Surface and Tribological Properties of Oxide Films on Aluminium Alloy through Fly-Ash Reinforcement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Hard Anodizing Process
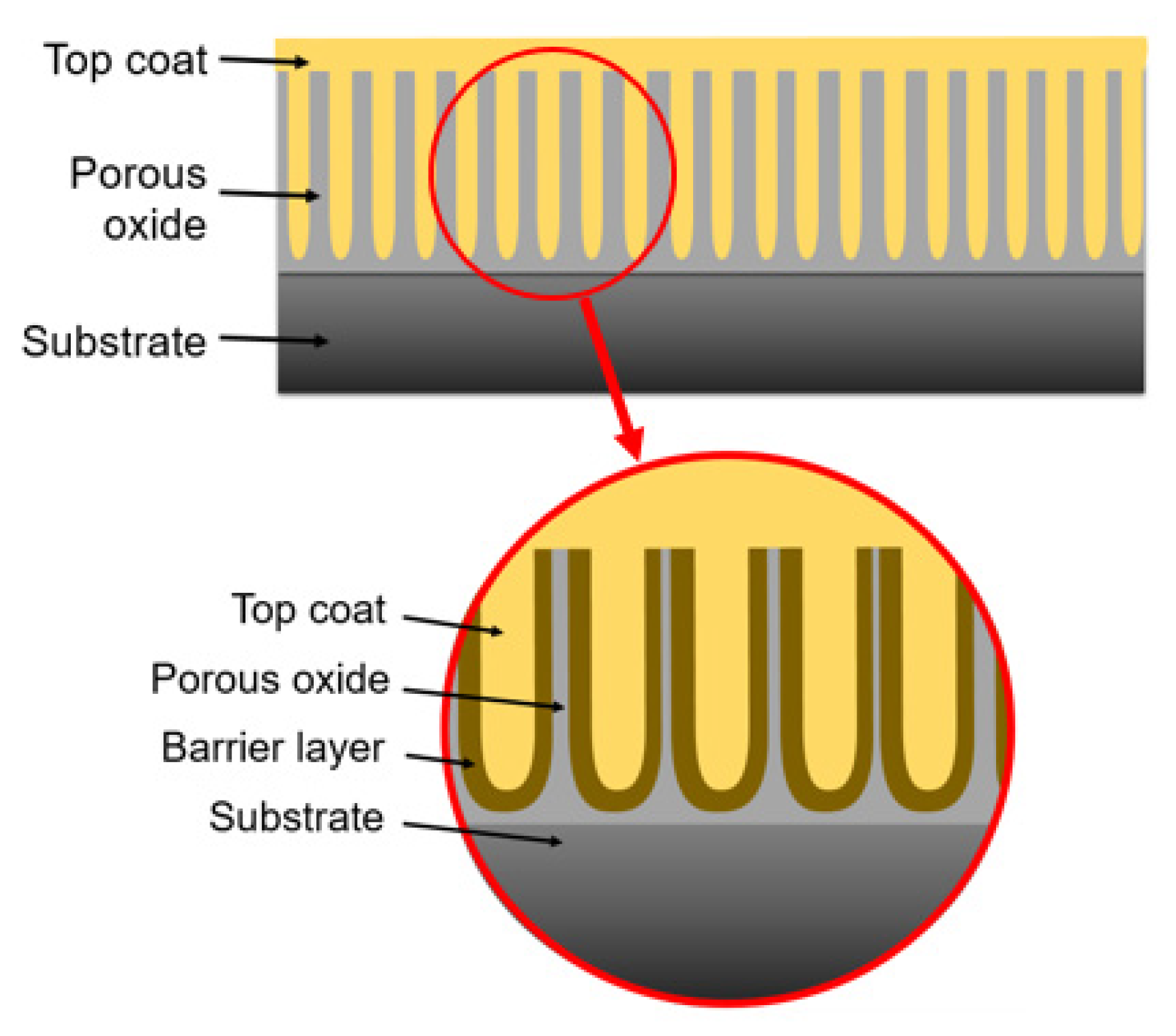
2.3. Hard Oxide Coating Characterization
3. Results and Discussion
3.1. Effect of Anodizing Time
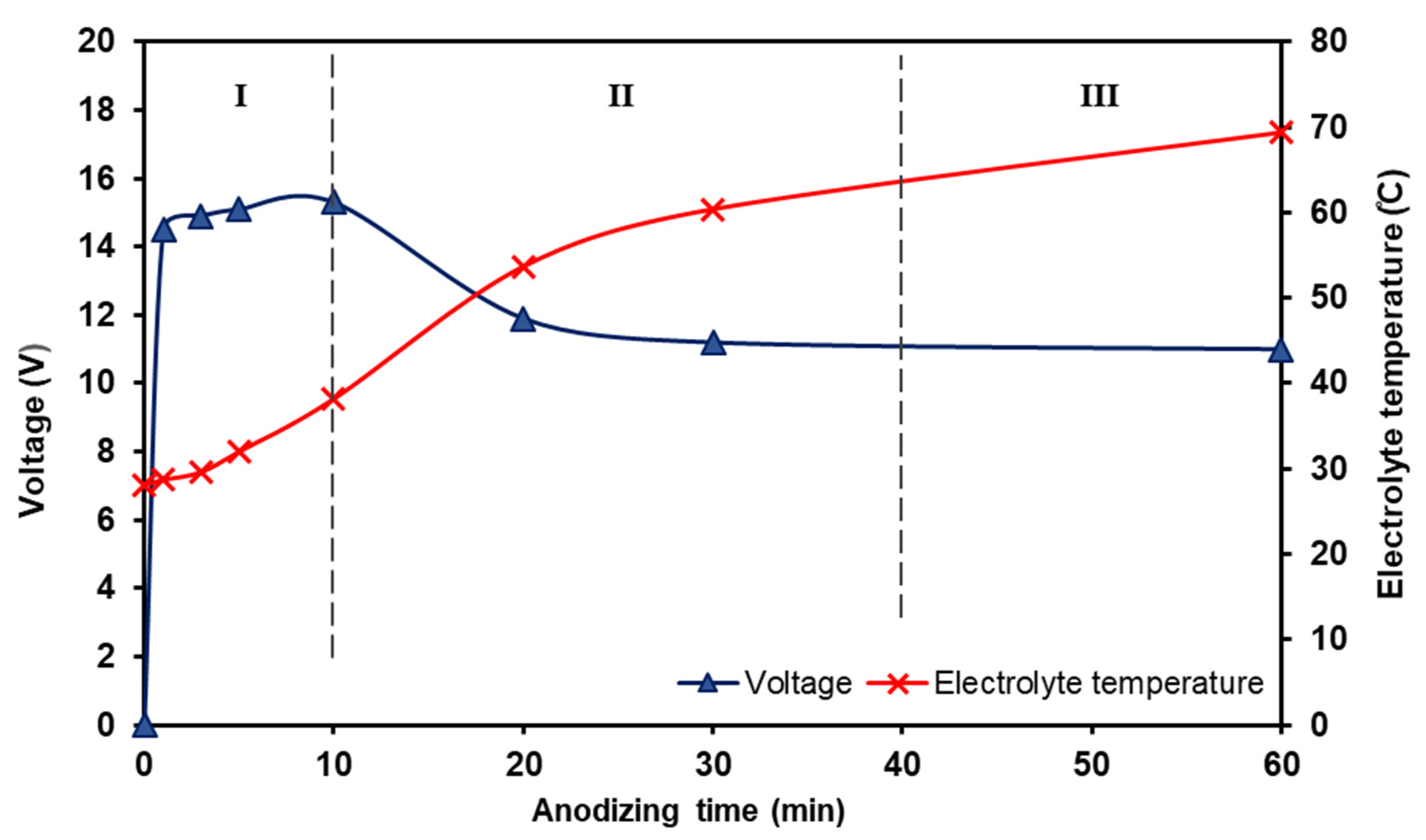
3.1.1. The effect of Anodizing Time on Voltage Behaviour and Growth Mechanism
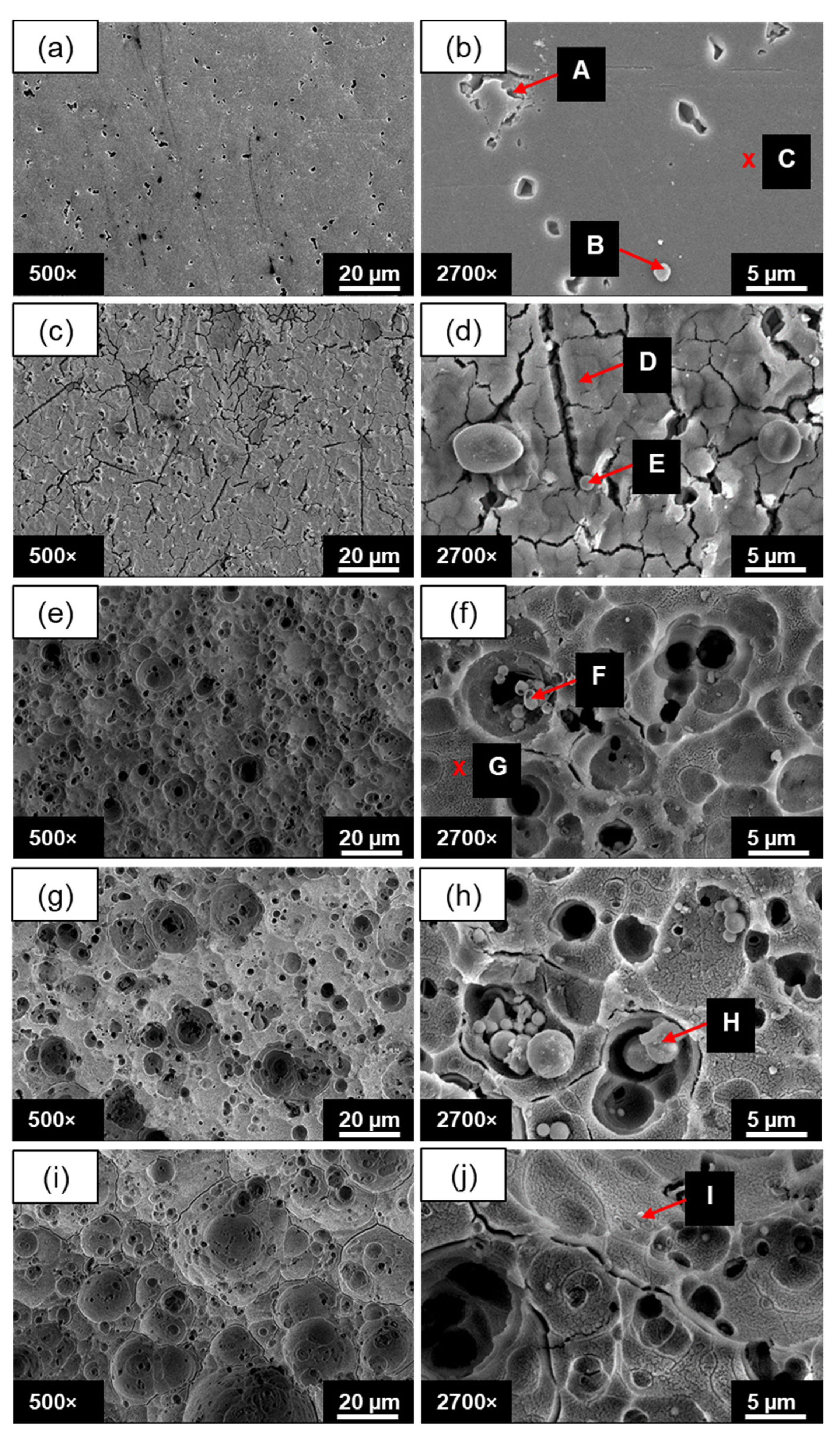
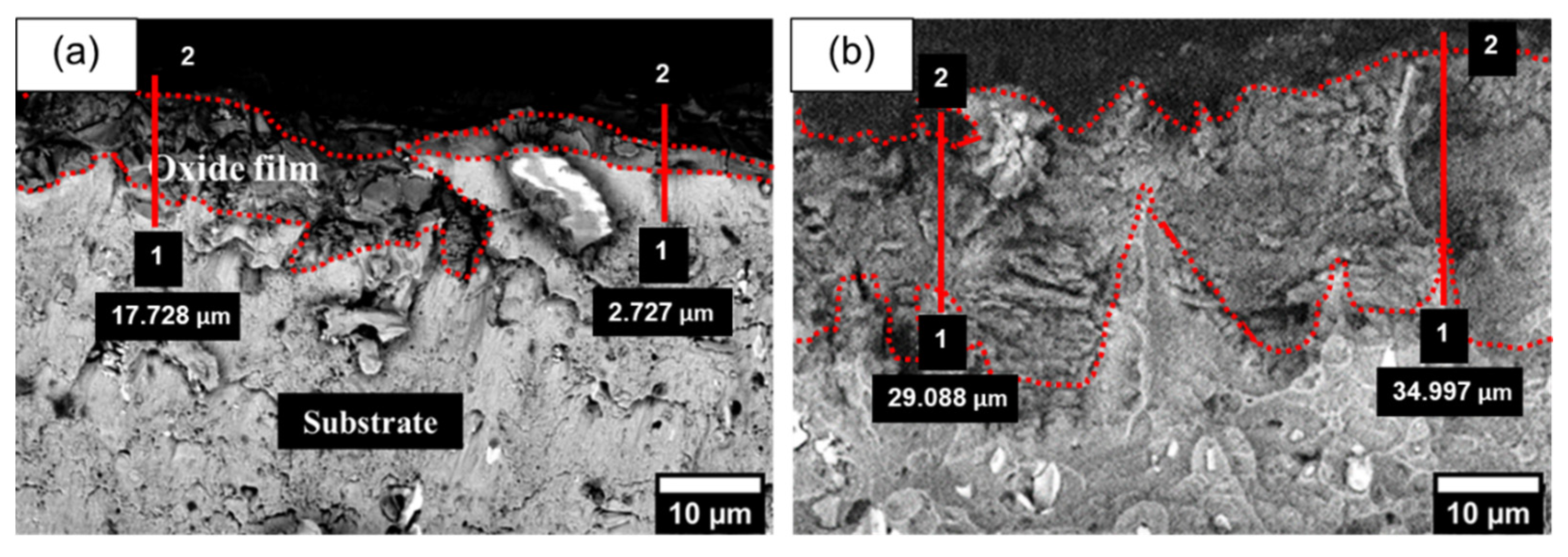
3.1.2. The Effect of Anodizing Time on Surface Morphology
3.1.3. The Effect of Anodizing Time on Surface Topographies
3.2. Effect of Fly-Ash Content
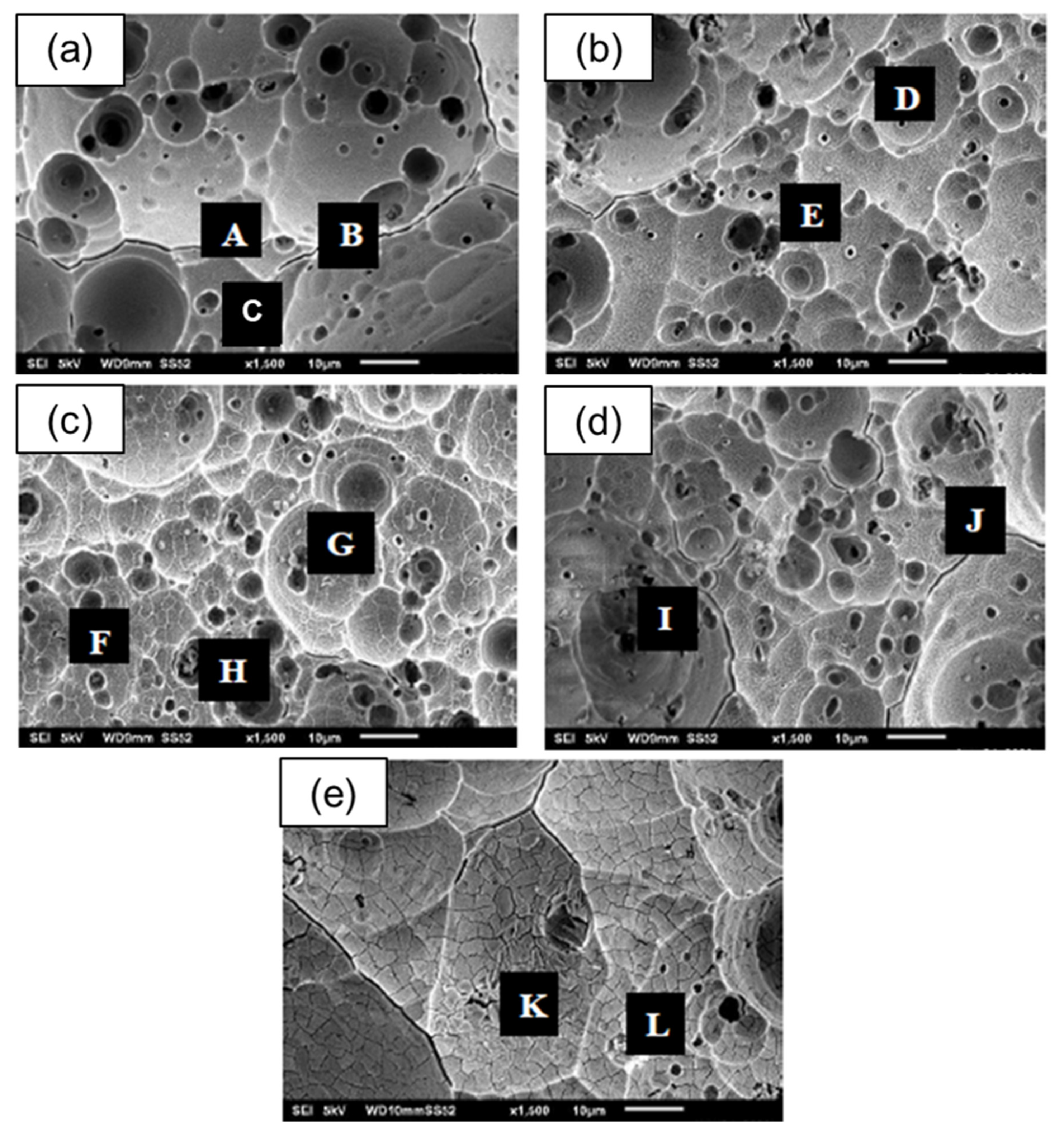
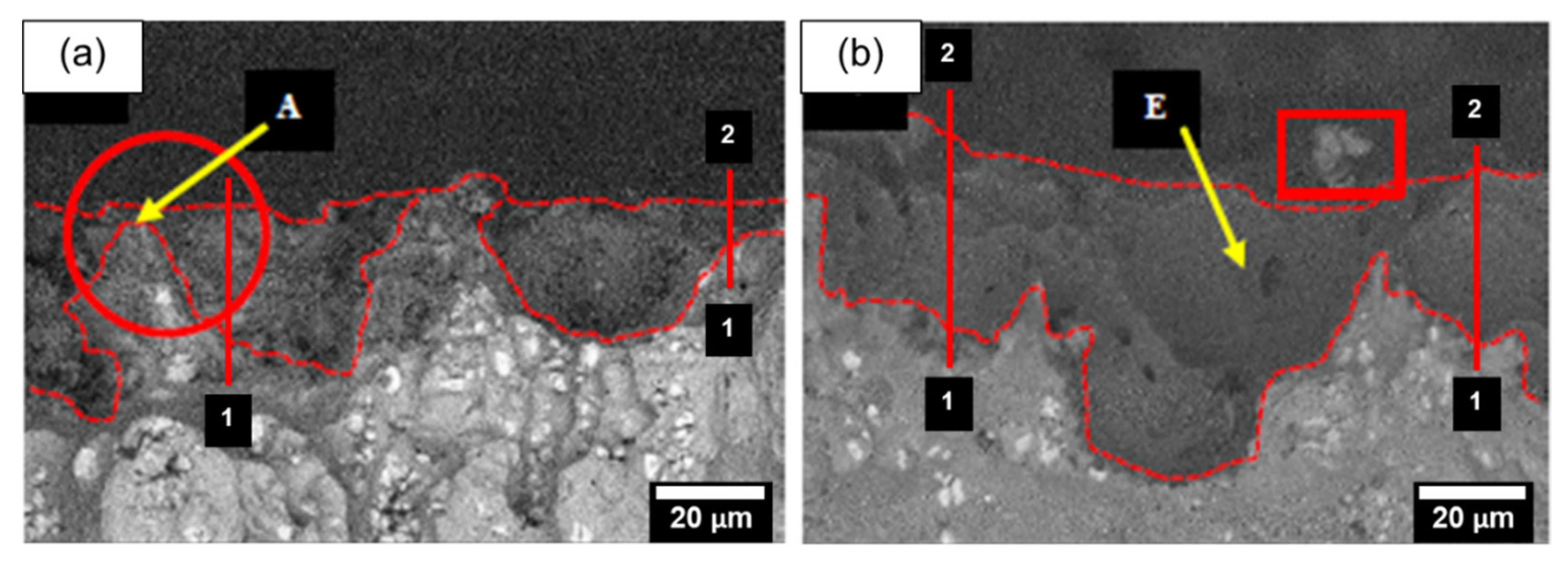
3.2.1. The Effect of Fly-Ash Content on Surface Morphology
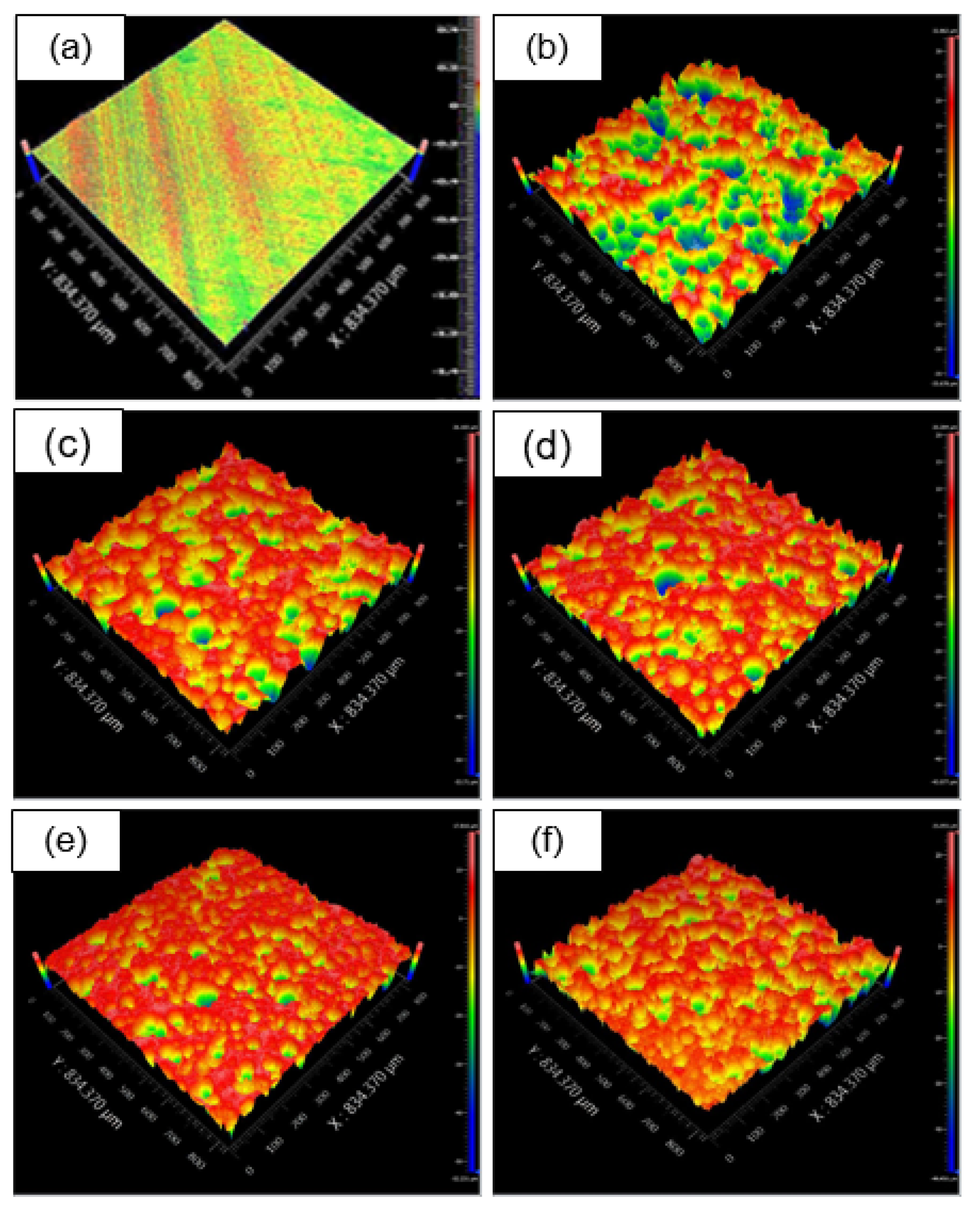
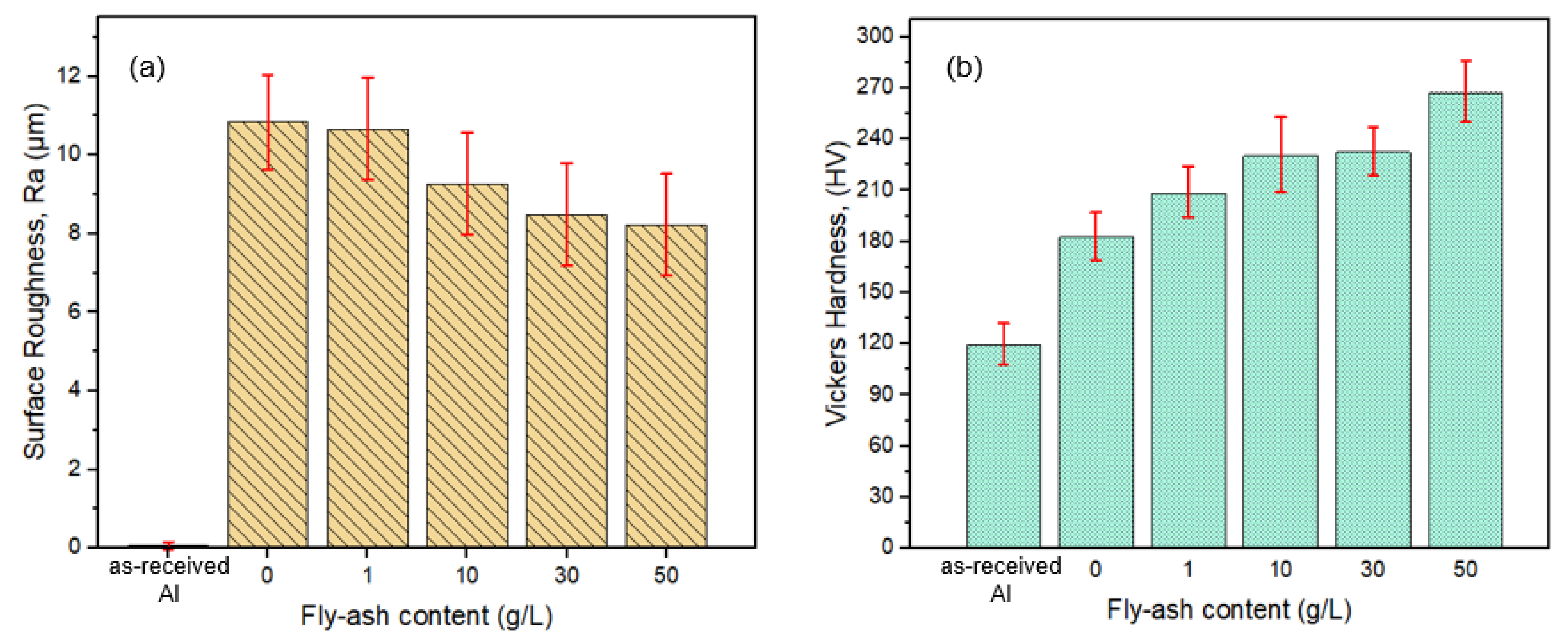
3.2.2. The Effect of Fly-Ash Content on Surface Topography
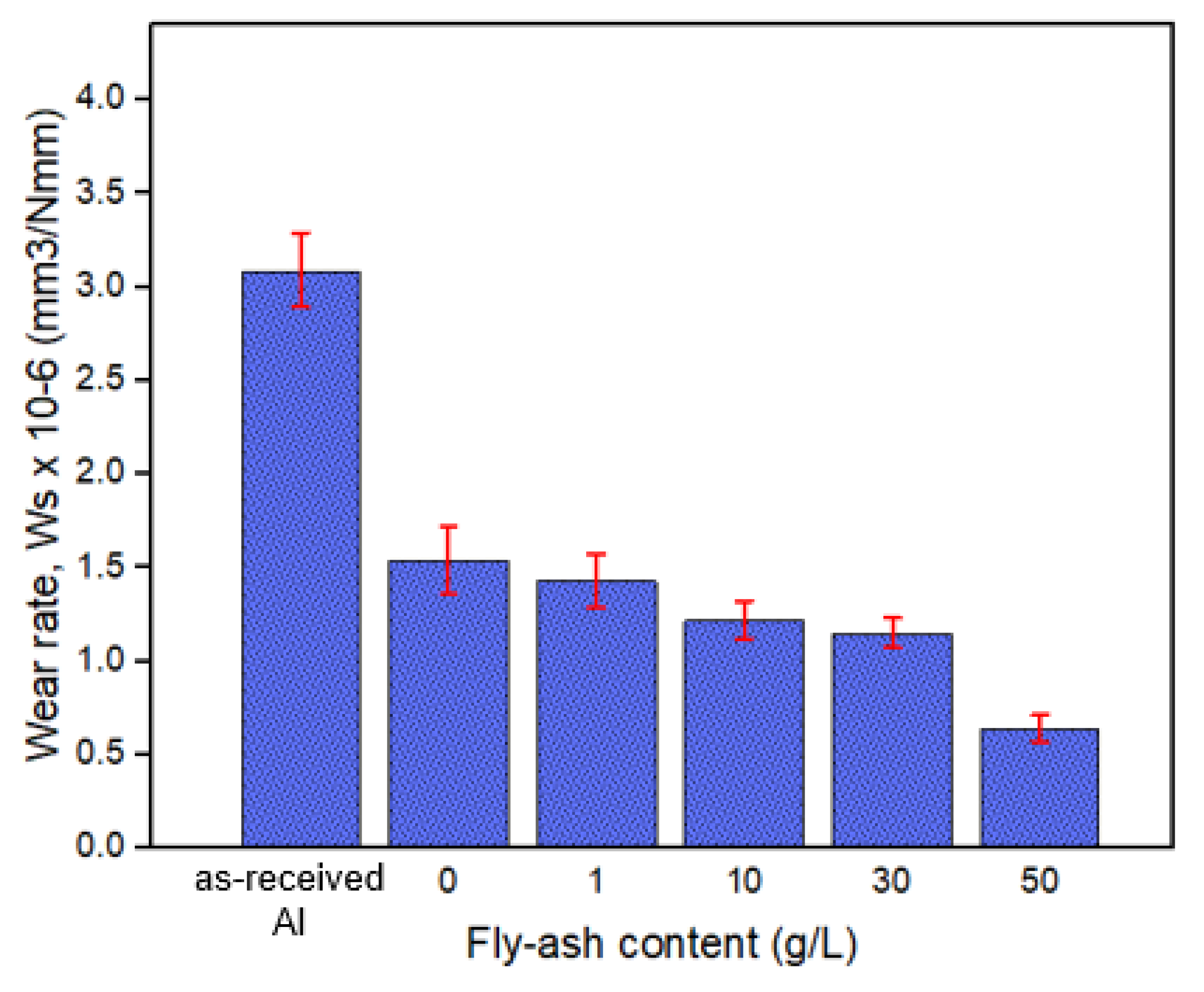
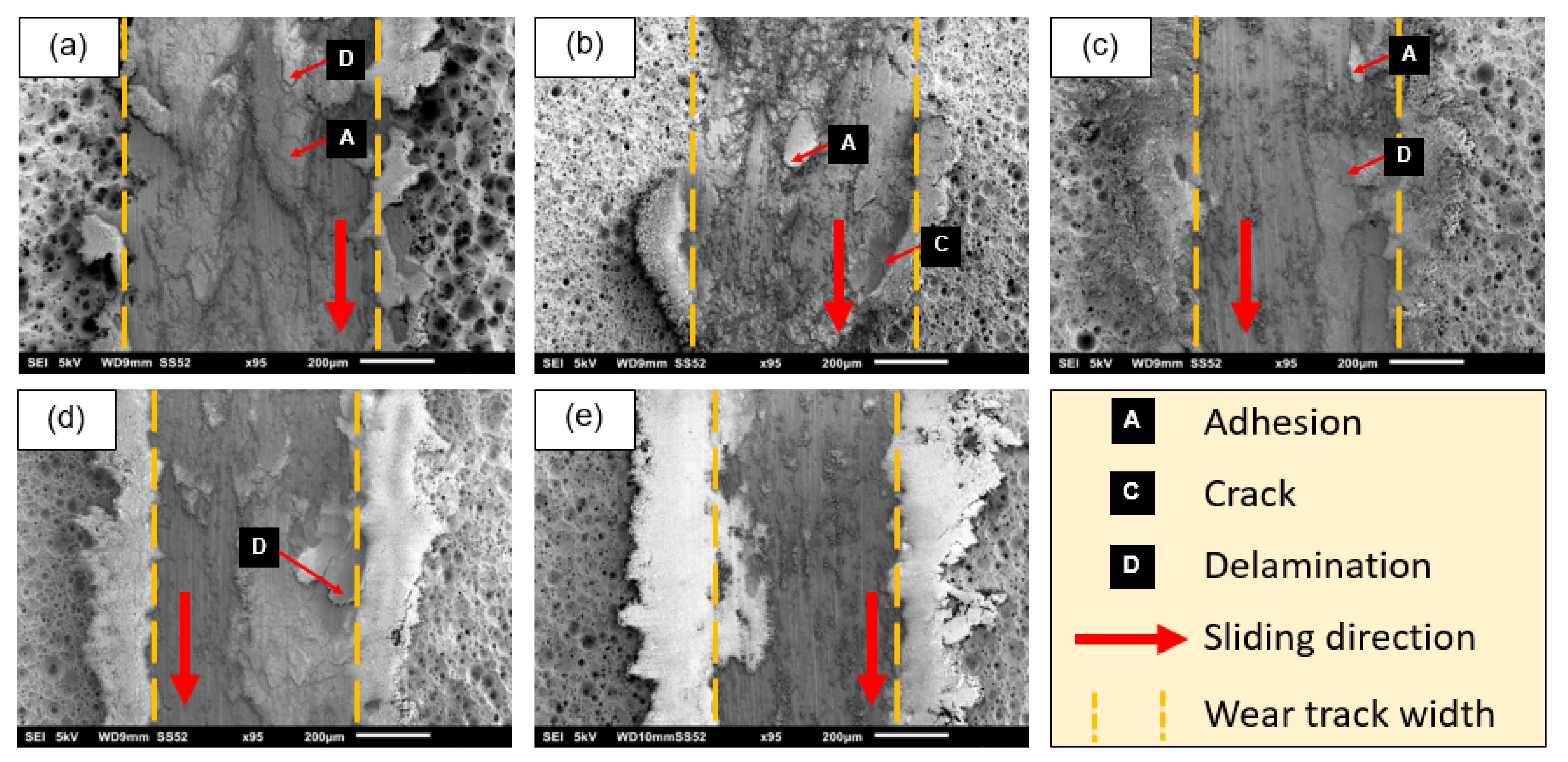
3.3. Tribological Properties of the Composite Oxide Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zalnezhad, E.; Sarhan, A.A.D.; Hamdi, M. Investigating the effects of hard anodizing parameters on surface hardness of hard anodized aerospace AL7075-T6 alloy using fuzzy logic approach for fretting fatigue application. Int. J. Adv. Manuf. Technol. 2013, 68, 453–464. [Google Scholar] [CrossRef]
