A Simple Scheme for Extraction of Asphaltenes from Asphalt at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Devices
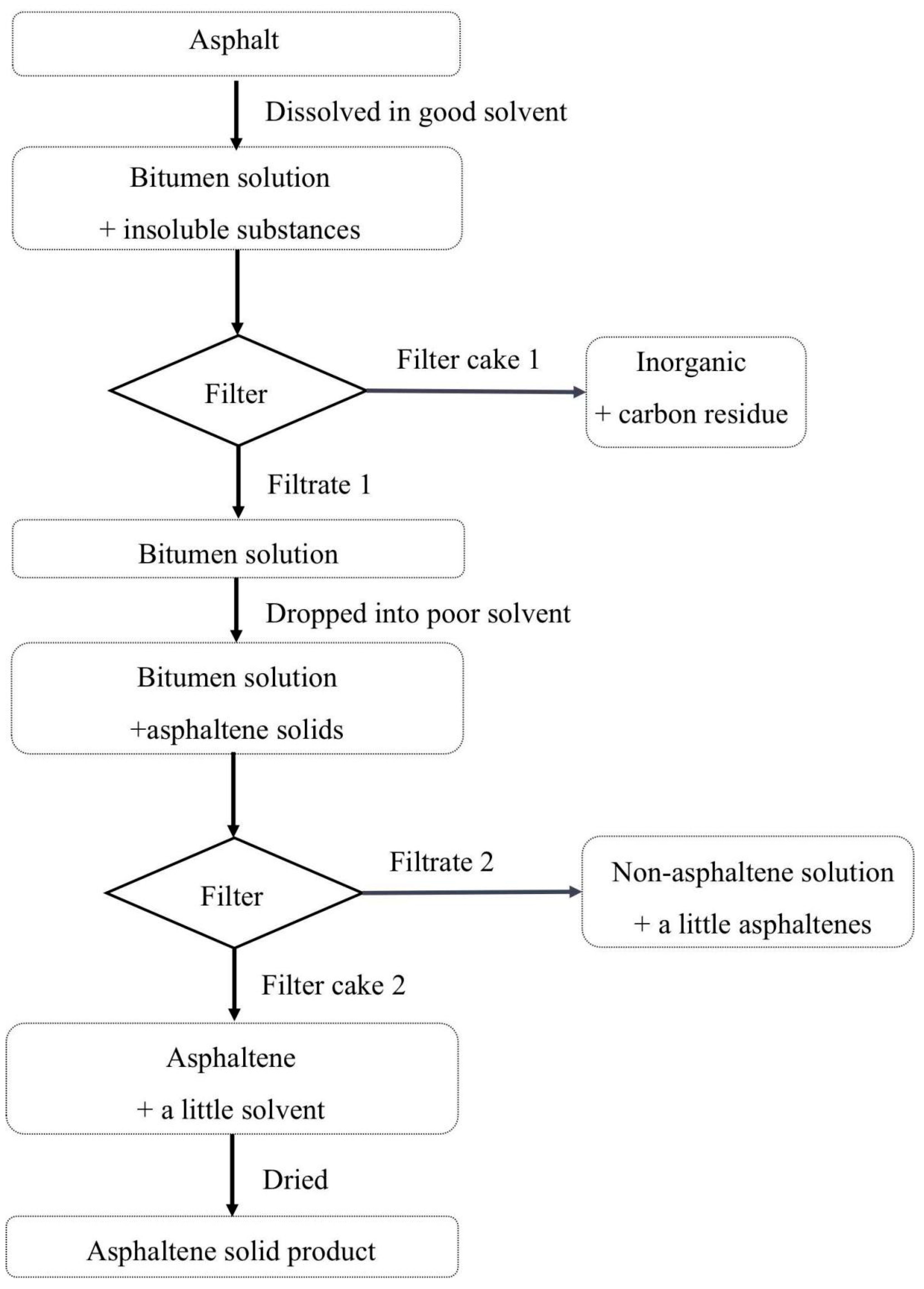
2.2. Experimental Procedures
- (1)
- A total of 5 g asphalt was weighed on a portable balance and then put into a 200 mL round-bottomed flask, as shown in Figure 2a.
- (2)
- After a PTFE coated magnetic stirrer bar with length 2.5 cm was added and 45 mL of xylene was poured into the flask, the solution was stirred vigorously for 30 min to dissolve the asphalt completely on a thermostatic magnetic stirrer, as shown in Figure 2b.
