Effect of Annealing Temperature on the Microstructure and Optical Properties of Lanthanum-Doped Hafnium Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation
2.3. Characterization
3. Results and Discussion
3.1. Structure Analysis
3.2. Bonding Structure Analysis
3.3. Raman Spectra Analysis
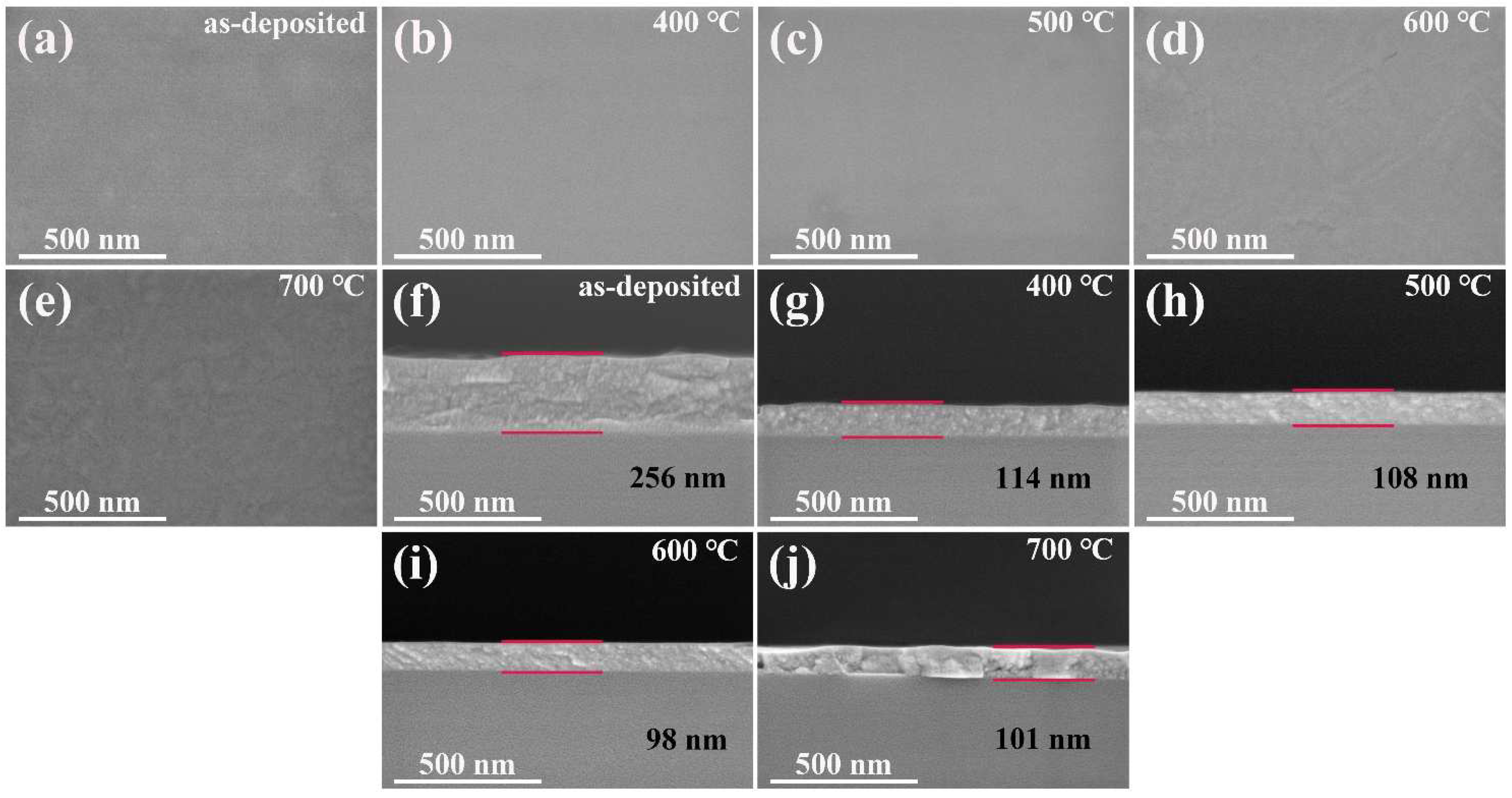
3.4. Morphology Analysis
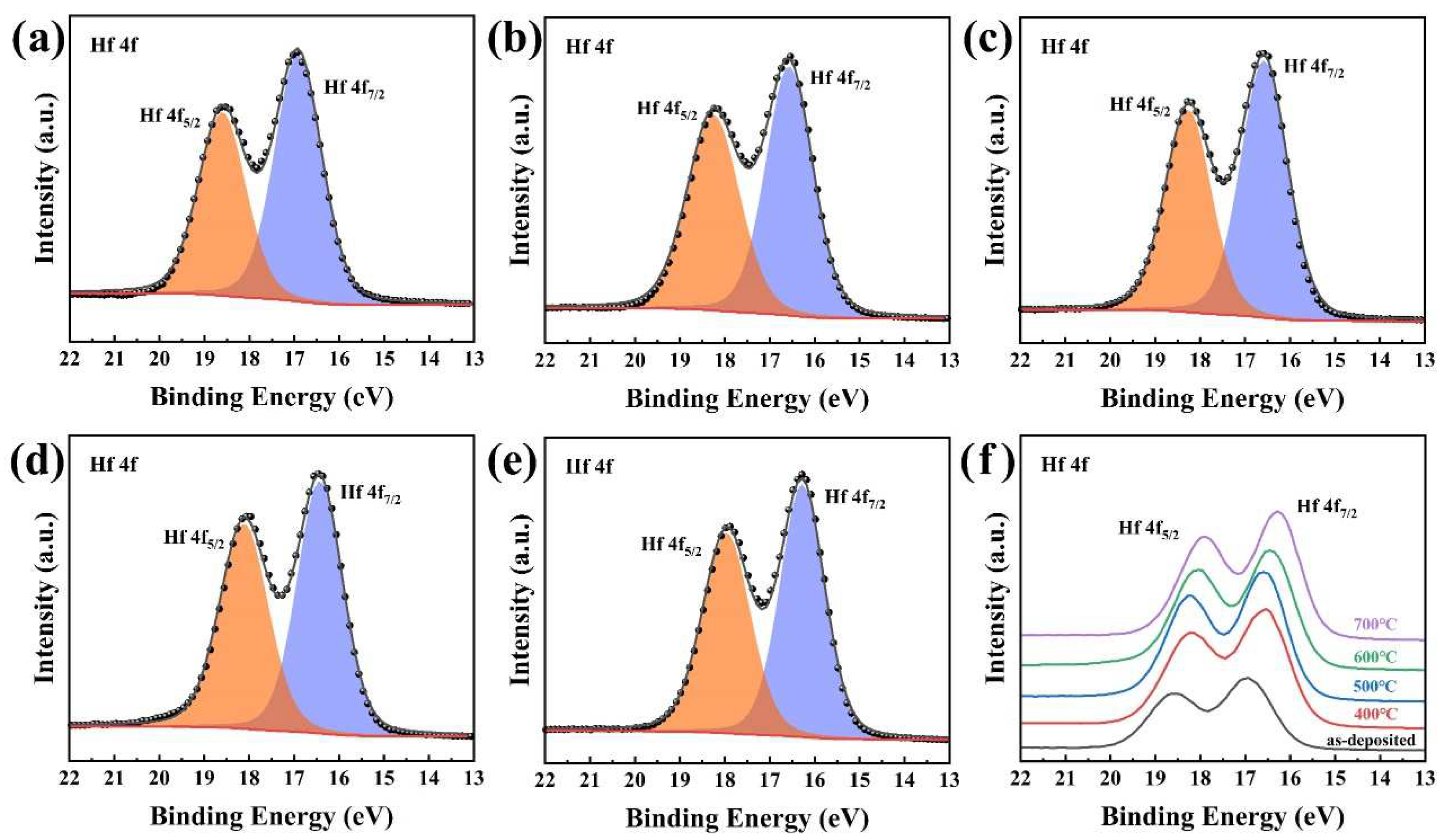
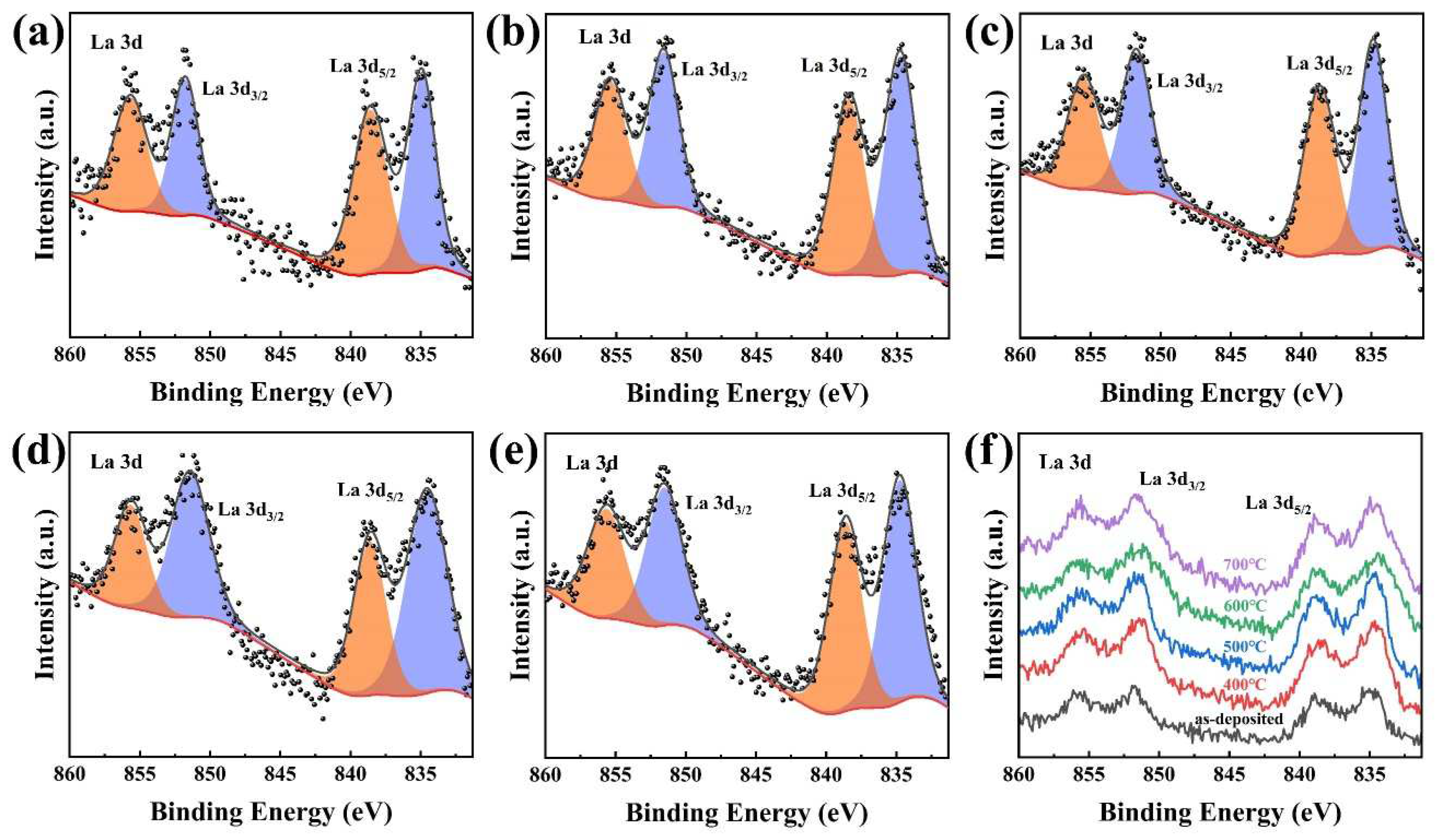
3.5. Chemical Compositions and States
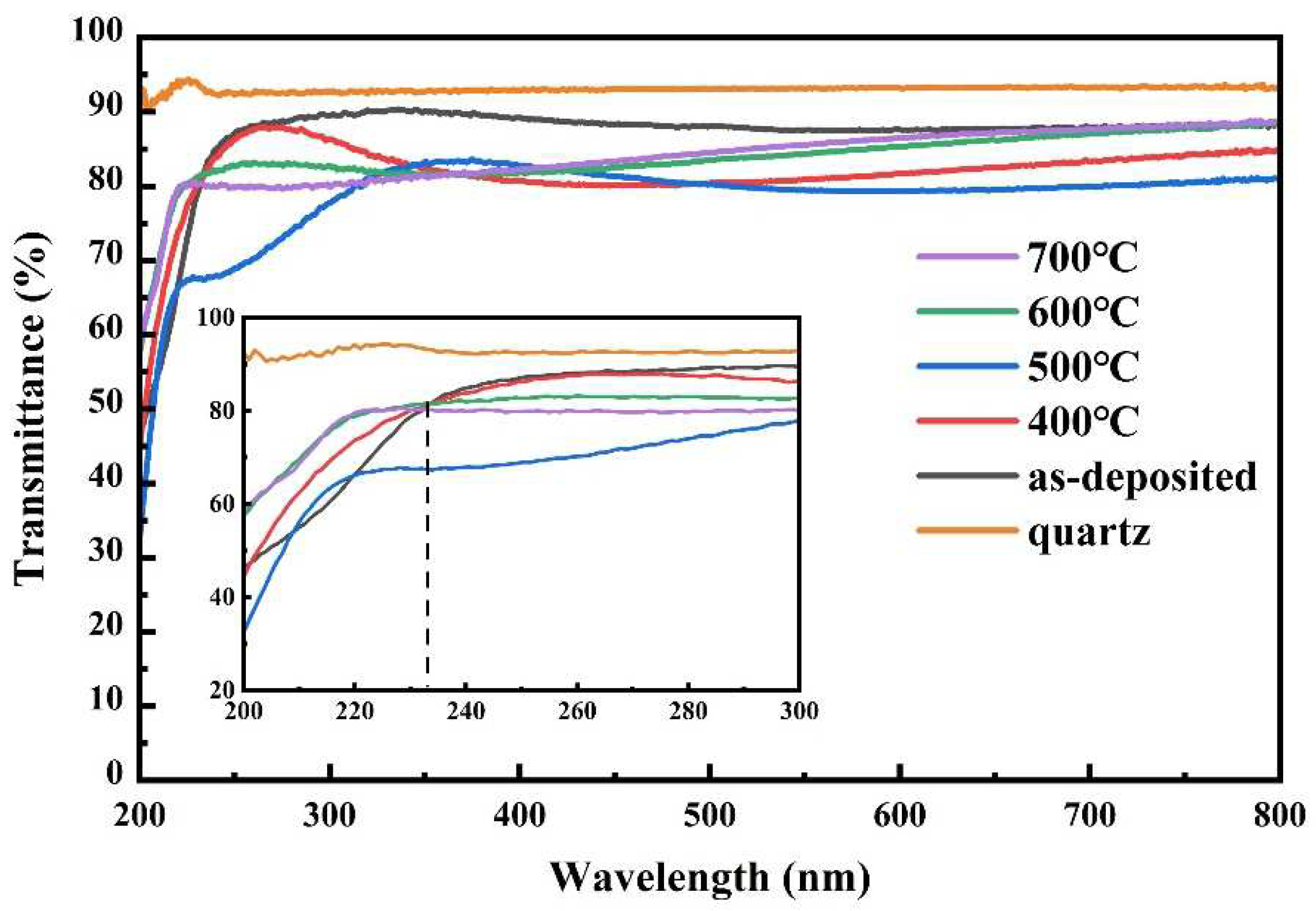
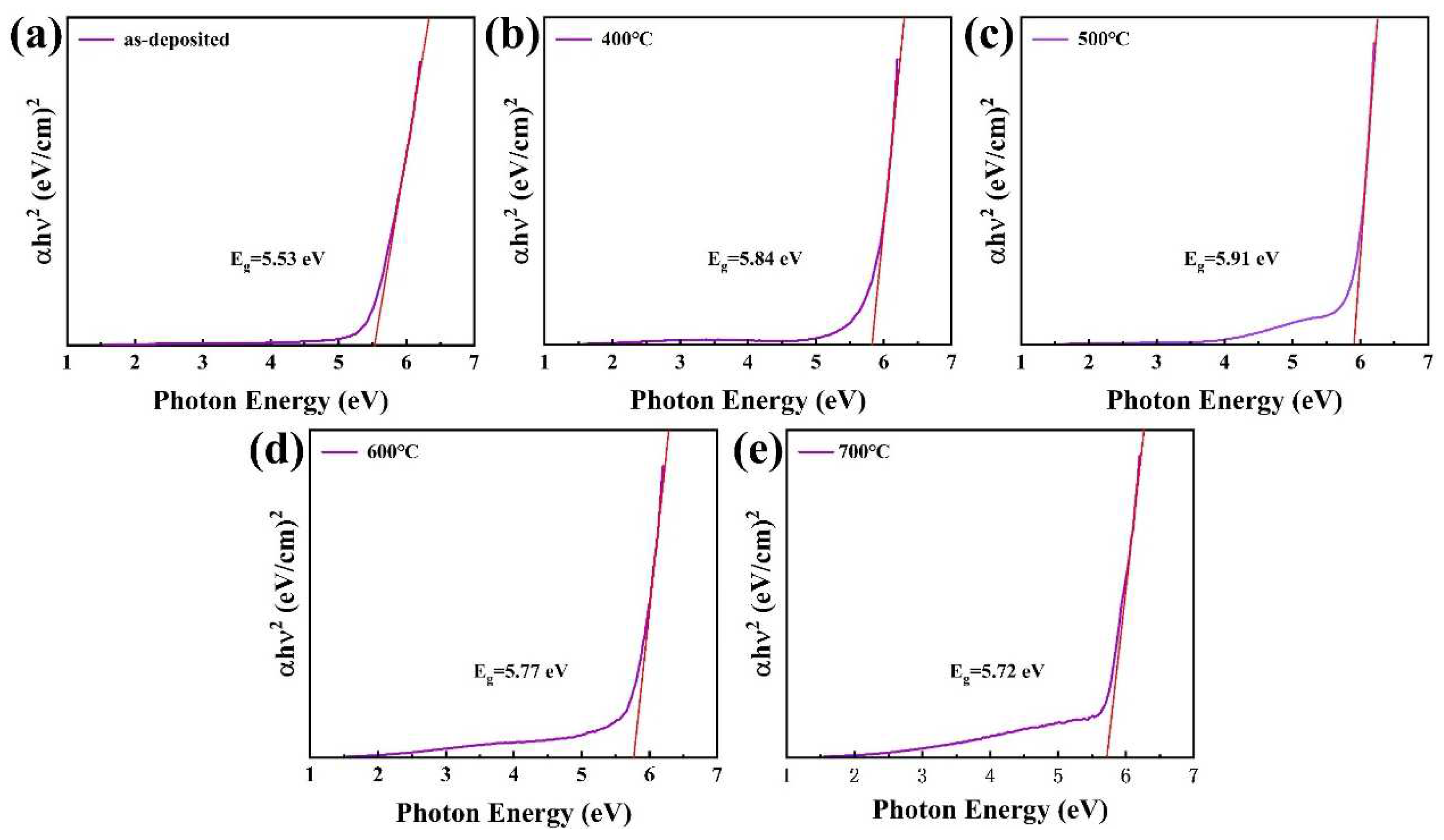
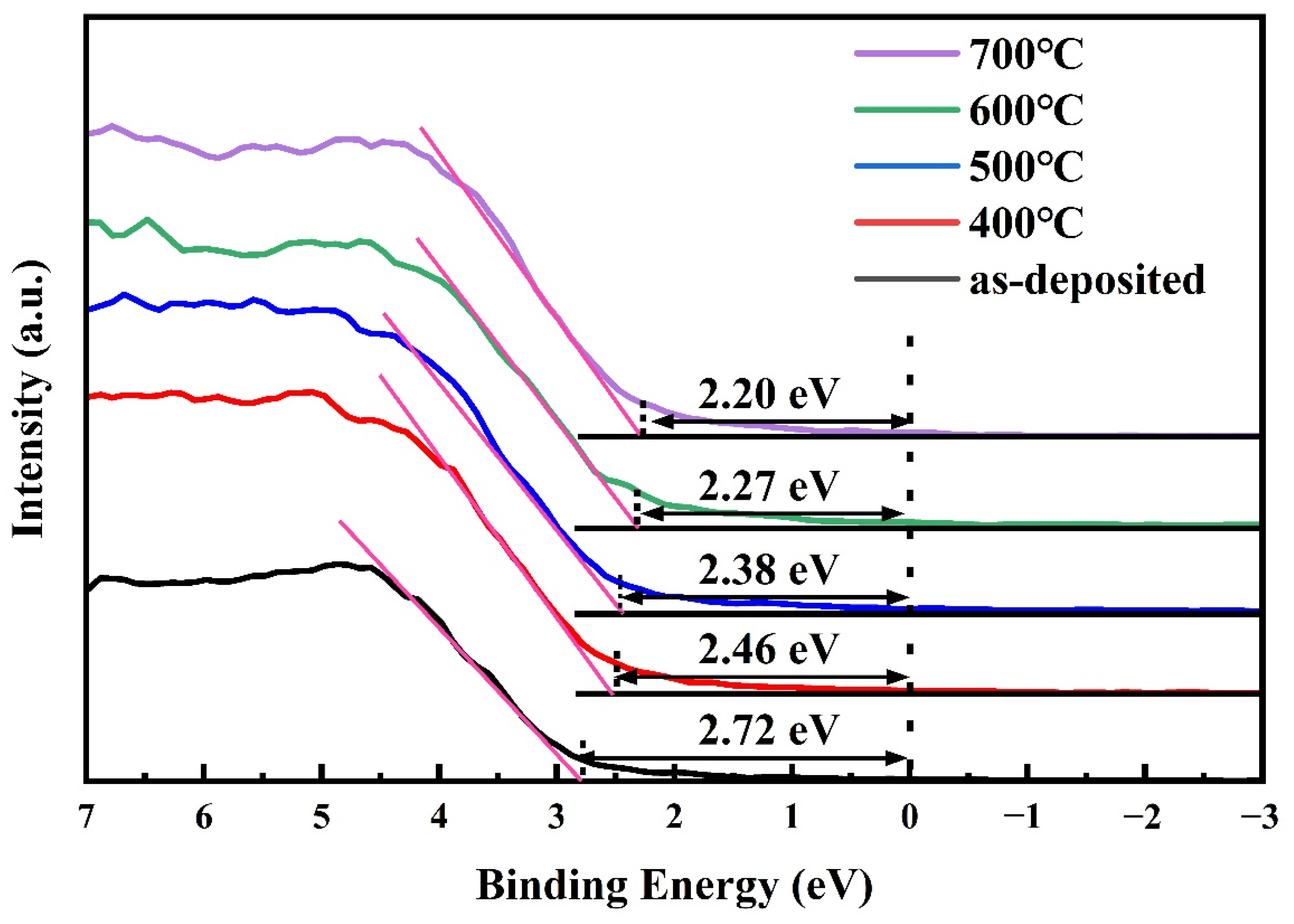
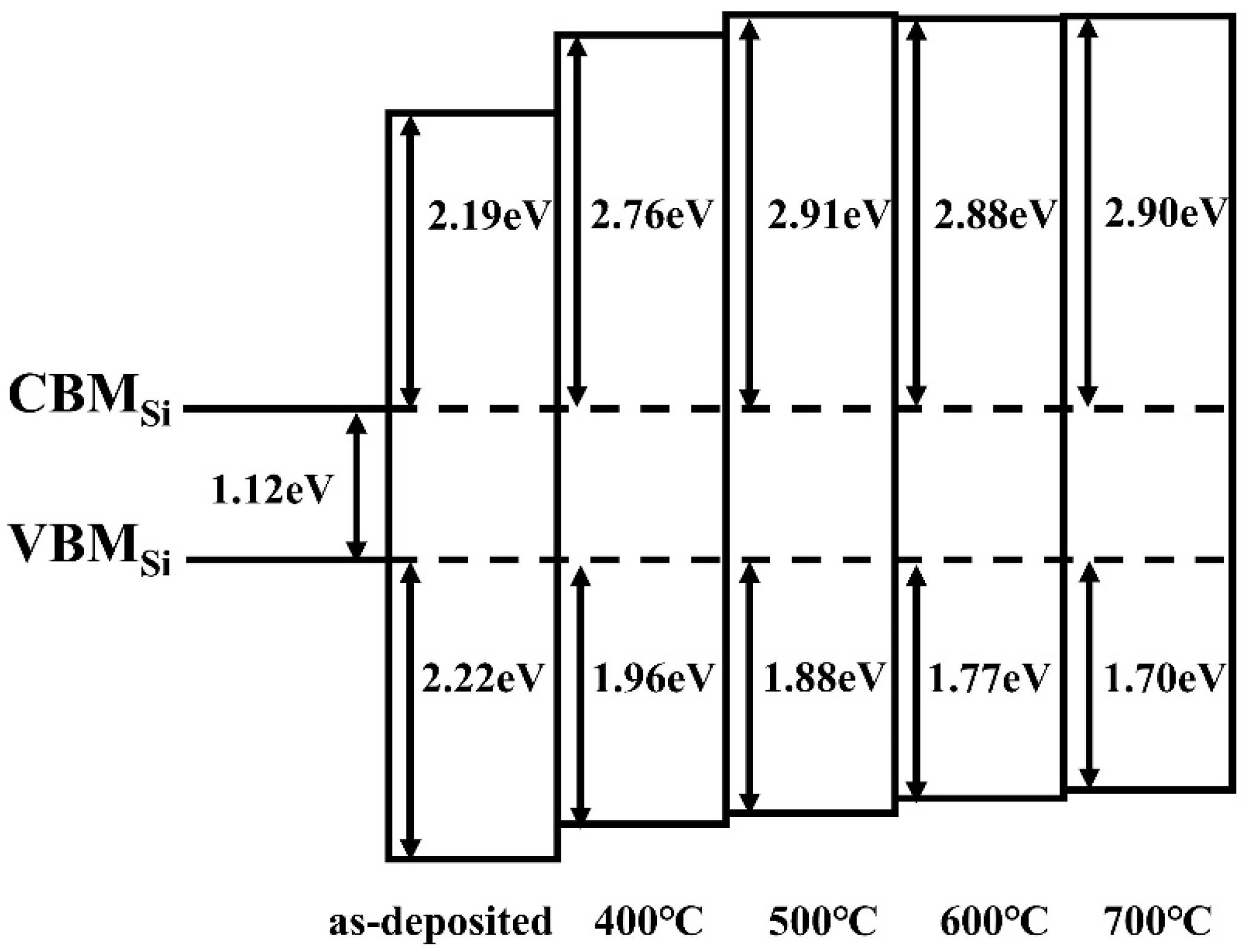
3.6. UV Spectral Analysis and Band Offset Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantas, A.; Aygun, G.; Turan, R. Impact of incorporated oxygen quantity on optical, structural and dielectric properties of reactive magnetron sputter grown high-κ HfO2/Hf/Si thin film. Appl. Surf. Sci. 2014, 318, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Qi, Z.; Zhang, G.; Chen, Y.; Li, T.; Pan, G.; Yin, M. The interface reaction of high-k La2Hf2O7/Si thin film grown by pulsed laser deposition. Appl. Surf. Sci. 2009, 256, 838–841. [Google Scholar] [CrossRef]
- Xiong, K.; Robertson, J.; Gibson, M.C.; Clark, S.J. Defect energy levels in HfO2 high-dielectric-constant gate oxide. Appl. Phys. Lett. 2005, 87, 183505. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.W.; Wang, Q.; Javey, A.; Tu, R.; Dai, H.J.; Kim, H.; McIntyre, P.C.; Krishnamohan, T.; Saraswat, K.C. Germanium nanowire field-effect transistors with SiO2 and high-κ HfO2 gate dielectrics. Appl. Phys. Lett. 2003, 83, 2432–2434. [Google Scholar] [CrossRef]
