Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions
Abstract
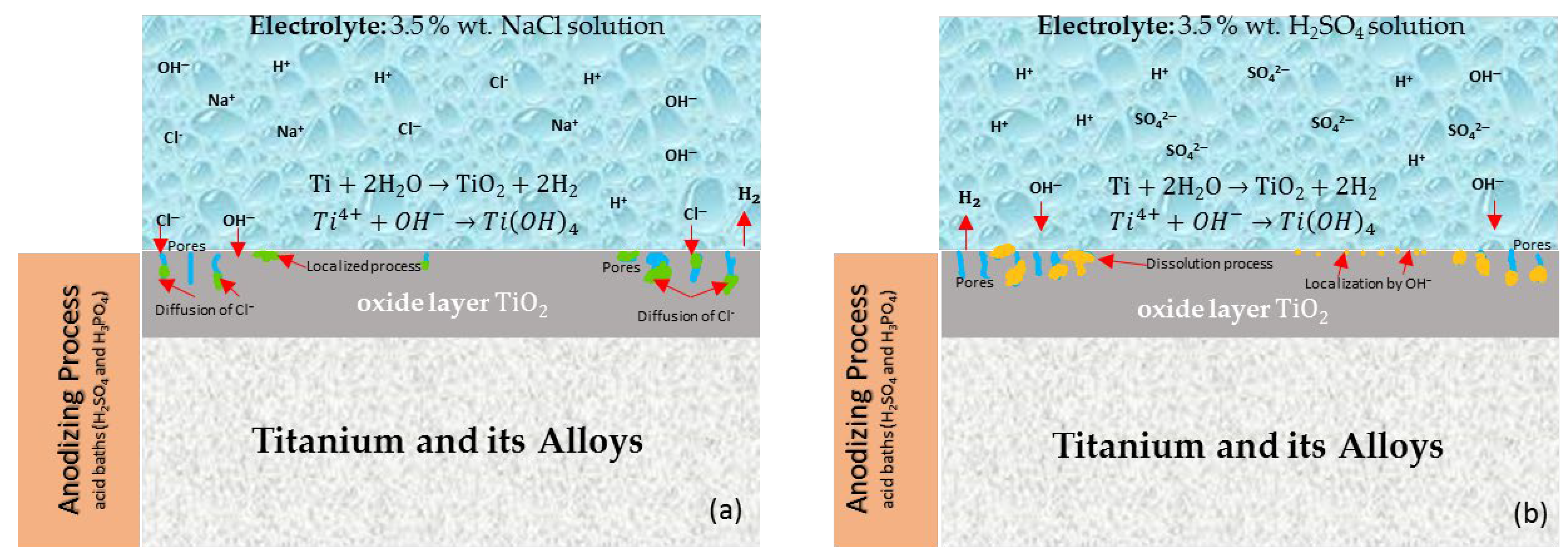
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Microstructural Characterization
2.3. Anodizing Process
2.4. Electrochemical Measurements
3. Results and Discussion
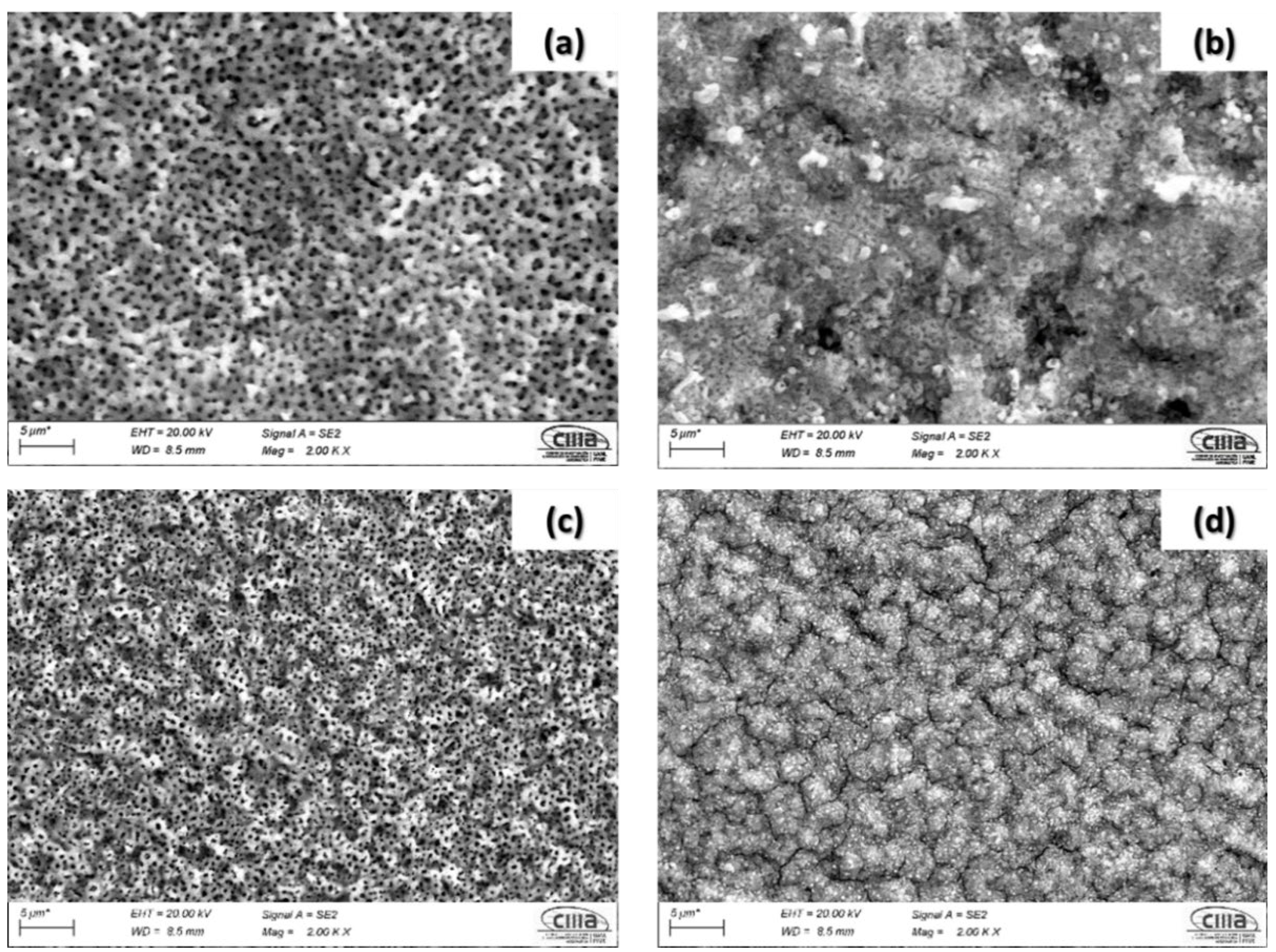
