Abstract
The thin Ti layers were inserted in the interfaces of base Al/Ni multilayer foils to form the Al/Ti/Ni/Ti (ATNT) foils through magnetron deposition. Al and Ni were determined in the as-deposited foils, while the absence of Ti was due to the strongly textured polycrystalline structure. TEM analysis implied an asymmetric interface structure between the Ni/Ti/Al interfaces and Al/Ti/Ni interfaces. After annealing at 473 K and 573 K for 3 h, the phase composition was the same as the initial state, which changed to be Al3Ni2, Ni3(AlTi), Ni and a small amount of Al3Ti when the treating temperature reached 673 K. Further increasing the annealing temperature to 773 K and 873 K leads to the appearance of stable AlNi. The obtained results implied that the inserted Ti layers impeded atomic interdiffusion and the formation of Al3Ni at the early stage, but had less impact on the final products. This further indicated that adding the inserted transition layer provides a reference to balance the storage stability and reaction performance of Al/Ni foils with regard to the applications.
1. Introduction
Nanoscale multilayer foils with extremely high ratios of interfacial area to volume tend to exhibit a self-propagating reaction when the heat released from local mixing is sufficient to activate the adjacent materials. Al/Ni multilayer foils consisting of alternating layers of Al and Ni are one of the most-studied systems due to a fairly large enthalpy of reaction, high energy-release rate, fast combustion, and the fact that they are relatively inexpensive and readily available through those physical vapor deposition techniques and mechanical processing [1,2,3,4]. These advantages are of interest as heat sources for bonding and joining [1,2], ignitors [5], and thermal battery [6].
Efforts have been devoted to studying the phase transformation, as well as its influence factors of the nanoscale Al/Ni multilayer foils under both extremely high and low heating rates, since the reaction mechanism varies significantly from the melting of Al to solid-state diffusion across metallic interfaces [7,8,9]. Apart from modulation periods [9,10,11], the intermixing region existing in the interfaces between the Al and Ni layers has a great impact on the phase transformation in the Al/Ni foils produced by magnetron sputter-deposition or ion-beam deposition [12]. In addition to the fabrication methods [4,10], easy interdiffusion between Al and Ni elements also contributed to the intensive intermixing [11]. The activation energy for the diffusion of Ni in Al is very similar to that for self-diffusion of Al, thus apparent interdiffusion happens under both deposition and storage [4,13]. These intermixing regions could act as a diffusion barrier as proved by both experiments and molecular dynamics (MD) simulation [9,14], and also change the primary phase from Al3Ni to Al9Ni2 in those foils with a modulation period larger than 25 nm [12,15,16]. Even worse, such regions reduced stored chemical energy and reaction rate since the premixed regions occupy a larger volume fraction [17]. From this viewpoint, it is highly important for the applications to alleviate the intermixing at the interfaces to diminish the detrimental effect but retain the self-sustaining reaction.
The so-called “sacrificial barriers” commonly used in the semiconductor provide a candidate for solving this problem. The thin Ti layers were added into the interfaces between the Si and Al layers, which reacted with Si to TiSi2 and with Al to form TiAl3 [18]. These compounds restrained the interdiffusion between Al and Si, but maintained adequate conductivity. Afterwards, enhanced reliability was obtained, although at the cost of increasing structural complexity and added processing expense. Here, the inserted layers must not change the self-propagating reaction of Al/Ni foils. Ti layers can be used as sacrificial barriers of the Al/Ni foils, since both the Al/Ti and Ti/Ni binary systems are also metal/metal reactive multilayers but with higher ignition temperatures compared to the Al/Ni system [19,20]. Additionally, the reaction mechanism for the three reactive systems is the diffusion of Ni and Ti into the melted Al with a much quicker atomic diffusion rate. In this aspect, the inserted Ti layers might help to avoid the intermixing caused by interdiffusion between Al and Ni during deposition and decrement of stored energy, while the self-propagating reaction of Al/Ni foils still maintains.
