Synthesis of ZnO/Bi2S3 Core/Shell Nanowire Array Photoanodes for Photocathodic Protection of Stainless Steel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photoanode Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
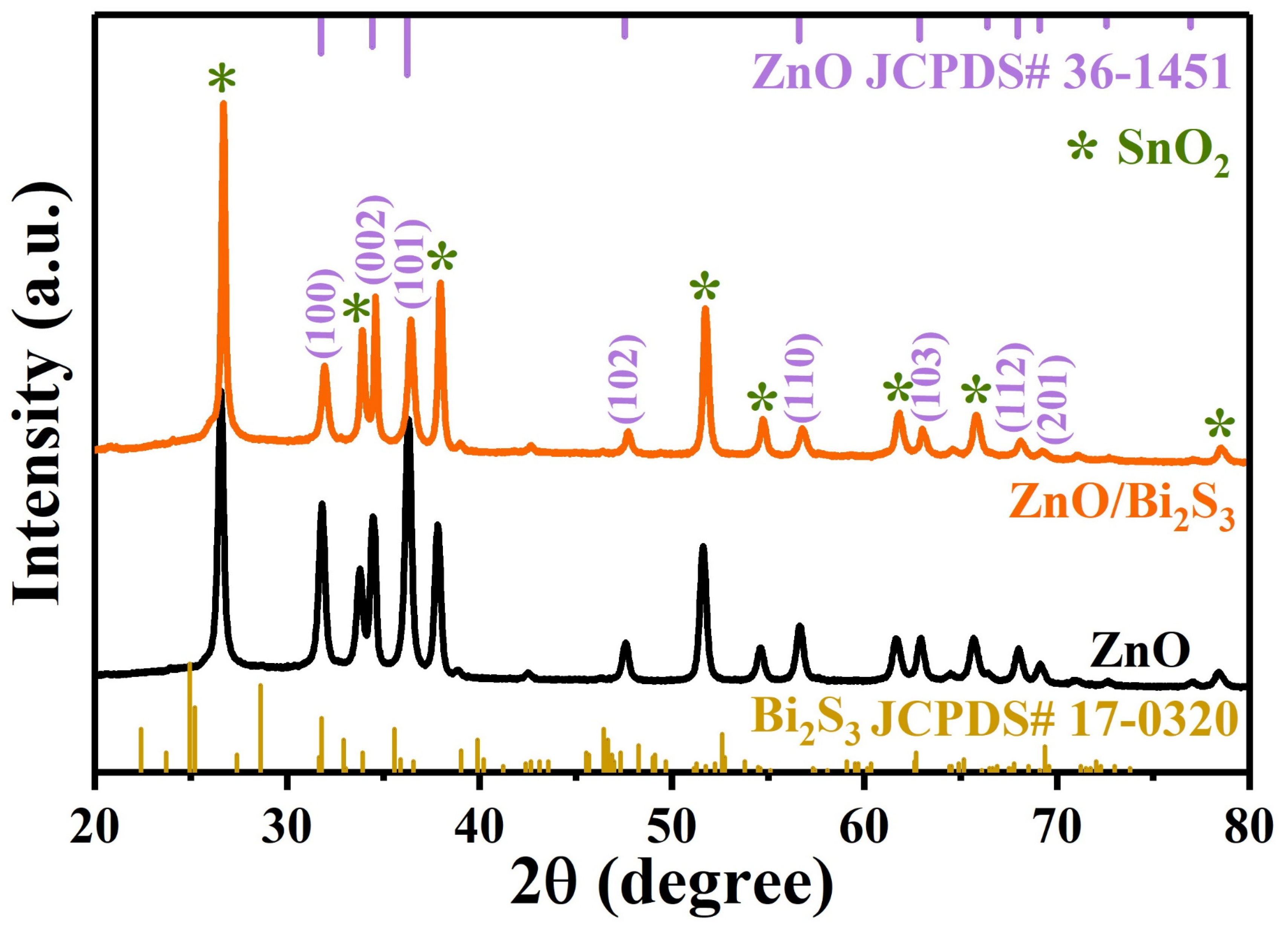
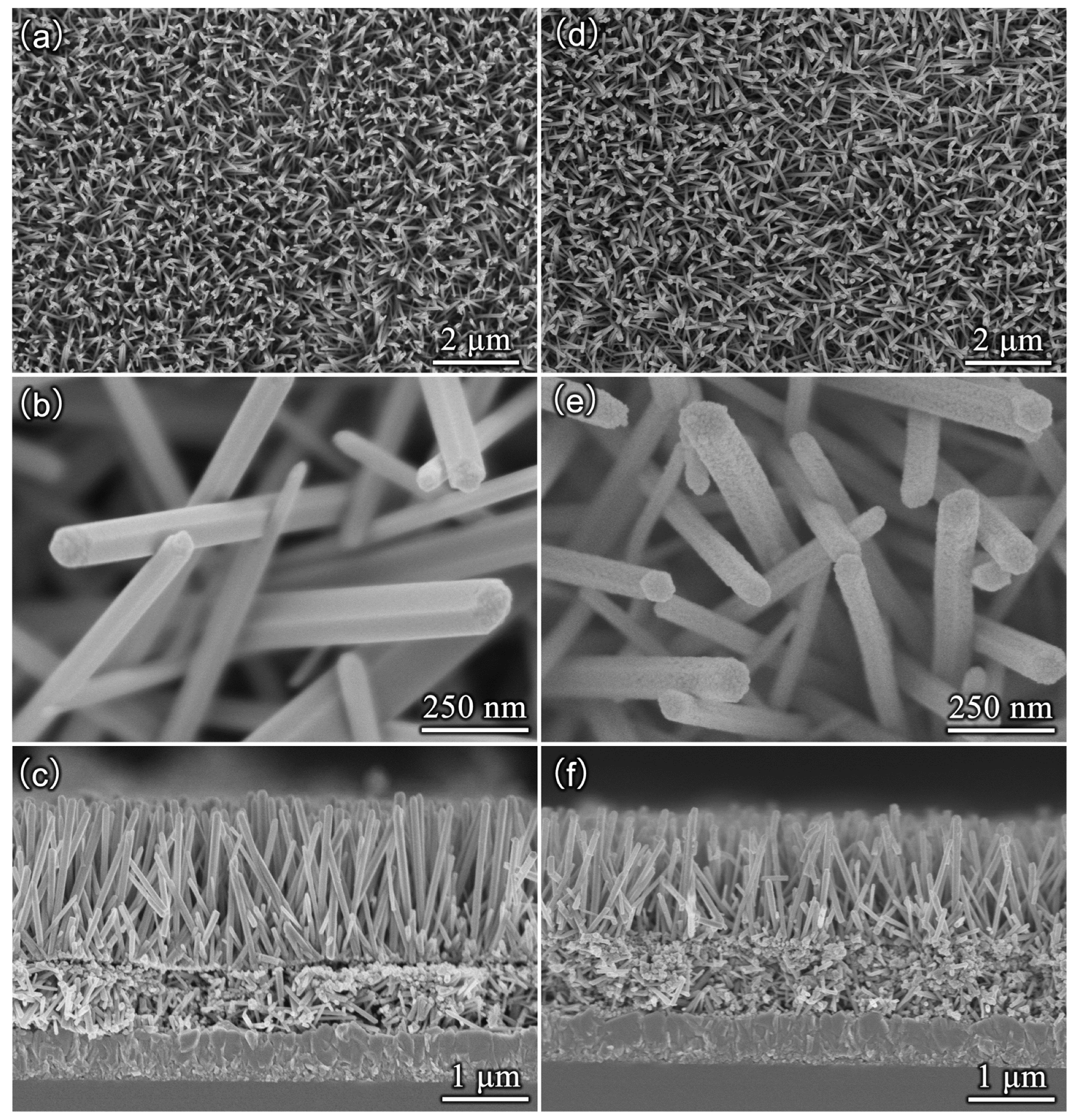
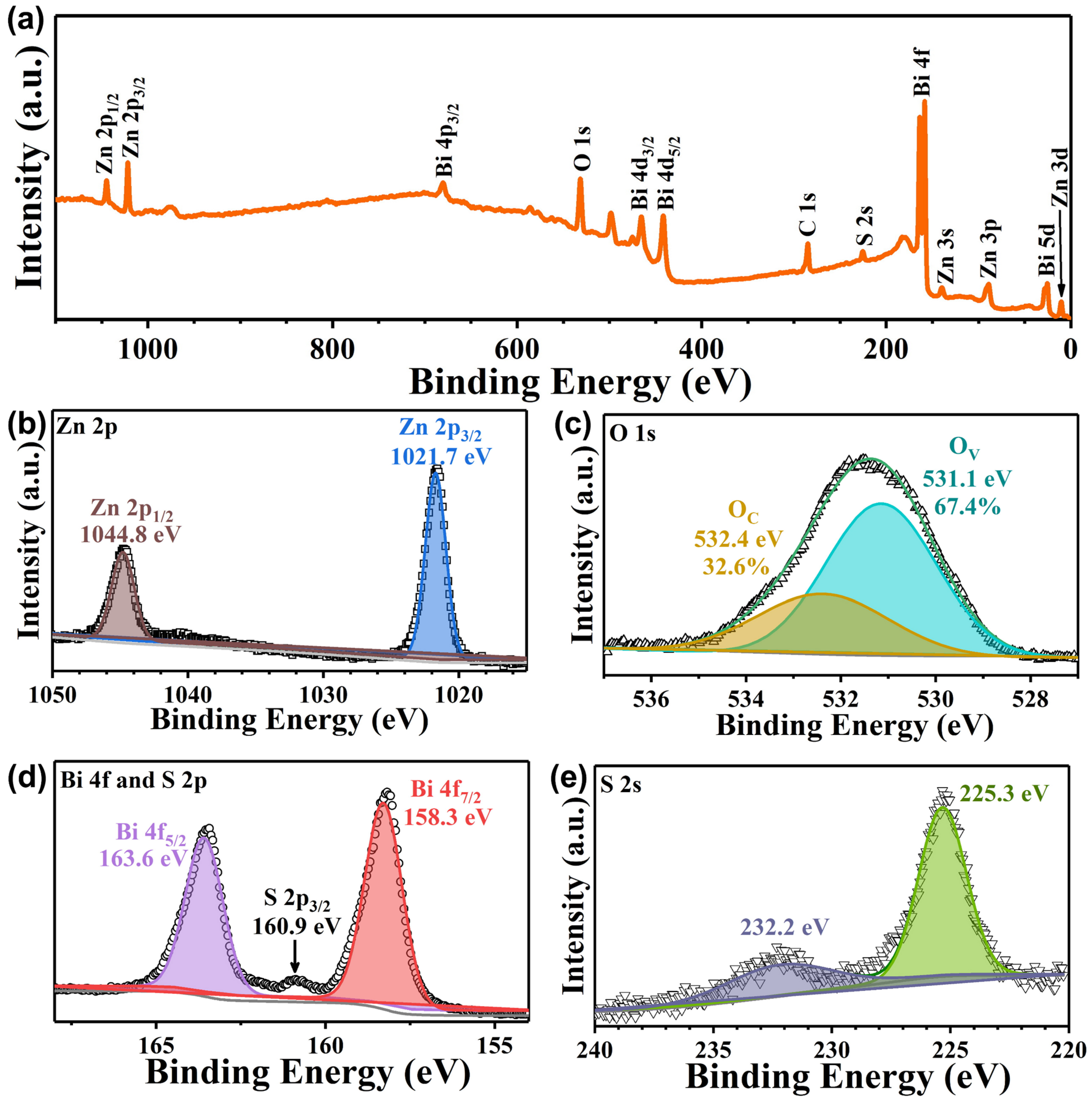
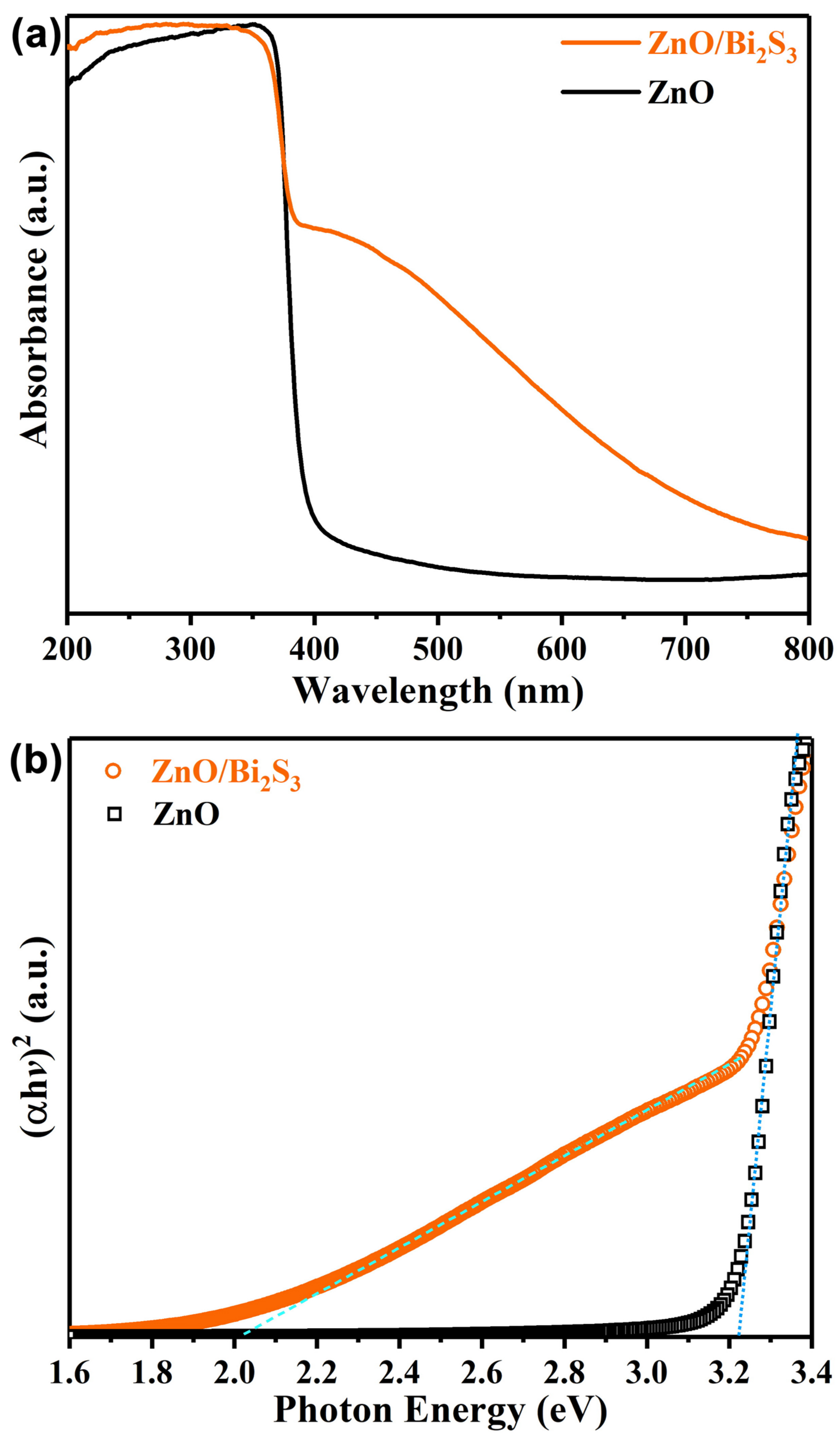
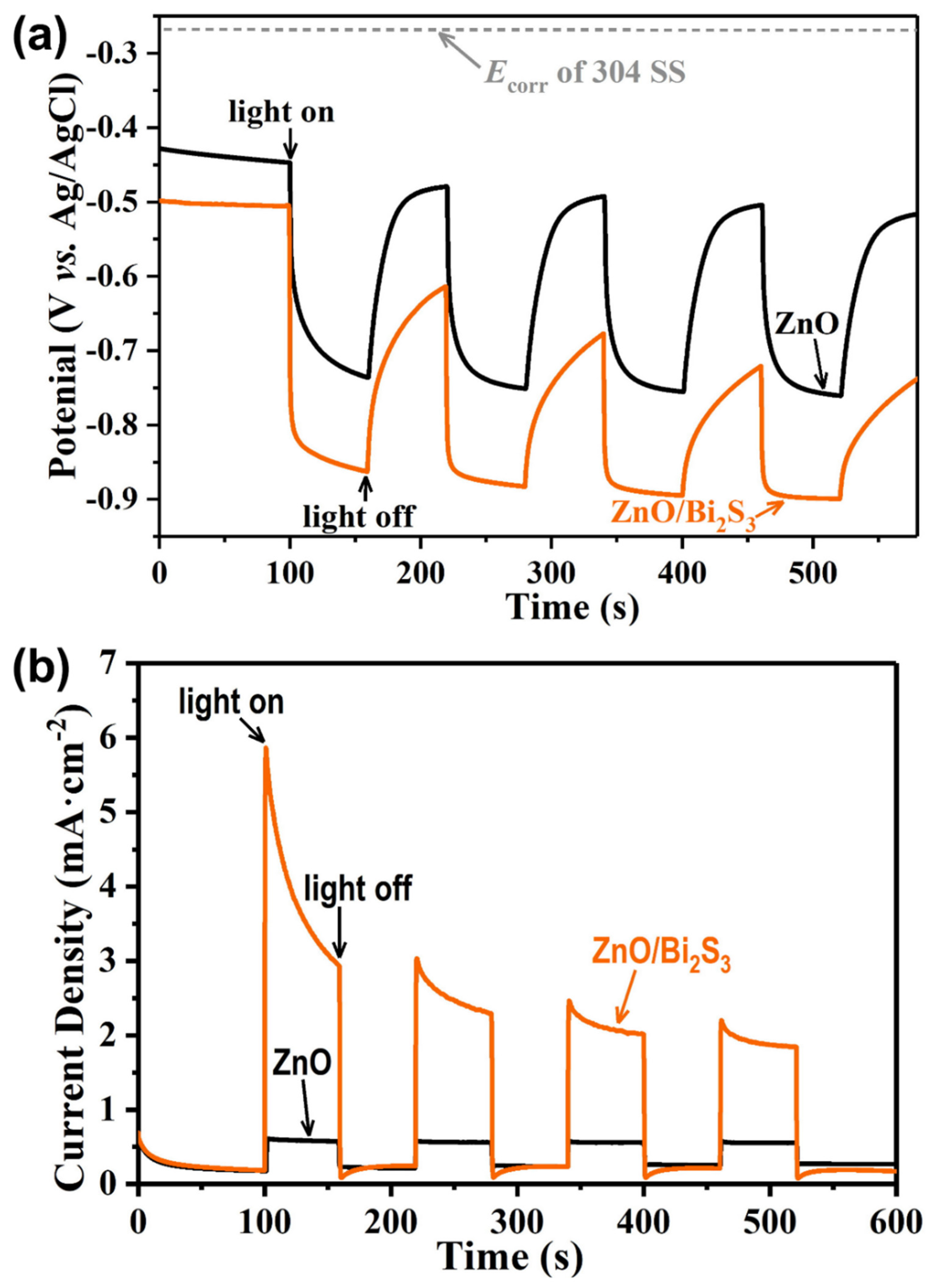
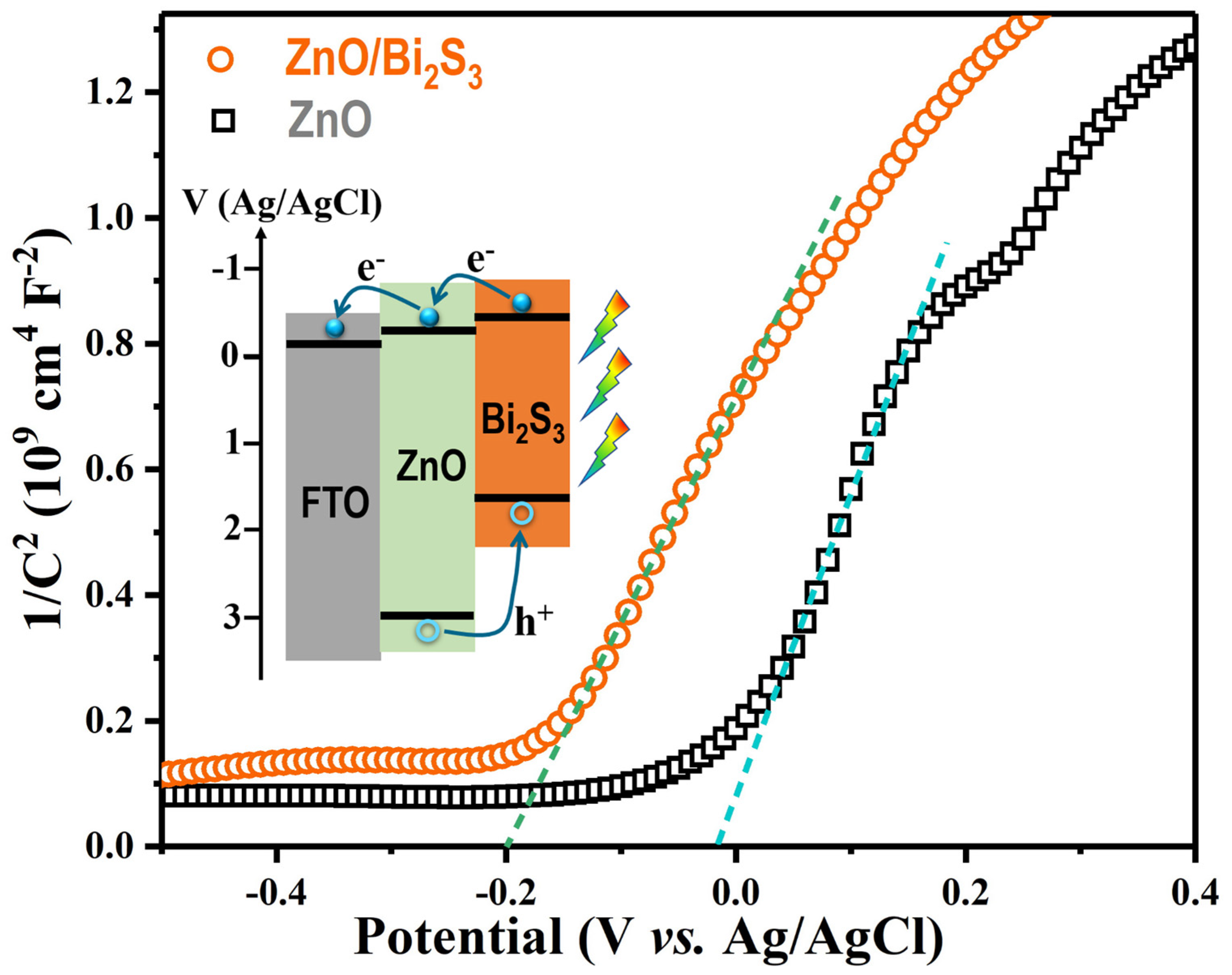
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mat. Sci. Eng. R 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Z.; Lu, D.; Jiang, Q.; Li, L.; Liao, T.; Hou, B. Enhanced photoelectrochemical cathodic protection performance of MoS2/TiO2 nanocomposites for 304 stainless steel under visible light. J. Mater. Sci. Technol. 2021, 64, 21–28. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Wang, Y.; Cui, X.; Wen, Z.; Liu, Y.; Fan, L.; Feng, J. Facile fabrication of SnO2 modified TiO2 nanorods film for efficient photocathodic protection of 304 stainless steel under simulated solar light. Corros. Sci. 2020, 176, 108927. [Google Scholar] [CrossRef]
- Bu, Y.; Ao, J.-P. A review on photoelectrochemical cathodic protection semiconductor thin films for metals. Green Energy Environ. 2017, 2, 331–362. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Liu, M.; Ge, C.; Hou, B.; Liu, N.; Ning, Y.; Hu, Y. Preparation of FeS2/TiO2 nanocomposite films and study on the performance of photoelectrochemistry cathodic protection. Sci. Rep. 2021, 11, 7509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gao, W.; Liu, N.; Xin, Y.; Liu, X.; Wang, X.; Tian, Y.; Chen, X.; Hou, B. Covalent organic framework decorated TiO2 nanotube arrays for photoelectrochemical cathodic protection of steel. Corros. Sci. 2020, 176, 108920. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Visible light illuminated high-performance WO3-TiO2-BiVO4 nanocomposite photoanodes capable of energy self-storage for photo-induced cathodic protection. Corros. Sci. 2020, 164, 108333. [Google Scholar] [CrossRef]
- Wei, N.; Lin, Y.; Li, Z.; Sun, W.; Zhang, G.; Wang, M.; Cui, H. One-dimensional Ag2S/ZnS/ZnO nanorod array films for photocathodic protection for 304 stainless steel. J. Mater. Sci. Technol. 2020, 42, 156–162. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, M.; Chen, Z.; Jing, J.; Feng, C. High-efficiency photoelectrochemical cathodic protection performance of the TiO2/AgInSe2/In2Se3 multijunction nanosheet array. Corros. Sci. 2020, 176, 108901. [Google Scholar] [CrossRef]
- Sun, W.; Wei, N.; Cui, H.; Lin, Y.; Wang, X.; Tian, J.; Li, J.; Wen, J. 3D ZnIn2S4 nanosheet/TiO2 nanowire arrays and their efficient photocathodic protection for 304 stainless steel. Appl. Surf. Sci. 2018, 434, 1030–1039. [Google Scholar] [CrossRef]
- Jin, P.; Guan, Z.-C.; Wang, H.-P.; Wang, X.; Liu, G.-K.; Du, R.-G. Bi2S3/rGO co-modified TiO2 nanotube photoanode for enhanced photoelectrochemical cathodic protection of stainless steel. J. Photochem. Photobiol. A 2021, 407, 113060. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, M.; Chen, Z.; Jing, J.; Lu, G.; Feng, C. Boosted photoinduced cathodic protection performance of ZnIn2S4/TiO2 nanoflowerbush with efficient photoelectric conversion in NaCl solution. J. Alloys Compd. 2021, 876, 160144. [Google Scholar] [CrossRef]