3.1. SEM Microstructural Analysis
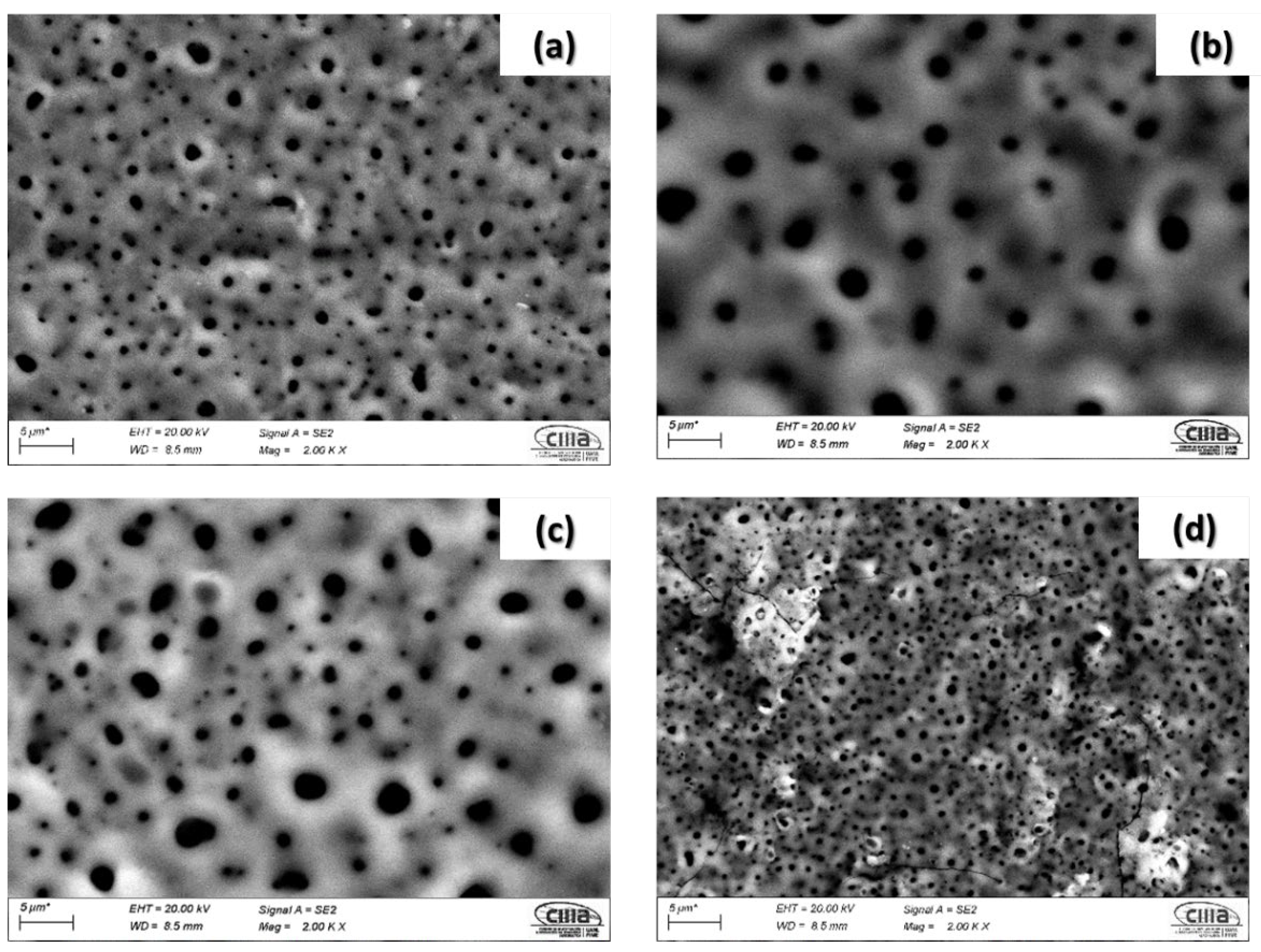
3.2. SEM–OM Surface Analysis of Anodized Alloys
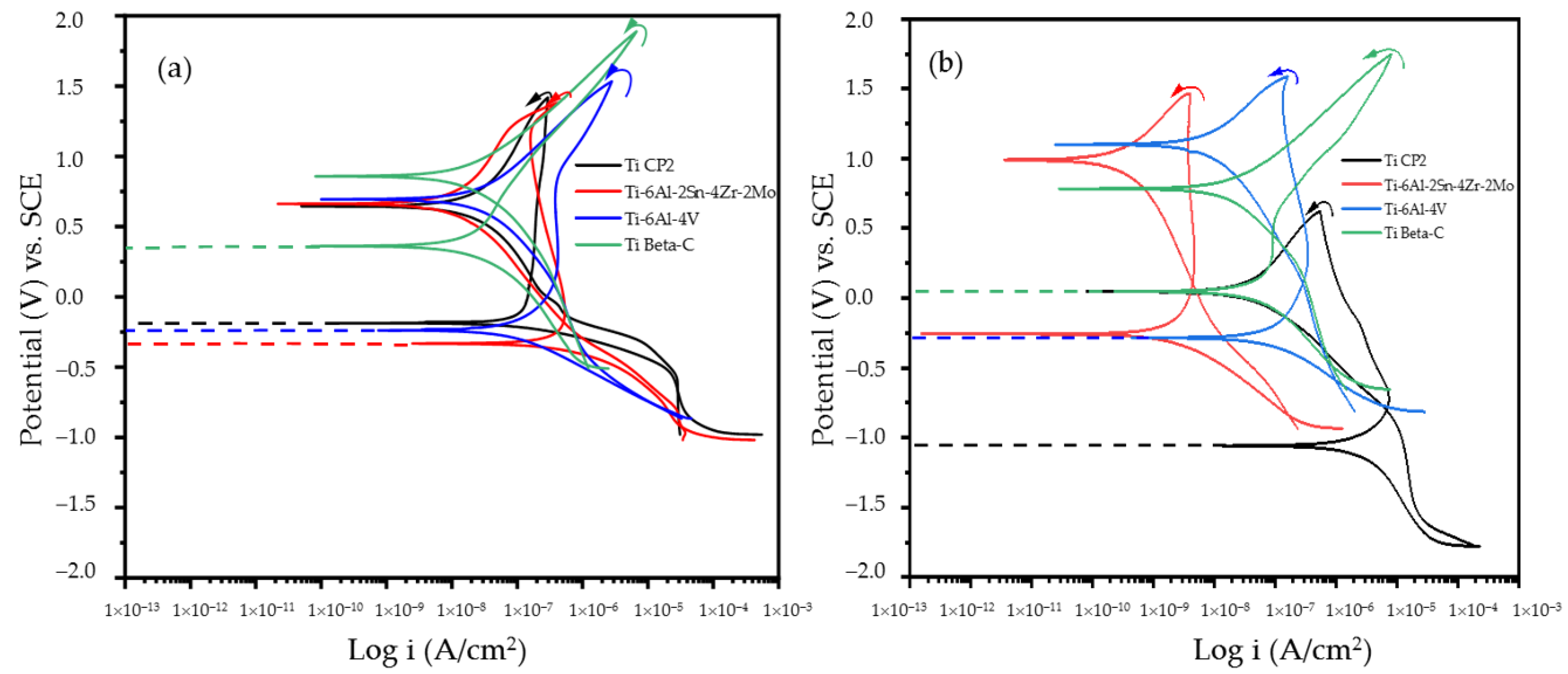
3.3. Cyclic Potentiodynamic Polarization (CPP)
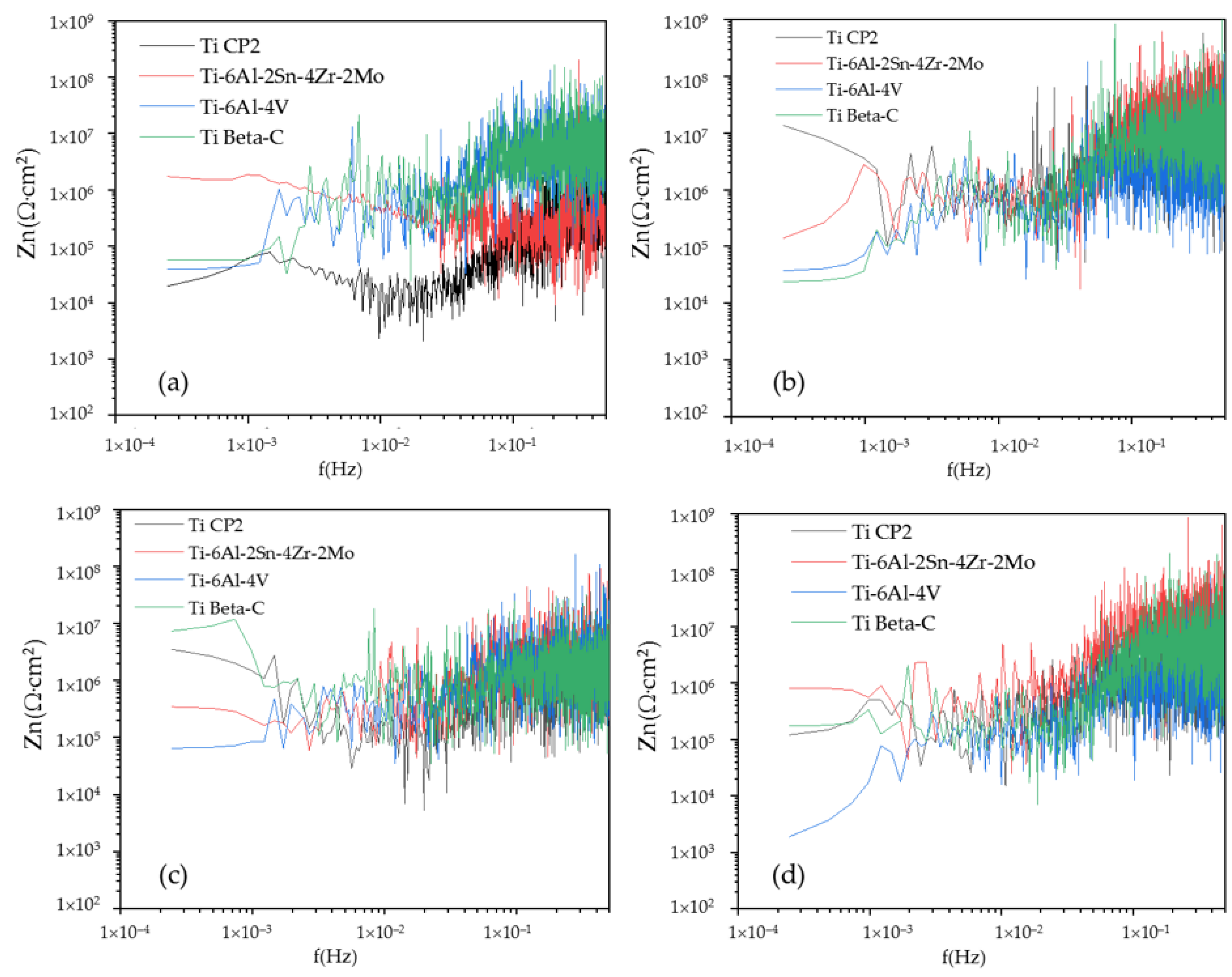
3.4. Electrochemical Noise