- (3)
- The solution after stirring was filtered to remove inorganic impurities and residual carbon (filter cake 1), as shown in Figure 2c. The filter membrane used was medium-speed setting filter paper. 5 mL of xylene was used to rinse the filter paper and combined with the filtrate 1. After filtering, the filtrate 1 was set aside for later use.
- (4)
- A total of 450 mL of n-heptane was poured into a 500 mL beaker with magnetic stirring. The above filtrate 1 was dropped into the beaker with a speed of 1–3 drops per second, as shown in Figure 2d.
- (5)
- After dropping, the solution in the beaker was left to stand for 30 min, as shown in Figure 2e.
- (6)
- The solution in the beaker was filtered to get filter cake 2 and filtrate 2, using a filter device with 0.45 um filter membrane, as shown in Figure 2f. The obtained filter cake 2 was washed with 50 mL of n-heptane. The washed solution was combined with the filtrate 2 and set aside for later use.
- (7)
- After filtering and washing, the filter cake 2 was obtained, as shown in Figure 2g. As it still contained a little solvent, it was dried at room temperature for 8 h to constant weight.
- (8)
- The asphaltene after drying was weighed on a PX224ZH/E electronic balance to calculate the yield and then sealed in a 20 mL glass bottle for further characterization, as shown in Figure 2h. The yield was defined as mass percentage of the obtained asphaltenes and matrix asphalt.
- (9)
- If needed, the solvents in filtrate 2 could be recovered by a reduced pressure rotary evaporation, as shown in Figure 2i. The 30–45 °C fraction was obtained as n-heptane 2, and the 60–80 °C fraction was obtained as xylene 2. The volumes for recovered solvents were measured to calculate the recovery rate (volume ratio between the recovered and the added solvents). If solvent recovery is not required, this step can be omitted.
2.3. Characterization Methods
3. Results and Discussion