- Adelmann, C.; Sriramkumar, V.; Van Elshocht, S.; Lehnen, P.; Conard, T.; De Gendt, S. Dielectric properties of dysprosium- and scandium-doped hafnium dioxide thin films. Appl. Phys. Lett. 2007, 91, 162902. [Google Scholar] [CrossRef]
- Wilk, G.D.; Wallace, R.M.; Anthony, J.M. High-κ gate dielectrics: Current status and materials properties considerations. J. Appl. Phys. 2001, 89, 5243–5275. [Google Scholar] [CrossRef]
- He, G.; Deng, B.; Liu, M.; Ma, Y.; Chen, X.; Lv, J.; Sun, Z. Oxynitridization Dependent Interface Control and Band Alignment of AlON/SiON/Si Gate Stacks Grown by Metalorganic Chemical Vapor Deposition. Sci. Adv. Mater. 2013, 5, 709–712. [Google Scholar] [CrossRef]
- Sim, H.; Hwang, H.S. Effect of deuterium postmetal annealing on the reliability characteristics of an atomic-layer-deposited HfO2/SiO2 stack gate dielectrics. Appl. Phys. Lett. 2002, 81, 4038–4039. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, S.; Hwang, H. Electrical characteristics of a Dy-doped HfO2 gate dielectric. Appl. Phys. Lett. 2001, 79, 2615–2617. [Google Scholar] [CrossRef]
- He, G.; Fang, Q.; Liu, M.; Zhu, L.; Zhang, L.Q.; Zhang, L.D. The structural and interfacial properties of HfO2/Si by the plasma oxidation of sputtered metallic Hf thin films. J. Cryst. Growth 2004, 268, 155–162. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kwon, S.-H. Sub-0.5 nm Equivalent Oxide Thickness Scaling for Si-Doped Zr1−xHfxO2 Thin Film without Using Noble Metal Electrode. ACS Appl. Mater. Interfaces 2015, 7, 15587–15592. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-K.; Wu, C.-H.; Liu, C.-C.; Lin, J.-F.; Yang, C.