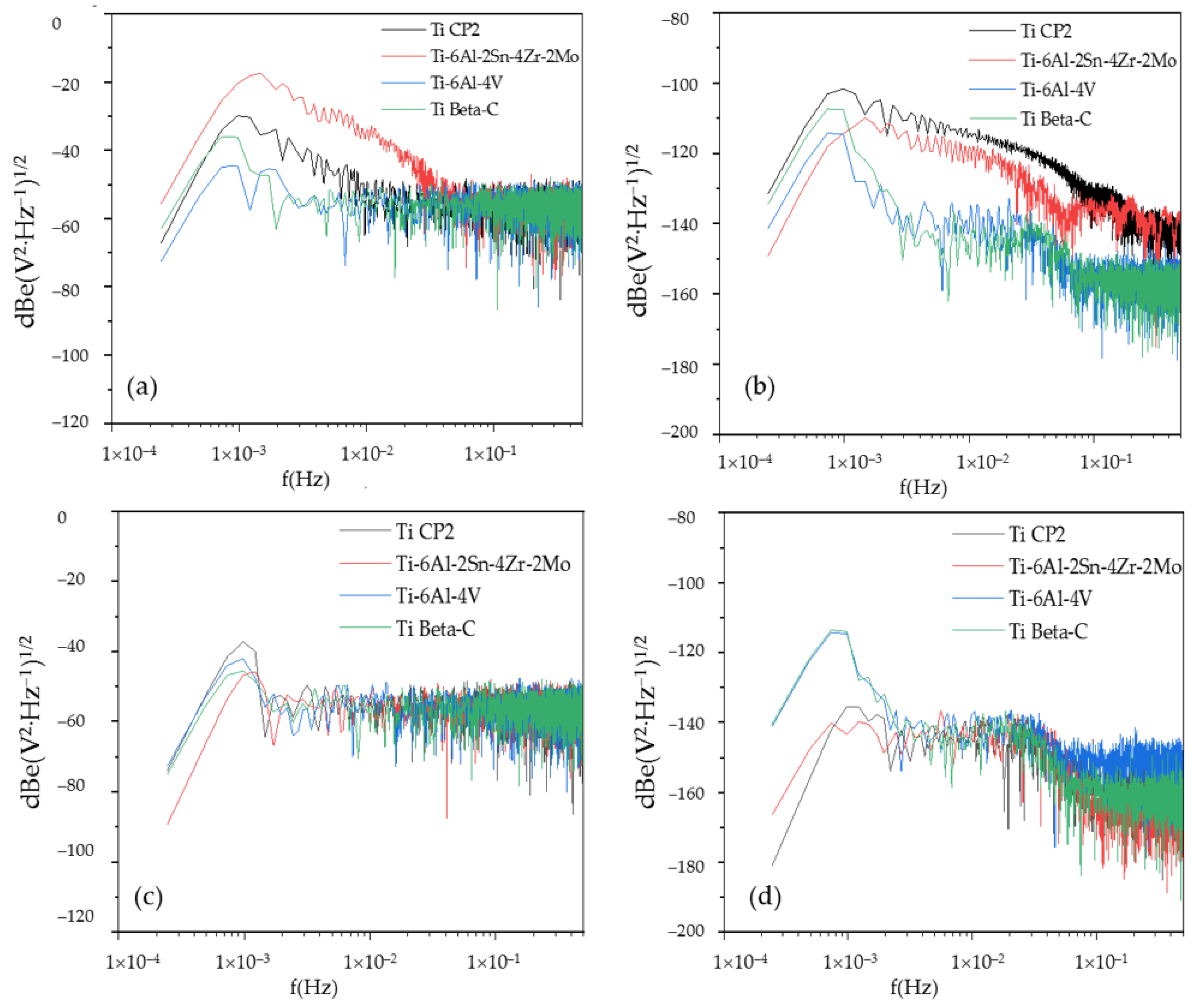
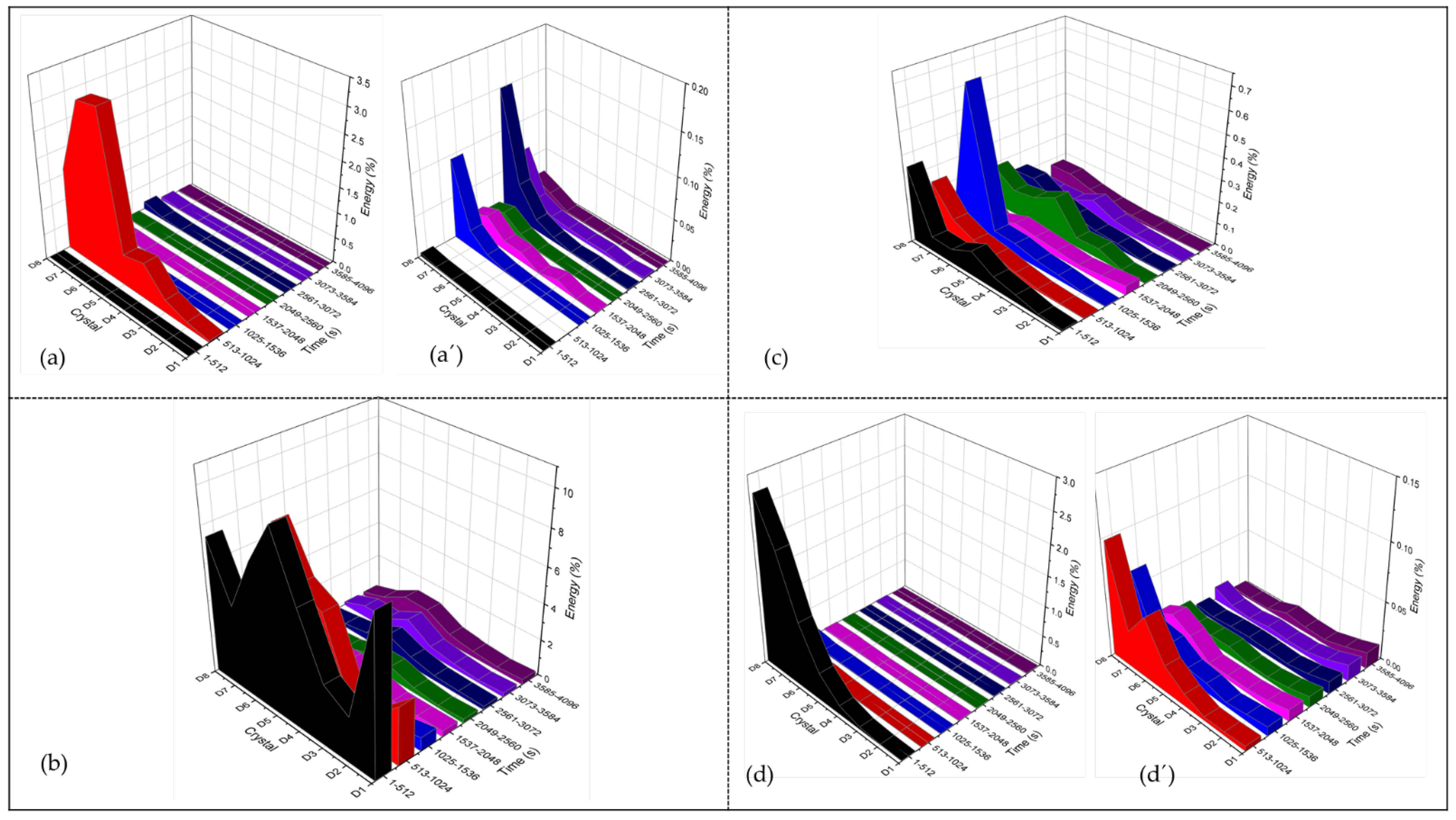
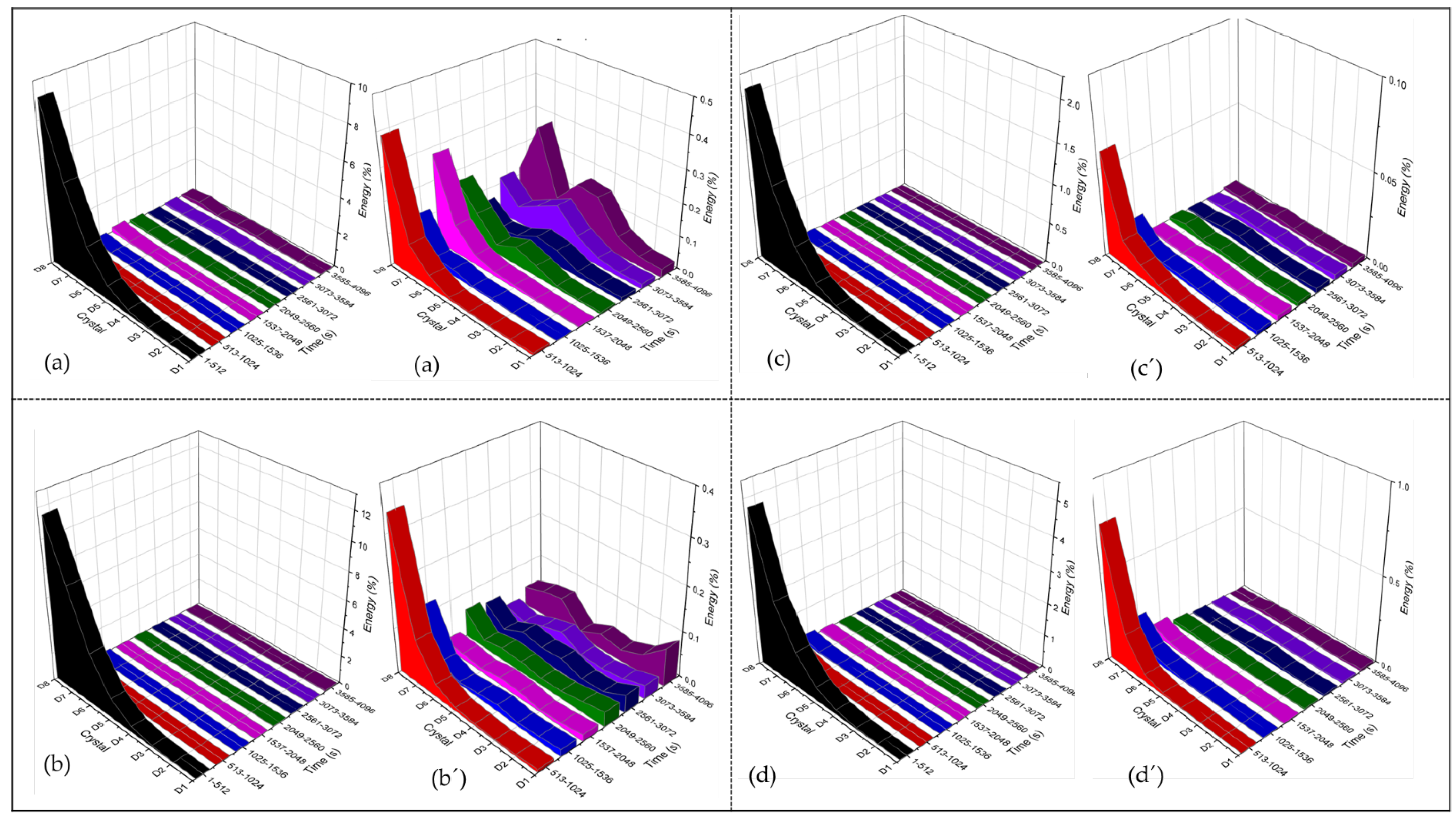
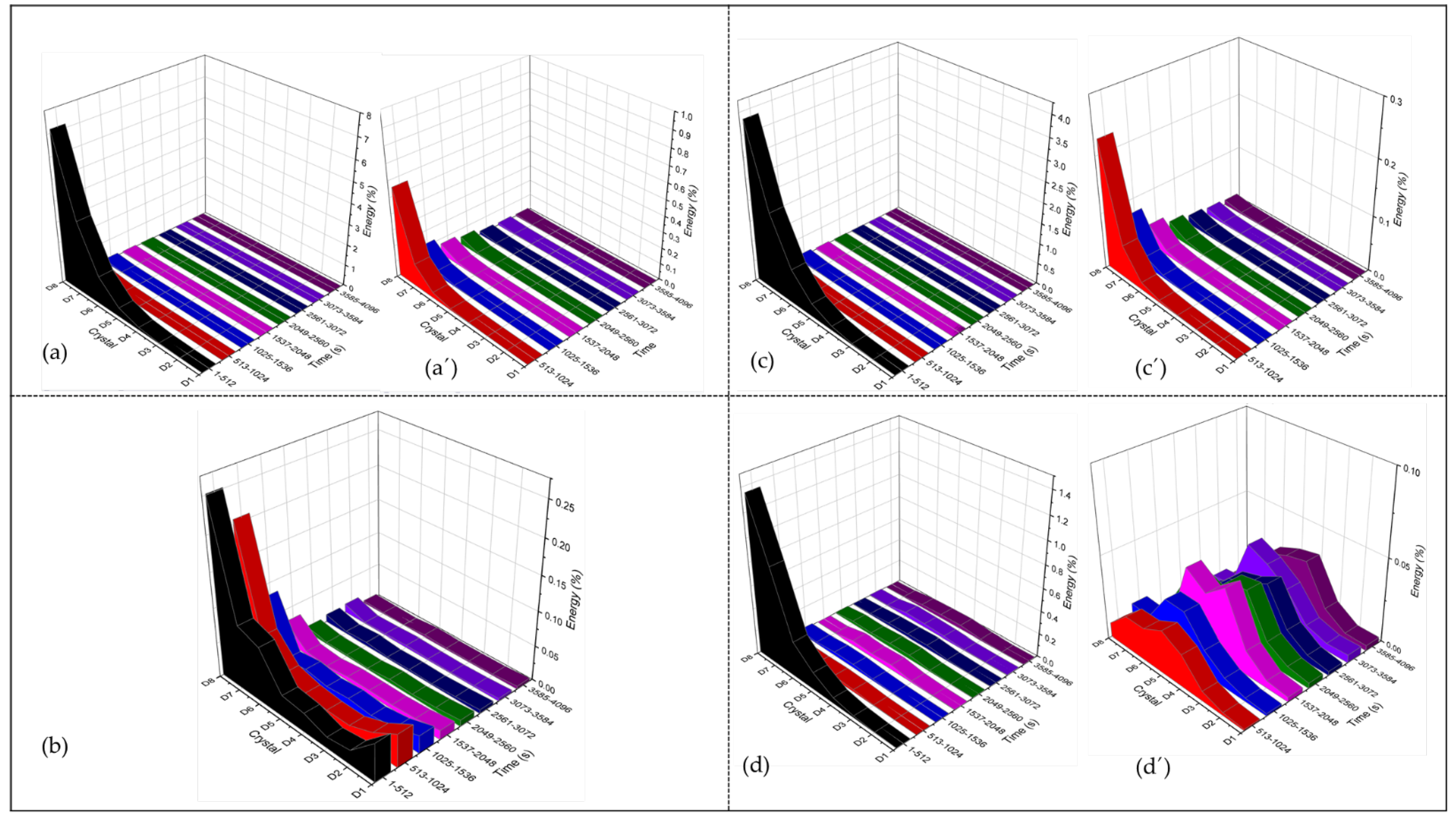
3.4.1. PSD Analysis
3.4.2. Wavelets Analysis
4. Discussion
5. Conclusions
- The results indicated that the titanium alloys anodized in H2SO4 showed a homogenous surface morphology with fine pores. However, samples anodized in H3PO4 showed a heterogeneous distribution with larger pore sizes but with fewer pores. This behavior is related to the reaction of oxygen in the H3PO4 bath. Additionally, pore nucleation is associated with a higher pH value in the H2SO4 bath.
