Preparation of Transparent Sandwich-like Superhydrophobic Coating on Glass with High Stability and Self-Cleaning Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
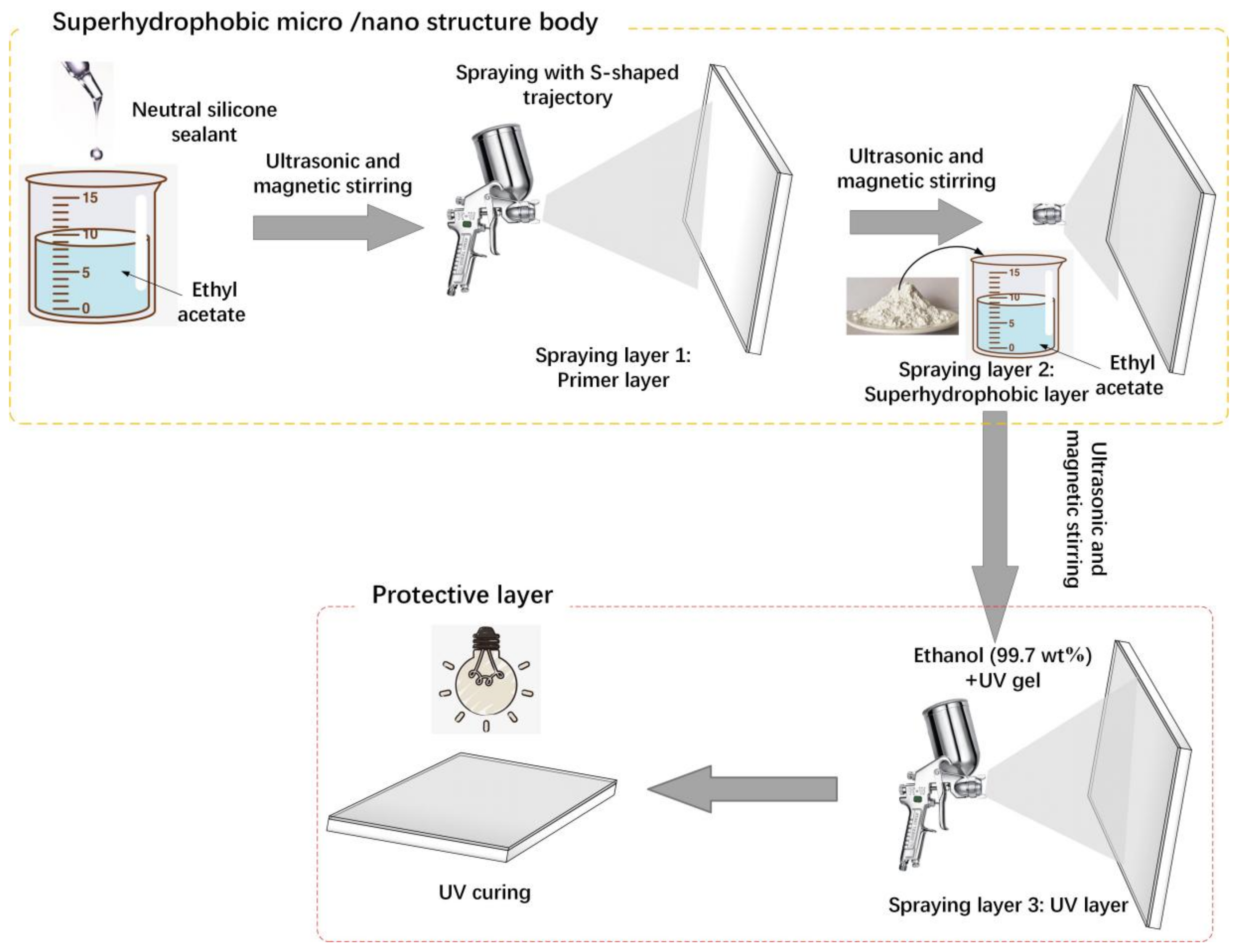
2.2. Fabrication Process of the Superhydrophobic Coating and Protective Layer