-L.; Wu, J.-Y.; Wang, S.-J. Characterization of Hf1−xZrxO2 Gate Dielectrics with 0≤ x ≤ 1 Prepared by Atomic Layer Deposition for Metal Oxide Semiconductor Field Effect Transistor Applications. Jpn. J. Appl. Phys. 2011, 51, 11101. [Google Scholar] [CrossRef]
- Ye, C.; Zhan, C.; Zhang, J.Q.; Wang, H.; Deng, T.F.; Tang, S.R. Influence of rapid thermal annealing temperature on structure and electrical properties of high permittivity HfTiO thin film used in MOSFET. Microelectron. Reliab. 2014, 54, 388–392. [Google Scholar] [CrossRef]
- Liang, S.; He, G.; Zhu, L.; Zheng, C.Y.; Gao, J.; Wang, D.; Zhang, C.; Liu, M. Modulation of the microstructure, optical, and electrical properties of HfYO gate dielectrics by annealing temperature. J. Alloys Compd. 2018, 735, 1427–1434. [Google Scholar] [CrossRef]
- Ma, R.; Liu, M.; He, G.; Fang, M.; Shang, G.; Zhang, J.; Chen, X.; Gao, J.; Fei, G.; Zhang, L. Effects of rapid thermal annealing on interfacial and electrical properties of Gd-doped HfO2 high-k gate dielectrics. J. Alloys Compd. 2015, 646, 310–314. [Google Scholar] [CrossRef]
- Tang, L.; Chen, C.; Wei, A.; Li, K.; Zhang, D.; Zhou, K. Regulating crystal structure and ferroelectricity in Sr doped HfO2 thin films fabricated by metallo-organic decomposition. Ceram. Int. 2019, 45, 3140–3147. [Google Scholar] [CrossRef]
- Nohira, H.; Shiraishi, T.; Takahashi, K.; Hattori, T.; Kashiwagi, I.; Ohshima, C.; Ohmi, S.; Iwai, H.; Joumori, S.; Nakajima, K.; et al. Atomic-scale depth profiling of composition, chemical structure and electronic band structure of La2O3/Si(100) interfacial transition layer. Appl. Surf. Sci. 2004, 234, 493–496. [Google Scholar] [CrossRef]