- Xu, D.; Yang, M.; Liu, Y.; Zhu, R.; Lv, X.; Zhang, C.; Liu, B. Fabrication of an innovative designed TiO2 nanosheets/CdSe/polyaniline/graphene quaternary composite and its application as in-situ photocathodic protection coatings on 304SS. J. Alloys Compd. 2020, 822, 153685. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, X.; Liu, N.; Wang, X.; Wang, L.; Hou, B. Study on the photocathodic protection of 304 stainless steel by Ag and In2S3 co-sensitized TiO2 composite. Appl. Surf. Sci. 2020, 507, 145088. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, M.; Chen, Z.; Jing, J.; Feng, C. An ultrafine hyperbranched CdS/TiO2 nanolawn photoanode with highly efficient photoelectrochemical performance. J. Alloys Compd. 2020, 816, 152533. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Wang, X.; Hou, B. 3D ZnIn2S4 nanosheets/TiO2 nanotubes as photoanodes for photocathodic protection of Q235 CS with high efficiency under visible light. J. Alloys Compd. 2019, 771, 892–899. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Ning, X.; Lei, J.; Shao, J.; Wang, W.; Huang, Y.; Hou, B. Sb2S3/Sb2O3 modified TiO2 photoanode for photocathodic protection of 304 stainless steel under visible light. Appl. Surf. Sci. 2018, 462, 155–163. [Google Scholar] [CrossRef]
- Hu, J.; Guan, Z.-C.; Liang, Y.; Zhou, J.-Z.; Liu, Q.; Wang, H.-P.; Zhang, H.; Du, R.-G. Bi2S3 modified single crystalline rutile TiO2 nanorod array films for photoelectrochemical cathodic protection. Corros. Sci. 2017, 125, 59–67. [Google Scholar] [CrossRef]