- The percentage of porosity decreased in alloys with a higher amount of beta stabilizers.
- CPP results revealed that the Ti Beta-C alloy had a higher corrosion resistance but a lower passivity range than the other anodized alloys; this behavior may be related to the formation of cracks in the anodized surface. Although the EN technique shows a stable behavior in wavelets analysis, and since this is a non-perturbative technique, the energy necessary to start the anodizing dissolution was not enough. However, the anodizing in H3PO4 showed higher activity in the middle crystal than in the H2SO4 bath because pores are larger, generating diverse reactions more easily.
- The CPP technique indicated that for anodized titanium and anodized titanium alloys, lower corrosion current densities (icorr), and negative hysteresis was observed for all samples. Thus, the process provides excellent corrosion resistance.
- Increasing porosity in titanium alloys increases the corrosion kinetics, particularly if pores are tiny. This behavior is observed in a more significant way for the anodized Ti-6Al-2Sn-4Zr-2Mo alloy in H2SO4, which presented higher porosity, fine pores, and an increase in the corrosion kinetics.
- Results show that the roughness of the anodize increase with the presence of beta-phase forming alloying elements in titanium.
- Electrochemical noise is a suitable technique to characterize the corrosion behavior, as well as the uniformity of the anodizing surface. The technique can also determine how homogenous the anodized layer is in the first seconds of the process. The wavelet method (in the time-frequency domain) allows us to determine that the energy accumulated in the middle crystal may be related to a heterogeneous pore distribution.
- Noise impedance (Zn) shows similar behavior for Ti-6Al-2Sn-4Zr-2Mo (anodized on H3PO4) and Ti Beta-C (anodized in both media) in relation to corrosion resistance as determined by the CPP technique. Therefore, the application of both methods is suitable to determine the corrosion kinetics of passive systems.
- The results by PSD in current for the anodized Ti CP2 and Ti-6Al-4V alloys showed the higher dissolution. These results correlate well with those obtained with the CPP technique.
- Anodization of Ti CP2 and Ti-6Al-4V alloys exposed in both media (NaCl and H2SO4) showed homogenous porosity and the largest pore size. This characteristic may be associated with the high dissolution of the anodizing shown by the PSD in current and CPP methods.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2019, 13, 28. [Google Scholar] [CrossRef]
- Gloria, A.; Montanari, R.; Richetta, M.; Varone, A. Alloys for aeronautic applications: State of the art and perspectives. Metals 2019, 9, 662. [Google Scholar] [CrossRef] [Green Version]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Lleyends, C. Titanium alloys for aerospace applications. Adv. Eng. Mater. 2003, 5, 419. [Google Scholar] [CrossRef]
- Veiga, C.; Davim, J.P.; Loureiro, A.J.R. Properties and applications of titanium alloys: A brief review. Rev. Adv. Mater. Sci. 2012, 32, 133. [Google Scholar]
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: A scoping review. J. Periodontal Res. 2017, 52, 946–954. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.H.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C.M. Can degradation products released from dental implants affect peri-implant tissues? J. Periodontal Res. 2017, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.D.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti-6Al-4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Alam, M.J.; Cameron, D.C. Preparation and characterization of TiO2 thin films by sol-gel method. J. Sol-Gel Sci. Technol. 2002, 25, 137–145. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Codeluppi, S.; Cordioli, A.; Pedeferri, M.P. Effect of thermal oxidation on titanium oxides characteristics. J. Exp. Nanosci. 2009, 4, 365–372. [Google Scholar] [CrossRef]
- Lobl, P.; Huppertz, M.; Mergel, D. Nucleation and growth in TiO2 films prepared by sputtering and evaporation. Thin Solid Film 1994, 251, 72–79. [Google Scholar] [CrossRef]
- Dziewonski, P.M.; Grzeszczuk, M. Deposition of thin TiO2 layers on platinum by means of cyclic voltammetry of selected complex Ti (IV) media leading to anatase. Electrochim. Acta 2009, 54, 4045–4055. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, R.; Sun, J.; Liu, S. Oxidation mechanism of biomedical titanium alloy surface and experiment. Int. J. Corros. 2020, 2020, 1678615. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Reactivity of porous titanium oxidefilm and chitosan layer electrochemically formed on Ti-6Al-4V alloy in biological solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface modification of titanium and titanium alloys: Technologies, developments, and future interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Dias Corpa Tardelli, J.; Lima da Costa Valente, M.; Theodoro de Oliveira, T.; Cândido dos Reis, A. Influence of chemical composition on cell viability on titanium surfaces: A systematic review. J. Prosthet. Dent. 2021, 125, 421–425. [Google Scholar] [CrossRef]
- Peñarrieta-Juanito, G.; Sordi, M.B.; Henriques, B.; Dotto, M.E.R.; Teughels, W.; Silva, F.S.; Magini, R.S.; Souza, J.C.M. Surface damage of dental implant systems and ions release after exposure to fluoride and hydrogen peroxide. J. Periodontal Res. 2018, 54, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Michalska-Domanska, M.; Łazinska, M.; Łukasiewicz, J.; Mol, J.M.C.; Durejko, T. Self-organized anodic oxides on titanium alloys prepared from glycol- and glycerol-based electrolytes. Materials 2020, 13, 4743. [Google Scholar] [CrossRef]
- Gulati, K.; Sinn Aw, M.; Findlay, D.; Losic, D. Local drug delivery to the bone by drug-releasing implants: Perspectives of nano-engineered titania nanotube arrays. Ther. Deliv. 2012, 3, 857–873. [Google Scholar] [CrossRef]
- Luz, A.R.; Santos, L.S.; Lepienski, C.M.; Kuroda, P.B.; Kuromoto, N.K. Characterization of the morphology, structure and wettability of phase dependent lamellar and nanotube oxides on anodized Ti-10Nb alloy. Appl. Surf. Sci. 2018, 448, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Dikicia, T.; Erola, M.; Toparlia, M.; Celika, E. Characterization and photocatalytic properties of nanoporous titanium dioxide layer fabricated on pure titanium substrates by the anodic oxidation process. Ceram. Int. 2014, 40, 1587–1591. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion behavior of AISI 409Nb stainless steel manufactured by powder metallurgy exposed in H2SO4 and NaCl solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Hai, L.; Gou-qiang, X.; Pan, Z.; Hua-sen, Z.; Kan, M.Y. The Hilbert–Huang transform-based denoising method for the TEM response of a PRBS source signal. Pure Appl. Geophys. 2016, 173, 2777–2789. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Igual-Muñoz, A.; García Antón, J.; García-García, D. Galvanic corrosion of titanium coupled to welded titanium in LiBr solutions at different temperatures. Corros. Sci. 2009, 51, 1095–1102. [Google Scholar] [CrossRef]