- Mart, C.; Kühnel, K.; Kämpfe, T.; Zybell, S.; Weinreich, W. Ferroelectric and pyroelectric properties of polycrystalline La-doped HfO2 thin films. Appl. Phys. Lett. 2019, 114, 102903. [Google Scholar] [CrossRef]
- Schroeder, U.; Richter, C.; Park, M.H.; Schenk, T.; Pešić, M.; Hoffmann, M.; Fengler, F.P.G.; Pohl, D.; Rellinghaus, B.; Zhou, C.; et al. Lanthanum-Doped Hafnium Oxide: A Robust Ferroelectric Material. Inorg. Chem. 2018, 57, 2752–2765. [Google Scholar] [CrossRef]
- Pasko, S.; Abrutis, A.; Hubert-Pfalzgraf, L.G.; Kubilius, V. Cobalt (II) β-diketonate adducts as new precursors for the growth of cobalt oxide films by liquid injection MOCVD. J. Cryst. Growth 2004, 262, 653–657. [Google Scholar] [CrossRef]
- Chu, L.K.; Merckling, C.; Alian, A.; Dekoster, J.; Kwo, J.; Hong, M.; Caymax, M.; Heyns, M. Low interfacial trap density and sub-nm equivalent oxide thickness in In0.53Ga0.47As (001) metal-oxide-semiconductor devices using molecular beam deposited HfO2/Al2O3 as gate dielectrics. Appl. Phys. Lett. 2011, 99, 042908. [Google Scholar] [CrossRef]
- Aguilar-Castillo, A.; Aguilar-Hernández, J.R.; García-Hipólito, M.; López-Romero, S.; Swarnkar, R.; Báez-Rodríguez, A.; Fragoso-Soriano, R.J.; Falcony, C. White light generation from HfO2 films co-doped with Eu3++ Tb3+ ions synthesized by pulsed laser ablation technique. Ceram. Int. 2017, 43, 355–362. [Google Scholar] [CrossRef]
- Islamov, D.R.; Zalyalov, T.M.; Orlov, O.M.; Gritsenko, V.A.; Krasnikov, G.Y. Impact of oxygen vacancy on the ferroelectric properties of lanthanum-doped hafnium oxide. Appl. Phys. Lett. 2020, 117, 162901. [Google Scholar] [CrossRef]
