Research Status of Graphene Polyurethane Composite Coating
Abstract
:1. Introduction
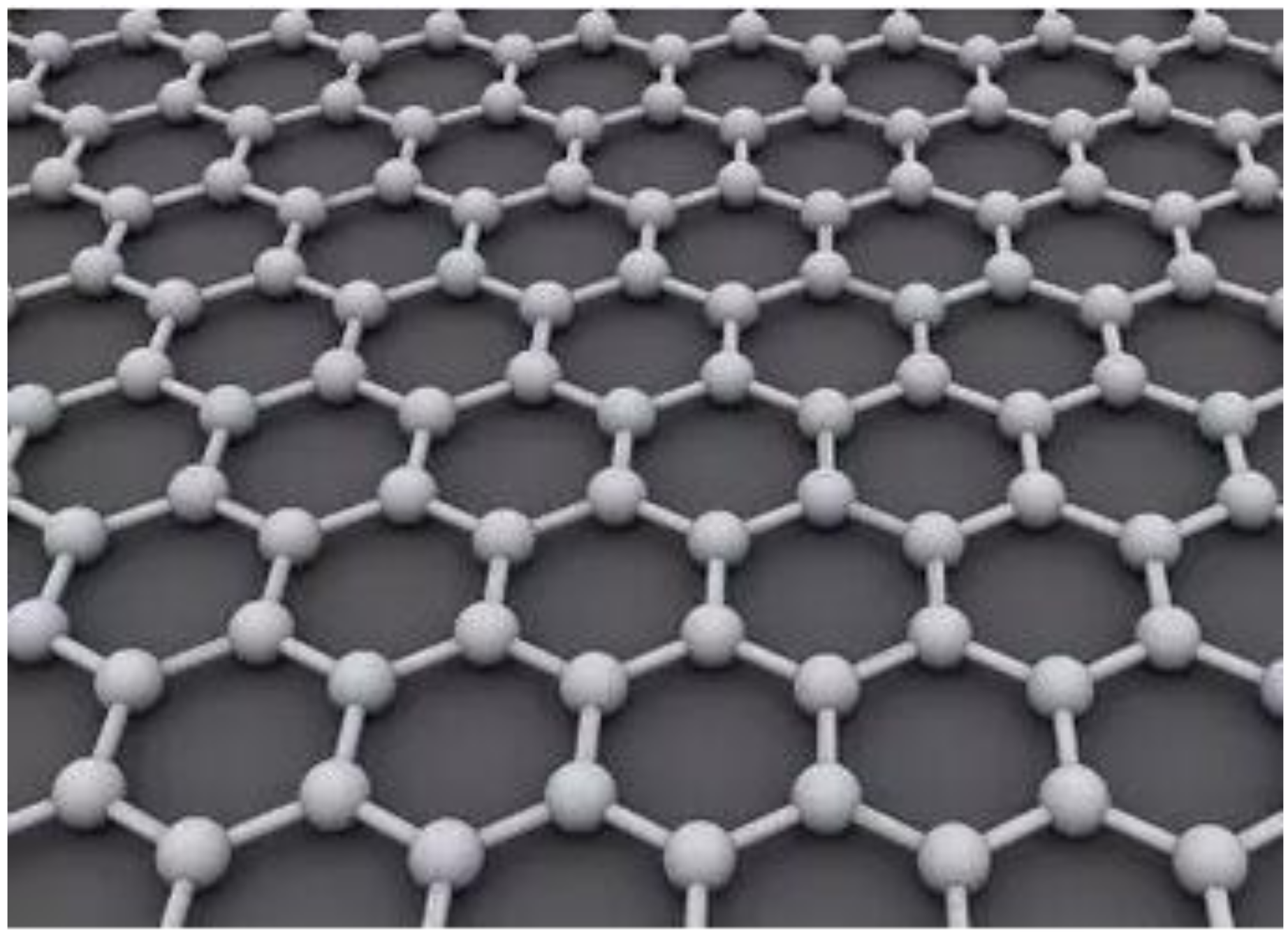
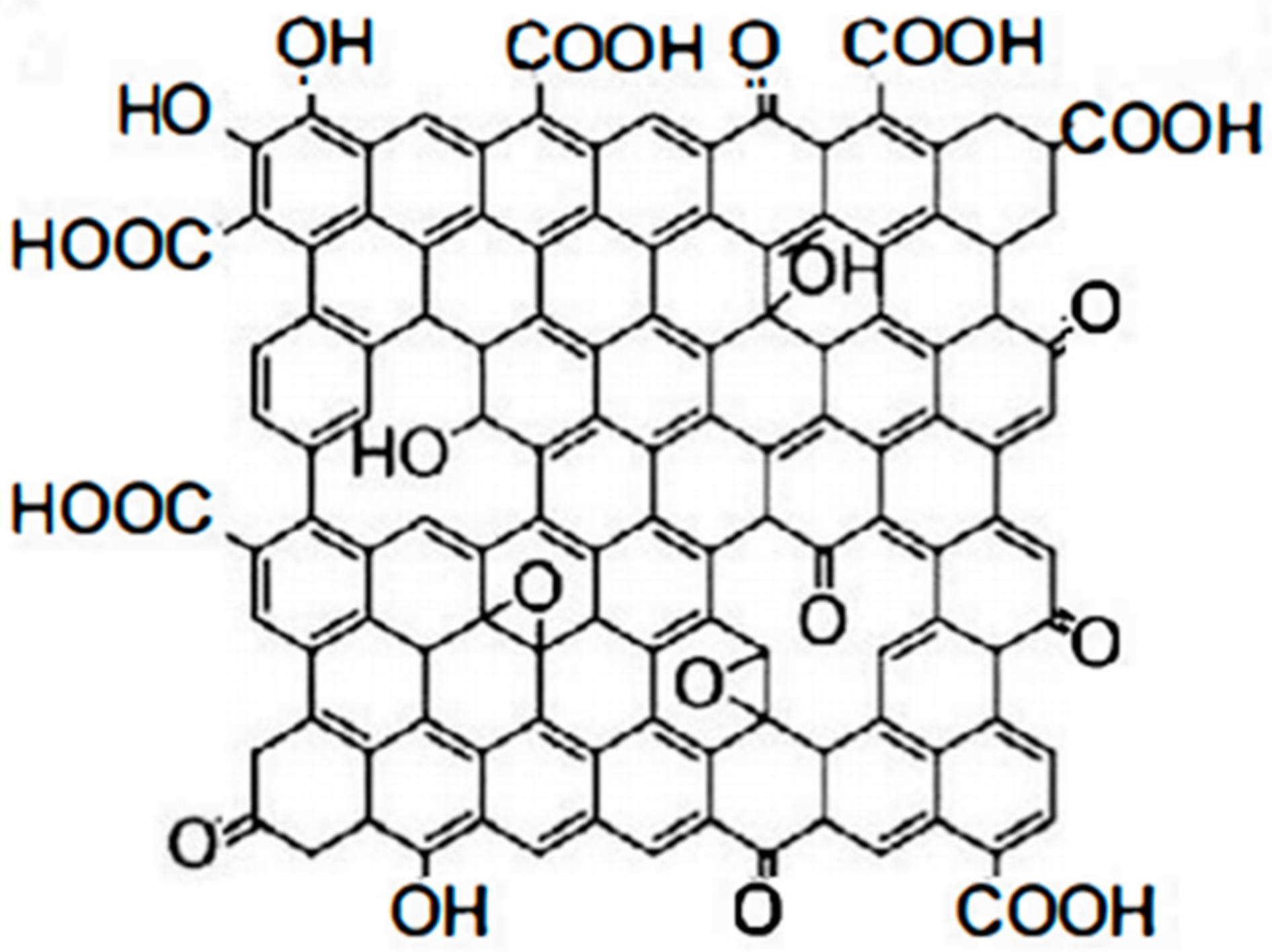
2. Graphene Material
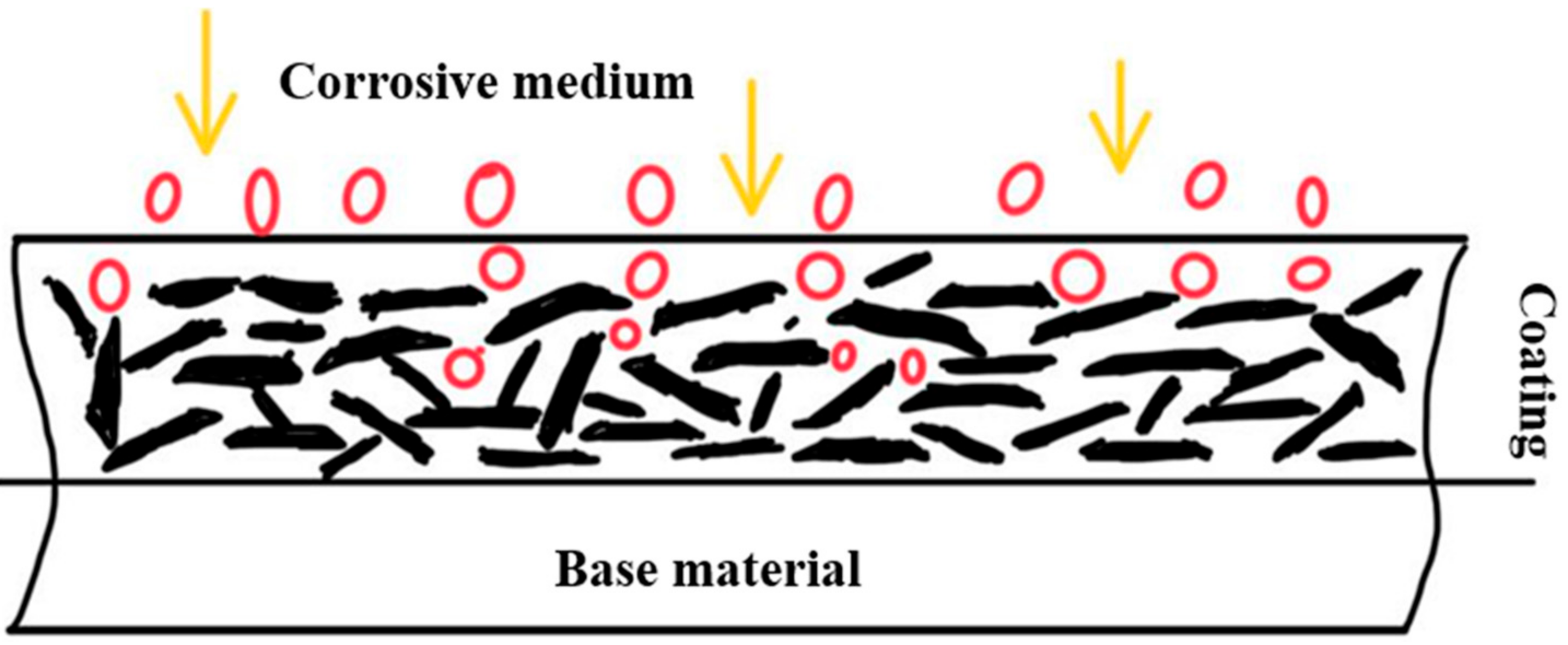
3. Anti-Corrosion Mechanism of Graphene Nanocomposites
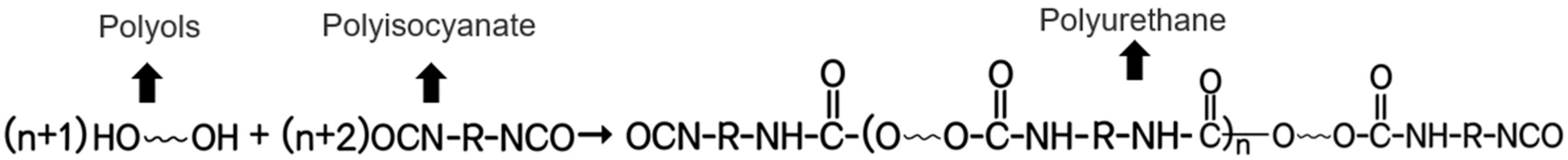
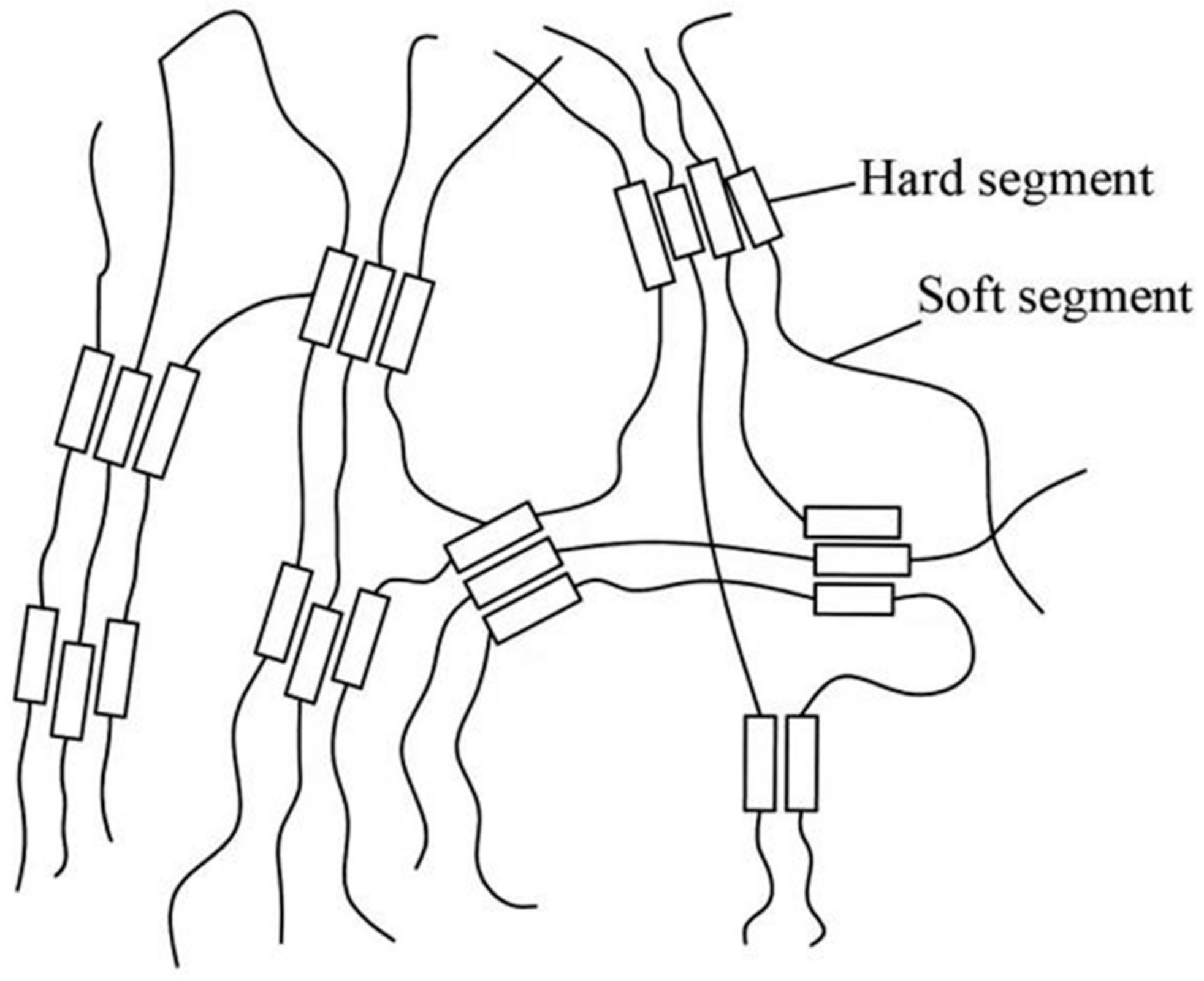
4. Polyurethane Anticorrosive Coating
5. Modification of Graphene Nanomaterials
5.1. Noncovalent Modification of GO
5.2. Covalent Modification of GO
6. Preparation Method of Graphene PU Composite Material
6.1. Solution Blending Method
6.2. Melt Blending Method
6.3. In Situ Polymerization
6.4. Photopolymerization
6.5. Sol-Gel Method
6.6. Self-Assembly
7. Coating Preparation Processes
7.1. Laser Cladding Technology
7.2. Electron Beam Melting Technology
7.3. Thermal Spraying Technology
7.4. Brazing Technology
7.5. Vapor Deposition Technology
7.6. Surface Composite Ion Treatment Technology
8. Conclusions and Prospect
- (1)
- Further research should be carried out on the optimization of the modification mode of GO. The non-covalent modification retains the complete structure and properties of graphene materials, but the relationship with the substrate is unstable and prone to being destroyed. While covalent modification can effectively improve the reaction and compatibility between GO and polymer, it will inevitably destroy the internal structure, thus affecting the performance of graphene materials. Therefore, it is an important research direction to study a new modification method that can modify GO without destroying its structure.
