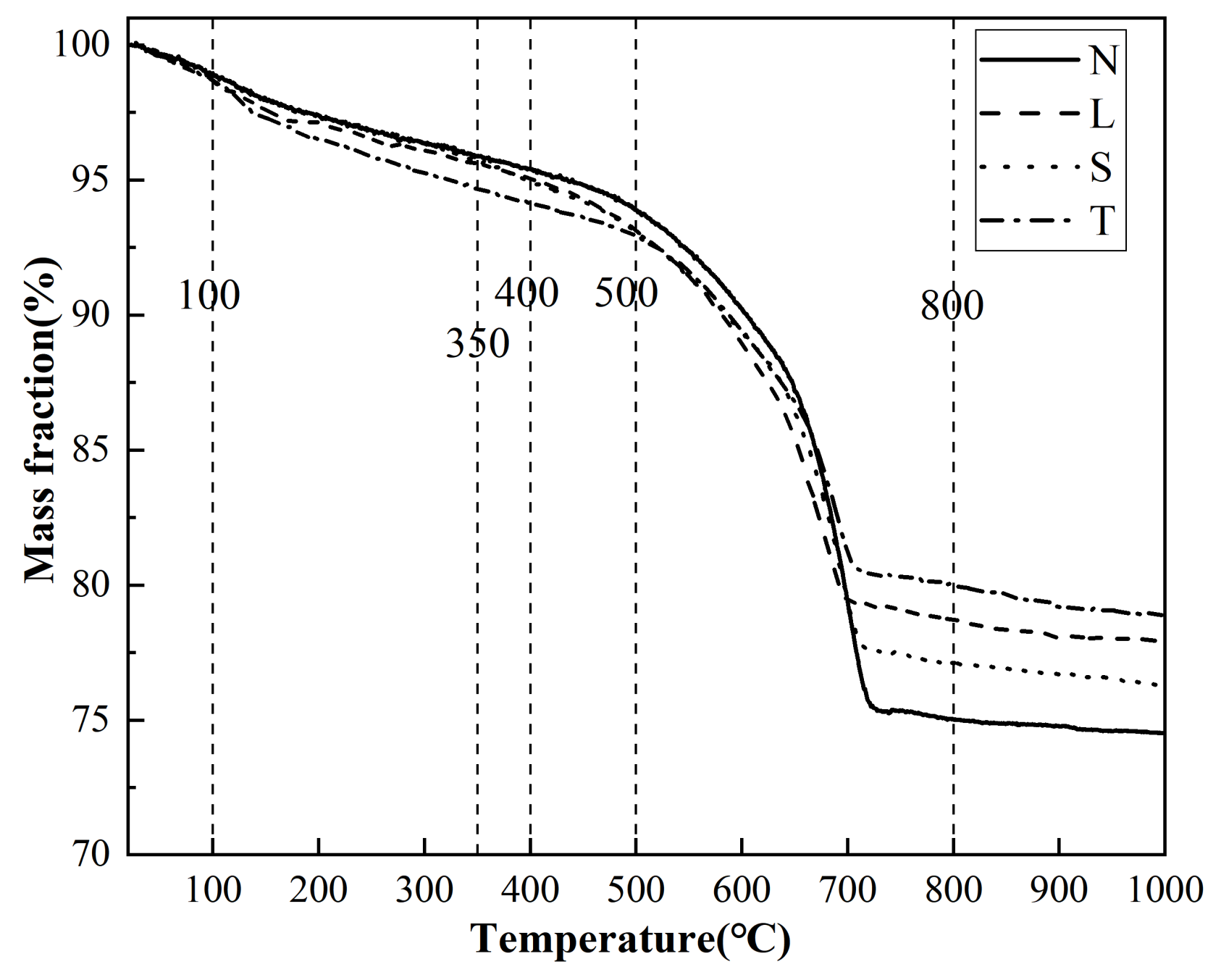
3.7. XRD, FTIR and TGA Test Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zm, A.; Xga, B.; As, C. Mechanical performances and microstructures of metakaolin contained UHPC matrix under steam curing conditions—ScienceDirect. Constr. Build. Mater. 2020, 268, 121112. [Google Scholar]
- Wang, L.; Yong, H.; Lu, J.; Shu, C.; Wang, H. influence of coarse aggregate type on the mechanical strengths and durability of cement concrete. Coatings 2021, 11, 1036. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Wu, Y.; Liu, Q.; Yu, H.; Zhang, C. Assessing recycled pavement concrete mechanical properties under joint action of freezing and fatigue via RSM. Constr. Build. Mater. 2018, 164, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Liu, J.; Hou, D.; Geng, Y.; Chen, X.; Gao, Y.; Jin, Z.; Yin, B. Protective mechanism of silane on concrete upon marine exposure. Coatings 2019, 9, 558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Durability of airport concrete pavement improved by four novel coatings. Adv. Cem. Res. 2018, 31, 214–224. [Google Scholar] [CrossRef]
- Safiuddin, M. Concrete damage in field conditions and protective sealer and coating systems. Coatings 2017, 7, 90. [Google Scholar] [CrossRef]
- Han, Y.M.; Dong, G.S.; Choi, D.S. Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system. Constr. Build. Mater. 2007, 21, 362–369. [Google Scholar]
- Wang, L.; Shu, C.; Jiao, T.; Han, Y.; Wang, H. Effect of assembly unit of expansive agents on the mechanical performance and durability of cement-based materials. Coatings 2021, 11, 731. [Google Scholar] [CrossRef]
- Liu, B.J.; Qin, J.L.; Sun, M.H. Influence of silane-based impregnation agent on the permeability of concretes. KSCE J. Civ. Eng. 2019, 23, 3443–3450. [Google Scholar] [CrossRef]
- Li, G.; Dong, L.; Bai, Z.A.; Lei, M.; Du, J.M. Predicting carbonation depth for concrete with organic film coatings combined with ageing effects. Constr. Build. Mater. 2017, 142, 59–65. [Google Scholar] [CrossRef]
- Pan, X.; Shi, Z.; Shi, C.; Ling, T.C.; Ning, L. A review on surface treatment for concrete—Part 2: Performance. Constr. Build. Mater. 2017, 133, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Shi, Z.; Shi, C.; Hu, X.; Wu, L. Interactions between inorganic surface treatment agents and matrix of Portland cement-based materials. Constr. Build. Mater. 2016, 113, 721–731. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, M.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Yang, C.C.; Wang, L.C.; Weng, T.L. Using charge passed and total chloride content to assess the effect of penetrating silane sealer on the transport properties of concrete. Mater. Chem. Phys. 2004, 85, 238–244. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Brenna, A.; Bolzoni FA BI, O.; Berra, M.; Pastore, T.; Ormellese, M. Effect of polymer modified cementitious coatings on water and chloride permeability in concrete. Constr. Build. Mater. 2013, 49, 720–728. [Google Scholar] [CrossRef]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Pavlidou, E.; Achilias, D.S.; Karapanagiotis, I. TEOS-Based superhydrophobic coating for the protection of stone-built cultural heritage. Coatings 2021, 11, 135. [Google Scholar] [CrossRef]