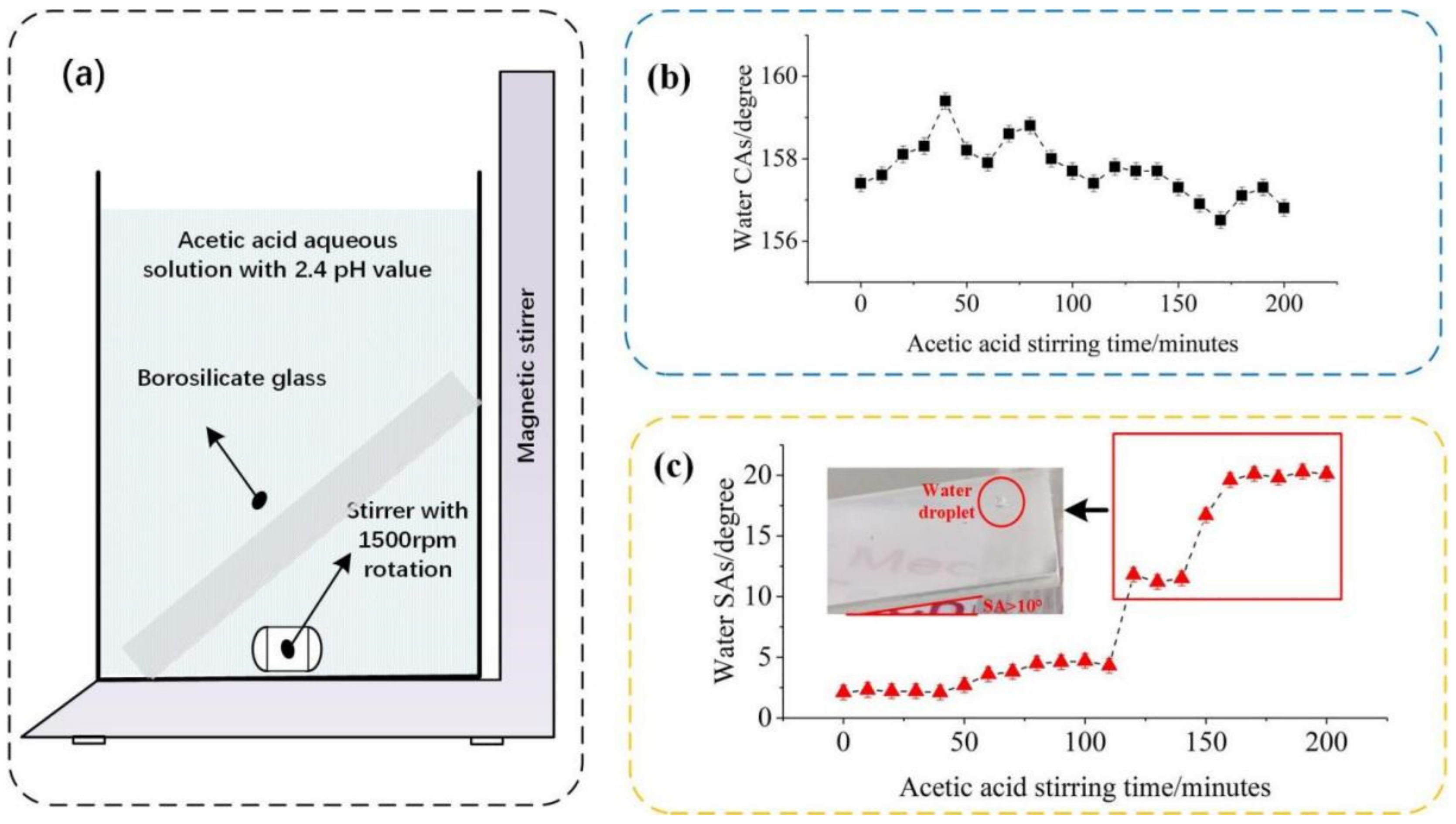
2.2.1. Pretreatment
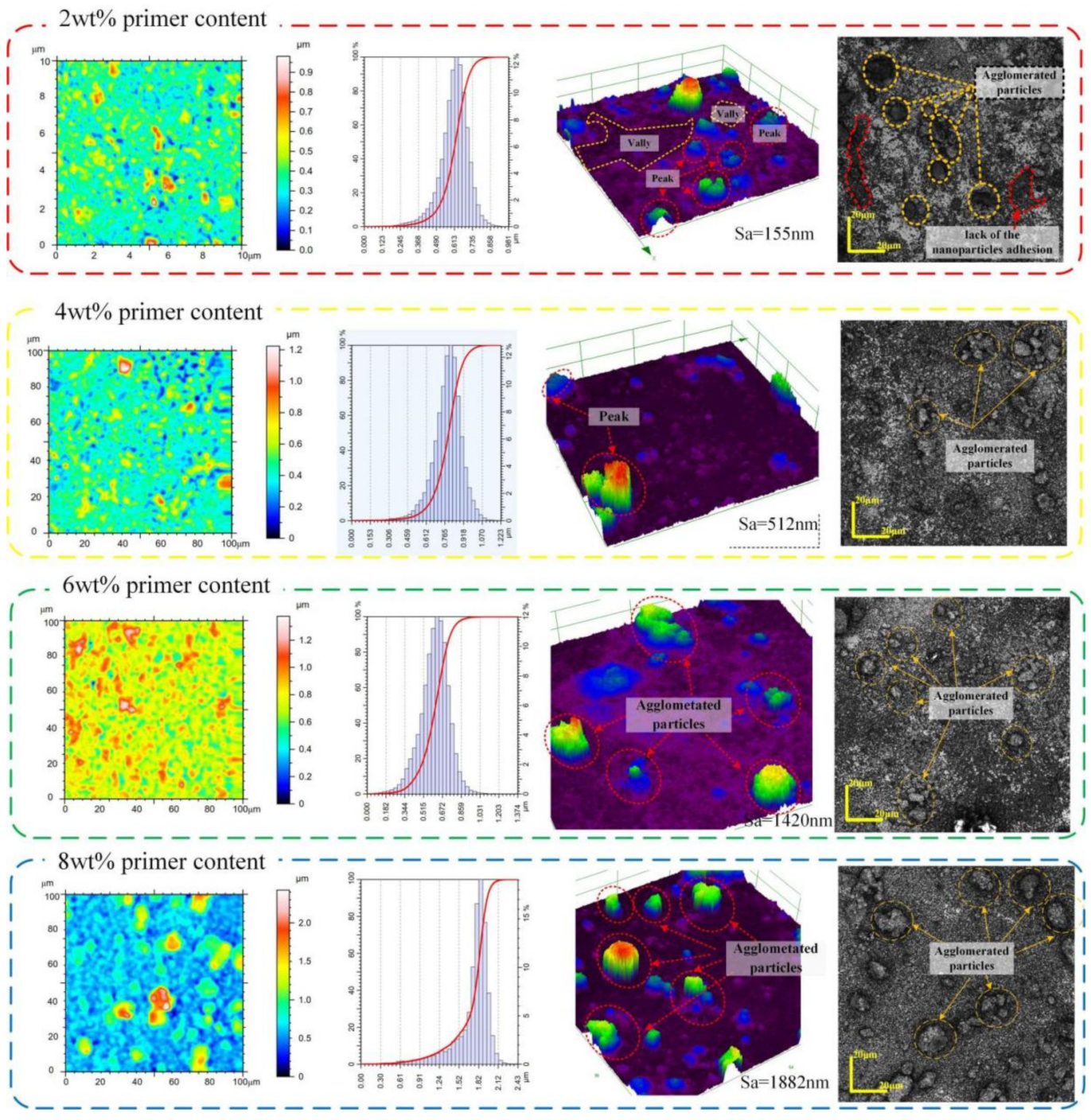
2.2.2. Fabrication of Superhydrophobic Micro/Nanostructure Body
2.2.3. Fabrication of UV Gel Protective Layer