- Kale, V.; Hunge, Y.M.; Kamble, S.A.; Deshmukh, M.; Bhoraskar, S.V.; Mathe, V.L. Modification of energy level diagram of nano-crystalline ZnO by its composites with ZnWO4 suitable for sunlight assisted photo catalytic activity. Mater. Today Commun. 2021, 26, 102101. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Mathe, V.L. Ultrasound assisted synthesis of WO3-ZnO nanocomposites for brilliant blue dye degradation. Ultrason. Sonochem. 2018, 45, 116–122. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sensors Actuat. B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. One-step facile preparation of ZnO nanorods as high-performance photoanodes for photoelectrochemical cathodic protection. Electrochim. Acta 2018, 276, 311–318. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Z.; Cheng, Y. An in-depth study of photocathodic protection of SS304 steel by electrodeposited layers of ZnO nanoparticles. Surf. Coat. Technol. 2020, 399, 126158. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S. Robust ZnO nanowire photoanodes with oxygen vacancies for efficient photoelectrochemical cathodic protection. Appl. Surf. Sci. 2021, 566, 150694. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z.; Bu, Y.; Yu, J.; Hou, B. Effect of ZnO on the corrosion of zinc, Q235 carbon steel and 304 stainless steel under white light illumination. Corros. Sci. 2014, 82, 77–84. [Google Scholar] [CrossRef]
- Techapiesancharoenkij, R.; Sripianem, W.; Tongpul, K.; Peamjharean, C.; Wichean, T.N.; Meesak, T.; Eiamchai, P. Investigation of the photocathodic protection of a transparent ZnO coating on an AISI type 304 stainless steel in a 3% NaCl solution. Surf. Coat. Technol. 2017, 320, 97–102. [Google Scholar] [CrossRef]