- (2)
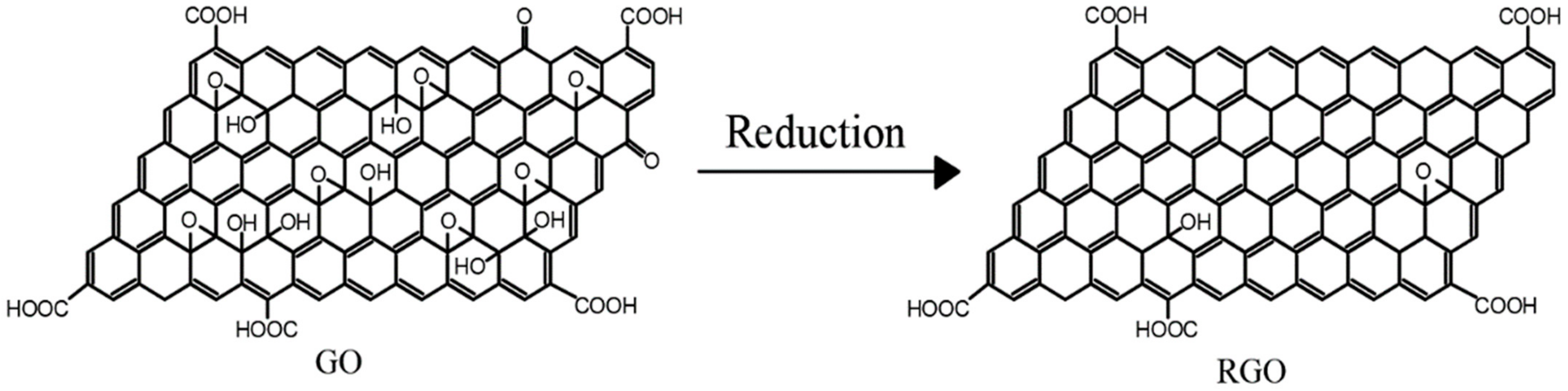
- Among the currently adopted modification methods, a large part of the research is to prepare RGO by reducing GO as precursors and using reducing agents to repair the GO structure and restore its properties. However, most of the previous reducing agents are toxic substances, which cannot meet the environmental requirements in the new era, so it is necessary to try to develop the use of new, green, and non-toxic reducing agents to restore GO. Second, restoring the structure and properties of GO by reduction reaction is also an important research direction. It is necessary to strengthen the research on the mechanism of structural changes of graphene materials and use it as a guide to explore new and more effective reducing agents that can restore the structure of GO to the maximum.
- (3)
- The application mechanism of graphene materials in coating is mainly the barrier effect generated by its lamellar structure. When the graphene nanomaterials are arranged horizontally in the layer, that is, parallel to the surface of the substrate, they have the best barrier performance. Therefore, the directional arrangement of graphene materials in coatings plays an important role in the performance of graphene nanomaterials. In the future, researchers should pay more attention to the directional arrangement of graphene nanomaterials.