- Wang, Z.B.; Hu, H.X.; Zheng, Y.G. Synergistic effects of fluoride and chloride on general corrosion behavior of AISI 316 stainless steel and pure titanium in H2SO4 solutions. Corros. Sci. 2018, 130, 203–217. [Google Scholar] [CrossRef]
- Rao, B.M.; Torabi, A.; Varghese, O.K. Anodically grown fuctional oxide nanotubes and applications. MRS Commun. 2016, 6, 375–396. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Corrosion behavior and surface characterization of titanium in solution containing fluoride and albumin. Biomaterials 2005, 26, 829–837. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Jaroenworaluck, A.; Regonini, D.; Bowen, C.; Stevens, R.; Allsopp, D. Macro, micro and nanostructure of TiO2 anodised films prepared in a fluorine-containing electrolyte. J. Mater. Sci. 2007, 42, 6729–6734. [Google Scholar] [CrossRef]
- Jelliti, S.; Richard, C.; Retraint, D.; Roland, T.; Chemkhi, M.; Demangel, C. Effect of surface nanocrystallization on the corrosion behavior of Ti-6Al-4V titanium alloy. Surf. Coat. Technol. 2013, 224, 82–87. [Google Scholar] [CrossRef]
- Ji, R.; Wang, B.; Jin, H.; Liu, Y.; Cheng, W.; Cai, B.; Li, X. Removing loose oxide layer and producing dense α-phase layer simultaneously to improve corrosion resistance of Ti-6Al-4V titanium alloy by coupling electrical pulse and ultrasonic treatment. Surf. Coat. Technol. 2020, 384, 125329. [Google Scholar] [CrossRef]
- Risking, J.; Khentov, A. Electrocorrosion and Protection of Metals, 2nd ed.; Risking, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–248. [Google Scholar]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical corrosion of titanium and titanium-based alloys. J. Prothet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef]
- Pink, H.; Rui, S.; Kuaishe, W.; Fan, Y.; Boliang, H.; Zhen-Lu, C.; Qinwe, L.; Weicheng, C.; Dongxin, L.; Lei, G.; et al. Electrochemical corrosion behavior of titanium-zirconium-molybdenum alloy. Rare Met. Mater. Eng. 2017, 46, 1225–1230. [Google Scholar] [CrossRef] [Green Version]
- Jaquez-Muñoz, J.; Gaona-Tiburcio, C.; Lira-Martinez, A.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Samaniego-Gamez, O.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderon, F. Susceptibility to pitting corrosion of Ti-CP2, Ti-6Al-2Sn-4Zr-2Mo, and Ti-6Al-4V alloys for aeronautical applications. Metals 2021, 11, 1002. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical noise analysis of the corrosion of titanium alloys in NaCl and H2SO4 solutions. Metals 2021, 11, 105. [Google Scholar] [CrossRef]
- Galván-Martínez, R.; Cabrera-de la Cruz, D.; Contreras, A.; Orozco-Cruz, R. A novel experimental arrangement for corrosión study of X60 pipeline steel weldments at turbulent flow conditions. Corros. Eng. Sci. Technol. 2016, 51, 400–407. [Google Scholar] [CrossRef]
- Galván-Martinez, R.; Orozco-Cruz, R.; Torres-Sanchez, R.; Martinez, E.A. Corrosion study of the X52 steel immersed in seawater with a corrosion inhibitor using a rotating cylinder electrode. Mater. Corros. 2010, 61, 872–876. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Noel, J.J.; Shoesmith, D.W.; Ebrahimi, N. Corrosion of Titanium, and Its Alloys, 1st ed.; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 193–199. [Google Scholar]
- Seo, D.; Lee, J.B. Effects of competitive anion adsorption (Br− or Cl−) and semiconducting properties of the passive films on the corrosion behavior of the additively manufactured Ti-6Al-4V alloys. Corros. Sci. 2020, 173, 108789. [Google Scholar] [CrossRef]
- Tafel, J. Über die polarization bei kathodischer wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Attabi, S.; Mokhtari, M.; Taibi, Y.; Abdel-Rahman, I.; Hafez, B.; Elmsellem, H. Electrochemical and tribological behavior of surface-treated titanium alloy Ti-6Al-4V. J. Bio-Tribo-Corros. 2019, 5, 2. [Google Scholar] [CrossRef]
- Orazem, E.N.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Wagner, C.; Traud, W. Über die Deutung von Korrosionsvorgängen durch Überlagerung von Elektrochemischen Teilvorgängen und über die Potentialbildung an Mischelektroden, Z. Elektrochem; Springer: Berlin/Heidelberg, Germany, 1951; pp. 391–454. [Google Scholar]
- Butler, J.A.V. Studies in heterogeneous equilibria. Part II—The kinetic interpretation on the Nernst theory of electromotive force. Trans. Faraday Soc. 1924, 19, 729–733. [Google Scholar] [CrossRef]
- Monticelli, C. Evaluation of corrosion inhibitors by electrochemical noise analysis. J. Electrochem. Soc. 1992, 139, 706. [Google Scholar] [CrossRef]
- Park, C.J.; Kwon, H.S. Electrochemical noise analysis of localized corrosion of duplex stainless steel aged at 475 °C. Mater. Chem. Phys. 2005, 91, 355–360. [Google Scholar] [CrossRef]
- Suresh, G.U.; Kamachi, M.S. Electrochemical noise analysis of pitting corrosion of type 304L stainless steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef]
- Cottis, R.; Turgoose, S.; Mendoza-Flores, J. The effects of solution resistance on electrochemical noise resistance measurements: A theorical analysis. In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: West Conshohocken, PA, USA, 1996; pp. 93–100. [Google Scholar]
- Eden, D.A. Electrochemical noise—The first two octaves. In Corrosion/98; NACE: San Diego, CA, USA, 1998; pp. 1–31. [Google Scholar]
- Coakley, J.; Vorontsov, V.A.; Littlell, K.C.; Heenan, R.K.; Ohnuma, G.; Jones, N.G.; Dye, D. Nanoprecipitation in a beta-titanium alloy. J. Alloys Compd. 2015, 623, 146. [Google Scholar] [CrossRef] [Green Version]
- ASTM E3-95. Standard Practice for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- ASTM E407-07. Standard Practice for Microetching Metals and Alloys; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- AMS2487. Anodic Treatment of Titanium and Titanium Alloys Solution pH 12.4 Maximum; SAE International: Warrendaly, PA, USA, 2018. [Google Scholar]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion resistance of hard coat anodized AA 6061 in citric–sulfuric solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Estupiñan-Lopez, H.F.; Pedraza-Basulto, G.; Poblano-Salas, C. Parameter studies on high-velocity oxy-fuel spraying of CoNiCrAlY coatings used in the aeronautical industry. Int. J. Corros. 2014, 2014, 703806. [Google Scholar] [CrossRef]
