Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Base Material and Coating Powder
2.2. Development of Microcrystalline and Nanocrystalline HEA Coatings
2.3. Characterization of the HEA Coatings
2.4. High-Temperature Oxidation Study under Cyclic Conditions
3. Results and Discussion
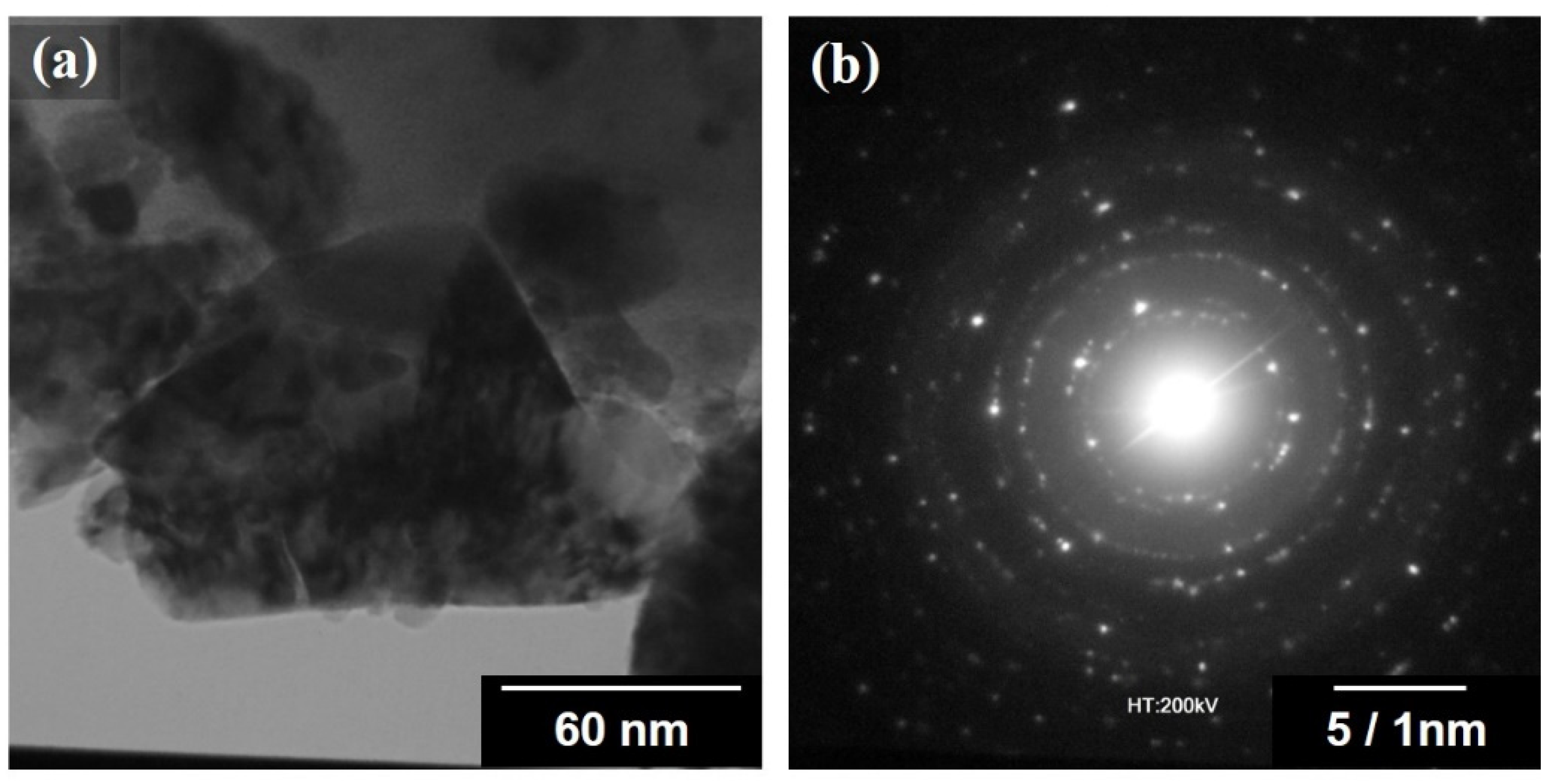
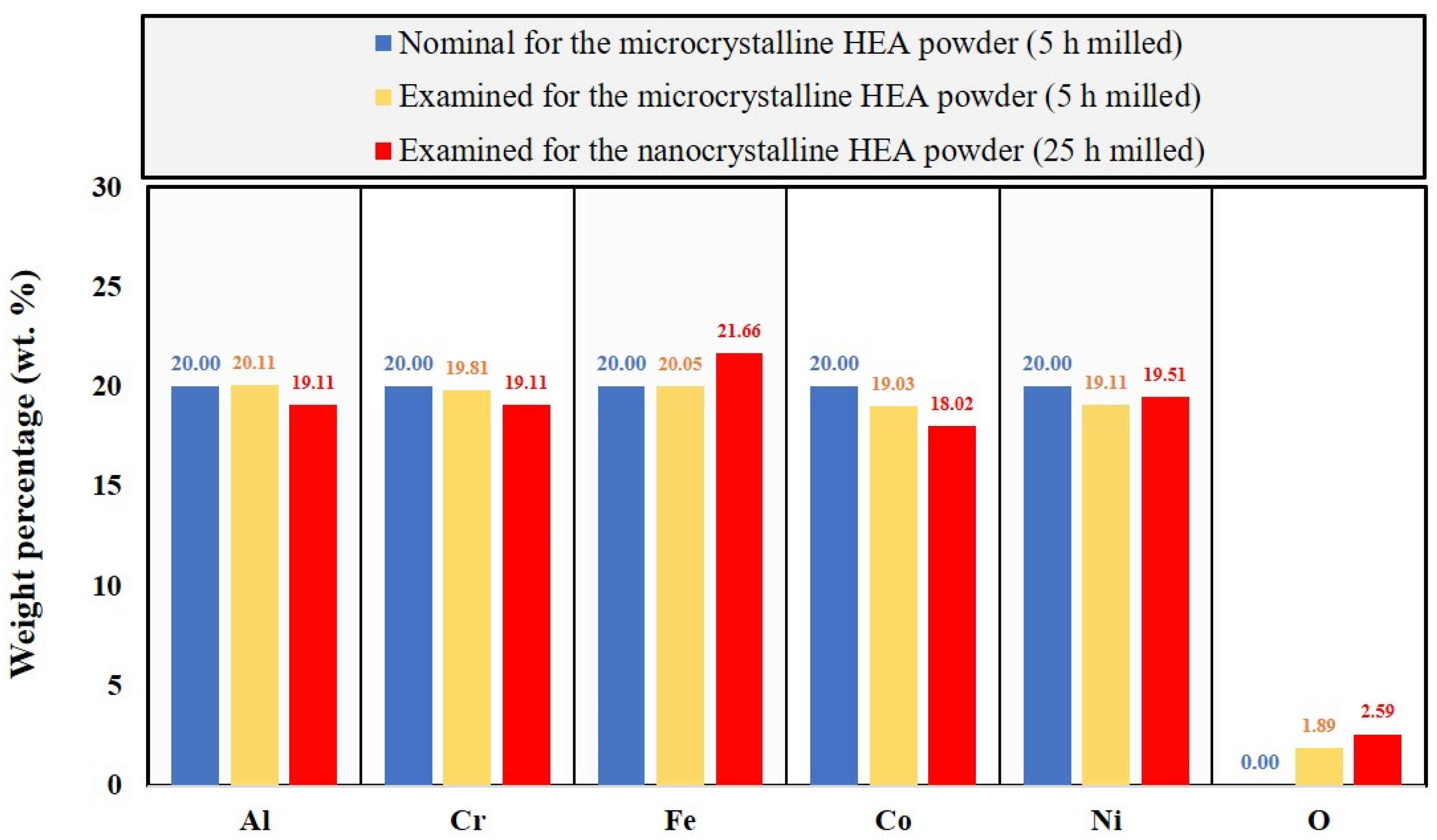
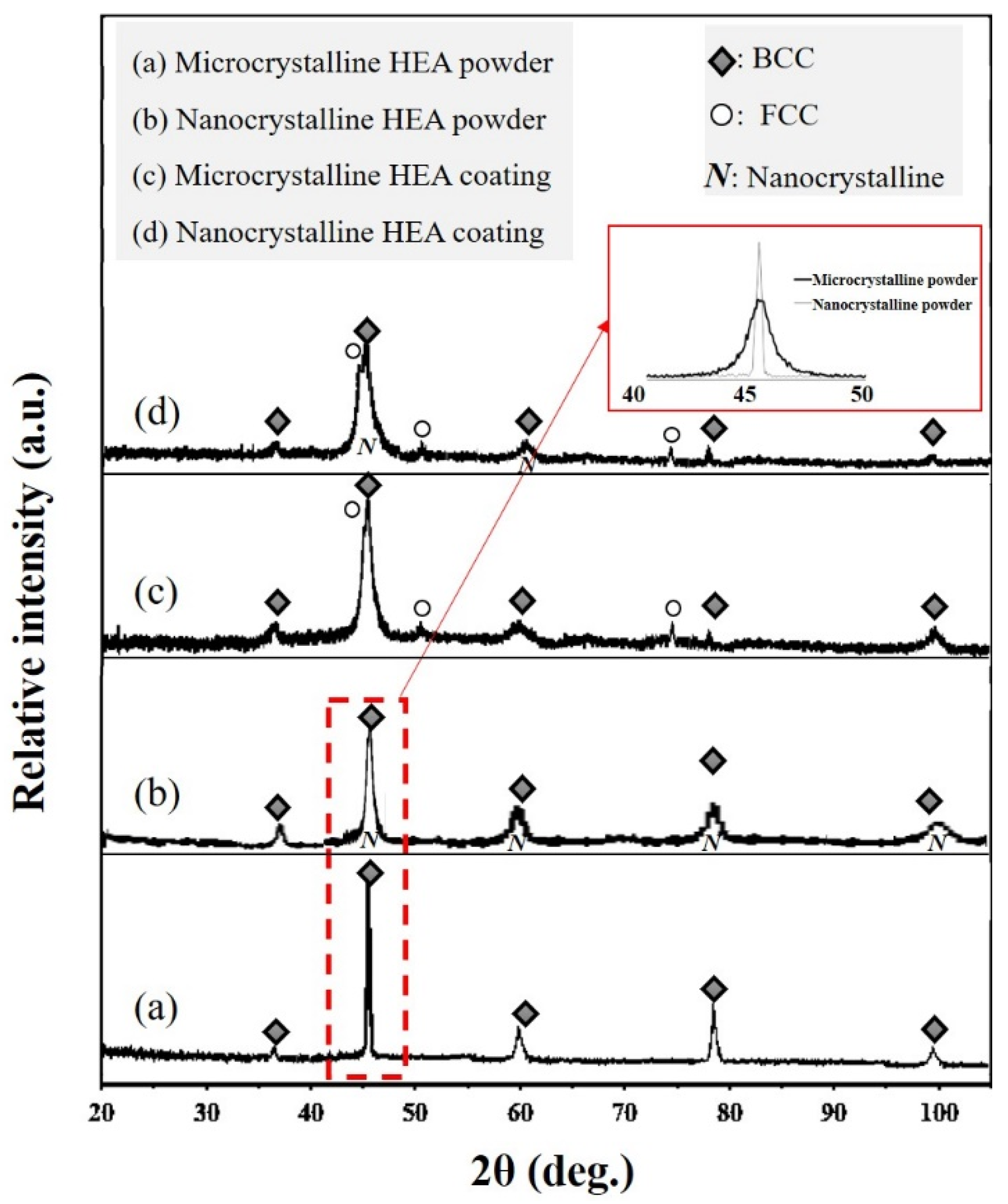
3.1. Characterization of the Nanocrystalline HEA Powder
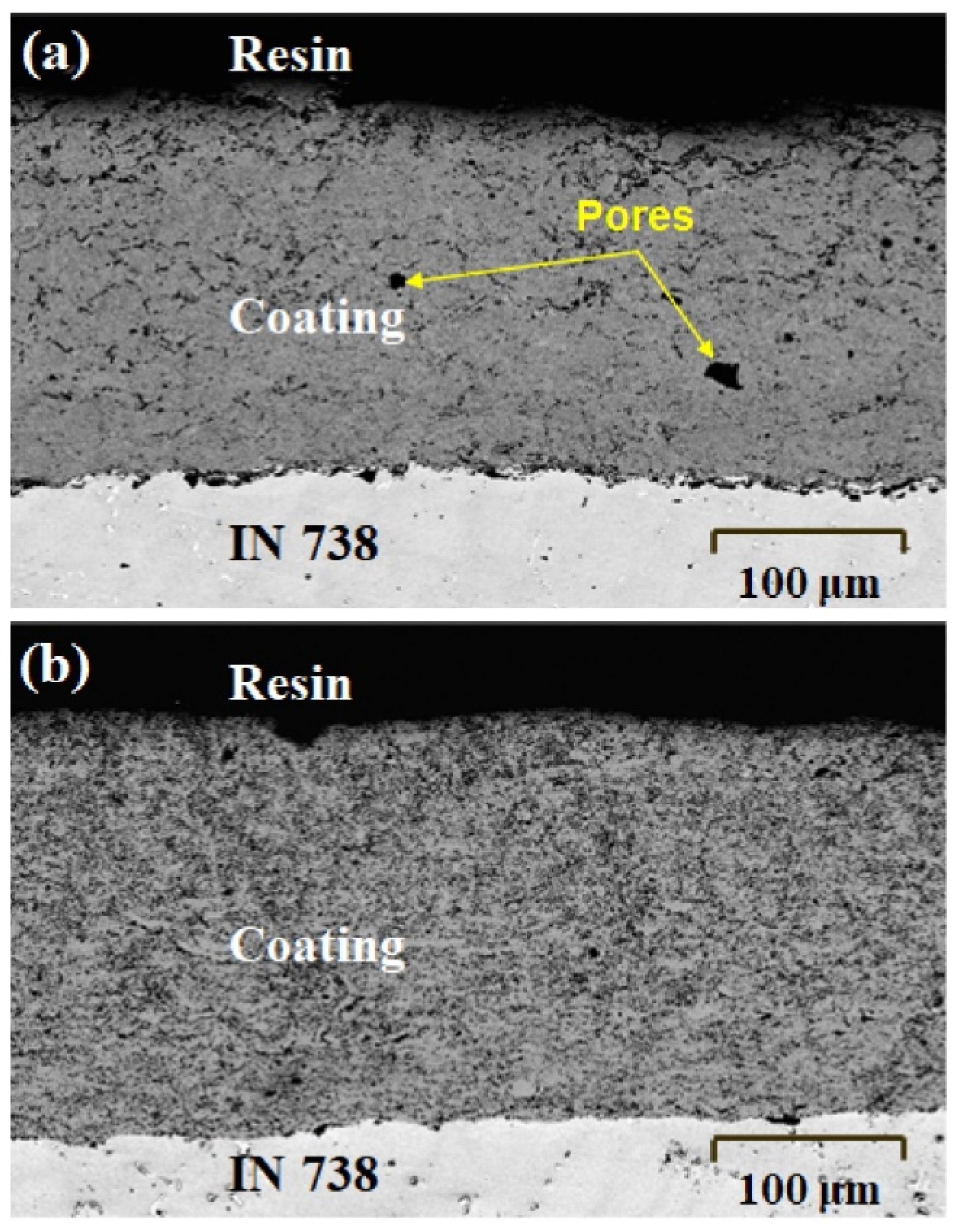
3.2. Characterization of the Nanocrystalline HEA Coating
3.3. Oxidation Behavior of the Nanocrystalline HEA Coating
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.-X.; Cheng, C.-Q.; Shang, J.-L.; Wang, R.; Li, P.; Zhao, J. Oxidation behavior of high-entropy alloys AlxCoCrFeNi (x = 0.15, 0.4) in supercritical water and comparison with HR3C steel. Trans. Nonferrous Met. Soc. China 2015, 25, 1341–1351. [Google Scholar] [CrossRef]
- Ghadami, S.; Taheri-Nassaj, E.; Baharvandi, H.R.; Ghadami, F. Improvement of mechanical properties of HfB2-based composites by incorporating in situ SiC reinforcement through pressureless sintering. Sci. Rep. 2021, 11, 9835. [Google Scholar] [CrossRef]