- (4)
- The superior and inferior properties of graphene polyurethane composites are closely related to their dispersion properties, and the preparation processes such as mechanical stirring time, ultrasonic dispersion degree, graphene content, and type of solvent added directly affect the dispersion properties of graphene. Therefore, optimization of the preparation process can improve the performance of composites to some extent. At present, for the characterization of the dispersity of composites, electron microscopy is mainly used to observe the morphology and structure of the particles. Although possessing the advantage of being intuitive and convenient, this qualitative analysis method is too subjective to allow quantitative analysis of the dispersity of materials. Therefore, using a quantitative way of characterization to precisely evaluate the dispersion performance of composites, establishing the link between individual preparation processes and dispersion performance, is a future research direction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sangaj, N.S.; Malshe, V.C. Permeability of polymers in protective organic coatings. Prog. Org. Coat. 2004, 50, 28–39. [Google Scholar] [CrossRef]
- Liu, Q.-P.; Zhang, L.-Y.; Qiu, Y.-R. Advances in the application of graphene in anti-corrosion coatings. Miner. Conserv. Util. 2020, 40, 153–160. (In Chinese) [Google Scholar]
- Morcillo, M.; Chico, B.; Díaz, I.; Cano, H.; de la Fuente, D. Atmospheric corrosion data of weathering steels. A review. Corros. Sci. 2013, 77, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Song, N.; Wang, W. Synthesis of graphene/epoxy resin composite via 1, 8-diaminooctane by ultrasonication approach for corrosion protection. Ultrason. Sonochem. 2018, 42, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Alhumade, H.; Abdala, A.; Yu, A.; Elkamel, A.; Simon, L. Corrosion inhibition of copper in sodium chloride solution using polyetherimide/graphene composites. Can. J. Chem. Eng. 2016, 94, 896–904. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Jie, Z.; Yinghua, L.I. Development status of elastomer market in China. Polyurethane Ind. 2019, 34, 1–5. (In Chinese) [Google Scholar]
- Eigler, S. Graphene. An introduction to the fundamentals and industrial applications edited by Madhuri Sharon and Maheshwar Sharon. Angew. Chem. Int. Ed. 2016, 55, 5122. [Google Scholar] [CrossRef]
- Bai, S.; Shen, X. Graphene–inorganic nanocomposites. RSC Adv. 2012, 2, 64–98. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Guo, Z.; Chen, Y. Ultrafast coating procedure for graphene on solid-phase microextraction fibers. Talanta 2013, 119, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.C. Application and Uses of Graphene; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Lee, K.E.; Oh, J.J.; Yun, T.; Kim, S.O. Liquid crystallinity driven highly aligned largegraphene oxide composites. Solid State Chem. 2015, 224, 115–119. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2015, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/epoxy coating as multifunctional material for aircraft structures. Aerospace 2015, 2, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Mahanta, N.K.; Abramson, A.R. Thermal conductivity of graphene and graphene oxide nanoplatelets. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomech anical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2012; pp. 1–6. [Google Scholar]
- Pei, S.F.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem. Pap. 2016, 71, 459–487. [Google Scholar] [CrossRef]
