Microstructure and Corrosion Resistance of an HVAF-Sprayed Al-Based Amorphous Coating on Magnesium Alloys
Abstract
:1. Introduction
2. Materials and Methods
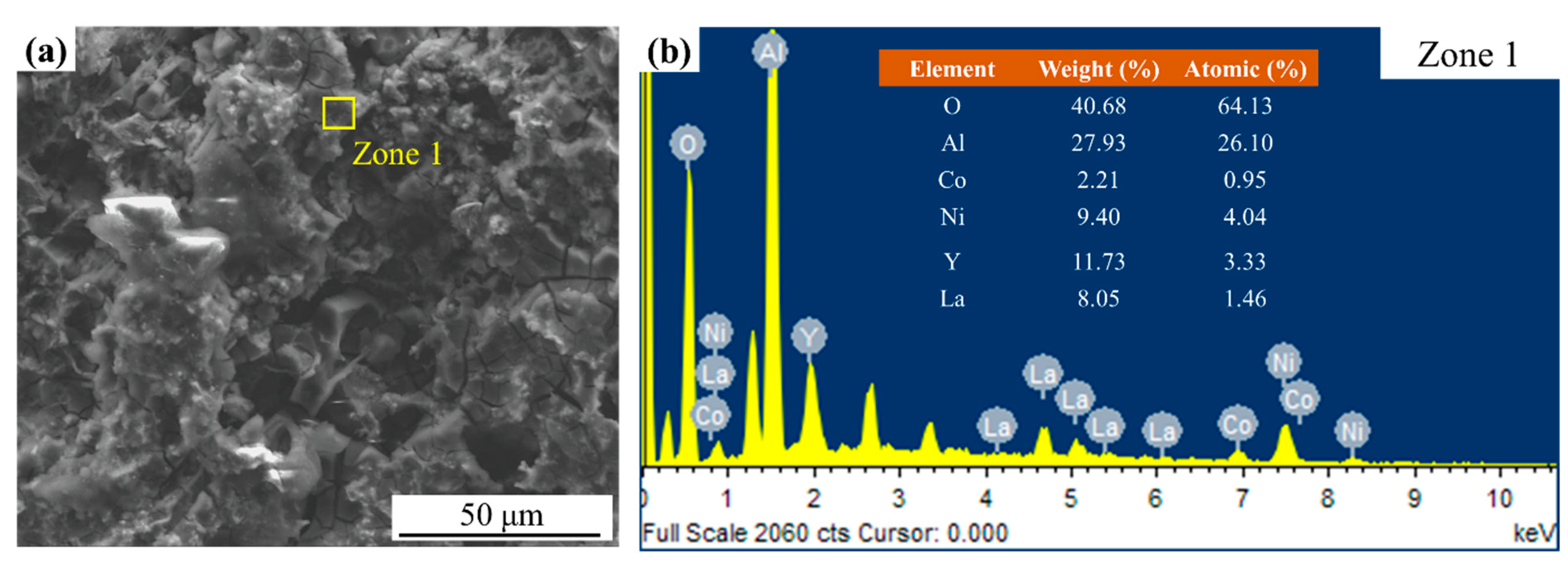
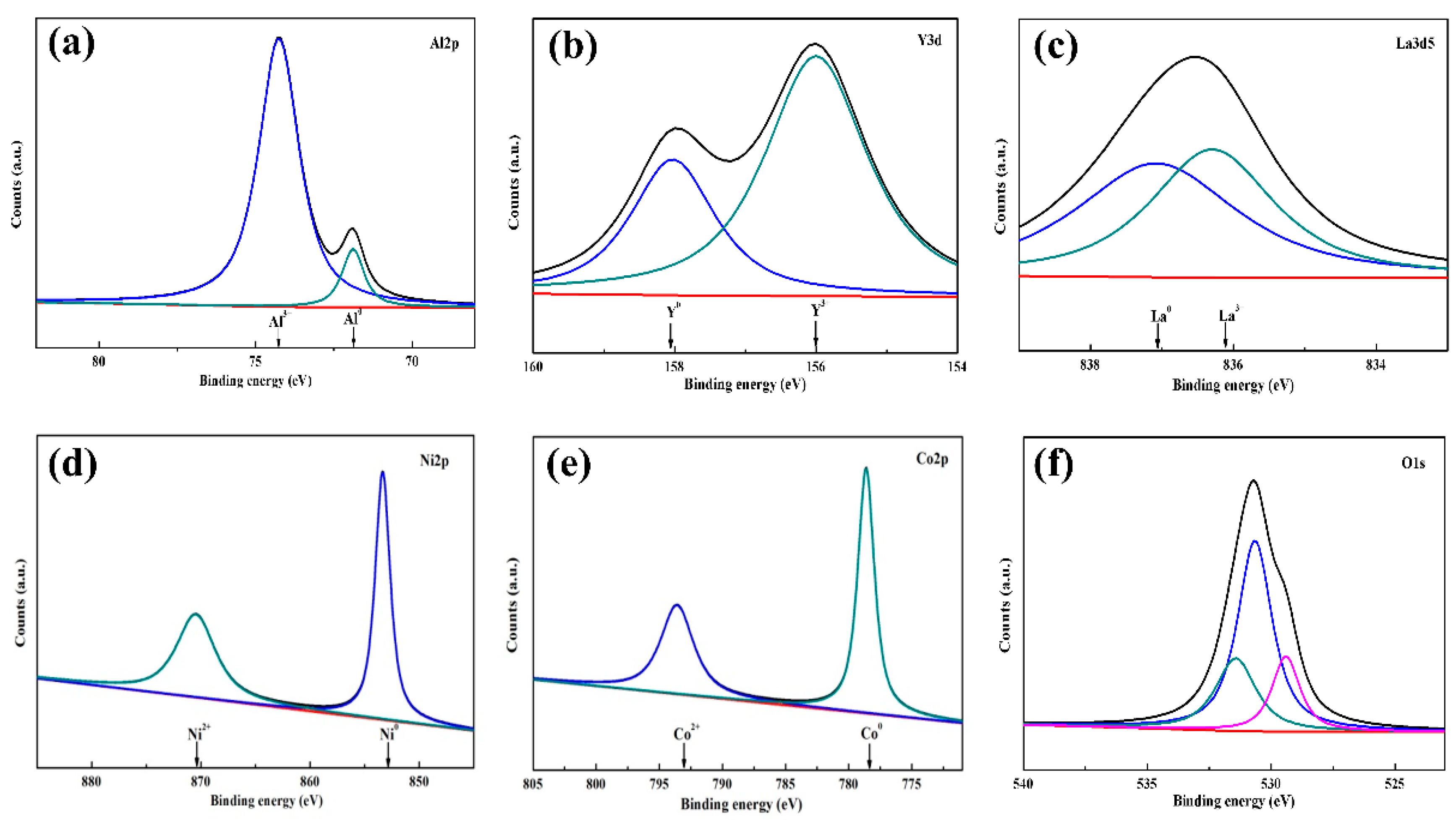
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2021, 3, 104–116. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, X.; Lei, J.; Li, L. Brome-like rare-earth film for durable protection of magnesium alloy. J. Taiwan Inst. Chem. Eng. 2021, 128, 409–416. [Google Scholar] [CrossRef]
- Heimann, R.B. Magnesium alloys for biomedical application: Advanced corrosion control through surface coating. Surf. Coat. Technol. 2021, 405, 126521. [Google Scholar] [CrossRef]
- Fang, R.; Liu, R.; Xie, Z.-H.; Wu, L.; Ouyang, Y.; Li, M. Corrosion-resistant and superhydrophobic nickel-phosphorus/nickel/PFDTMS triple-layer coating on magnesium alloy. Surf. Coat. Technol. 2022, 432, 128054. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, S.; Liang, G.; Tian, G. Improvement in corrosion resistance of micro-arc oxidized AZ91 alloy sealed with cement-mixed paraffin wax. J. Mater. Res. Technol. 2021, 15, 6956–6973. [Google Scholar] [CrossRef]
- Seifzadeh, D.; Farhoudi, L. Electroless Co–P plating on magnesium alloy and its anti-corrosion properties. Surf. Eng. 2016, 32, 348–355. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Xu, A.; Liu, X. Understanding pitting corrosion behavior of AZ91 alloy and its MAO coating in 3.5% NaCl solution by cyclic potentiodynamic polarization. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- Zheng, T.; Hu, Y.; Pan, F.; Zhang, Y.; Tang, A. Fabrication of corrosion-resistant superhydrophobic coating on magnesium alloy by one-step electrodeposition method. J. Magnes. Alloy. 2019, 7, 193–202. [Google Scholar] [CrossRef]
- Zhai, H.; Yuan, H.; Li, W.; Zhang, X.; Li, X.; Cai, A. Corrosion resistance mechanisms of detonation sprayed Fe-based amorphous coating on AZ31B magnesium alloy. J. Non-Cryst. Solids 2021, 576, 121276. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Yang, X.; Hao, S.; Chen, Y.; Li, H.; Pan, D. Interfacial characteristic and microstructure of Fe-based amorphous coating on magnesium alloy. Surf. Coat. Technol. 2021, 425, 127659. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, R.; Xie, L.; Wang, W.; Li, Y.; Wang, S.; Li, H.; Zhang, J. Interfacial bonding mechanism and properties of HVOF-sprayed Fe-based amorphous coatings on LA141 magnesium alloy substrate. Surf. Coat. Technol. 2021, 426, 127801. [Google Scholar] [CrossRef]
- Liang, D.; Ma, J.; Cai, Y.; Liu, X.; Xie, S.; Wei, X.; Xu, G.; Shen, J. Characterization and elevated-temperature tribological performance of AC–HVAF-sprayed Fe-based amorphous coating. Surf. Coat. Technol. 2020, 387, 125535. [Google Scholar] [CrossRef]