- ASTM G5-11. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM G61-86 (2018). Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado, E.M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to nitric acid passivation of 15-5 and 17-4PH stainless steel using electrochemical techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef] [PubMed]
- Homborg, A.M.; Cottis, R.A.; Mol, J.M.C. An integrated approach in the time, frequency and time-frequency domain for the identification of corrosion using electrochemical noise. Electrochim. Acta 2016, 222, 627–640. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Aguilar, L.M.R.; Zambrano-Robledo, P.; Estupiñán-López, F.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Castillo-González, E.; Almeraya-Calderón, F. Electrochemical noise analysis of nickel based superalloys in acid solutions. Int. J. Electrochem. Sci. 2014, 9, 523–533. [Google Scholar]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical noise measurements of advanced high-strength steels in different solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Dawson, D.L. Electrochemical noise measurement: The definitive in-situ technique for corrosion applications? In Electrochemical Noise Measurement for Corrosion Applications STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Reirchert, D.L., Dawson, L., Eds.; ASTM International, Materials Park: West Conshohocken, PA, USA, 1996; pp. 3–39. [Google Scholar]
- ASTM G199-09. Standard Guide for Electrochemical Noise Measurement; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Froes, F.; Quian, M. Titanium for Consumer Applications. Real World Use of Titanium; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 27–65. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Mehdipour, M.; Naderi, R.; Markhali, B.P. Electrochemical study of effect of the concentration of azole derivatives on corrosion behavior of stainless steel in H2SO4. Prog. Org. Coat. 2014, 77, 1761–1767. [Google Scholar] [CrossRef]
- Bertocci, U.; Huet, F. Noise analysis applied to electrochemical systems. Corrosion 1995, 51, 131–144. [Google Scholar] [CrossRef]
- Bertucci, U.; Gabrielli, C.; Huet, F.; Keddam, M.; Rousseau, P. Noise resistance applied to corrosion measurements: II. Experimental tests. J. Electrochem. Soc. 1997, 144, 37. [Google Scholar] [CrossRef]
- Xia, D.-H.; Song, S.-Z.; Behnamian, Y. Detection of corrosion degradation using electrochemical noise (EN): Review of signal processing methods for identifying corrosion forms. Corros. Eng. Sci. Technol. 2016, 51, 527–544. [Google Scholar] [CrossRef]
- Lee, C.C.; Mansfeld, F. Analysis of electrochemical noise data for a passive system in the frequency domain. Corros. Sci. 1998, 40, 959–962. [Google Scholar] [CrossRef]
- Legat, A.; Dolecek, V. Corrosion monitoring system based on measurement and analysis of electrochemical noise. Corrosion 1995, 51, 295–300. [Google Scholar] [CrossRef]
- Estupiñán-López, H.F.; Almeraya-Calderón, F.; Bautista Margulis, G.R.; Baltazar Zamora, M.A.; Martínez-Villafañe, A.; Uruchurtu, C.J.; Gaona-Tiburcio, C. Transient analysis of electrochemical noise for 316 and duplex 2205 stainless steels under pitting corrosion. Int. J. Electrochem. Sci. 2011, 6, 1785–1796. [Google Scholar]
- Xia, D.; Qin, Z.; Song, Z.; Macdonald, D.; Luo, J. Combating marine corrosion on engineered oxide surface by repelling, blocking and capturing Cl−: Mini review. Corros. Commun. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Corral-Higuera, R.; Arredondo-Rea, P.; Neri-Flores, M.A.; Gómez-Soberón, J.M.; Almaral-Sánchez, J.L.; Castorena-González, J.C.; Almeraya-Calderón, F. Chloride ion penetrability and corrosion behavior of steel in concrete with sustainability characteristics. Int. J. Electrochem. Sci. 2011, 6, 958–970. [Google Scholar]
- Martinez-Villafañe, A.; Chacon-Nava, J.G.; Gaona-Tiburcio, C.; Almeraya-Calderon, F.; Domínguez-Patiño, G.; Gonzalez-Rodríguez, G. Oxidation performance of a Fe–13Cr alloy with additions of rare earth elements. Mater. Sci. Eng. A 2003, 363, 15–19. [Google Scholar] [CrossRef]
- Corral, H.R.; Arredondo, R.S.P.; Neri, F.M.; Gómez, S.J.M.; Almeraya, C.F.; Castorena, G.J.H.; Almaral, S.J. Sulfate attack and reinforcement corrosion in concrete with recycled concrete aggregates and supplementary cementing materials. Int. J. Electrochem. Sci. 2011, 6, 613–621. [Google Scholar]
- Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G.; Campillo, B.; Gaona-Tiburcio, C.; Dominguez-Patiño, G.; Leduc Lezama, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. An electrochemical study of the corrosion behavior of a dual phase steel in 0.5m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786–1798. [Google Scholar]
- Pan, C.; Wang, X.; Behnamian, Y.; Wu, Z.; Qin, Z.; Xia, D.; Hu, W. Monododecyk phosphate film on LY12 aluminum alloy: pH-Controlled self-assembly and corrosion resistance. J. Electrochem. Soc. 2020, 167, 164510. [Google Scholar] [CrossRef]
- Prando, D.; Branna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 1: Aggressive environments and man forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kim, M.; Jung, G.; Vang, M.; Park, Y. The effects of spark anodizing treatment of pure titanium metals and titanium alloys on corrosion characteristics. Surf. Coat. Technol. 2007, 201, 8738–8745. [Google Scholar] [CrossRef]
- Homborg, A.M.; Tinga, T.; Zhang, X.; Van Westing, E.P.M.; Ferrari, G.M.; Wit, J.H.W.; Mol, J.M.W. A critical appraisal of the interpretation of electrochemical noise for corrosion studies. Corrosion 2017, 70, 971–987. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Attarzadeh, F.R.; Vakili-Azghandi, M. Effect of multi-pass friction stir processing on the electrochemical and corrosion behavior of pure titanium in strongly acidic solutions. Metall. Mater. Trans. A 2017, 48, 403–411. [Google Scholar] [CrossRef]
- Martínez, C.; Guerra, C.; Silva, D.; Cubillos, M.; Briones, F.; Muñoz, L.; Páez, M.A.; Aguilar, C.; Sancy, M. Effect of porosity on mechanical and electrochemical properties of Ti-6Al-4V alloy. Electrochim. Acta 2020, 338, 135858. [Google Scholar] [CrossRef]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure commercial titanium color anodizing and corrosion resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Prando, D.; Nicolis, D.; Pedeferri, M.; Ormellese, M. Pitting corrosion on anodized titanium: Effect of halides. Mater. Corros. 2018, 29, 1441–1446. [Google Scholar] [CrossRef]