- Wang, H.; Xie, J.; Jiang, X.; Zhu, Z.; Wang, Y.; Chamas, M.; Duan, M.; Tang, J. Photoelectrochemical Behavior of Microrod ZnO Coated 316L Stainless Steel Prepared by Hydrothermal Method. Int. J. Electrochem. Sc. 2016, 11, 10278–10286. [Google Scholar] [CrossRef]
- Das Mahapatra, A.; Basak, D. Enhanced ultraviolet photosensing properties in Bi2S3 nanoparticles decorated ZnO nanorods’ heterostructure. J. Alloys Compd. 2019, 797, 766–774. [Google Scholar] [CrossRef]
- Xitao, W.; Rong, L.; Kang, W. Synthesis of ZnO@ZnS-Bi2S3 core-shell nanorod grown on reduced graphene oxide sheets and its enhanced photocatalytic performance. J. Mater. Chem. A 2014, 2, 8304–8313. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B-Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B-Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2 reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-scheme 2D/3D g-C3N4@ZnO with enhanced photocatalytic activity for cephalexin oxidation under solar light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Wang, D.; Han, D.; Shi, Z.; Wang, J.; Yang, J.; Li, X.; Song, H. Optimized design of three-dimensional multi-shell Fe3O4/SiO2/ZnO/ZnSe microspheres with type II heterostructure for photocatalytic applications. Appl. Catal. B Environ. 2018, 227, 61–69. [Google Scholar] [CrossRef]
- Jiang, Y.; Liao, J.-F.; Xu, Y.-F.; Chen, H.-Y.; Wang, X.-D.; Kuang, D.-B. Hierarchical CsPbBr3 nanocrystal-decorated ZnO nanowire/macroporous graphene hybrids for enhancing charge separation and photocatalytic CO2 reduction. J. Mater. Chem. A 2019, 7, 13762–13769. [Google Scholar] [CrossRef]