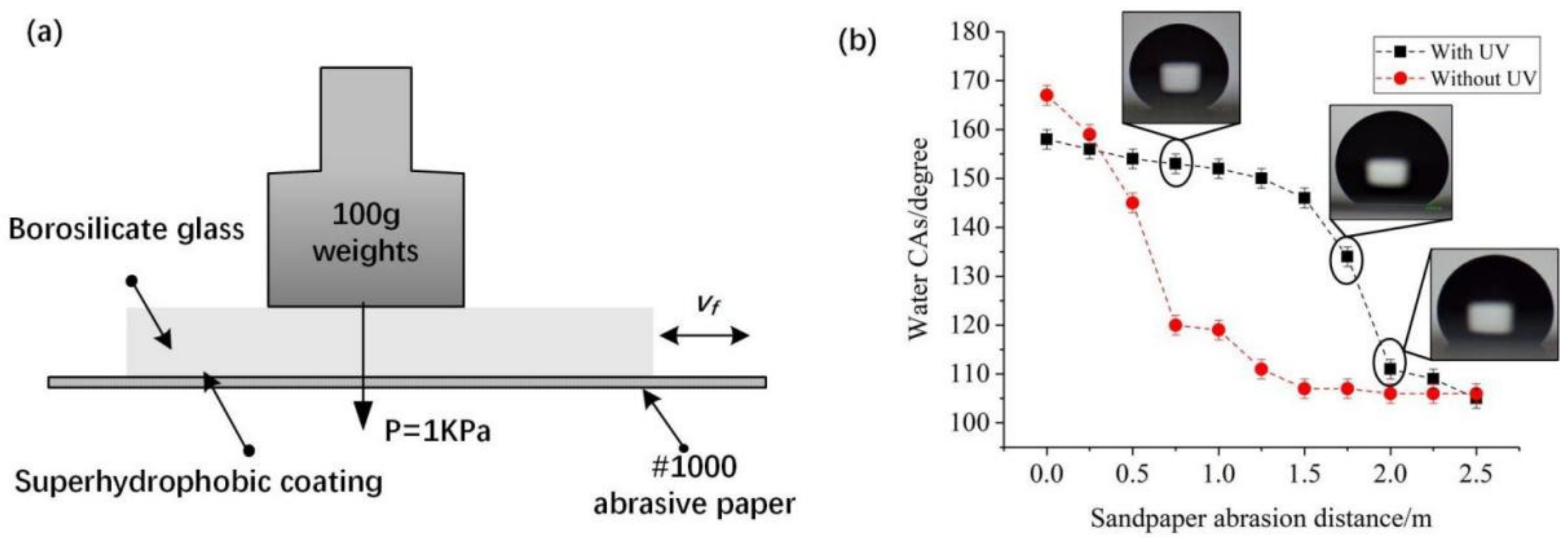
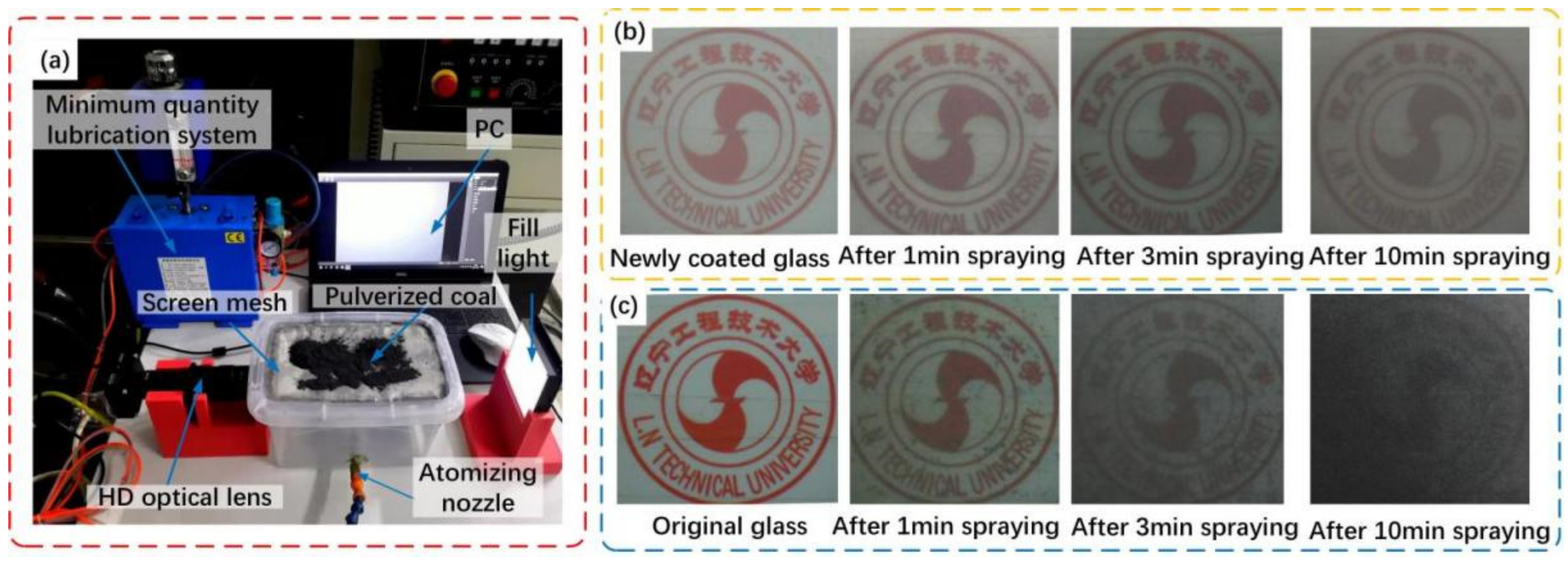
2.3. Characterization
3. Results and Discussion
3.1. Wettability and Transparency of Coating
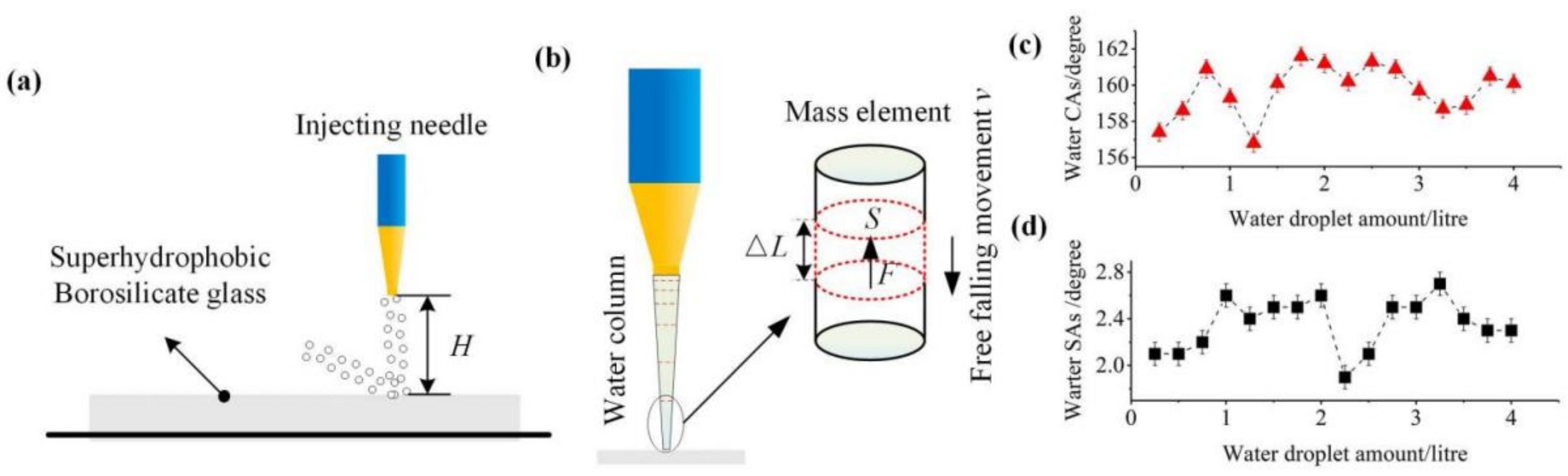
3.2. Stability and Mechanical Durability
- The water column maintains a free falling movement and continuous flow;
- The mechanical action mode of the coated surface and water column is inelastic vertical collision, and the rebound velocity v′ is located between v and 0;
- The water column mass distribution is uniform.