- Sayyadi-Shahraki, A.; Rafiaei, S.M.; Ghadami, S.; Nekouee, K.A. Densification and mechanical properties of spark plasma sintered Si3N4/ZrO2 nano-composites. J. Alloys Compd. 2019, 776, 798–806. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, X.; Chen, S.; Ma, Z.; Wang, W.; Wang, C.; Yue, L.; Sun, H.; Shao, Q.; Murugadoss, V. Long-term antibacterial stable reduced graphene oxide nanocomposites loaded with cuprous oxide nanoparticles. J. Colloid Interface Sci. 2019, 533, 13–23. [Google Scholar] [CrossRef]
- Ghadami, F.; Dadfar, M.R.; Zeraati, A.S. Mixed mode I/II delamination analysis of rubber-modified glass-reinforced epoxy composites. J. Reinf. Plast. Compos. 2014, 33, 1634–1643. [Google Scholar] [CrossRef]
- Jin, K.; Pan, S.; Wang, T.; Zhang, Z. Non-negligible corrosion process in a novel sulfur-based energy storage system. J. Power Sources 2021, 490, 229529. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Xu, Z.; Li, D.Y. Effect of Ti addition on the sliding wear behavior of AlCrFeCoNi high-entropy alloy. Wear 2020, 462, 203493. [Google Scholar] [CrossRef]
- Erdogan, A.; Doleker, K.M.; Zeytin, S. Effect of Al and Ti on High-Temperature Oxidation Behavior of CoCrFeNi-Based High-Entropy Alloys. JOM 2019, 71, 3499–3510. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-entropy alloys: Emerging materials for advanced functional applications. J. Mater. Chem. A 2021, 9, 663–701. [Google Scholar] [CrossRef]