- Suvorova, E.I.; Uvarov, O.V.; Arkharova, N.A.; Ibrayeva, A.D.; Skuratov, V.A.; Buffat, P.A. Structure evolution, bandgap, and dielectric function in La-doped hafnium oxide thin layer subjected to swift Xe ion irradiation. J. Appl. Phys. 2020, 128, 164103. [Google Scholar] [CrossRef]
- Gong, Y.-P.; Li, A.-D.; Qian, X.; Zhao, C.; Wu, D. Interfacial structure and electrical properties of ultrathin HfO2 dielectric films on Si substrates by surface sol-gel method. J. Phys. D Appl. Phys. 2008, 42, 015405. [Google Scholar] [CrossRef]
- Jin, P.; He, G.; Xiao, D.; Gao, J.; Liu, M.; Lv, J.; Liu, Y.; Zhang, M.; Wang, P.; Sun, Z. Microstructure, optical, electrical properties, and leakage current transport mechanism of sol-gel-processed high-k HfO2 gate dielectrics. Ceram. Int. 2016, 42, 6761–6769. [Google Scholar] [CrossRef]
- Kariper, I.A. Production of HfO2 thin films using different methods: Chemical bath deposition, SILAR and sol-gel process. Int. J. Miner. Met. Mater. 2014, 21, 832–838. [Google Scholar] [CrossRef]
- Kidchob, T.; Malfatti, L.; Serra, F.; Falcaro, P.; Enzo, S.; Innocenzi, P. Hafnia sol-gel films synthesized from HfCl4: Changes of structure and properties with the firing temperature. J. Sol-Gel Sci. Technol. 2007, 42, 89–93. [Google Scholar] [CrossRef]
- Leu, C.-C.; Chen, S.-T.; Liu, F.-K. Metal nanocrystal memory with sol–gel derived HfO2 high-κ tunnel oxide. Thin Solid Films 2011, 519, 5629–5633. [Google Scholar] [CrossRef]
- Tetzner, K.; Schroder, K.A.; Bock, K. Photonic curing of sol-gel derived HfO2 dielectrics for organic field-effect transistors. Ceram. Int. 2014, 40, 15753–15761. [Google Scholar] [CrossRef]