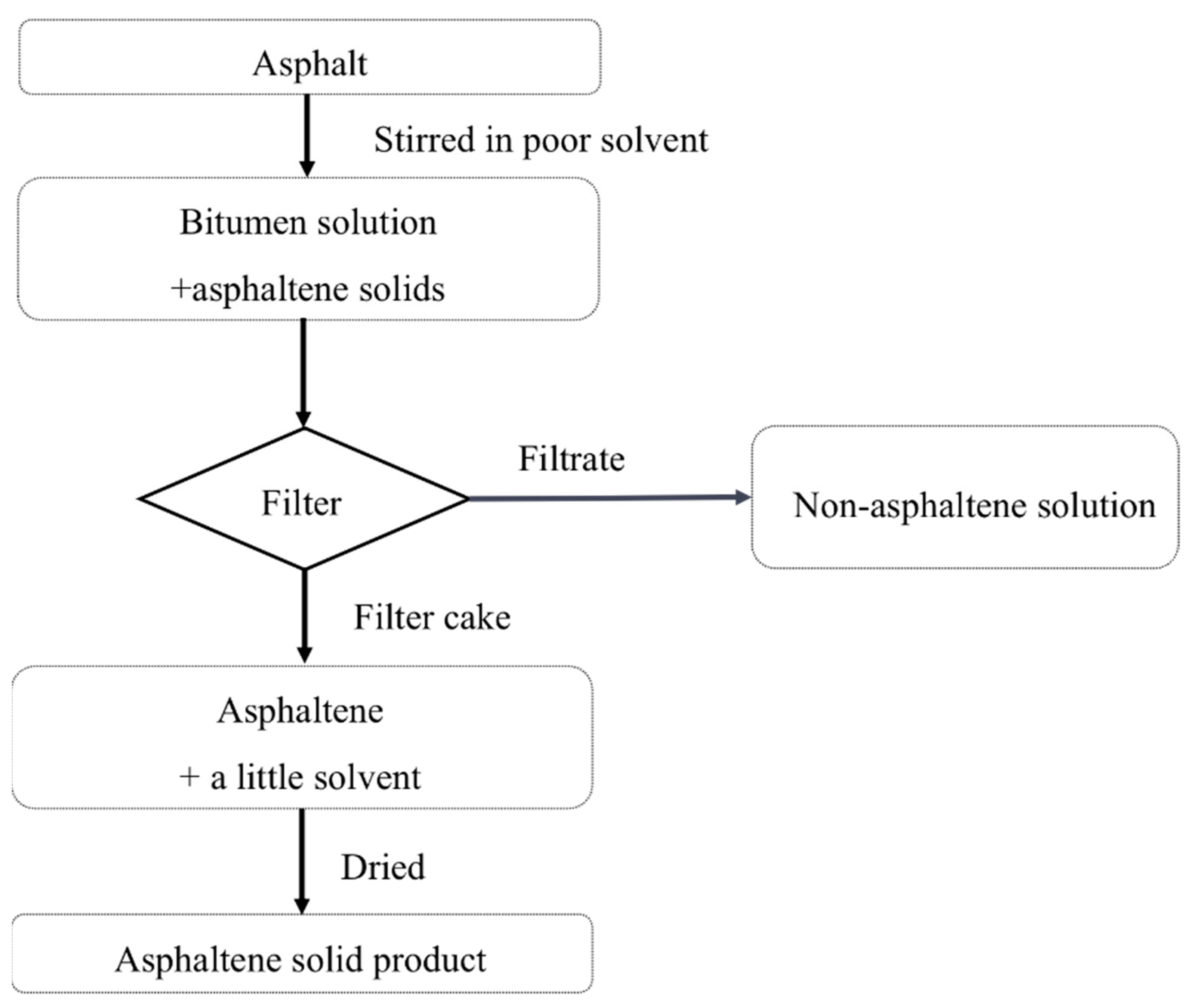
3.1. Experiment Process
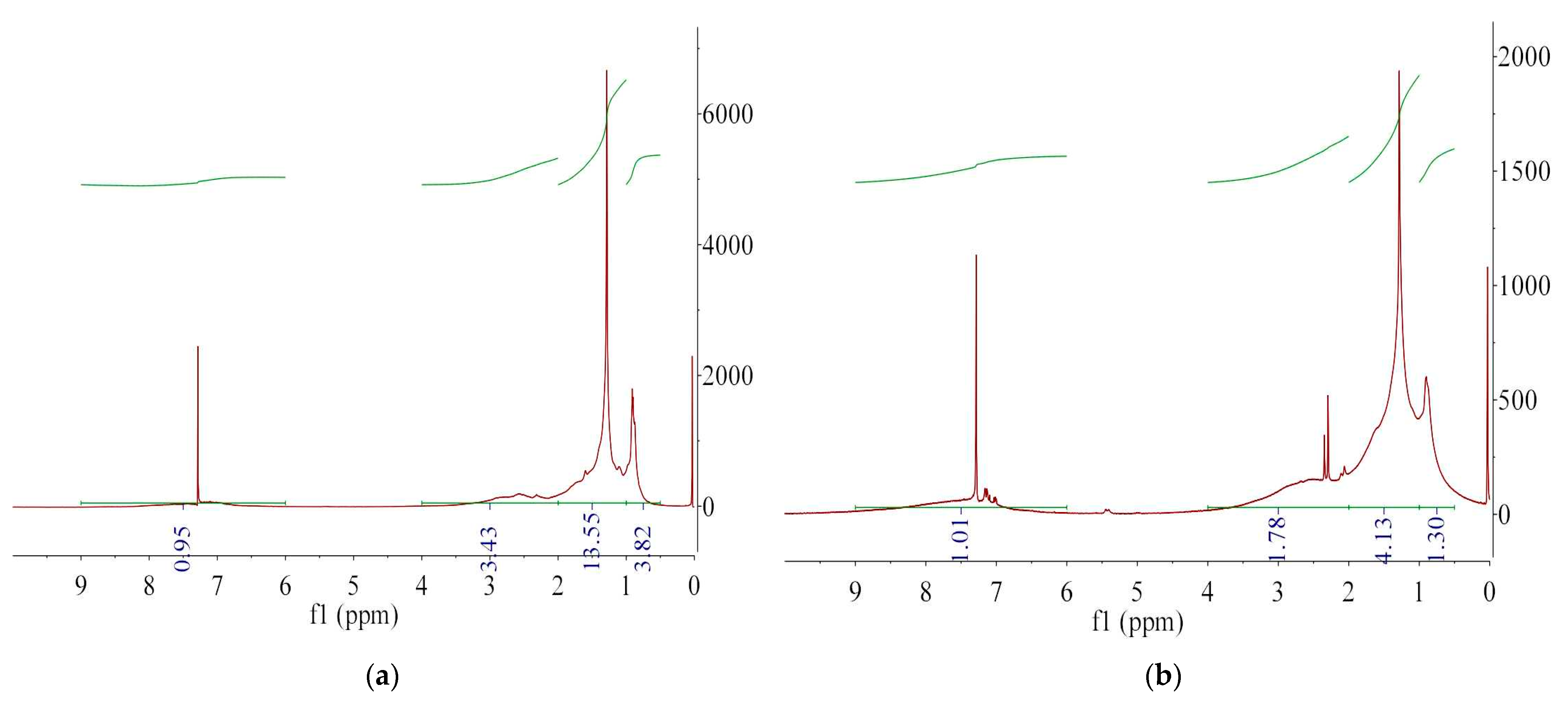
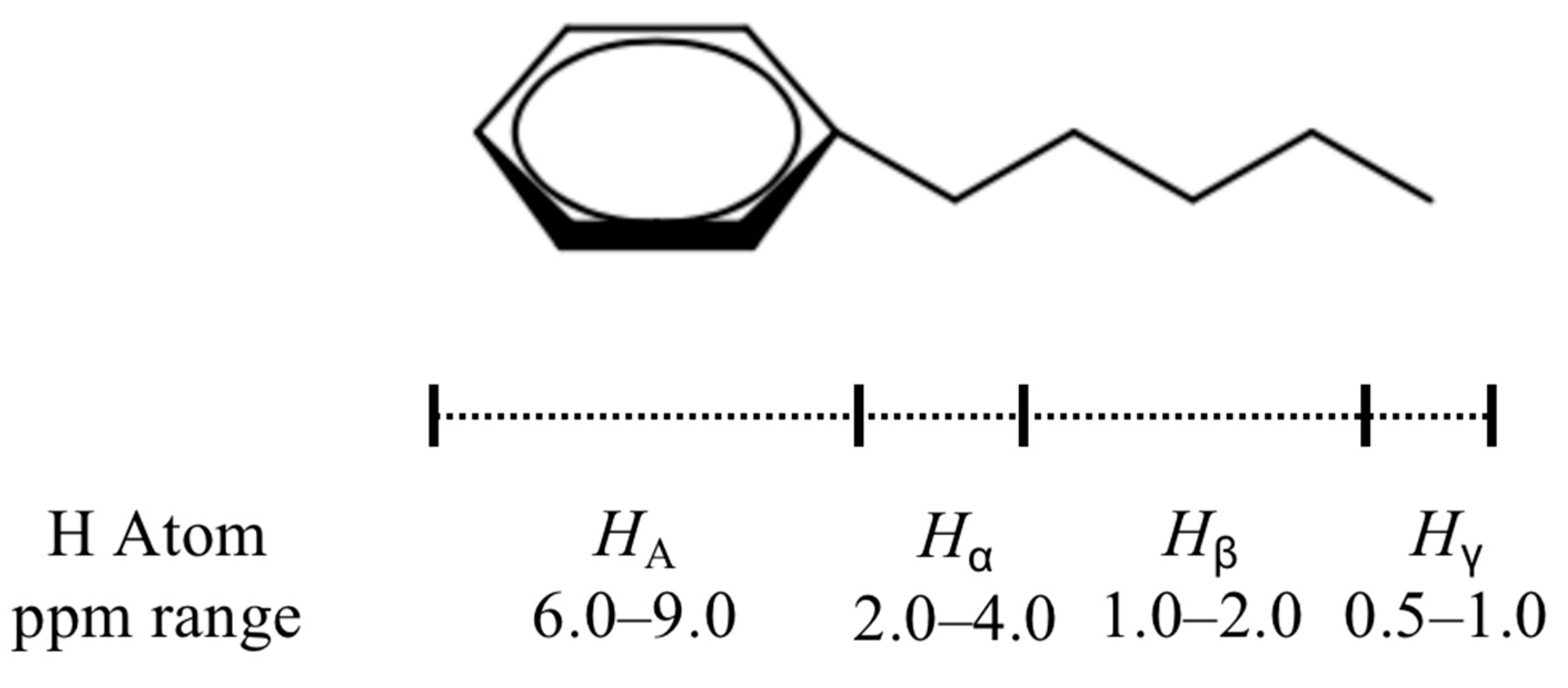
3.2. Characterization of Extracted Asphaltenes
3.3. Choice of Solvent and Its Effect on Asphaltene Yield
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, M.T.; Nguyen, D.L.T.; Xia, C.; Nguyen, T.B.; Shokouhimehr, M.; Sana, S.S.; Grace, A.N.; Aghbashlo, M.; Tabatabaei, M.; Sonne, C.; et al. Recent advances in asphaltene transformation in heavy oil hydroprocessing: Progress, challenges, and future perspectives. Fuel Process. Technol. 2021, 213, 106681. [Google Scholar] [CrossRef]
- Hassanzadeh, M.; Abdouss, M. A comprehensive review on the significant tools of asphaltene investigation. Analysis and characterization techniques and computational methods. J. Pet. Sci. Eng. 2022, 208, 109611. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Rossi, C.O. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Lemarchand, C.A.; Greenfield, M.L.; Dyre, J.C.; Hansen, J.S. ROSE bitumen: Mesoscopic model of bitumen and bituminous mixtures. J. Chem. Phys. 2018, 149, 214901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, D.A.; Yan, Y.; Rodrigues, J.K.G.; Roque, R. Optimizing the use of reactive terpolymer, polyphosphoric acid and high-density polyethylene to achieve asphalt binders with superior performance. Constr. Build. Mater. 2018, 169, 522–529. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, P.; Dong, S.; Li, Y.; Yuan, G. Asphalt binder micro-characterization and testing approaches: A review. Measurement 2020, 151, 107255. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Zhang, Y.; Zhang, H.; Zhang, S. Influence of multi-dimensional nanomaterials composite form on thermal and ultraviolet oxidation aging resistances of SBS modified asphalt. Constr. Build. Mater. 2021, 273, 122054. [Google Scholar] [CrossRef]