- Mu, Y.K.; Jia, Y.D.; Xu, L.; Jia, Y.F.; Tan, X.H.; Yi, J.; Wang, G.; Liaw, P.K. Nano oxides reinforced high-entropy alloy coatings synthesized by atmospheric plasma spraying. Mater. Res. Lett. 2019, 7, 312–319. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153. [Google Scholar] [CrossRef] [Green Version]
- Yue, T.M.; Xie, H.; Lin, X.; Yang, H.; Meng, G. Microstructure of Laser Re-Melted AlCoCrCuFeNi High Entropy Alloy Coatings Produced by Plasma Spraying. Entropy 2013, 15, 2833. [Google Scholar] [CrossRef] [Green Version]
- Ang, A.S.M.; Berndt, C.C.; Sesso, M.L.; Anupam, A.; Praveen, S.; Kottada, R.S.; Murty, B.S. Plasma-Sprayed High Entropy Alloys: Microstructure and Properties of AlCoCrFeNi and MnCoCrFeNi. Metall. Mater. Trans. A 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R. Improvement of high velocity oxy-fuel spray coatings by thermal post-treatments: A critical review. Thin Solid Films 2019, 678, 42–52. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Abrasive wear behavior of nano-ceria modified NiCoCrAlY coatings deposited by the high-velocity oxy-fuel process. Mater. Res. Express 2020, 6, 1250d6. [Google Scholar] [CrossRef]