- Martínez-Viademonte, M.P.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Mohamad, S.; Liza, S.; Yaakob, Y. Technology strengthening of the mechanical and tribological properties of composite oxide film formed on aluminum alloy with the addition of graphite. Surf. Coat. Technol. 2020, 403, 126435. [Google Scholar] [CrossRef]
- Bononi, M.; Giovanardi, R.; Bozza, A. Pulsed current hard anodizing of heat treated aluminum alloys: Frequency and current amplitude influence. Surf. Coat. Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Ossowska, A.; Zieliński, A.; Olive, J.-M.; Wojtowicz, A.; Szweda, P. Influence of Two-Stage Anodization on Properties of the Oxide Coatings on the Ti–13Nb–13Zr Alloy. Coatings 2020, 10, 707. [Google Scholar] [CrossRef]
- Aydogan, D.T.; Muhaffel, F.; Acar, O.K.; Topcuoglu, E.N.; Kulekci, H.G.; Kose, G.T.; Baydogan, M.; Cimenoglu, H. Surface modification of Ti6Al4V by micro-arc oxidation in AgC2H3O2-containing electrolyte. Surf. Innov. 2018, 6, 277–285. [Google Scholar] [CrossRef]
- Ghorbanian, B.; Tajally, M.; Khoie, S.M.M.; Tavakoli, H. Corrosion behavior of MoS2-incorporated PEO coatings prepared on Al alloy. Surf. Innov. 2020, 8. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Ramana, C.V. Plasma electrolytic oxidation ceramic coatings on zirconium (Zr) and Zr alloy: Part I—Growth mechanisms, microstructure, and chemical composure. Composites 2021, 11, 634. [Google Scholar]