- Pigino, B.; Leemann, A.; Franzoni, E.; Lura, P. Ethyl silicate for surface treatment of concrete—Part II: Characteristics and performance. Cem. Concr. Compos. 2011, 34, 313–321. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E.; Pigino, B. Ethyl silicate for surface treatment of concrete—Part I: Pozzolanic effect of ethyl silicate. Cem. Concr. Compos. 2012, 34, 306–312. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, R.; Cai, Y.; Cheng, X.; Shah, S.P. In situ Ca(OH)2 consumption of TEOS on the surface of hardened cement-based materials and its improving effects on the Ca-leaching and sulfate-attack resistivity. Constr. Build. Mater. 2016, 113, 890–896. [Google Scholar] [CrossRef]
- Shcherban’, E.M.; Stel’makh, S.A.; Beskopylny, A.; Mailyan, L.R.; Meskhi, B.; Varavka, V. Nanomodification of lightweight fiber reinforced concrete with micro silica and its influence on the constructive quality coefficient. Materials 2021, 14, 7347. [Google Scholar] [CrossRef]
- Barberena-Fernández, A.M.; Blanco-Varela, M.T.; Carmona-Quiroga, P.M. Use of nanosilica- or nanolime-additioned TEOS to consolidate cementitious materials in heritage structures: Physical and mechanical properties of mortars. Cem. Concr. Compos. 2018, 95, 271–276. [Google Scholar] [CrossRef]
- Scarfato, P.; Maio, L.D.; Fariello, M.L.; Russo, P.; Incarnato, L. Preparation and evaluation of polymer/clay nanocomposite surface treatments for concrete durability enhancement. Cem. Concr. Compos. 2012, 34, 297–305. [Google Scholar] [CrossRef]
- Pan, X.Y.; Shi, C.J.; Zhang, J.K.; Jia, L.F.; Lin, M. Effect of inorganic surface treatment on air permeability of cement-based materials. J. Mater. Civ. Eng. 2016, 28, 04015145. [Google Scholar] [CrossRef]
- Pan, X.Y.; Shi, C.J.; Zhang, J.K.; Jia, L.F.; Chong, L.L. Effect of inorganic surface treatment on surface hardness and carbonation of cement-based materials. Cem. Concr. Compos. 2018, 90, 218–224. [Google Scholar] [CrossRef]
- Kuang, Y.; Takashima, N.; Sakoi, Y.; Zhang, M.; Tsukinaga, Y. Salt scaling resistance and microstructure analysis of fly-ash cement-based concrete treated with different surface penetrants. Mater. Lett. 2021, 297, 129999. [Google Scholar] [CrossRef]
- Matijević, E. Chemistry of silica. J. Colloid Interface Sci. 1980, 77, 290–291. [Google Scholar] [CrossRef]
- GJB 1112A-2004. Construction and Acceptance Specifications for Flying Area Engineering of the Military Airfield. 2004. Available online: https://www.dugen.com/p-11204.html (accessed on 29 December 2021).