- Sohi, M.H.; Ghadami, F. Comparative tribological study of air plasma sprayed WC–12% Co coating versus conventional hard chromium electrodeposit. Tribol. Int. 2010, 43, 882–886. [Google Scholar] [CrossRef]
- Toma, D.; Brandl, W.; Köster, U. The Characteristics of Alumina Scales Formed on HVOF-Sprayed MCrAlY Coatings. Oxid. Met. 2000, 53, 125–137. [Google Scholar] [CrossRef]
- Mercier, D.; Gauntt, B.D.; Brochu, M. Thermal stability and oxidation behavior of nanostructured NiCoCrAlY coatings. Surf. Coat. Technol. 2011, 205, 4162–4168. [Google Scholar] [CrossRef]
- Jiang, H.; Lau, M.; Tellkamp, V.L.; Lavernia, E.J. Chapter 3—Synthesis of nanostructured coatings by high-velocity oxygen-fuel thermal spraying. In Handbook of Nanostructured Materials and Nanotechnology; Nalwa, H.S., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 159–213. [Google Scholar]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Zajusz, M.; Jawańska, M.; Gil, A.; Jedliński, J.; Mroczka, K.; Matsuda, K.; Kulik, T.; et al. Oxidation Behavior of Alx(CoCrFeNi)100-x High-Entropy Alloys Under Thermal-Cycling Conditions. Oxid. Met. 2021, 96, 307–321. [Google Scholar] [CrossRef]
- Gorr, B.; Schellert, S.; Müller, F.; Christ, H.-J.; Kauffmann, A.; Heilmaier, M. Current Status of Research on the Oxidation Behavior of Refractory High Entropy Alloys. Adv. Eng. Mater. 2021, 23, 2001047. [Google Scholar] [CrossRef]