- Malinovschi, V.; Marin, A.H.; Ducu, C.; Moga, S.; Andrei, V.; Coaca, E.; Craciun, V.; Lungu, M.; Lungu, C.P. Improvement of Mechanical and Corrosion Properties of Commercially Pure Titanium Using Alumina PEO Coatings. Coatings 2021, 12, 29. [Google Scholar] [CrossRef]
- Bozza, A.; Giovanardi, R.; Manfredini, T.; Mattioli, P. Pulsed current effect on hard anodizing process of 7075-T6 aluminium alloy. Surf. Coat. Technol. 2015, 270, 139–144. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.; Terryn, H.; Skeldon, P.; Thompson, G.; Duszczyk, J.; Katgerman, L. Influence of substrate microstructure on the growth of anodic oxide layers. Electrochim. Acta 2003, 49, 1127–1140. [Google Scholar] [CrossRef]
- Saenz de Miera, M.; Curioni, M.; Skeldon, P.; Thompson, G.E. The behaviour of second phase particles dur-ing anodizing of aluminium alloys. Corros. Sci. 2010, 52, 2489–2497. [Google Scholar] [CrossRef]
- Iglesias-Rubianes, L.; Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Ferguson, J.; Beneke, M. Cyclic oxidation processes during anodizing of Al-Cu alloys. Electrochim. Acta 2007, 52, 7148–7157. [Google Scholar] [CrossRef]
- Santecchia, E.; Cabibbo, M.; Hamouda, A.M.S.; Musharavati, F.; Popelka, A.; Spigarelli, S. Dry Sliding Tribological Properties of a Hard Anodized AA6082 Aluminum Alloy. Metals 2020, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Mehdizade, M.; Soltanieh, M.; Eivani, A.R. Investigation of anodizing time and pulse voltage modes on the corrosion behavior of nanostructured anodic layer in commercial pure aluminum. Surf. Coat. Technol. 2018, 358, 741–752. [Google Scholar] [CrossRef]