- Li, M.; Tu, X.; Wang, Y.; Su, Y.; Hu, J.; Cai, B.; Lu, J.; Yang, Z.; Zhang, Y. Highly Enhanced Visible-Light-Driven Photoelectrochemical Performance of ZnO-Modified In2S3 Nanosheet Arrays by Atomic Layer Deposition. Nano-Micro Lett. 2018, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Sadhasivam, S.; Anbarasan, N.; Mukilan, M.; Manivel, P.; Jeganathan, K. Bi2S3 anchored ZnS/ZnO nanorod arrays photoanode for enhanced visible light driven photo electrochemical properties. Int. J. Hydrogen Energy 2020, 45, 30080–30090. [Google Scholar] [CrossRef]
- Sun, X.; Wu, H.; Tang, Y.; Zheng, Z.; Li, Y.; Li, L.; Zhang, Q. Enhanced photoelectrocatalytic degradation of tetrabromobisphenol a from tip-decorated ZnO nanorod electrode with Bi2S3 nanoparticles. Mater. Sci. Semicond. Process. 2021, 128, 105724. [Google Scholar] [CrossRef]
- Chen, P.; Gu, L.; Cao, X. From single ZnO multipods to heterostructured ZnO/ZnS, ZnO/ZnSe, ZnO/Bi2S3 and ZnO/Cu2S multipods: Controlled synthesis and tunable optical and photoelectrochemical properties. CrystEngComm 2010, 12, 3950–3958. [Google Scholar] [CrossRef]
- Balachandran, S.; Swaminathan, M. The simple, template free synthesis of a Bi2S3-ZnO heterostructure and its superior photocatalytic activity under UV-A light. Dalton T 2013, 42, 5338–5347. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shi, R.; Ma, Y.; Cai, F.; Wang, X.; Yuan, Z. Enhanced thermoelectric performance of n-type Bi2S3 with added ZnO for power generation. RSC Adv. 2015, 5, 31004–31009. [Google Scholar] [CrossRef]
- Bera, S.; Ghosh, S.; Basu, R.N. Fabrication of Bi2S3/ZnO heterostructures: An excellent photocatalyst for visible-light-driven hydrogen generation and photoelectrochemical properties. New J. Chem. 2018, 42, 541–554. [Google Scholar] [CrossRef]