- Ghadami, F.; Ghadami, S.; Davoudabadi, M.A. Sliding Wear Behavior of the Nanoceria-Doped AlCrFeCoNi High-Entropy Alloy Coatings Deposited by Air Plasma Spraying Technique. J. Therm. Spray Technol. 2022, 1–13. [Google Scholar] [CrossRef]
- Zakeri, A.; Ghadami, F.; Rouhaghdam, A.S.; Saeedi, B. Study on production of modified MCrAlY powder with nano oxide dispersoids as HVOF thermal spray feedstock using mechanical milling. Mater. Res. Express 2020, 7, 015030. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Mechanism of the oxide scale formation in thermally-sprayed NiCoCrAlY coatings modified by CeO2 nanoparticles. Mater. Today Commun. 2020, 24, 101357. [Google Scholar] [CrossRef]
- Ghadami, F.; Sabour Rouh Aghdam, A.; Ghadami, S. A comprehensive study on the microstructure evolution and oxidation resistance of conventional and nanocrystalline MCrAlY coatings. Sci. Rep. 2021, 11, 875. [Google Scholar] [CrossRef]
- Bai, M.; Song, B.; Reddy, L.; Hussain, T. Preparation of MCrAlY–Al2O3 composite coatings with enhanced oxidation resistance through a novel powder manufacturing process. J. Therm. Spray Technol. 2019, 28, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Ghadami, F.; Aghdam, A.S.R.; Ghadami, S. Characterization of MCrAlY/nano-Al2O3 nanocomposite powder produced by high-energy mechanical milling as feedstock for high-velocity oxygen fuel spraying deposition. Int. J. Miner. Metall. Mater. 2021, 28, 1534–1543. [Google Scholar] [CrossRef]