AlN is chosen as the deposition substrate so as to avoid the delamination of the multilayers from the substrate [21]. Considering that a much thicker inserted layer might lead to the termination of self-propagating reaction [22], thus Ti layers with a thickness of 20 nm was added into the interfaces to form the new Al/Ti/Ni/Ti (ATNT) multilayer foils, which is much thinner than the layer thickness of Al and Ni. In order to gain a better understanding of the interface structure and atomic diffusion in the ATNT foils, the as-deposited foils were treated at temperature from 473 K to 873 K. These scientific investigations feed back into practical applications by enabling improved predictions of performance and by identifying ways that performance can be tuned for specific applications [22]. However, it should be emphasized that a higher heating rate is required with regard to the application of multilayer foils [7]. The phase composition of the sample after annealing at 873 K was AlNi and a few Al3Ni2, Al3Ti, NiTi, which means that the inserted Ti layer has less effect on the final products although it impedes the interdiffusion and the formation of Al3Ni at the early stage. The findings also suggest that adding the inserted layer provides a reference to balance the storage stability and reaction performance of Al/Ni multilayers with regard to the applications.
2. Materials and Methods
Al/Ti/Ni/Ti multilayer foils were magnetron deposited from Al (99.999%, Beijing Dream Material Technology Co., Beijing, China), Ni (99.999%, Beijing Dream Material Technology Co., Beijing, China) and Ti (99.999%, Beijing Dream Material Technology, Beijing, China) targets. AlN with dimensions of 5 × 5 × 1 mm3 was used as a deposition substrate (Beijing Dream Material Technology Co., Beijing, China). The structure for a single modulation period is Al/Ti/Ni/Ti with an individual thickness of 375/20/250/20 nm, which is referred to as ATNT multilayer foils. The relative thickness of Al and Ni layers was maintained at a 3:2 ratio to obtain a final product with an average stoichiometry of AlNi. It should be stressed that, although thick layers of Al and Ni are used to study the effect of inserted Ti layers on phase transformation, those foils for welding and brazing usually had a thickness ranging from a few nm to around 100 nm [23,24,25]. The distance between the deposition target and the deposition platform is 120 mm (FJL560, SKY Technology Development Co., Shenyang, China). The deposition power was 120 W, the Ar pressure during deposition was 0.5 Pa, and the deposition rate for Al, Ni, and Ti elements was 15, 9, and 13 nm·min−1, respectively.
The as-deposited multilayers sealed in the vacuum quartz tube were annealed at 473, 573, 673, 773, and 873 K for 3 h before being quenched to room temperature to investigate the phase transformation. The vacuum degree for the sealed quartz tube is at the level of 10−3 Pa. In order to avoid the impact of remnant oxygen, two pure Ti plates were also placed at each side of the as-prepared multilayer foils simultaneously. Considering the thinness of the multilayers, a D8 Advance DaVinci grazing incidence X-ray diffractometer (D8 Advance, Bruker, Rheinstetten, Germany) equipped with a goniometer and Cu Kα source was applied to characterize the phases before and after the heat treatment. The incidence angle was fixed to be 2°, and the scanning rate was 2°·min−1 with a speed of 0.6 s per step and sampling steps of 0.02°. The interface structure, element distribution, and phase distribution were studied by using Tecnai G2 F30 TEM (FEI, Hillsboro, OR, USA). The samples were prepared using focused ion beam milling on the Zeiss Auriga Dual-beam FIB (Carl Zeiss, Jena, Germany).