- Prando, D.; Branna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M. Corrosion of titanium: Part 1: Effects of surface treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Prando, D.; Branna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M. Electrochemical anodizing treatment to enhance localized corrosion resistance of pure titanium. J. Appl. Biomater. Funct. Mater. 2017, 15, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.; Hung, Y.; Hong, T.; Wu, C.; Kuo, T.; Lee, T.; Liao, T.; Lin, H.; Chuang, C. Preparation and characterization of porous bioceramic layers on pure titanium surface obtained by micro-arc oxidation process. Appl. Phys. A 2017, 123, 204. [Google Scholar] [CrossRef]
- Chamanzadeh, Z.; Noormohammadi, M.; Zahedifar, M. Self-organized and uniform TiO2 nanotube arrays with optimized NH4F concentration in electrolyte by high voltage electrochemical anodization. Mater. Res. Express 2018, 5, 55025. [Google Scholar] [CrossRef]
- Mizukoshi, Y.; Masahashi, N. Fabrication of a TiO2 photocatalyst by anodic oxidation of Ti in an acetic acid electrolyte. Surf. Coat. Technol. 2014, 240, 226–232. [Google Scholar] [CrossRef]
- Laurindo, C.A.H.; Torres, R.D.; Mali, S.A.; Gilbert, J.L.; Soares, P. Incorporation of Ca and P on anodized titanium surface: Effect of high current density. Mater. Sci. Eng. C 2014, 37, 223–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, H.; Ding, X.; Yan, Q.; Wang, L.; Ma, W. Simulation of anodizing current-time curves and morphology evolution of TiO2 nanotubes anodized in electrolytes with different NH4F concentrations. Electrochim. Acta 2015, 176, 1083–1091. [Google Scholar] [CrossRef]
- Mazzarolo, A.; Curioni, M.; Vicenzo, A.; Skeldon, P.; Thompson, G.E. Anodic growth of titanium oxide: Electrochemical behaviour and morphological evolution. Electrochim. Acta 2012, 75, 288–295. [Google Scholar] [CrossRef]
- Matykina, E.; Montuori, M.; Gough, J.; Monfor, F.; Berkani, A.; Skeldon, P.; Thomson, G.E.; Habazaki, H. Spark anodising of titanium for biomedical applications. Int. J. Surf. Eng. Coat. 2006, 84, 125–133. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pedeferri, M.P. EVect of anodic oxidation parameters on the titanium oxides formatio. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]





| Elements | Ti CP2 | Alloys | ||
|---|---|---|---|---|
| Ti-6Al-2Sn-4Zr-2Mo | Ti-6Al-4V | Ti Beta-C | ||
| Fe | 0.038 ± 0.005 | – | 0.21 ± 0.01 | 0.08 ± 0.01 |
| Al | – | 6.75 ± 0.20 | 7.14 ± 0.37 | 4.2 ± 0.13 |
| V | – | – | 4.03 ± 0.08 | 8.1 ± 0.07 |
| Zr | – | 4.18 ± 0.01 | – | 4.3 ± 0.01 |
| Cr | – | – | – | 3.3 ± 0.07 |
| Mo | – | 1.99 ± 0.008 | – | 3.9 ± 0.01 |
| Sn | – | 2.08 ± 0.01 | – | – |
| Ti | 99.94 ± 0.005 | 84.65 ± 0.19 | 87.71 ± 0.36 | 75.2 ± 0.14 |
| Alloy | Ecorr (V) | icorr (A/cm2) | Active–Passive Trans (V) | Hysteresis | Range Passive (V) | Passive Breakdown (V) |
|---|---|---|---|---|---|---|
| Titanium and alloys anodized in H2SO4 | ||||||
| 3.5 wt % NaCl | ||||||
| Ti CP2 | 0.19 | 1.04 × 10−7 | – | Negative | 0.85 | 0.83 |
| Ti-6Al-2Sn-4Zr-2Mo | −0.33 | 3.07 × 10−7 | – | Negative | 1.29 | 1.19 |
| Ti-6Al-4V | −0.24 | 6.65 × 10−8 | – | Negative | 0.48 | 0.77 |
| Ti Beta-C | 0.15 | 9.12 × 10−9 | – | Negative | – | – |
| 3.5 wt % H2SO4 | ||||||
| Ti CP2 | 0.07 | 7.98 × 10−7 | – | Negative | 0.94 | 1.53 |
| Ti-6Al-2Sn-4Zr-2Mo | −0.31 | 1.16 × 10−5 | – | Negative | 0.96 | 1.02 |
| Ti-6Al-4V | −0.14 | 1.69 × 10−6 | – | Negative | 1.27 | 1.28 |
| Ti Beta-C | 0.31 | 4.1 × 10−8 | – | Negative | 0.35 | 0.96 |
| Titanium and alloys anodized in H3PO4 | ||||||
| 3.5 wt % NaCl | ||||||
| Ti CP2 | −1.05 | 1.06 × 10−7 | – | Negative | 1.26 | 0.59 |
| Ti-6Al-2Sn-4Zr-2Mo | −0.25 | 5.09 × 10−7 | – | Negative | 1.24 | 1.42 |
| Ti-6Al-4V | −0.28 | 4.24 × 10−7 | – | Negative | 1.17 | 1.44 |
| Ti Beta-C | 0.05 | 2.24 × 10−8 | – | Negative | 0.20 | 0.48 |
| 3.5 wt % H2SO4 | ||||||
| Ti CP2 | −0.34 | 3.43 × 10−8 | – | Negative | 1.42 | 1.28 |
| Ti-6Al-2Sn-4Zr-2Mo | 0.01 | 3.24 × 10−8 | – | Negative | 1.6 | 1.75 |
| Ti-6Al-4V | −0.31 | 4.60 × 10−7 | – | Negative | 1.25 | 1.78 |
| Ti Beta-C | 0.22 | 3.1 × 10−8 | – | Negative | 0.37 | 0.94 |
| Corrosion Type | dB(V)·Decade−1 | dB(A)·Decade−1 | ||
|---|---|---|---|---|
| Minimum | Maximum | Minimum | Maximum | |
| Uniform | 0 | −7 | 0 | −7 |
| Pitting | −20 | −25 | −7 | −14 |
| Passive | −15 | −25 | −1 | 1 |
| Titanium and Alloys Anodized in H2SO4 | ||||
| 3.5 wt % NaCl | ||||
| Alloys | Ψ0 (dBi) | Β (dB (V)) | Β (dB (A)) | Zn0 (Ω·cm2) |
| Ti CP2 | −131.48 | −2.13 | −16.34 | 19.46 × 103 |
| Ti-6Al-2Sn-4Zr-2Mo | −149.19 | −8.50 | −9.00 | 17.29 × 105 |
| Ti-6Al-4V | −141.30 | −0.10 | −8.36 | 38.75 × 103 |
| Ti Beta-C | −134.42 | −0.20 | −8.88 | 57.80 × 103 |
| 3.5 wt % H2SO4 | ||||
| Ti CP2 | −159.42 | −1.15 | −10.02 | 34.94 × 105 |
| Ti-6Al-2Sn-4Zr-2Mo | −155.25 | 0.08 | −6.38 | 34.37 × 104 |
| Ti-6Al-4V | −148.33 | 0.44 | −5.12 | 64.72 × 103 |
| Ti Beta-C | −176.70 | 0.07 | −2.59 | 72.97 × 105 |
| Titanium and Alloys Anodized in H3PO4 | ||||
| 3.5 wt % NaCl | ||||
| Alloys | Ψ0 (dBi) | Β (dB (V)) | Β (dB (A)) | |
| Ti CP2 | −180.98 | −0.47 | −6.84 | 13.63 × 106 |
| Ti-6Al-2Sn-4Zr-2Mo | −166.41 | 0.71 | −13.77 | 13.89 × 104 |
| Ti-6Al-4V | −141.21 | 0.08 | −5.74 | 36.98 × 103 |
| Ti Beta-C | −140.51 | 0.39 | −9.97 | 23.68 × 103 |
| 3.5 wt % H2SO4 | ||||
| Ti CP2 | −146.53 | −0.08 | −5.63 | 12.03 × 104 |
| Ti-6Al-2Sn-4Zr-2Mo | −171.24 | 0.99 | −9.53 | 79.92 × 104 |
| Ti-6Al-4V | −135.63 | 1.11 | −7.04 | 18.77 × 102 |
| Ti Beta-C | −154.68 | 0.15 | −12.00 | 17.24 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Delgado, A.D.; Flores-De los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings 2022, 12, 325. https://doi.org/10.3390/coatings12030325
Jáquez-Muñoz JM, Gaona-Tiburcio C, Chacón-Nava J, Cabral-Miramontes J, Nieves-Mendoza D, Maldonado-Bandala E, Delgado AD, Flores-De los Rios JP, Bocchetta P, Almeraya-Calderón F. Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings. 2022; 12(3):325. https://doi.org/10.3390/coatings12030325
Chicago/Turabian StyleJáquez-Muñoz, Jesús Manuel, Citlalli Gaona-Tiburcio, José Chacón-Nava, Jose Cabral-Miramontes, Demetrio Nieves-Mendoza, Erick Maldonado-Bandala, Anabel D. Delgado, Juan Pablo Flores-De los Rios, Patrizia Bocchetta, and Facundo Almeraya-Calderón. 2022. "Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions" Coatings 12, no. 3: 325. https://doi.org/10.3390/coatings12030325
APA StyleJáquez-Muñoz, J. M., Gaona-Tiburcio, C., Chacón-Nava, J., Cabral-Miramontes, J., Nieves-Mendoza, D., Maldonado-Bandala, E., Delgado, A. D., Flores-De los Rios, J. P., Bocchetta, P., & Almeraya-Calderón, F. (2022). Electrochemical Corrosion of Titanium and Titanium Alloys Anodized in H2SO4 and H3PO4 Solutions. Coatings, 12(3), 325. https://doi.org/10.3390/coatings12030325