- Odhiambo, J.G.; Li, W.; Zhao, Y.; Li, C. Porosity and Its Significance in Plasma-Sprayed Coatings. Coatings 2019, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Yao, G.; Guan, Z.; Yu, N.; Sokoluk, M.; Li, X. Kinetics and dynamics of surface thermal oxidation in Al-ZrB2 nanocomposites. Corros. Sci. 2020, 176, 108890. [Google Scholar] [CrossRef]
- Ghadami, F.; Sabour Rouh Aghdam, A.; Ghadami, S. Isothermal and Cyclic Oxidation Behavior of HVOF-Sprayed NiCoCrAlY Coatings: Comparative Investigations on the Conventional and Nanostructured Coatings. J. Therm. Spray Technol. 2020, 29, 1926–1942. [Google Scholar] [CrossRef]
- Saeedi, B.; Sabour Rouh Aghdam, A.; Gholami, G. A study on nanostructured in-situ oxide dispersed NiAl coating and its high temperature oxidation behavior. Surf. Coat. Technol. 2015, 276, 704–713. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Hussain, M.S.; Yajid, M.A.M. The role of formation of continues thermally grown oxide layer on the nanostructured NiCrAlY bond coat during thermal exposure in air. Appl. Surf. Sci. 2012, 261, 287–297. [Google Scholar] [CrossRef]