3. Results
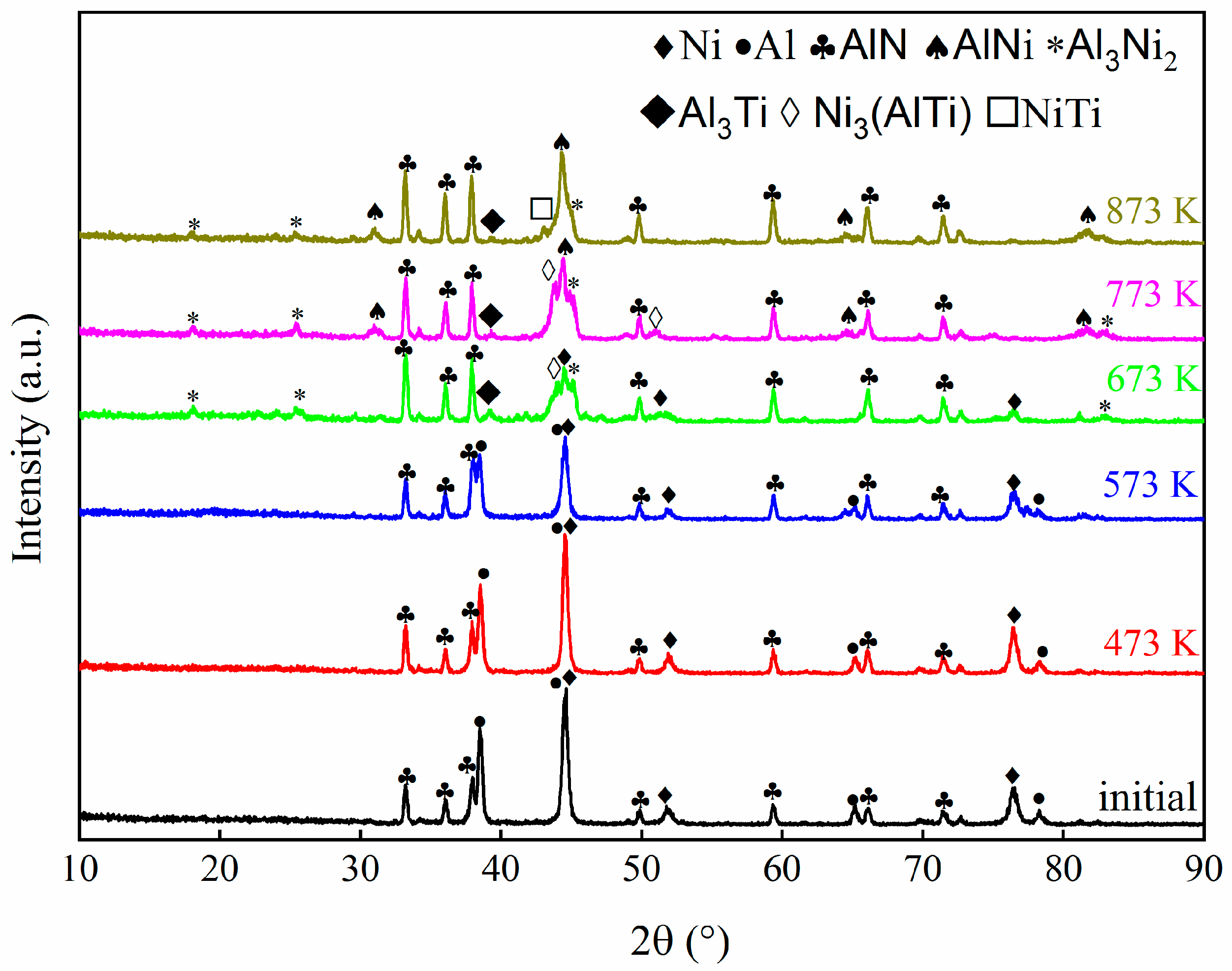
Figure 1 displays the grazing incidence XRD patterns of the as-deposited ATNT multilayer foil. The diffraction peaks mainly belonging to Al and Ni can be clearly distinguished, but those corresponding to hcp-Ti disappear. A similar phenomenon has been reported in the Al-Ti binary system, the strongest peak of the (101) plane for Ti was not observed while an overlapped peak between the (002) plane of Ti and the (111) plane of Al formed [26]. Both results indicate a strongly textured polycrystalline structure of the as-deposited foil. Here, the appearance of peaks from AlN in the XRD patterns was attributed to the inferior compactness of the foils due to the rougher surface of this substrate as pointed in a previous study [21].
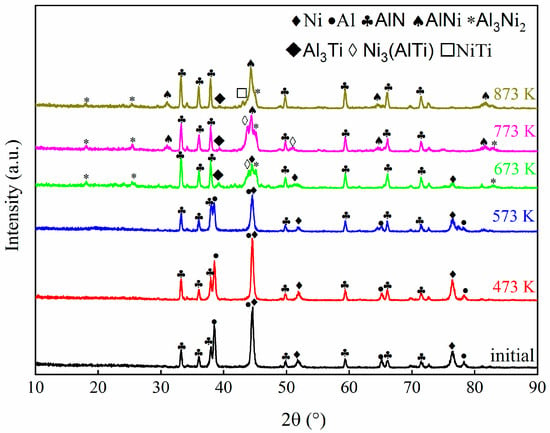
Figure 1.