- Velanganni, S.; Manikandan, A.; Prince, J.J.; Mohan, C.N.; Thiruneelakandan, R. Nanostructured ZnO coated Bi2S3 thin films: Enhanced photocatalytic degradation of methylene blue dye. Phys. B 2018, 545, 383–389. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wei, Q.; Hou, B. Photocathodic protection of 304 stainless steel by Bi2S3/TiO2 nanotube films under visible light. Nanoscale Res. Lett. 2017, 12, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bao, H.; Liu, X.; Zhang, X.; He, H.; Li, T.; Yang, H.; Shah, S.P.; Li, W. Bi2S3 nanoparticles/ZnO nanowire heterojunction films for improved photoelectrochemical cathodic protection for 304 SS under visible light. J. Appl. Electrochem. 2022, 52, 559–571. [Google Scholar] [CrossRef]
- Majumder, S.; Quang, N.D.; Kim, C.; Kim, D. Anion exchange and successive ionic layer adsorption and reaction-assisted coating of BiVO4 with Bi2S3 to produce nanostructured photoanode for enhanced photoelectrochemical water splitting. J. Colloid Interface Sci. 2021, 585, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Z.; Jin, R.; Wang, Y.; Gao, S. SILAR preparation of Bi2S3 nanoparticles sensitized TiO2 nanotube arrays for efficient solar cells and photocatalysts. Sep. Purif. Technol. 2019, 210, 798–803. [Google Scholar] [CrossRef]
- Kang, L.; Shi, L.; Zeng, Q.; Liao, B.; Wang, B.; Guo, X. Melamine resin-coated lignocellulose fibers with robust superhydrophobicity for highly effective oil/water separation. Sep. Purif. Technol. 2021, 279, 119737. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Zhang, Y.; Shi, Z.; Yang, M.; Zhang, C.; Liu, B. Fabrication of pyramid-BiVO4/CdSe composite with controlled surface oxygen vacancies boosting efficient carriers’ separation for photocathodic protection. Chem. Eng. J. 2020, 393, 124693. [Google Scholar] [CrossRef]