- Wang, Z.J.; Kumagai, T.; Kokawa, H.; Ichiki, M.; Maeda, R. Preparation of hafnium oxide thin films by sol-gel method. J. Electroceram. 2007, 21, 499–502. [Google Scholar] [CrossRef]
- Obstarczyk, A.; Kaczmarek, D.; Mazur, M.; Wojcieszak, D.; Domaradzki, J.; Kotwica, T.; Morgiel, J. The effect of post-process annealing on optical and electrical properties of mixed HfO2-TiO2 thin film coatings. J. Mater. Sci. Mater. Electron. 2019, 30, 6358–6369. [Google Scholar] [CrossRef] [Green Version]
- Battu, A.K.; Zade, V.B.; Deemer, E.; Ramana, C.V. Microstructure-Mechanical Property Correlation in Size Controlled Nanocrystalline Molybdenum Films. Adv. Eng. Mater. 2018, 20, 1800496. [Google Scholar] [CrossRef]
- Battu, A.K.; Makeswaran, N.; Ramana, C.V. Fabrication, characterization and optimization of high conductivity and high quality nanocrystalline molybdenum thin films. J. Mater. Sci. Technol. 2019, 35, 2734–2741. [Google Scholar] [CrossRef]
- Yan, K.; Yao, W.; Zhao, Y.; Yang, L.; Cao, J.; Zhu, Y. Oxygen vacancy induced structure change and interface reaction in HfO2 films on native SiO2/Si substrate. Appl. Surf. Sci. 2016, 390, 260–265. [Google Scholar] [CrossRef]
- Carlos, E.; Branquinho, R.; Kiazadeh, A.; Martins, J.; Barquinha, P.; Martins, R.; Fortunato, E. Boosting Electrical Performance of High-κ Nanomultilayer Dielectrics and Electronic Devices by Combining Solution Combustion Synthesis and UV Irradiation. ACS Appl. Mater. Interfaces 2017, 9, 40428–40437. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.F.; Quah, H.J. Wet oxidation growth of hafnium doped tantalum oxide films with different composition deposited on silicon substrate. Appl. Surf. Sci. 2020, 526, 146722. [Google Scholar] [CrossRef]