The grazing incidence XRD patterns of the as-deposited ATNT multilayer foil and those foils after annealing at 473 K, 573 K, 673 K, 773, and 873 K for 3 h.
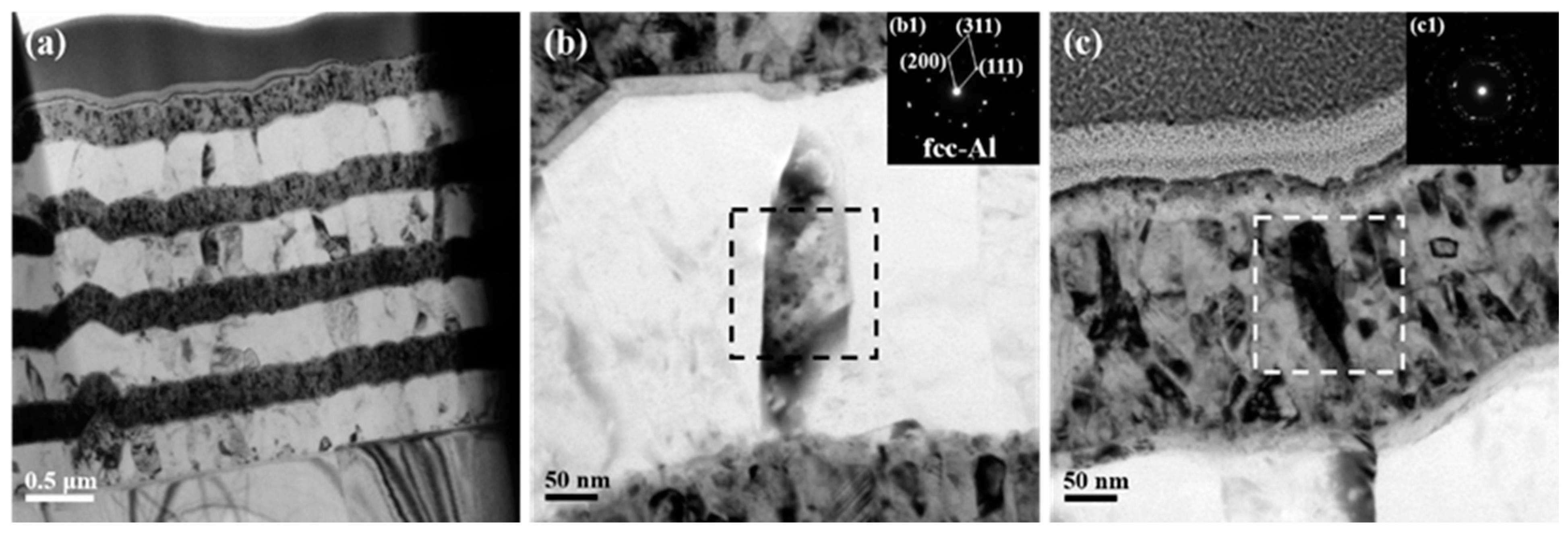
The microstructure of as-deposited ATNT foil is shown in Figure 2. The thickness of a single Al and Ni layer is around 380 nm and 250 nm, respectively. A transition Ti layer with a thickness of around 20 nm is observed between the Al and Ni layer, as seen in Figure 2a. As revealed in the previous report, the polycrystalline layers in foils typically had a strong crystallographic texture and a columnar grain structure. Low melting temperature elements, such as Al, typically tend to form wide grains that match the layer thickness [27], which is consistent with the observation in Figure 2b. The selected area electron diffraction patterns (SADPs) taken from a region with a diameter of 200 nm gave a set of diffraction spots of fcc-Al, while only some diffraction rings were found in the top Ni layer as shown in Figure 2c. One aspect that should be stressed is the asymmetric interface structure. A much clearer and flatter interface formed when Ti was deposited on the pre-formed Al layer followed by a new Ni layer deposited on Ti, which was named as Ni/Ti/Al interface hereinafter. On the contrary, a vague interface defined as Al/Ti/Ni interface was observed on the opposite side for a given Al layer as shown in Figure 2b.