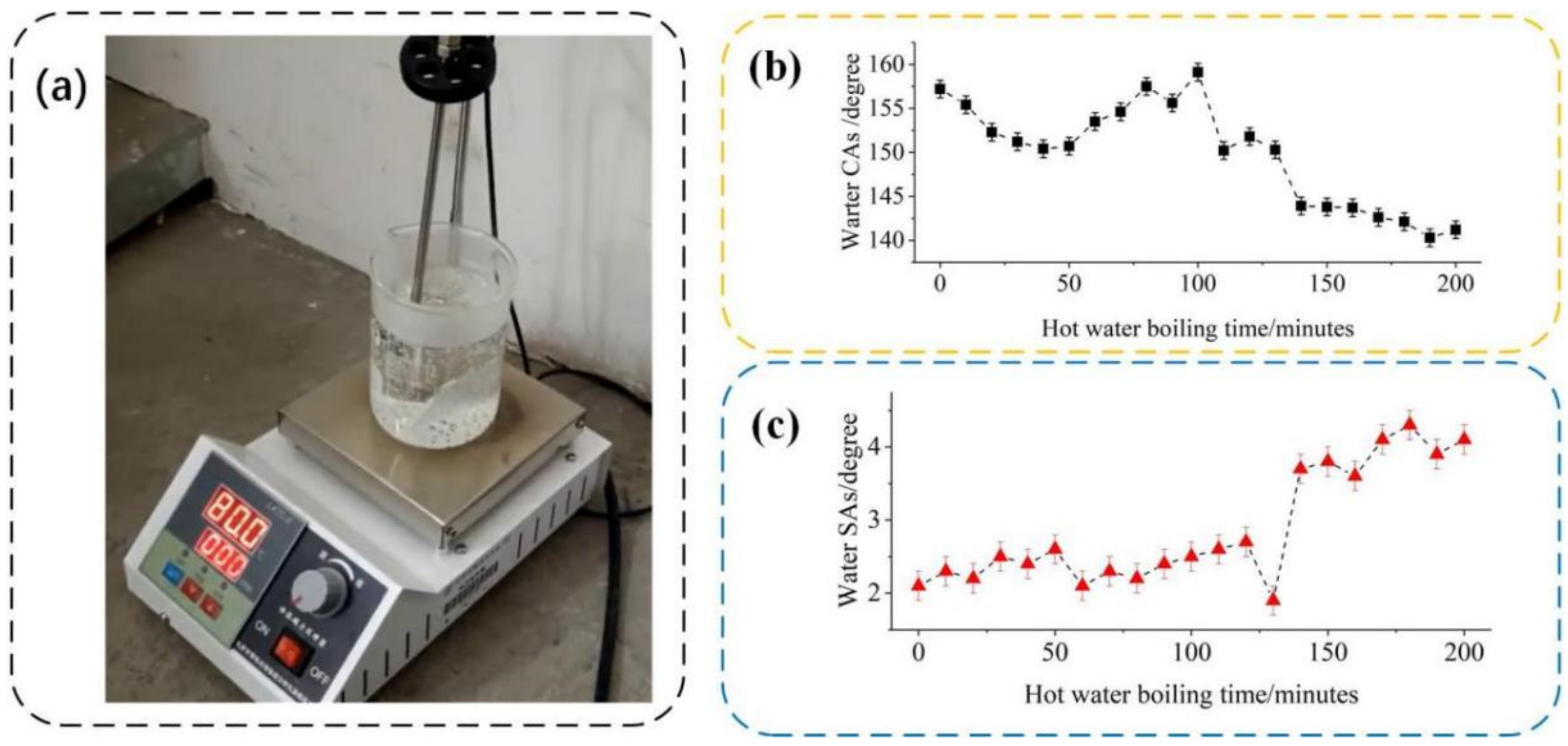
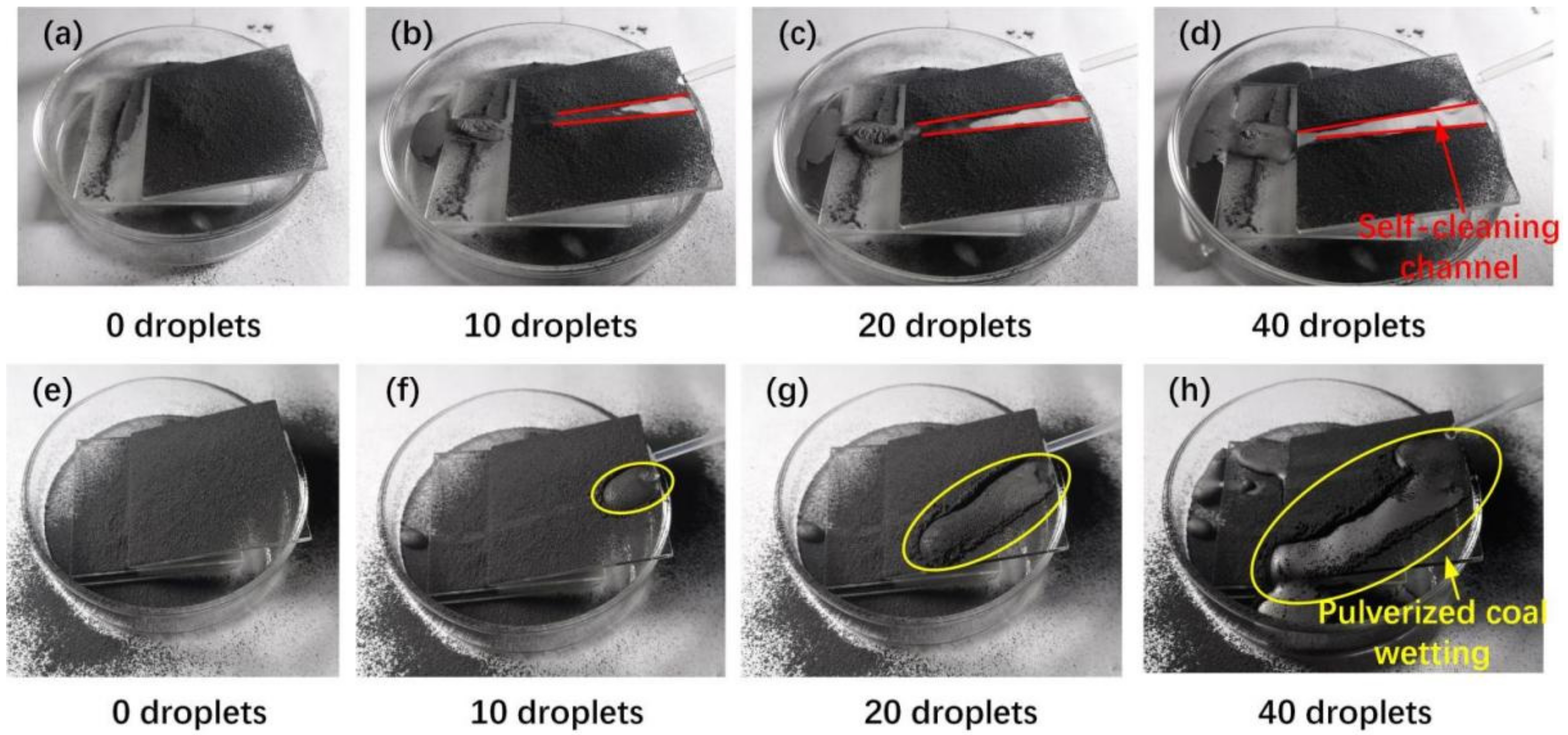
3.3. The Self-Cleaning Performance Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Liu, S. The impacts of coal dust on miners’ health: A review. Environ. Res. 2013, 190, 109849. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Shao, H.; Jiang, S.; Wang, Y.; Wang, K.; Wu, Z. Effect of low-concentration coal dust on gas explosion propagation law. Powder Technol. 2020, 367, 243–252. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Gao, W.; Bi, M.; Ma, L.; Qin, Q.; Shu, C. Effects of particle size on the self-ignition behaviour of a coal dust layer on a hot plate. Fuels 2020, 260, 116269. [Google Scholar] [CrossRef]
- Han, F.; Zhang, J.; Zhao, Y.; Li, J. Dynamic wetting characteristics of droplets on spherical dust surface. J. China Coal. Soc. 2021, 46, 2614–2622. [Google Scholar] [CrossRef]
- Chai, Z.; Zhang, H.; Yang, P.; Yang, Z.; Zhang, B. Molecular dynamics simulation of the effect of temperature and pressure on water absorption characteristics of kaolinite. J. China Coal. Soc. 2021, 46, 2557–2564. [Google Scholar] [CrossRef]
- Alshehri, A.; Champagne, P.; Keirsbulck, L.; Dogheche, E.H. Nanotechnology to Improve the Performances of Hydrodynamic Surfaces. Coatings 2019, 9, 808. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent Progress in Preparation and Anti-Icing Applications of Superhydrophobic Coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Song, J.; Wang, T.; Wang, H.; Wang, Q. Laser Texturing for Superwetting Titanium Alloy and Investigation of Its Erosion Resistance. Coatings 2021, 12, 1547. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N.; Zheng, X. Fabrication of superhydrophobic green surfaces with good self-cleaning, chemical stability and anti-corrosion properties. J. Mater. Sci. 2019, 54, 13006–13016. [Google Scholar] [CrossRef]
- Yi, Z.; Zhao, B.; Liao, M.; Qin, Z. Fabrication of Superhydrophobic Wood Surface by Etching Polydopamine Coating with Sodium Hydroxide. Coatings 2020, 10, 847. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Z.; Wu, H.; Li, L.; Fu, X. Study on Preparation of Superhydrophobic Ni-Co Coating and Corrosion Resistance by Sandblasting–Electrodeposition. Coatings 2020, 12, 1164. [Google Scholar] [CrossRef]
- Kim, M.K.; Yao, W.; Cho, Y.R. Fabrication of superhydrophobic surface with hierarchical structure by thermal imprinting and spraying. Colloid Surface A 2022, 634. [Google Scholar] [CrossRef]