- Wu, L.; Wen, C.; Zhang, G.; Liu, J.; Ma, K. Influence of anodizing time on morphology, structure and tribological properties of composite anodic films on titanium alloy. Vacuum 2016, 140, 176–184. [Google Scholar] [CrossRef]
- Arun, S.; Hariprasad, S.; Saikiran, A.; Ravisankar, B.; Parfenov, E.V.; Mukaeva, V.R.; Rameshbabu, N. The effect of graphite particle size on the corrosion and wear behaviour of the PEO-EPD coating fabricated on commercially pure zirconium. Surf. Coat. Technol. 2019, 363, 301–313. [Google Scholar]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. Highly corrosion protection proper-ties of plasma electrolytic oxidized titanium using rGO nanosheets. Appl. Surf. Sci. 2019, 486, 153–165. [Google Scholar] [CrossRef]
- Aydin, F.; Ayday, A.; Turan, M.E.; Zengin, H. ROle of graphene additive on wear and electrochemical corrosion behaviour of plasma electrolytic oxidation (PEO) coatings on Mg–MWCNT nanocomposite. Surf. Eng. 2019, 36, 791–799. [Google Scholar] [CrossRef]
- Chen, S.; Kang, C.; Wang, J.; Liu, C.; Sun, K. Synthesis of anodizing composite films containing superfine Al2O3 and PTFE particles on Al alloys. Appl. Surf. Sci. 2010, 256, 6518–6525. [Google Scholar] [CrossRef]
- Jin, R.; Fan, H.; Liu, Y.; Ma, W.; Lu, H.; Yang, P.; Ma, W. Formation Mechanism of Lotus-root-shaped Nanostructure during Two-step Anodization. Electrochim. Acta 2016, 188, 421–427. [Google Scholar] [CrossRef]