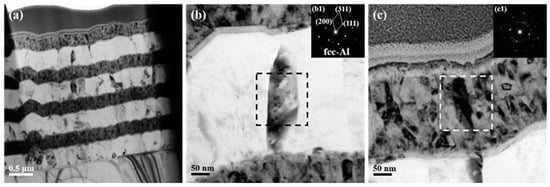
Figure 2.
TEM images together with selected area electron diffraction patterns of the as-deposited ATNT foil: (a) the whole morphology; (b) the Al layer in the middle region; (c) the Ni layer in the top region.
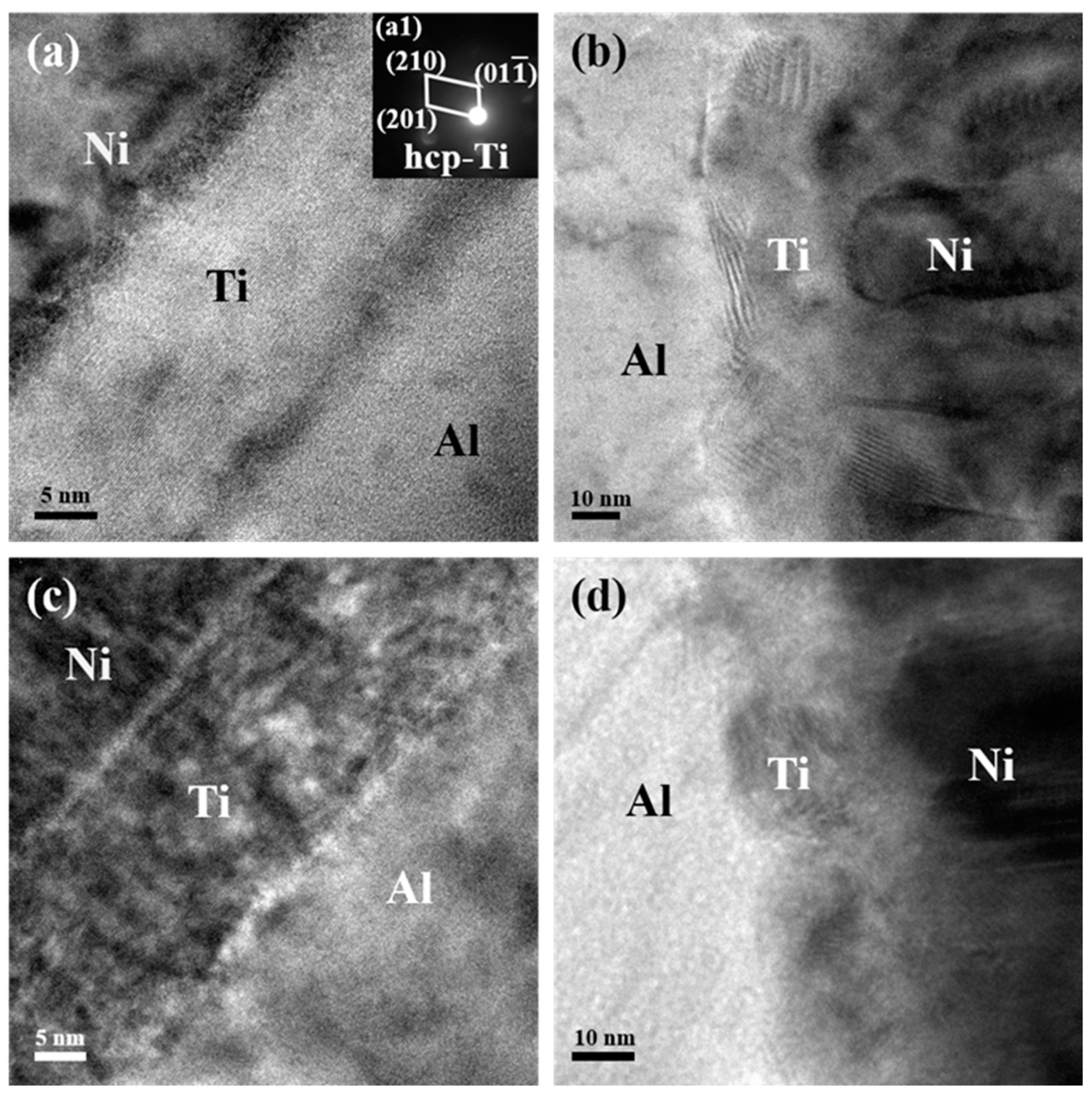
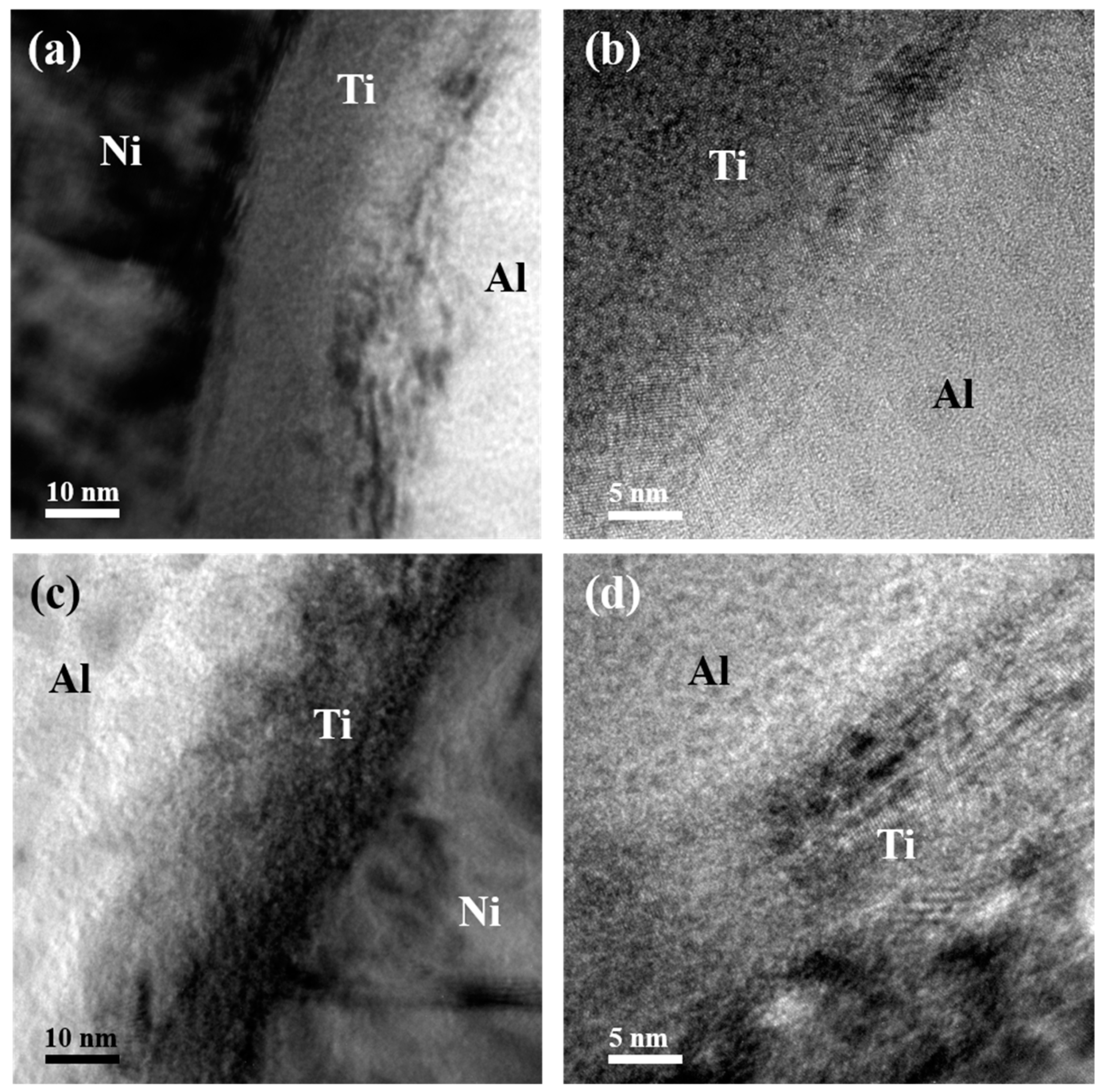
This asymmetric feature could be more distinctly observed in the enlarged TEM images in Figure 3. Apparent interfaces could be found either between the Ni and Ti layers or between the Ti and Al layers in the Ni/Ti/Al interface selected from both the bottom and upper region of the as-deposited ATNT foil as shown in Figure 3a,c. As for the Al/Ti/Ni interface, this characteristic is not much unambiguous (Figure 3b,d). At the same time, the nanobeam electron diffraction pattern was introduced to analyze the phase inner the Ti layers as seen in the inserted Figure of Figure 3a. The SADPs could be indexed to be hcp-Ti, which indicates that no intermetallic phase formed during sample preparation.