- Qu, J.E.; Yu, C.; Nie, C.; Wang, H.; Cao, Z.; Li, Y.; Wang, X. A new environmentally friendly approach to prepare superhydrophobic colored stainless steel surface for decoration, anti-corrosion and self-cleaning. J. Mater. Sci. 2020, 56, 854–869. [Google Scholar] [CrossRef]
- Zheng, Z.; Liao, C.; Xia, Y.; Cai, W.; Xie, C.; Zhang, W.; Liu, Y. Facile fabrication of robust, biomimetic and superhydrophobic polymer/graphene-based coatings with self-cleaning, oil-water separation, anti-icing and corrosion resistance properties. Colloid Surf. A 2021, 627. [Google Scholar] [CrossRef]
- Shen, K.; Lv, X.; Jia, Y.; Jin, G.; Huang, Y.; Hou, R.; Fu, J.; Shi, S. Research progress of transparent superhydrophobic surface. Surf. Technol. 2021, 50, 108–119. [Google Scholar] [CrossRef]
- Huang, W.H.; Lin, C.S. Robust superhydrophobic transparent coatings fabricated by a low-temperature sol–gel process. Appl. Surf. Sci. 2014, 305, 702–709. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, J.; Hong, X. Transparent superhydrophobic hollow films (TSHFs) with superior thermal stability and moisture resistance. RSC Adv. 2018, 8, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.C.; Simonaitis, J.W.; Gadelrab, K.R.; Tahir, M.; Ding, Y.; Alexander-Katz, A.; Ross, C.A. Imparting Superhydrophobicity with a Hierarchical Block Copolymer Coating. Small 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yi, P.; Gao, J.; Deng, Y.; Peng, L.; Lai, X. Large-Area Stable Superhydrophobic Poly(dimethylsiloxane) Films Fabricated by Thermal Curing via a Chemically Etched Template. ACS Appl. Mater. Interfaces 2020, 12, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hua, Y.; Ye, Y.; Chen, R.; Li, Z. Transparent superhydrophobic solar glass prepared by fabricating groove-shaped arrays on the surface. Appl. Surf. Sci. 2017, 426, 957–964. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, J.; Wen, M.; Yao, M.; Wang, F.; Huang, F.; Zhang, Q.; Cheng, Y.; Zhong, J. Sequentially Reinforced Additive Coating for Transparent and Durable Superhydrophobic Glass. Langmuir 2018, 34, 11316–11324. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Zhao, Y.; Liu, L.; Wang, L.; Wang, J.; Yu, S. Facile fabrication and evaluation of self-healing Zn-Al layered double hydroxide superhydrophobic coating on aluminum alloy. J. Mater. Sci. 2021, 56, 14803–14820. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; McCarty, C.W. How Wenzel and Cassie Were Wrong. Langmuir 2007, 23, 3762–3765. [Google Scholar] [CrossRef]
- Sebastian, D.; Yao, T.J.; Nipa, L.; Lian, I.; Twu, G. Corrosion Behavior and Mechanical Properties of a Nanocomposite Superhydrophobic Coating. Coatings 2021, 11, 652. [Google Scholar] [CrossRef]