- GB/T 50080-2016; Standard for Test Method of Performance on Ordinary Fresh Concrete. China Architecture & Building Press: Beijing, China, 2016.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- GB/T 16925-1997; Test Method for Abrasion Resistance of Concrete and Its Products (Ball Bearing Method). State Bureau of Technical Supervision: Beijing, China, 1997.
- Barberena-Fernandez, A.M.; Blanco-Varela, M.T.; Carmona-Quiroga, P.M. Interaction of TEOS with cementitious materials: Chemical and physical effects. Cem. Concr. Compos. 2015, 55, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Astm, C. Standard test method for electrical indication of concrete’s ability to resist chloride ion penetration. Annu. Book ASTM Stand. 1997, 4, 639–644. [Google Scholar]
- Hou, P.; Xin, C.; Qian, J.; Shah, S.P. Effects and mechanisms of surface treatment of hardened cement-based materials with colloidal nanoSiO2 and its precursor. Constr. Build. Mater. 2014, 53, 66–73. [Google Scholar] [CrossRef]
- Franzoni, E.; Pigino, B.; Pistolesi, C. Ethyl silicate for surface protection of concrete: Performance in comparison with other inorganic surface treatments. Cem. Concr. Compos. 2013, 44, 69–76. [Google Scholar] [CrossRef]
- Wu, Z.W.; Lian, H.Z. High Performance Concrete; China Railway Publishing House: Beijing, China, 1999. [Google Scholar]
- Mehta, P.K.; Monteiro, P. Concrete: Microstructure, properties, and materials. In Concrete: Microstructure, Properties, and Materials; The McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Pan, X.; Shi, C.; Hu, X.; Ou, Z. Effects of CO2 surface treatment on strength and permeability of one-day-aged cement mortar. Constr. Build. Mater. 2017, 154, 1087–1095. [Google Scholar] [CrossRef]





| Density/(g/cm3) | pH | Solid Content/% | Viscosity/Pa·s | Surface Tension/(mN/m) |
|---|---|---|---|---|
| 1.18 ± 0.03 | 11.0 ± 1.0 | 22 ± 2.2 | 11.0 ± 1.0 | ≤30.0 |
| Particle Size/nm | Solid Content/% | Density/(g/cm3) | pH | Appearance |
|---|---|---|---|---|
| 10 ± 1 | 12.5 ± 1 | 1.125 ± 0.007 | 11 ± 0.5 | Semitransparent |
| Silicon Content/% | Acidity/ppm | Viscosity/(CPs/25 °C) | Density/(g/mL) | VOC/% |
|---|---|---|---|---|
| 40.61 | 40 | 5.04 | 1.058 | <3.0 |
| Type | Cement/(kg/m3) | Water/(kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate /(kg/m3) | Water Reducing Agent | Water Cement Ratio | Vebe Consistometer/s | Flexural Strength at 28 d/MPa |
|---|---|---|---|---|---|---|---|---|
| W4 | 320 | 140.8 | 622 | 1391 | - | 0.44 | 22 | 4.7 |
| W5 | 330 | 135.3 | 630 | 1328 | 0.2% | 0.41 | 22 | 5.6 |
| W6 | 330 | 125.4 | 612 | 1434 | 0.4% | 0.38 | 28 | 6.4 |
| Charge Passed (C) | >4000 | 2000–4000 | 1000–2000 | 100–1000 | <100 |
|---|---|---|---|---|---|
| Chloride Ion Penetrability | High | Moderate | Low | Very Low | Negligible |
| Hydration Products | Chemical Formula | Endothermic Dehydration Temperature (°C) |
|---|---|---|
| 1.1 nm Tobermorite | 5CaO·6SiO2·5H2O | 100–350 |
| Calcium silicate hydrate (B) | CaO·SiO2·H2O(B) | 200–250 |
| Calcium silicate hydrate (B) | CaO·SiO2·2H2O(B) | 100–300 |
| Portlandite | Ca(OH)2 | Around 500 |
| Dellaite (A) | 2CaO·SiO2·H2O(A) | 460–480 |
| Dellaite (B) | 2CaO·SiO2·H2O(B) | 560–600 |
| Dellaite (C) | 2CaO·SiO2·H2O(C) | 740 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wu, Y.; Wu, H. A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete. Coatings 2022, 12, 162. https://doi.org/10.3390/coatings12020162
Li T, Wu Y, Wu H. A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete. Coatings. 2022; 12(2):162. https://doi.org/10.3390/coatings12020162
Chicago/Turabian StyleLi, Tianlun, Yonggen Wu, and Haoxiang Wu. 2022. "A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete" Coatings 12, no. 2: 162. https://doi.org/10.3390/coatings12020162
APA StyleLi, T., Wu, Y., & Wu, H. (2022). A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete. Coatings, 12(2), 162. https://doi.org/10.3390/coatings12020162