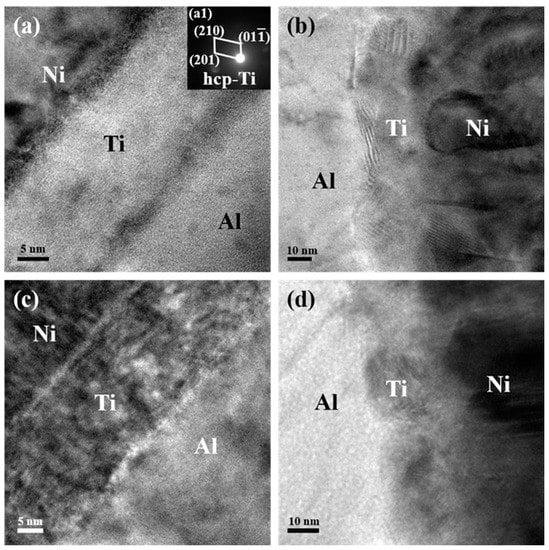
Figure 3.
TEM images of the Ni/Ti/Al interface and Al/Ti/Ni interface for the as-deposited ATNT foil: (a,b) the bottom region; (c,d) the upper region.
Subsequently, the initially deposited foil was heated to different temperatures and held for 3 h to study phase transformation. After annealing at 473 K and 573 K for 3 h, the intensity of mixed Al and Ti peak gradually decreased implying the atomic interdiffusion upon isothermal annealing; the samples have the same phase composition compared to the as-deposited foil, as shown in Figure 1. When the annealing temperature was raised to 673 K, the peaks corresponding to Al and Ti totally disappeared and the phase composition transformed into Al3Ni2, Ni3(AlTi), as well as a small amount of Al3Ti. Further increasing the annealing temperature to 773 K leads to the appearance of stable AlNi in addition to Al3Ni2, Ni3(AlTi), and Al3Ti. Afterwards, the peak of Ni3(AlTi) could not be detected in the specimen after treating at 873 K for 3 h, and the peak intensity of Al3Ni2 and Al3Ti becomes much weaker. The phase composition changes to be AlNi were accompanied by a few Al3Ni2, Al3Ti, and NiTi. Compared with the results in the literature [28], the formation of Al3Ni is significantly inhibited by adding a Ti layer into the base Al and Ni layers. Nevertheless, the emergence of Al3Ni2 at 673 K and AlNi at 773 K has not been impacted. Thus, TEM analysis was again performed to comprehend the mechanisms of phase transition.