- Ke, C.; Li, Z.; Zhang, C.; Wu, X.; Zhu, Z.; Jiang, Y. Investigation of the Effects of Component Ratios on the Properties of Superhydrophobic Polyurethane/Fluorinated Acrylic Co-Polymer/SiO2 Nanocomposite Coatings. Coatings 2021, 11, 174. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic Coatings Based on Siloxane Resin and Calcium Hydroxide Nanoparticles for Marble Protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic Composite Films Produced on Various Substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Niskanen, I.; Forsberg, V.; Zakrisson, D.; Reza, S.; Hummelgård, M.; Andres, B.; Fedorov, I.; Suopajärvi, T.; Liimatainen, H.; Thungström, G. Determination of nanoparticle size using Rayleigh approximation and Mie theory. Chem. Eng. Sci. 2019, 201, 222–229. [Google Scholar] [CrossRef]
- Hříbalová, S.; Pabst, W. Light scattering in monodisperse systems–from suspensions to transparent ceramics. J. Eur. Ceram. Soc. 2020, 40, 1522–1531. [Google Scholar] [CrossRef]
- León, J.M.; Pura, J.L.; Bernardo, V.; Reza, S.; Rodríguez-Pérez, M.Á. Transparent nanocellular PMMA: Characterization and modeling of the optical properties. Polymer 2019, 170, 16–23. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Xin, J. Can superhydrophobic surfaces repel hot water? J. Mater. Chem. 2009, 31, 5457–5668. [Google Scholar] [CrossRef]
- Lyu, J.; Wu, B.; Wu, N.; Peng, C.; Yang, J.; Meng, Y.; Xing, S. Green preparation of transparent superhydrophobic coatings with persistent dynamic impact resistance for outdoor applications. Chem. Eng. J. 2021, 15, 126456. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Xu, J.; Wu, Q.; Li, D.; Chen, G.; He, M.; Tian, J. Fabrication of transparent and superhydrophobic nanopaper via coating hybrid SiO2/MWCNTs composite. Carbohyd. Polym. 2019, 1, 115229. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, Z.; Li, B.; Feng, X.; Jiao, Z.; Zhang, J.; Zhao, J.; Niu, S.; Ren, L. Flexible Self-Cleaning Broadband Antireflective Film Inspired by the Transparent Cicada Wings. ACS Appl. Mater. Interfaces 2019, 11, 17019–17027. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Shieh, J. Growing invisible silica nanowires on fused silica plates provides highly transparent and superwetting substrates. Appl. Surf. Sci. 2019, 15, 619–625. [Google Scholar] [CrossRef]
- Yokoi, N.; Manabe, K.; Tenjimbayashi, M.; Shiratori, S. Optically Transparent Superhydrophobic Surfaces with Enhanced Mechanical Abrasion Resistance Enabled by Mesh Structure. ACS Appl. Mater. Interfaces 2015, 8, 4809–4816. [Google Scholar] [CrossRef]