- Ghadami, F.; Aghdam, A.S.R.; Zakeri, A.; Saeedi, B.; Tahvili, P. Synergistic effect of CeO2 and Al2O3 nanoparticle dispersion on the oxidation behavior of MCrAlY coatings deposited by HVOF. Ceram. Int. 2020, 46, 4556–4567. [Google Scholar] [CrossRef]
- Ghadami, F.; Sabour Rouh Aghdam, A.; Ghadami, S.; Zeng, Q. Effect of vacuum heat treatment on the oxidation kinetics of freestanding nanostructured NiCoCrAlY coatings deposited by high-velocity oxy-fuel spraying. J. Vac. Sci. Technol. A 2020, 38, 022601. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Chen, J.-H.; Stadler, S.; Chen, S.-H. Properties of atomized AlCoCrFeNi high-entropy alloy powders and their phase-adjustable coatings prepared via plasma spray process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]




| Element | Percentage (wt.%) | Element | Percentage (wt.%) |
|---|---|---|---|
| Ni | 52.3 | Al | 0.5 |
| Cr | 19.0 | Ti | 0.9 |
| Fe | 18.5 | Si | 0.2 |
| Ta | 5.1 | Mn | 0.2 |
| Mo | 3.1 | Cu | 0.5 |
| Parameter | Value |
|---|---|
| Nozzle type | GH |
| Nozzle size (mm) | 6 |
| Primary gas flow/Argon (L/min) | 65 |
| Secondary gas flow/Hydrogen (L/min) | 3 |
| Powder carrier gas flow/Argon (L/min) | 2 |
| Arc current (A) | 700 |
| Arc voltage (V) | 58 |
| Spraying angle (°) | 90 |
| Number of passes | 8 |
| Powder feed rate (g/min) | 36 |