- Siskin, M.; Kelemen, S.R.; Eppig, C.P.; Brown, L.D.; Afeworki, M. Asphaltene molecular structure and chemical influences on the morphology of coke produced in delayed coking. Energy Fuels 2006, 20, 1227–1234. [Google Scholar] [CrossRef]
- Guo, M.; Liang, M.; Fu, Y.; Sreeram, A.; Bhasin, A. Average molecular structure models of unaged asphalt binder fractions. Mater. Struct. 2021, 54, 173. [Google Scholar] [CrossRef]
- Gray, M.R.; Yarranton, H.W.; Chacón-Patiño, M.L.; Rodgers, R.P.; Bouyssiere, B.; Giusti, P. Distributed properties of asphaltene nanoaggregates in crude oils: A review. Energy Fuels 2021, 35, 18078–18103. [Google Scholar] [CrossRef]
- Ismail, M.; Yang, Y.; Chaisoontornyotin, W.; Ovalles, C.; Rogel, E.; Moir, M.E.; Hoepfner, M.P. Effect of chemical inhibitors on asphaltene precipitation and morphology using ultra-small-angle X-ray scattering. Energy Fuels 2019, 33, 3681–3693. [Google Scholar] [CrossRef]
- Ashoori, S.; Sharifi, M.; Masoumi, M.; Salehi, M.M. The relationship between SARA fractions and crude oil stability. Egypt J. Pet. 2017, 26, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Kraiwattanawong, K.; Fogler, H.S.; Gharfeh, S.G.; Singh, P.; Thomason, W.H.; Chavadej, S. Thermodynamic solubility models to predict asphaltene instability in live crude oils. Energy Fuels 2007, 21, 1248–1255. [Google Scholar] [CrossRef]
- Mirwald, J.; Werkovits, S.; Camargo, I.; Maschauer, D.; Hofko, B.; Grothe, H. Understanding bitumen ageing by investigation of its polarity fractions. Constr. Build. Mater. 2020, 250, 118809. [Google Scholar] [CrossRef]
- Sakib, N.; Bhasin, A. Measuring polarity-based distributions (SARA) of bitumen using simplified chromatographic techniques. Int. J. Pavement Eng. 2018, 20, 1371–1384. [Google Scholar] [CrossRef]
- Cimino, R.; Correra, S.; Sacomani, P.A.; Carniani, C. Thermodynamic modelling for prediction of asphaltene deposition in live oils. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar]