- Sheng, X.; Cai, W.; Zhong, L.; Xie, D.; Zhang, X. Synthesis of functionalized graphene/polyaniline nanocomposites with effective synergistic reinforcement on anticorrosion. Ind. Eng. Chem. Res. 2016, 55, 8576–8585. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Gupta, P.K. Graphite, Graphene, and Their Polymer Nanocomposites; Taylor and Francis CRC Press: London, UK, 2012. [Google Scholar]
- Tong, Y.; Bohm, S.; Song, M. The capability of graphene on improving the electrical conductivity and anti-corrosion properties of Polyurethane coatings. Appl. Surf. Sci. 2017, 424, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-D.; Ren, P.-G.; Xu, J.-Z.; Xu, L.; Zhong, G.-J.; Hsiao, B.S.; Li, Z.-M. Improved barrier properties of poly(lactic acid) with randomly dispersed graphene oxide nanosheets. J. Membr. Sci. 2014, 464, 110–118. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, Q.; Cheng, L.; Wu, H.; Zhao, H.; Wang, L. Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings. Chem. Eng. J. 2020, 389, 124435. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, E.N.; Liu, X.; Song, W.; Li, Y.; Wang, X.; Liu, C. Epoxy coating with in-situ synthesis of polypyrrole functionalized graphene oxide for enhanced anticorosive performance. Prog. Org. Coat. 2020, 140, 105488. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R. Enhancing corrosion and wear performance of PEO coatings on Mg alloys using graphene and graphene oxide additions: A review. FlatChem 2021, 27, 100241. [Google Scholar] [CrossRef]
- Rusu, L.-C.; Ardelean, L.C.; Jitariu, A.-A.; Miu, C.A.; Streian, C.G. An insight into the structural diversity and clinical applicability of polyurethanes in biomedicine. Polymers 2020, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Brzeska, J.; Tercjak, A.; Sikorska, W.; Kowalczuk, M.; Rutkowska, M. Predicted studies of branched and cross-linked polyurethanes based on polyhydroxybutyrate with polycaprolactone triol in soft segments. Polymers 2020, 12, 1068. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, X.; Soucek, M.D.; Castaneda, H. Inhibition of acid undercutting of inorganic/organic hybrid polyurethane coatings. Prog. Org. Coat. 2019, 134, 169–176. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, C.; Chen, J.; Li, S.; Li, Y.; Lei, W. Correlation of Raw Materials and Waterborne Polyurethane Properties by Sequence Similarity Analysis. J. Mater. Sci. Technol. 2016, 32, 687–694. [Google Scholar] [CrossRef]
- Guo, J.Q.; Li, J.L.; Liang, J.F. Advances in chemical reduction methods and mechanisms of go. Mater. Eng. 2020, 48, 24–35. (In Chinese) [Google Scholar]
- Zhang, Y.; Huang, W.; Lv, P. Research progress in the modification of polyurethane coatings to improve their anticorrosive properties. Polyurethane Ind. 2020, 35, 5–7. (In Chinese) [Google Scholar]
- Guo, Y. Factors influencing the performance of one-component polyurethane waterproof coating. Shanghai Coat. 2011, 49, 42–44. (In Chinese) [Google Scholar]
- Wei, L.F.; Zhang, W.B.; Ma, J.Z. x-n stacking interface design for improving the strength and electromagnetic interference shielding of ultrathin and flexible waterborne polymer/sulfonatedgraphene composite. Carbon 2019, 149, 679–692. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Lv, Q. Enhanced tribological properties of aligned reduced graphene oxide-Fe3O4@ polyphosphazene/bismaleimides composites. Carbon 2016, 102, 145–153. [Google Scholar] [CrossRef]
- Jing, L.-C.; Wang, T.; Cao, W.-W.; Wen, J.-G.; Zhao, H.; Ning, Y.-J.; Yuan, X.-T.; Tian, Y.; Teng, L.-H.; Geng, H.-Z. Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized graphene oxide in the presence of acidified multi-walled carbon nanotubes. Prog. Org. Coat. 2020, 146, 105734. [Google Scholar] [CrossRef]