- Zhao, X.; Vanderbilt, D. First-principles study of structural, vibrational, and lattice dielectric properties of hafnium oxide. Phys. Rev. B 2002, 65, 233106. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Liu, Z.; Lu, H.; Liu, W.; Tian, H. Structure and optical properties of HfO2 thin films on silicon after rapid thermal annealing. Opt. Mater. 2010, 32, 432–435. [Google Scholar] [CrossRef]
- Zhang, Y.; He, G.; Wang, W.; Yang, B.; Zhang, C.; Xia, Y. Aqueous-solution-driven HfGdO gate dielectrics for low-voltage-operated α-InGaZnO transistors and inverter circuits. J. Mater. Sci. Technol. 2020, 50, 1–12. [Google Scholar] [CrossRef]
- Lin, S.-S.; Liao, C.-S. Effects of the ratio of O2/Ar pressure on wettability and optical properties of HfO2 films before and after doping with Al. Appl. Surf. Sci. 2016, 380, 229–236. [Google Scholar] [CrossRef]
- Chung, J.; Tak, Y.J.; Kim, W.-G.; Park, J.W.; Kim, T.S.; Lim, J.H.; Kim, H.J. Low-temperature fabrication of solution-processed hafnium oxide gate insulator films using a thermally purified solution process. J. Mater. Chem. C 2018, 6, 4928–4935. [Google Scholar] [CrossRef]
- Xiong, Y.; Tu, H.; Du, J.; Ji, M.; Zhang, X.; Wang, L. Band structure and electrical properties of Gd-doped HfO2 high k gate dielectric. Appl. Phys. Lett. 2010, 97, 012901. [Google Scholar] [CrossRef]
- Battu, A.K.; Manandhar, S.; Shutthanandan, V.; Ramana, C.V. Controlled optical properties via chemical composition tuning in molybdenum-incorporated beta-Ga2O3 nanocrystalline films. Chem. Phys. Lett. 2017, 684, 363–367. [Google Scholar] [CrossRef]