- Chen, S.; Liao, M.; Yang, P.; Yan, S.; Jin, R.; Zhu, X. Simulation of anodizing current–time curves and the morphology evolution of TiO2 nanotubes obtained in phosphoric electrolytes. RSC Adv. 2016, 6, 84309–84318. [Google Scholar] [CrossRef]
- Razzaq, A.M.; Majid, D.L.; Ishak, M.R.; Basheer, U.M. Mathematical Modeling and Analysis of Tribological Properties of AA6063 Aluminum Alloy Reinforced with Fly Ash by Using Response Surface Methodology. Crystals 2020, 10, 403. [Google Scholar] [CrossRef]
- Magibalan, S. Wear Behaviour of Aluminium Alloy 8011 with 4% Fly Ash Composites by Using Sensitivity Analysis. Alum. In Aluminium Alloys and Composites; IntechOpen: London, UK, 2020; Chapter 7. [Google Scholar]
- Niraj, N.; Pandey, K.M.; Dey, A. Tribological behaviour of Magnesium Metal Matrix Composites reinforced with fly ash cenosphere. Mater. Today Proc. 2018, 5, 20138–20144. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, R.; Chaudhary, R. Effect of flyash particles with aluminium melt on the wear of aluminium metal matrix composites. Eng. Sci. Technol. Int. J. 2017, 20, 1318–1323. [Google Scholar] [CrossRef]
- Patil, N.A.; Pedapati, S.R.; Mamat, O.; Lubis, A.M.H.S. Effect of SiC/fly ash reinforcement on surface properties of aluminium 7075 hybrid composites. Coatings 2020, 10, 541. [Google Scholar] [CrossRef]