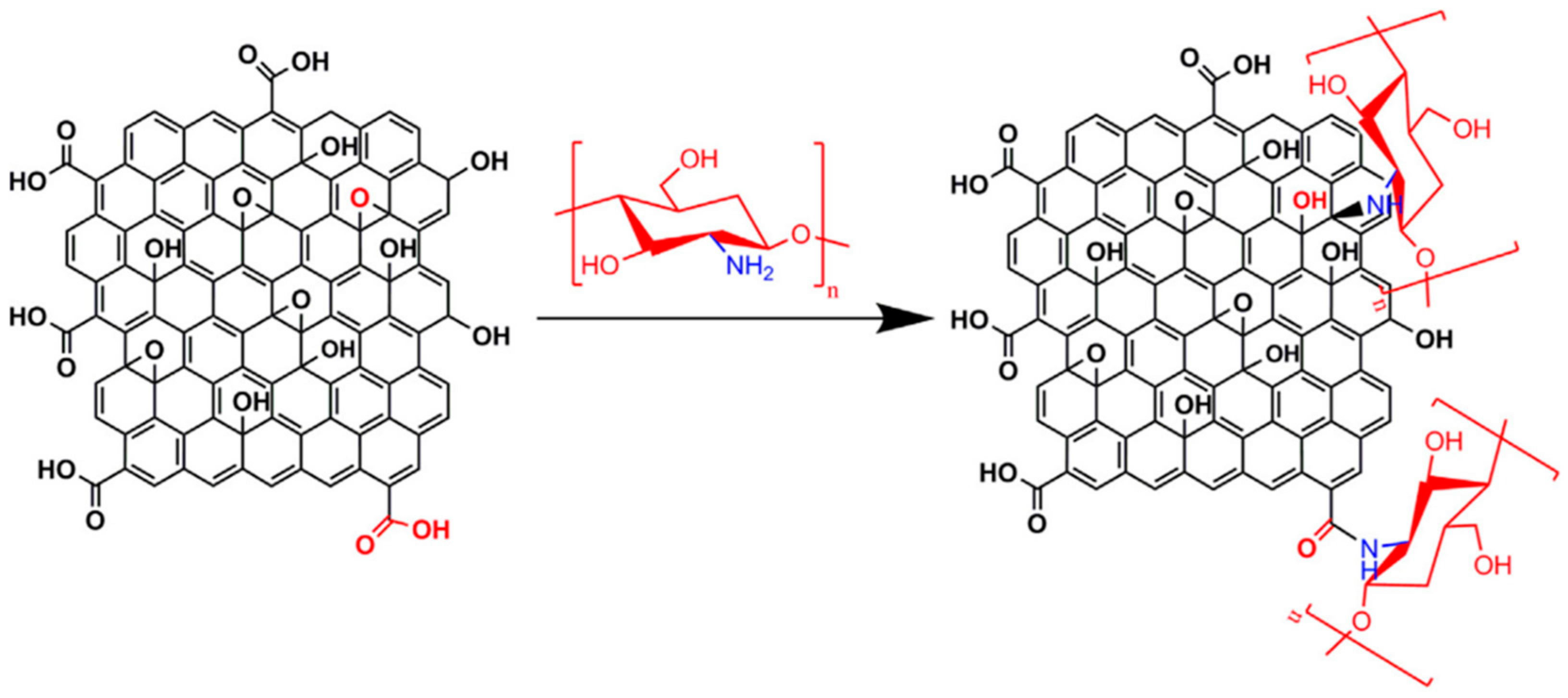
- Najafabadi, S.A.A.; Mohammadi, A.; Kharazi, A.Z. Polyurethane nanocomposite impregnated with chitosan-modified graphene oxide as a potential antibacterial wound dressing. Mater. Sci. Eng. C 2020, 115, 110899. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Jin, Y.; Lai, S.; Shi, L.; Shen, Y.; Yang, H. Multifunctional light-responsive graphene-based polyurethane composites with shape memory, self-healing, and flame retardancy properties. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105686. [Google Scholar] [CrossRef]
- Bera, M.; Prabhakar, A.; Maji, P.K. Nanotailoring of thermoplastic polyurethane by amine functionalized graphene oxide: Effect of different amine modifier on final properties. Compos. Part B Eng. 2020, 195, 108075. [Google Scholar] [CrossRef]
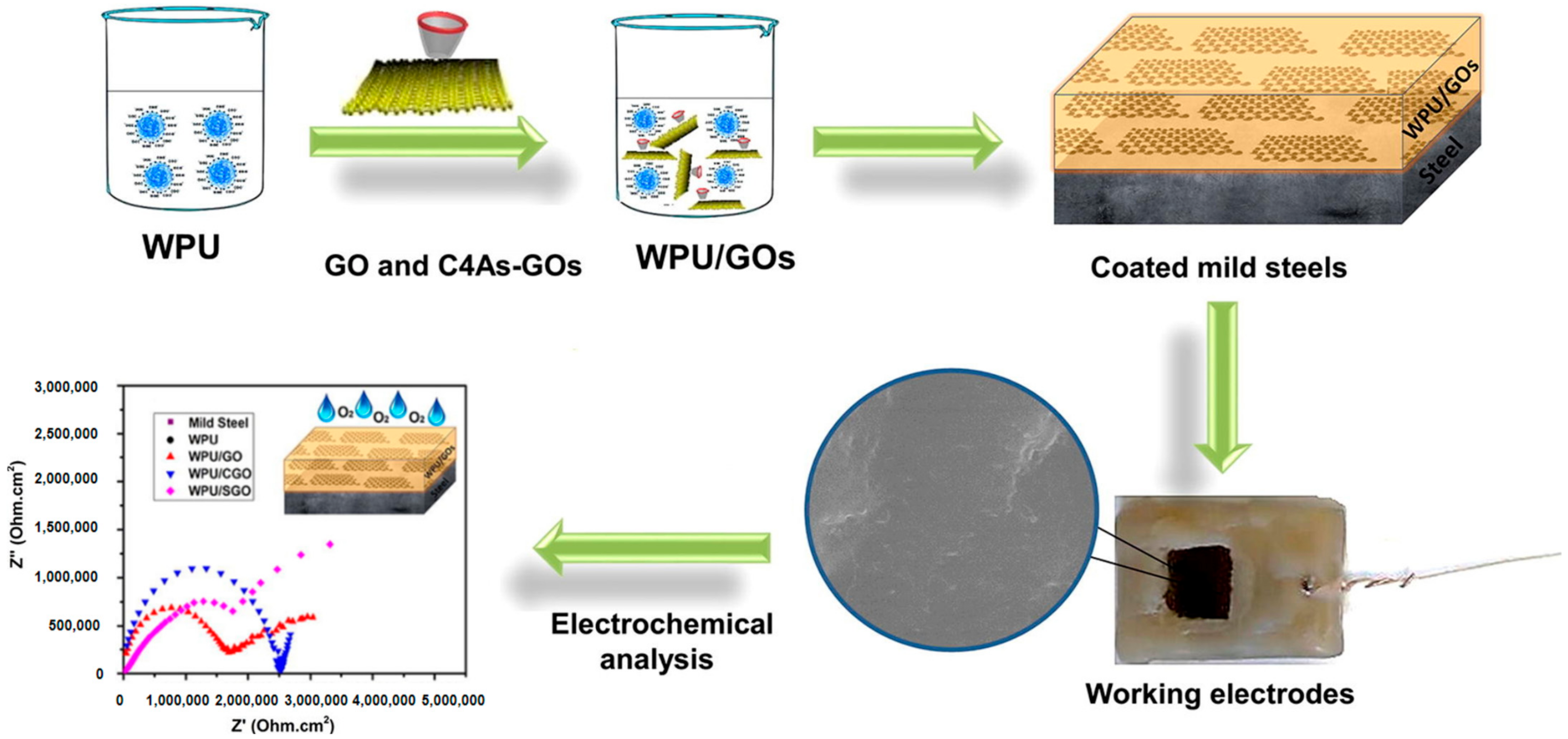
- Mohammadi, A.; Barikani, M.; Doctorsafaei, A.H.; Isfahani, A.P.; Shams, E.; Ghalei, B. Aqueous dispersion of polyurethane nanocomposites based on calix [4] arenes modified graphene oxide nanosheets: Preparation, characterization, and anti-corrosion properties. Chem. Eng. J. 2018, 349, 466–480. [Google Scholar] [CrossRef]
- Sun, Z.; He, Y.; Fan, H. Preparation and properties of graphene/polyurethane composite coating agent. Leather Sci. Eng. 2016, 26, 10–15. [Google Scholar]
- Wang, Q. Preparation of Graphene Oxide and its Modification of Polyurethane Materials; Yantai University: Yantai, China, 2012. [Google Scholar]
- Dong, J.; Fang, L.; Ni, Y. Study on properties of graphene oxide/polyurethane composites by in-situ polymerization. New Chem. Mater. 2017, 45, 32–36. [Google Scholar]
- Akhan, S.; Oktay, B.; Özdemir, O.K.; Madakbaş, S.; Apohan, N.K. Polyurethane graphene nanocomposites with self-healing properties by azide-alkyne click reaction. Mater. Chem. Phys. 2020, 254, 123315. [Google Scholar] [CrossRef]
- Chen, Y. Preparation and Properties of Polymer/Inorganic Nanoparticle Composites by Solution Casting; Hubei University of Technology: Hubei, China, 2017. (In Chinese) [Google Scholar]
- Wang, X.; Xing, W.; Song, L. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf. Coat. Technol. 2012, 206, 4778–4784. [Google Scholar] [CrossRef]
- Shi, Z.; Li, A.; Zhang, C.; Zhang, Y.-F. Reduced graphene oxide coated polyurethane composite foams as flexible strain sensors for large deformation. Mater. Sci. Eng. B 2021, 272, 115360. [Google Scholar] [CrossRef]
- Xiao, L.L.; Ren, Y.; Gao, Q.H. Introduction to the research progress of laser cladding technology. New Technol. New Technol. 2021, 7, 5–7. (In Chinese) [Google Scholar]
- Bai, Q.F.; Zhang, J.; Li, Q.H. Microstructure and corrosion resistance of Fe-based coatings prepared using high-speed laser cladding and powerful spinning treatment. Mater. Lett. 2022, 310, 131429. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.; Bai, P.; Du, W.; Li, Y.; Yang, X.; Wang, Q. In-situ synthesis of TiC/graphene/Ti6Al4V composite coating by laser cladding. Mater. Lett. 2020, 270, 127711, (prepublish). [Google Scholar] [CrossRef]
- Lu, R.X.; Liu, H.L.; Wang, X.Y. Research status and development of electron beam cladding technology. Therm. Process. Technol. 2022, 15–19. (In Chinese) [Google Scholar]
- Wang, W.Q.; Zhang, S.Q.; Xiao, S. Microstructure and properties of multilayer WC-40Co coating on Ti-6Al-4V by electron beam cladding. Mater. Charact. 2022, 183, 111585. [Google Scholar] [CrossRef]