- Kumar, S.S.; Rubio, E.J.; Noor-A-Alam, M.; Martinez, G.; Manandhar, S.; Shutthanandan, V.; Thevuthasan, S.; Ramana, C.V. Structure, Morphology, and Optical Properties of Amorphous and Nanocrystalline Gallium Oxide Thin Films. J. Phys. Chem. C 2013, 117, 4194–4200. [Google Scholar] [CrossRef]
- Li, W.D.; He, G.; Zheng, C.Y.; Liang, S.; Zhu, L.; Jiang, S.S. Solution-processed HfGdO gate dielectric thin films for CMOS application: Effect of annealing temperature. J. Alloys Compd. 2018, 731, 150–155. [Google Scholar] [CrossRef]





| Samples | Composition by XPS (at.%) | ||||
|---|---|---|---|---|---|
| Hf | Si | C | O | La | |
| As-deposited | 10.17 | 5.02 | 50.4 | 33.72 | 0.68 |
| 400 ℃ | 15.6 | 8.41 | 33.78 | 41.2 | 1 |
| 500 ℃ | 16.13 | 8.26 | 33.12 | 41.44 | 1.05 |
| 600 ℃ | 15.83 | 8.07 | 35.29 | 39.75 | 1.06 |
| 700 ℃ | 16.1 | 8.03 | 33.88 | 40.83 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Tuokedaerhan, K.; Cai, H.; Lu, Z. Effect of Annealing Temperature on the Microstructure and Optical Properties of Lanthanum-Doped Hafnium Oxide. Coatings 2022, 12, 439. https://doi.org/10.3390/coatings12040439
Cui X, Tuokedaerhan K, Cai H, Lu Z. Effect of Annealing Temperature on the Microstructure and Optical Properties of Lanthanum-Doped Hafnium Oxide. Coatings. 2022; 12(4):439. https://doi.org/10.3390/coatings12040439
Chicago/Turabian StyleCui, Xiangduo, Kamale Tuokedaerhan, Haotian Cai, and Zhenchuan Lu. 2022. "Effect of Annealing Temperature on the Microstructure and Optical Properties of Lanthanum-Doped Hafnium Oxide" Coatings 12, no. 4: 439. https://doi.org/10.3390/coatings12040439