- Li, F.; Yang, Y. The interfacial effect of mineral filler to asphalt binder and its influence on asphalt aging behavior. Mater. Struct. 2021, 54, 10. [Google Scholar] [CrossRef]
- Ali, S.I.; Awan, Z.; Lalji, S.M. Laboratory evaluation experimental techniques of asphaltene precipitation and deposition controlling chemical additives. Fuel 2022, 310, 122194. [Google Scholar] [CrossRef]
- Mohammed, I.; Mahmoud, M.; El-Husseiny, A.; Shehri, D.A.; Al-Garadi, K.; Kamal, M.S.; Alade, O.S. Impact of asphaltene precipitation and deposition on wettability and permeability. ACS Omega 2021, 6, 20091–20102. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Xu, J.; Yan, E. Intrinsic temperature and moisture sensitive adhesion characters of asphalt-aggregate interface based on molecular dynamics simulations. Constr. Build. Mater. 2021, 292, 123462. [Google Scholar] [CrossRef]
- Fan, M.; Zhao, S.; Sun, X.; Xu, Z. Comparison of properties of C5 and C7 asphaltenes from different heavy oils. China Pet. Process. Petrochem. Technol. 2019, 50, 96–102. [Google Scholar]
- Huang, J. Characterization of asphalt fractions by NMR spectroscopy. Pet. Sci. Technol. 2010, 28, 618–624. [Google Scholar] [CrossRef]
- Mozaffari, S.; Ghasemi, H.; Tchoukov, P.; Czarnecki, J.; Nazemifard, N. Lab-on-a-chip systems in asphaltene characterization: A review of recent advances. Energy Fuels 2021, 35, 9080–9101. [Google Scholar] [CrossRef]
- Strausz, O.P.; Mojelsky, T.W.; Lown, E.M. The molecular structure of asphaltene: An unfolding story. Fuel 1992, 71, 1355–1362. [Google Scholar] [CrossRef]
- Fan, M.; Sun, X.; Xu, Z.; Zhao, S. Characterization of residue (C5-C7) asphaltene (soluble in n-heptane but insoluble in n-pentane). J. Petrochem. Univ. 2011, 24, 8–12. [Google Scholar]


| Experiment No. | Good Solvent | Poor Solvent |
|---|---|---|
| 1 | Xylene | n-Heptane |
| 2 | Toluene | n-Heptane |