- Razzaq, A.M.; Majid, D.L.A.A.; Ishak, M.R.; Uday, M.B. Microstructural characterization of fly ash particulate reinforced AA6063 aluminium alloy for aerospace applications. IOP Conf. Ser. Mater. Sci. Eng. 2017, 270, 12028. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Surface characterization and in-fluence of anodizing process on fatigue life of Al 7050 alloy. Mater. Des. 2011, 32, 3328–3335. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Lv, G.-H.; Chen, H.; Gu, W.C.; Li, L.; Niu, E.W.; Zhang, X.H.; Yang, S.Z. Effects of graphite additives in electrolytes on the microstructure and corrosion resistance of Alumina PEO coatings. Curr. Appl. Phys. 2009, 9, 324–328. [Google Scholar] [CrossRef]
- Mu, M.; Zhou, X.; Xiao, Q.; Liang, J.; Hou, X. Preparation and tribological properties of self-lubricating TiO2 /graphite composite coating on Ti6Al4V alloy. Appl. Surf. Sci. 2012, 258, 8570–8576. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Blawert, C.; Zheludkevich, M.L.; Zhang, T.; Wang, F. Formation of self-lubricating PEO coating via in-situ incorporation of PTFE particles. Surf. Coat. Technol. 2018, 337, 379–388. [Google Scholar] [CrossRef]
- Kumar, K.A.R.; Balamurugan, K.; Gnanaraj, D. Hardness, tribology and microstructural studies on Aluminium-flyash metal matrix composites. J. Sci. Ind. Res. 2015, 74, 165–170. [Google Scholar]
- Wojciechowski, J.; Baraniak, M.; Pernak, J.; Lota, G. Nickel coatings electrodeposited from watts type baths containing quaternary ammonium sulphate salts. Int. J. Electrochem. Sci. 2017, 12, 3350–3360. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Technol. 2017, 309, 767–778. [Google Scholar] [CrossRef]