- Zingg, D.S.; Hercules, D.M. Electron spectroscopy for chemical analysis studies of lead sulfide oxidation. J. Phys. Chem. 1978, 82, 1992–1995. [Google Scholar] [CrossRef]
- Malakooti, R.; Cademartiri, L.; Akçakir, Y.; Petrov, S.; Migliori, A.; Ozin, G.A. Shape-controlled Bi2S3 nanocrystals and their plasma polymerization into flexible films. Adv. Mater. 2006, 18, 2189–2194. [Google Scholar] [CrossRef]
- Jing, J.; Chen, Z.; Bu, Y.; Sun, M.; Zheng, W.; Li, W. Significantly enhanced photoelectrochemical cathodic protection performance of hydrogen treated Cr-doped SrTiO3 by Cr6+ reduction and oxygen vacancy modification. Electrochim. Acta 2019, 304, 386–395. [Google Scholar] [CrossRef]
- Kuang, S.; Zheng, W.; Gu, Y.; Sun, Z.; Yang, Z.; Li, W.; Feng, C. Dual-functional ZnxMg1-xO solid solution nanolayer modified ZnO tussock-like nanorods with improved photoelectrochemical anti-corrosion performance. J. Electroanal. Chem. 2018, 815, 175–182. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Liu, S. Synthesis of ZnO/Bi2S3 Core/Shell Nanowire Array Photoanodes for Photocathodic Protection of Stainless Steel. Coatings 2022, 12, 244. https://doi.org/10.3390/coatings12020244
Lin Y, Liu S. Synthesis of ZnO/Bi2S3 Core/Shell Nanowire Array Photoanodes for Photocathodic Protection of Stainless Steel. Coatings. 2022; 12(2):244. https://doi.org/10.3390/coatings12020244
Chicago/Turabian StyleLin, Yichang, and Sheng Liu. 2022. "Synthesis of ZnO/Bi2S3 Core/Shell Nanowire Array Photoanodes for Photocathodic Protection of Stainless Steel" Coatings 12, no. 2: 244. https://doi.org/10.3390/coatings12020244