- Si, C.; Wu, W. Excellent corrosion resistant amorphous coating prepared by gas atomization followed by AC-HVAF spray technology. Mater. Res. Express 2019, 6, 055202. [Google Scholar] [CrossRef]
- Ma, H.R.; Li, J.W.; Jiao, J.; Chang, C.T.; Wang, G.; Shen, J.; Wang, X.M.; Li, R.W. Wear resistance of Fe-based amorphous coatings prepared by AC-HVAF and HVOF. Mater. Sci. Technol. 2016, 33, 65–71. [Google Scholar] [CrossRef]
- Ţălu, Ş. Micro and Nanoscale Characterization of Three Dimensional Surfaces. Basics and Applications; Napoca Star Publishing House: Cluj-Napoca, Romania, 2015; pp. 21–77. [Google Scholar]
- Wang, Y.; Jiang, S.L.; Zheng, Y.G.; Ke, W.; Sun, W.H.; Chang, X.C.; Hou, W.L.; Wang, J.Q. Effect of processing parameters on the microstructures and corrosion behaviour of high-velocity oxy-fuel (HVOF) sprayed Fe-based amorphous metallic coatings. Mater. Corros. 2013, 64, 801–810. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.-J.; Yue, W.; Fu, Z.-Q.; Zhu, L.