| Standoff distance (mm) | 140 |
| Structural Mode | Porosity (%) | Microhardness (HV200) |
|---|---|---|
| Microcrystalline | 1.3 | 542 ± 19 |
| Nanocrystalline | 1.6 | 492 ± 12 |
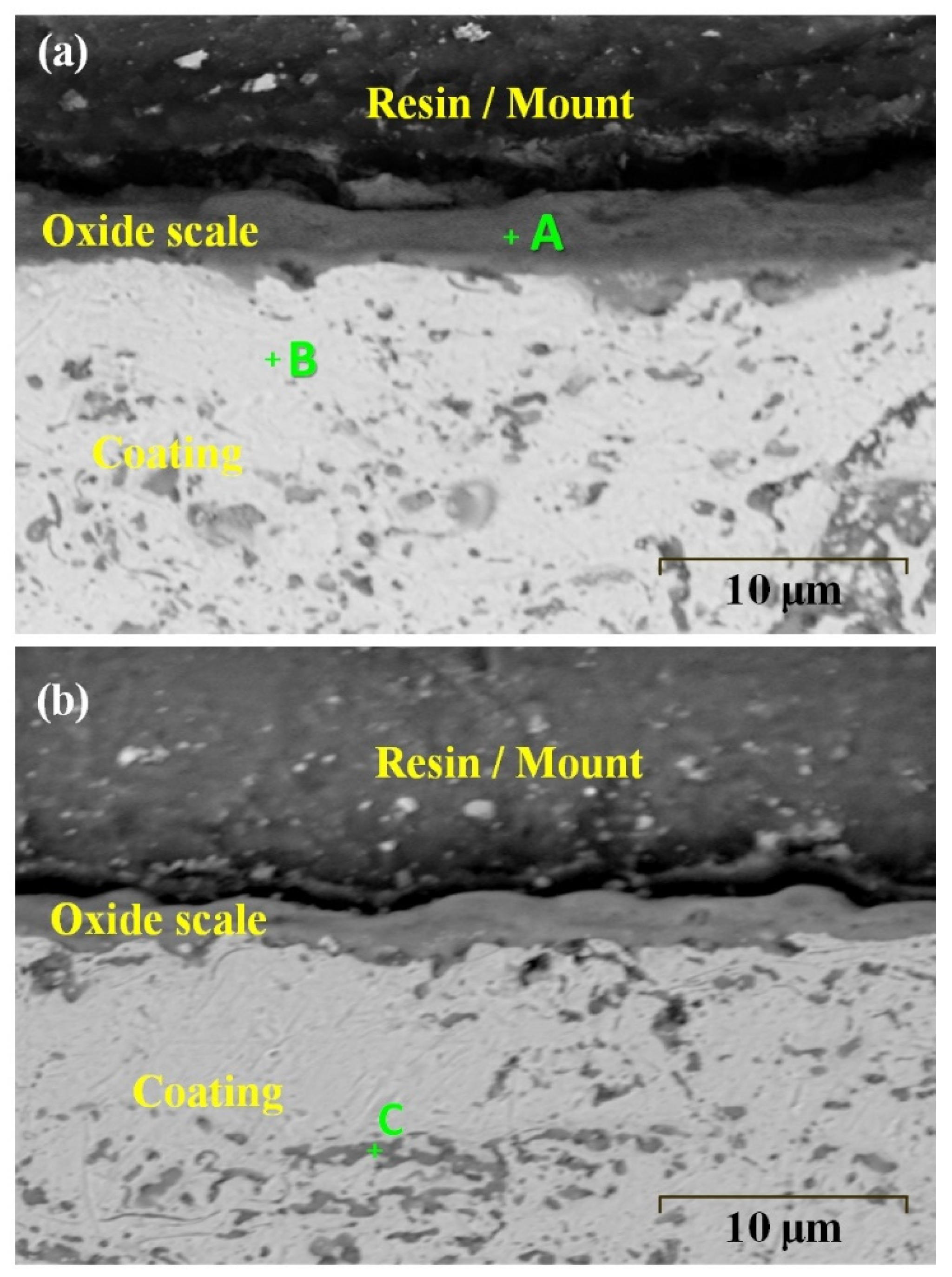
| Indexed Region | Al | Cr | Fe | Co | Ni | O |
|---|---|---|---|---|---|---|
| A | 16.2 | 12.5 | 13.8 | 11.2 | 9.5 | 36.8 |
| B | 19.9 | 21.5 | 19.3 | 18.3 | 19.8 | 1.2 |
| C | 16.2 | 16.8 | 18.2 | 15.6 | 17.3 | 15.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghadami, F.; Davoudabadi, M.A.; Ghadami, S. Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings 2022, 12, 372. https://doi.org/10.3390/coatings12030372
Ghadami F, Davoudabadi MA, Ghadami S. Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings. 2022; 12(3):372. https://doi.org/10.3390/coatings12030372
Chicago/Turabian StyleGhadami, Farzin, Mohammad Amin Davoudabadi, and Soheil Ghadami. 2022. "Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique" Coatings 12, no. 3: 372. https://doi.org/10.3390/coatings12030372
APA StyleGhadami, F., Davoudabadi, M. A., & Ghadami, S. (2022). Cyclic Oxidation Properties of the Nanocrystalline AlCrFeCoNi High-Entropy Alloy Coatings Applied by the Atmospheric Plasma Spraying Technique. Coatings, 12(3), 372. https://doi.org/10.3390/coatings12030372