| Element | Composition (%) |
|---|---|
| Al | 93.9 |
| Cu | 3.96 |
| Mn | 0.62 |
| Mg | 0.58 |
| Si | 0.53 |
| Fe | 0.19 |
| others | 0.22 |
| Properties | Value |
|---|---|
| Tensile strength | 427 MPa |
| Yield strength | 276 MPa |
| Elongation | 22% |
| Hardness | 119 HV |
| Element | Composition (%) |
|---|---|
| SiO2 | 44.1 |
| Al2O3 | 19.1 |
| CaO | 13.5 |
| Fe2O3 | 12.4 |
| MgO | 4.7 |
| Na2O | 2.9 |
| K2O | 1.4 |
| SO3 | 1.0 |
| others | 0.9 |
| Properties | Phase 1 | Phase 2 |
|---|---|---|
| Cathode | AA2017 | |
| Anode | Copper sheet | |
| Electrolyte | 20% H2SO4 | |
| Voltage (V) | 15 | |
| Current (A) | 2.0 | |
| Time (min) | 5, 10, 20, 30, 60 | 60 |
| Fly-ash content (g/L) | 1 | 0, 1, 10, 30, 50 |
| Point | Weight % | ||||||
|---|---|---|---|---|---|---|---|
| Al | O | Si | Fe | Mg | Na | K | |
| A | 28.32 | 51.68 | 20.00 | - | - | - | - |
| B | 16.47 | 34.28 | - | 37.63 | 11.62 | - | - |
| C | 50.90 | 49.10 | - | - | - | - | - |
| D | 14.96 | 39.24 | 20.33 | 21.45 | 1.69 | - | 2.32 |
| E | 50.18 | 49.82 | - | - | - | - | - |
| F | 16.72 | 33.94 | 1.46 | 41.27 | 6.62 | - | - |
| G | 48.92 | 51.08 | - | - | - | - | - |
| H | 12.47 | 40.67 | 44.24 | - | 2.62 | - | - |
| I | 7.53 | 65.31 | 27.15 | - | - | - | - |
| Point | Weight % | |||
|---|---|---|---|---|
| Al | O | C | Si | |
| A | 25.46 | 53.62 | 20.92 | - |
| B | 39.99 | 40.06 | 19.95 | - |
| C | 49.50 | 43.98 | 6.52 | - |
| D | 26.54 | 49.38 | 18.66 | 12.47 |
| E | 23.06 | 54.11 | 10.36 | 12.47 |