-N.; She, D.-S.; Liang, J.; Wang, C.-B. Corrosion Behavior of FeCrMoCBY Amorphous Coating Fabricated by High-Velocity Air Fuel Spraying. J. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Gao, M.; Lu, W.; Yang, B.; Zhang, S.; Wang, J. High corrosion and wear resistance of Al-based amorphous metallic coating synthesized by HVAF spraying. J. Alloy. Compd. 2018, 735, 1363–1373. [Google Scholar] [CrossRef]
- Tailleart, N.; Gauthier, B.; Eidelman, S.; Scully, J. Metallurgical and Physical Factors Controlling the Multi-Functional Corrosion Properties of Pulsed Thermal-Sprayed Al-Co-Ce Coatings. Corros. J. Sci. Eng. 2012, 68, 035006-1–035006-26. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Ma, A.; Hu, H.; Zheng, Y.; Yang, B.; Wang, J. Influence of sealing treatment on the corrosion behavior of HVAF sprayed Al-based amorphous/nanocrystalline coating. Surf. Coat. Technol. 2018, 353, 263–273. [Google Scholar] [CrossRef]
- Liao, J.; Yang, B.; Zhang, Y.; Lu, W.; Gu, X.; Wang, J. Evaluation of glass formation and critical casting diameter in Al-based metallic glasses. Mater. Des. 2015, 88, 222–226. [Google Scholar] [CrossRef]
- Yang, B.; Yao, J.; Zhang, J.; Yang, H.; Wang, J.; Ma, E. Al-rich bulk metallic glasses with plasticity and ultrahigh specific strength. Scr. Mater. 2009, 61, 423–426. [Google Scholar] [CrossRef]
- Mahade, S.; Aranke, O.; Björklund, S.; Dizdar, S.; Awe, S.; Mušálek, R.; Lukáč, F.; Joshi, S. Influence of processing conditions on the microstructure and sliding wear of a promising Fe-based coating deposited by HVAF. Surf. Coat. Technol. 2021, 409, 126953. [Google Scholar] [CrossRef]
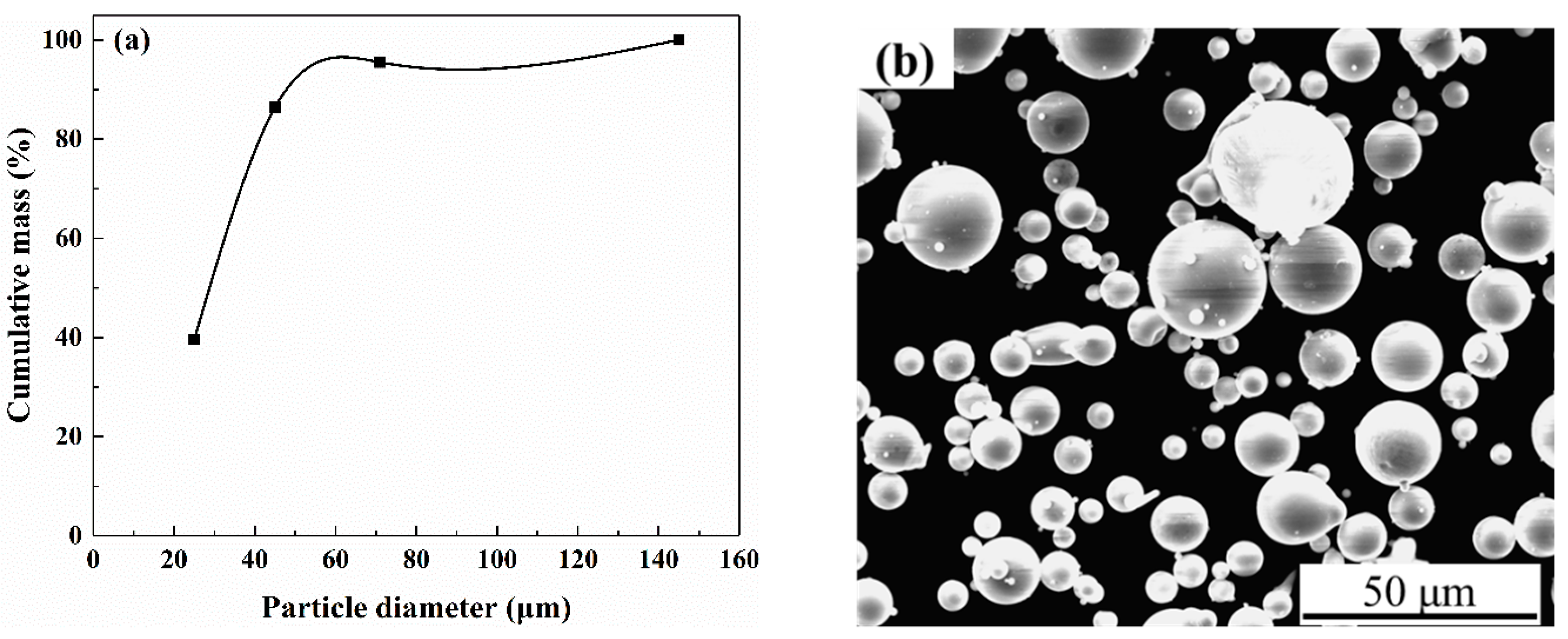



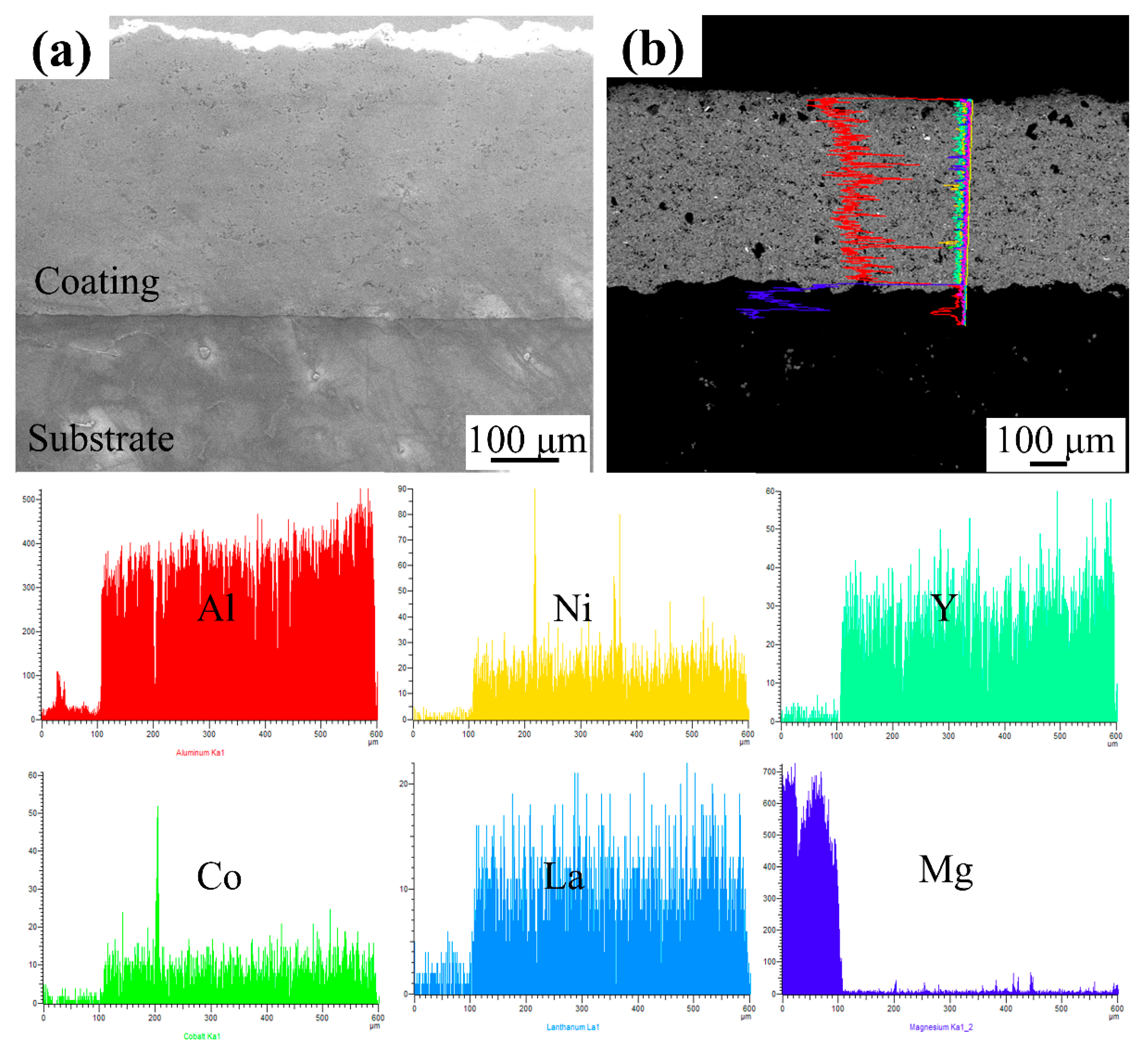

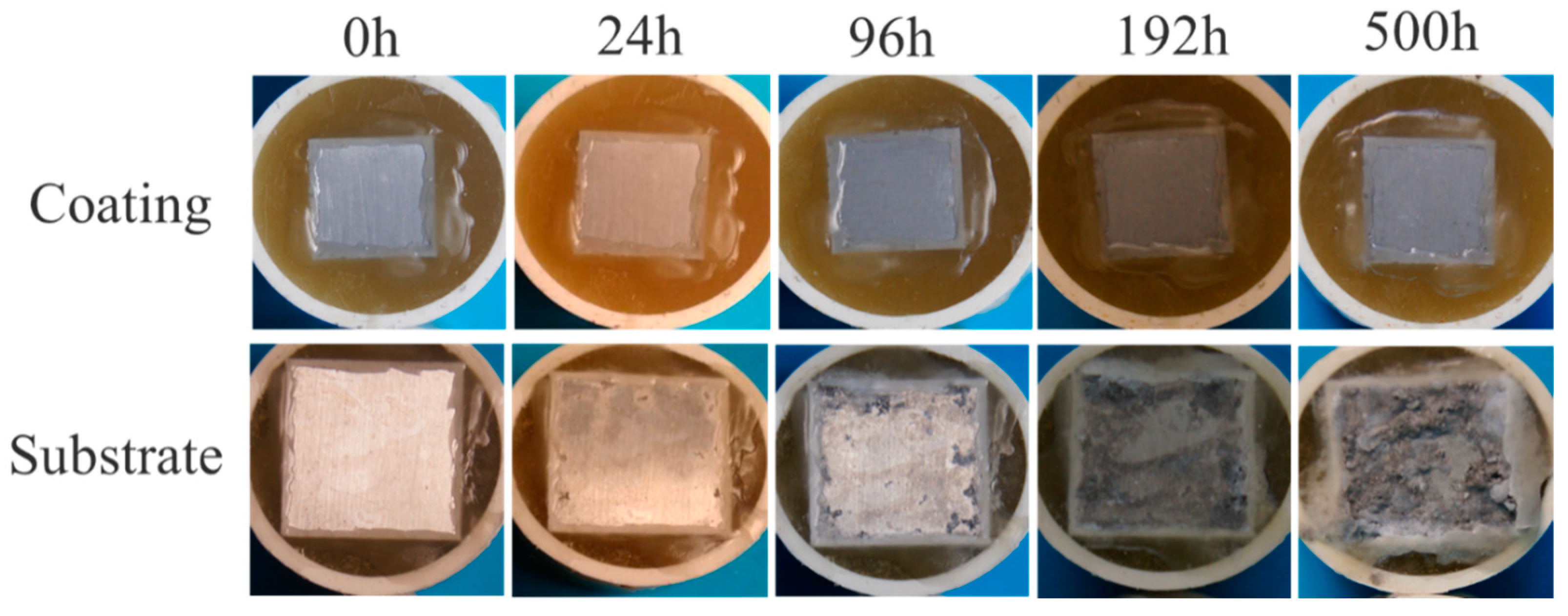
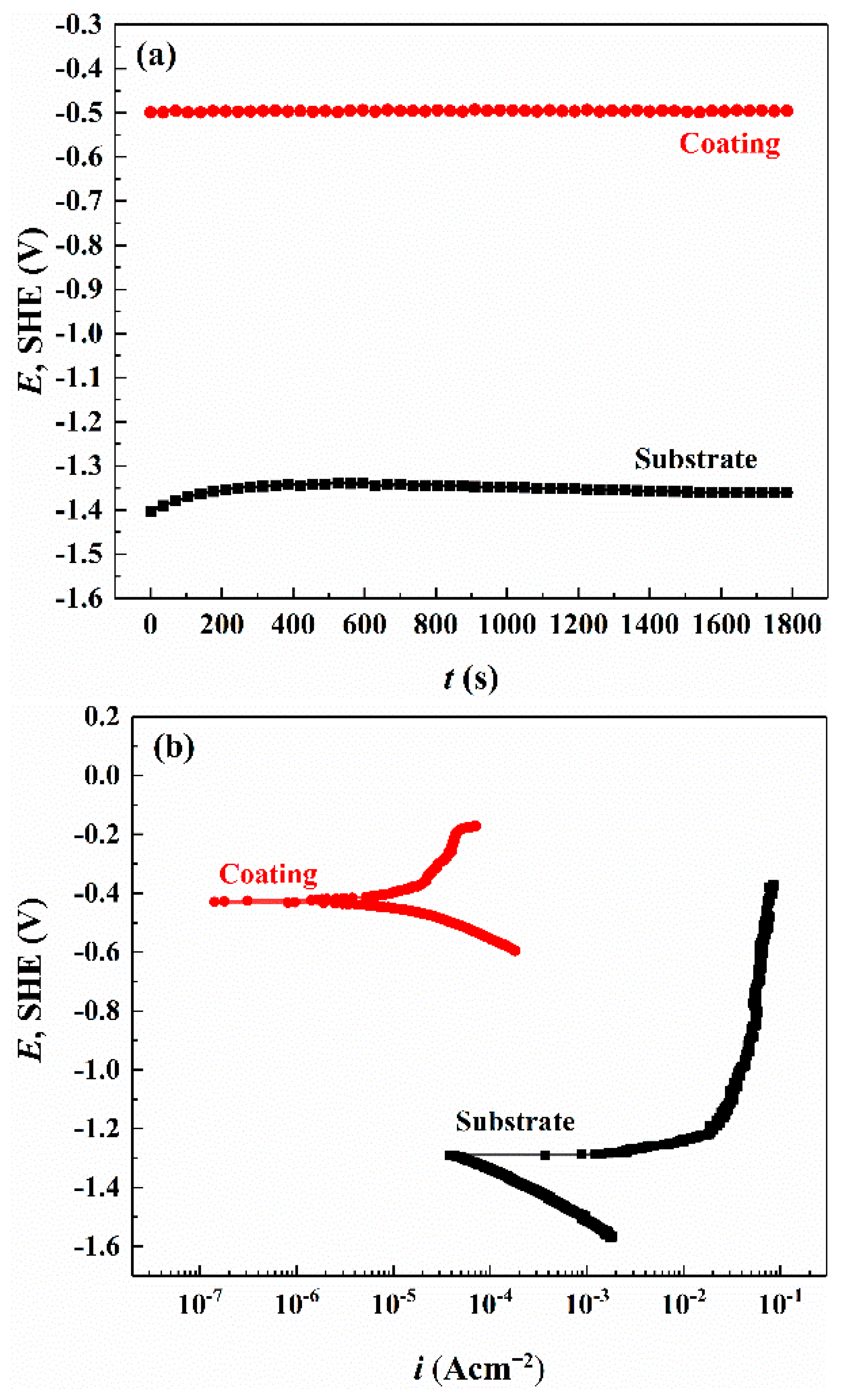
| ZM5 Alloy | Al | Mn | Zn | Mg | - |
| 7.5~9.0 | 0.2~0.8 | 0.15~0.5 | Bal | - | |