- Gurav, A.B.; Shi, H.; Duan, M.; Pang, X.; Li, X. Highly transparent, hot water and scratch resistant, lubricant-infused slippery surfaces developed from a mechanically-weak superhydrophobic coating. Chem. Eng. J. 2021, 15, 127809. [Google Scholar] [CrossRef]
- Lin, Y.; Han, J.; Cai, M.; Liu, W.; Luo, X.; Zhang, H.; Zhong, M. Durable and robust transparent superhydrophobic glass surfaces fabricated by a femtosecond laser with exceptional water repellency and thermostability. J. Mater. Chem. A 2018, 6, 9049–9056. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Lyongs, A.M. Durable, optically transparent, superhydrophobic polymer films. Appl. Surf. Sci. 2019, 15, 187–195. [Google Scholar] [CrossRef]




| Materials | Methods | CAs (°) | Transmittance (%) | Stability |
|---|---|---|---|---|
| Modified SiO2 [33] | Self-assembly process | 161.5 | 83.13 | Excellent sand, water jet and corrosive media resistance |
| Fluorinated silica multi-walled carbon nanotubes [34] | Spray-drying | 159 | 75 | Low |
| SiO2, PMMA [35] | Sol–gel and Template-based method | 152 | 93 | Low |
| Silica nanowires [36] | CVD | 158 | 89 | Displayed antifogging properties |
| Silica nanoparticles, FAS [37] | Alkaline etching | 150 | 79 | Exhibited a good resistance to acidic and basic solutions over a wide range of pH values |
| Silicone oils, SiO2 [38] | LISS | 110 | - | Highly hot water, pH liquids and scratch resistance |
| Silica glass [39] | Femtosecond laser and modify | 150 | 92 | Excellent sand and water jet resistance |
| SiO2 and COP [40] | Dip-coating | 150 | 87 | Excellent sand and water jet resistance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liang, H.; Song, J.; Guo, C.; Tang, J. Preparation of Transparent Sandwich-like Superhydrophobic Coating on Glass with High Stability and Self-Cleaning Properties. Coatings 2022, 12, 228. https://doi.org/10.3390/coatings12020228
Li Q, Liang H, Song J, Guo C, Tang J. Preparation of Transparent Sandwich-like Superhydrophobic Coating on Glass with High Stability and Self-Cleaning Properties. Coatings. 2022; 12(2):228. https://doi.org/10.3390/coatings12020228
Chicago/Turabian StyleLi, Qiang, Hongming Liang, Jinlong Song, Chenguang Guo, and Jinbao Tang. 2022. "Preparation of Transparent Sandwich-like Superhydrophobic Coating on Glass with High Stability and Self-Cleaning Properties" Coatings 12, no. 2: 228. https://doi.org/10.3390/coatings12020228
APA StyleLi, Q., Liang, H., Song, J., Guo, C., & Tang, J. (2022). Preparation of Transparent Sandwich-like Superhydrophobic Coating on Glass with High Stability and Self-Cleaning Properties. Coatings, 12(2), 228. https://doi.org/10.3390/coatings12020228