| F | - | 10.10 | 21.86 | 68.04 |
| G | 11.04 | 29.37 | 21.82 | 37.76 |
| H | 15.19 | 15.99 | 11.62 | 57.21 |
| I | 32.52 | 51.48 | 12.67 | 3.33 |
| J | 14.76 | 40.51 | 32.61 | 12.13 |
| K | 62.43 | 12.90 | 8.05 | 8.79 |
| L | 4.86 | 24.95 | 24.03 | 6.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mat Tahir, N.A.; Liza, S.; Fukuda, K.; Mohamad, S.; Hashimi, M.Z.F.; Yunus, M.S.M.; Yaakob, Y.; Othman, I.S. Surface and Tribological Properties of Oxide Films on Aluminium Alloy through Fly-Ash Reinforcement. Coatings 2022, 12, 256. https://doi.org/10.3390/coatings12020256
Mat Tahir NA, Liza S, Fukuda K, Mohamad S, Hashimi MZF, Yunus MSM, Yaakob Y, Othman IS. Surface and Tribological Properties of Oxide Films on Aluminium Alloy through Fly-Ash Reinforcement. Coatings. 2022; 12(2):256. https://doi.org/10.3390/coatings12020256
Chicago/Turabian StyleMat Tahir, Noor Ayuma, Shahira Liza, Kanao Fukuda, Syazwani Mohamad, Mohd Zakir Fathi Hashimi, Mohd Saifulnizam Mohd Yunus, Yazid Yaakob, and Intan Sharhida Othman. 2022. "Surface and Tribological Properties of Oxide Films on Aluminium Alloy through Fly-Ash Reinforcement" Coatings 12, no. 2: 256. https://doi.org/10.3390/coatings12020256
APA StyleMat Tahir, N. A., Liza, S., Fukuda, K., Mohamad, S., Hashimi, M. Z. F., Yunus, M. S. M., Yaakob, Y., & Othman, I. S. (2022). Surface and Tribological Properties of Oxide Films on Aluminium Alloy through Fly-Ash Reinforcement. Coatings, 12(2), 256. https://doi.org/10.3390/coatings12020256