| Powder | Al | Ni | Y | Co | La |
| 68.1 | 10.4 | 11.8 | 3.4 | 6.3 |
| Samples | Ecorr V (SHE) | icorr A/cm2 | ba | bc |
|---|---|---|---|---|
| ZM5 alloy (average) | −1.306 | 6.74 × 10−4 | 34.6 | 150.6 |
| Al-based coating (average) | −0.430 | 7.53 × 10−6 | 221.9 | −132.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Wang, X.; Ren, Z. Microstructure and Corrosion Resistance of an HVAF-Sprayed Al-Based Amorphous Coating on Magnesium Alloys. Coatings 2022, 12, 425. https://doi.org/10.3390/coatings12040425
Wen S, Wang X, Ren Z. Microstructure and Corrosion Resistance of an HVAF-Sprayed Al-Based Amorphous Coating on Magnesium Alloys. Coatings. 2022; 12(4):425. https://doi.org/10.3390/coatings12040425
Chicago/Turabian StyleWen, Shu, Xiaoming Wang, and Zhiqiang Ren. 2022. "Microstructure and Corrosion Resistance of an HVAF-Sprayed Al-Based Amorphous Coating on Magnesium Alloys" Coatings 12, no. 4: 425. https://doi.org/10.3390/coatings12040425