- Rao, M.S.; Ramesh, M.R.; Ravikiran, K. Developing partially oxidized NiCr coatings using the combined flame spray and plasma spray process for improved wear behaviour at high temperature. Wear 2021, 478, 203885. [Google Scholar]
- Chen, H.X.; Kong, D.J. Comparison on electrochemical corrosion performances of arc and laser thermal sprayed Al–Ti–Ni coatings in marine environment. Mater. Chem. Phys. 2020, 251, 123200. [Google Scholar]
- Lukáš, M.; Michal, Š.; Šárka, H.; Petra, Š. Application of flash-pulse thermography methods for quantitative thickness inspection of coatings made by different thermal spraying technologies. Surf. Coat. Technol. 2021, 406, 126748. [Google Scholar]
- Tahmineh, F.; Navid, S.; Tatiana, K.; Ben, E.F.; Nima, M.; Martin, P.; Ali, D.; Christian, M. Wetting and corrosion characteristics of thermally sprayed copper-graphene nanoplatelet coatings for enhanced dropwise condensation application. Carbon Trends 2021, 3, 100018. [Google Scholar]
- Wu, N.; Li, Y.; Ma, Q. Microstructure evolution and shear strength of vacuum brazed joint for super-Ni/NiCr laminated composite with Ni–Cr–Si–B amorphous interlayer. Mater. Des. 2014, 53, 816–821. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.Q.; Chen, X.G.; Chi, C.T.; Wang, M.S.; Han, X.; Xie, Y.J. Influence of Cr addition on the interface purification of vacuum brazed NiCr-Cr3C2 coatings on single crystal superalloy. Surf. Coat. Technol. 2017, 325, 200–209. [Google Scholar] [CrossRef]
- Gu, J.F.; Li, P.; Zhong, Q.D. Application of physical vapor deposition in corrosion resistant coatings. Mater. Guide 2016, 30, 75–80. (In Chinese) [Google Scholar]
- Jiang, Q.; Liao, X.; Yang, J.; Wang, G.; Chen, J.; Tian, C.; Li, G. A two-step process for the preparation of thermoplastic polyurethane/graphene aerogel composite foams with multi-stage networks for electromagnetic shielding. Compos. Commun. 2020, 21, 100416. [Google Scholar] [CrossRef]
- Niu, Y. Research and application progress of chemical vapor deposition technology. Sci. Technol. Wind. 2020, 13, 161. (In Chinese) [Google Scholar]
- Aamir, N.; Faheem, M.M.; Ali, R.M.; Tasaduq, I.M.; Javaid, I.M.; Ur, R.Z. Binder free boron nitride-based coatings deposited on mild steel by chemical vapour deposition: Anti-corrosion performance analysis. Phys. B Condens. Matter 2020, 602, 412600, (prepublish). [Google Scholar]
- Naghdi, S.; Nešović, K.; Mišković-Stanković, V.; Rhee, K.Y. Comprehensive electrochemical study on corrosion performance of graphene coatings deposited by chemical vapour deposition at atmospheric pressure on platinum-coated molybdenum foil. Corros. Sci. 2018, 130, 31–44. [Google Scholar] [CrossRef]
- Xu, Q.; Deng, Z.; Sun, Q.; Tu, R.; Zhang, S.; Yang, M.; Li, Q.; Zhang, L.; Goto, T.; Ohmori, H. Electrically conducting graphene/SiC(111) composite coatings by laser chemical vapor deposition. Carbon 2018, 139, 76–84. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Huang, L.; Fu, J. Research Status of Graphene Polyurethane Composite Coating. Coatings 2022, 12, 264. https://doi.org/10.3390/coatings12020264
Fang Z, Huang L, Fu J. Research Status of Graphene Polyurethane Composite Coating. Coatings. 2022; 12(2):264. https://doi.org/10.3390/coatings12020264
Chicago/Turabian StyleFang, Zhou, Lijin Huang, and Junjie Fu. 2022. "Research Status of Graphene Polyurethane Composite Coating" Coatings 12, no. 2: 264. https://doi.org/10.3390/coatings12020264
APA StyleFang, Z., Huang, L., & Fu, J. (2022). Research Status of Graphene Polyurethane Composite Coating. Coatings, 12(2), 264. https://doi.org/10.3390/coatings12020264