| 3 | Xylene | n-Hexane |
| 4 | Xylene | n-Pentane |
| Fractions | N (%) | C (%) | H (%) | S (%) | Others (%) |
|---|---|---|---|---|---|
| Asphalt | 0.74 ± 0.07 | 83.09 ± 0.12 | 8.83 ± 0.05 | 4.19 ± 0.07 | 3.14 ± 0.31 |
| Asphaltene | 1.55 ± 0.06 | 82.33 ± 0.12 | 7.25 ± 0.06 | 5.97 ± 0.06 | 2.88 ± 0.30 |
| Structural Parameters | Asphaltene | Asphalt |
|---|---|---|
| q | 0.94 | 0.78 |
| hA | 0.12 | 0.04 |
| hα | 0.22 | 0.15 |
| hβ | 0.50 | 0.63 |
| hγ | 0.16 | 0.17 |
| fA | 0.53 | 0.39 |
| Solvents | Chemical Formula | C/H Mass Ratio | C/H Atomic Ratio |
|---|---|---|---|
| Xylene | C8H10 | 9.61 | 0.80 |
| Toluene | C7H8 | 10.51 | 0.88 |
| n-Pentane | C5H12 | 5.00 | 0.42 |
| n-Hexane | C6H14 | 5.15 | 0.43 |
| n-Heptane | C7H16 | 5.25 | 0.44 |
| Experiment No. | Good Solvent | Poor Solvent | Asphaltene Yield |
|---|---|---|---|
| 1 | Xylene | n-Heptane | 13.2 ± 0.4% |
| 2 | Toluene | n-Heptane | 11.3 ± 0.4% |
| 3 | Xylene | n-Hexane | 13.7 ± 0.4% |
| 4 | Xylene | n-Pentane | 16.8 ± 0.4% |
| Extraction Times | Good Solvent | Poor Solvent | Asphaltene Yield |
|---|---|---|---|
| 1 | Xylene | n-Heptane | 13.2 ± 0.4% |
| 2 | Xylene 2 | n-Heptane 2 | 10.8 ± 0.4% |
| 3 | Xylene 3 | n-Heptane 3 | 8.3 ± 0.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D. A Simple Scheme for Extraction of Asphaltenes from Asphalt at Room Temperature. Coatings 2022, 12, 407. https://doi.org/10.3390/coatings12030407
Sun D. A Simple Scheme for Extraction of Asphaltenes from Asphalt at Room Temperature. Coatings. 2022; 12(3):407. https://doi.org/10.3390/coatings12030407
Chicago/Turabian StyleSun, Dachuan. 2022. "A Simple Scheme for Extraction of Asphaltenes from Asphalt at Room Temperature" Coatings 12, no. 3: 407. https://doi.org/10.3390/coatings12030407
APA StyleSun, D. (2022). A Simple Scheme for Extraction of Asphaltenes from Asphalt at Room Temperature. Coatings, 12(3), 407. https://doi.org/10.3390/coatings12030407