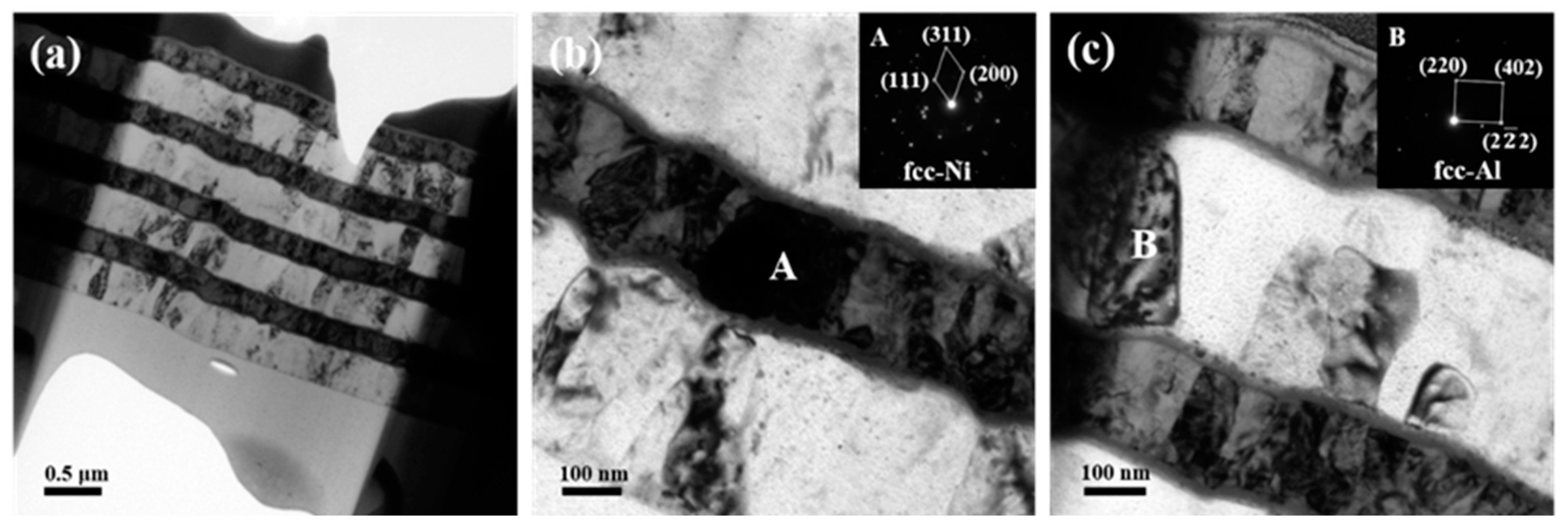
Figure 4 demonstrates the cross-sectional TEM images of the foil after annealing at 573 K for 3 h. It can be found that the interdiffusion of Al and Ni atoms in the foil was hampered after adding Ti layers as seen in Figure 4a. Unlike with diffraction rings of the Ni layers for the initial foil, some columnar Ni grain marked as grain A with height matching the layer thickness was observed, which was confirmed by the inserted SADPs in Figure 4b. Grain B in Figure 4c was still identified to be Al. This result implies that the inserted Ti-layer hinders the interdiffusion between Al and Ni and simultaneously restrains the formation of intermetallic Al3Ni which always precipitated at this temperature [28]. However, intensive intermixing among these three elements happens at both the Ni/Ti/Al interface and Al/Ti/Ni interface, which is more clearly observed in Figure 5.
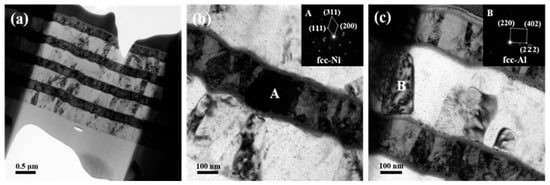
Figure 4.
TEM images together with selected area electron diffraction patterns of the ATNT foil after annealing at 573 K for 3 h: (a) the whole morphology; (b) the bottom region; (c) the top region.
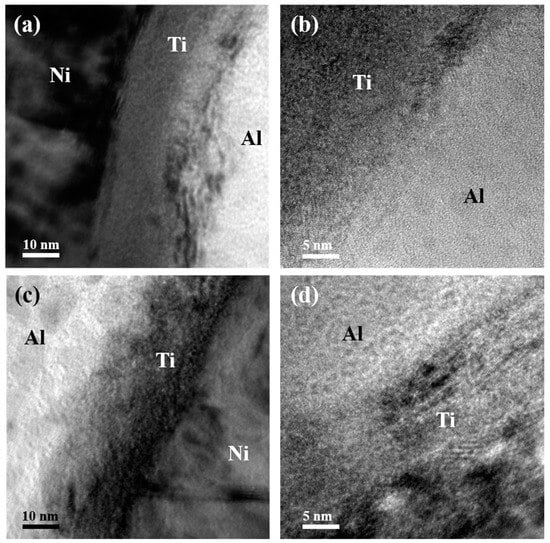
Figure 5.
TEM images of the interfaces for the ATNT foil after annealing at 573 K for 3 h: (a,b) the Ni/Ti/Al interface; (c,d) Al/Ti/Ni interface.
Figure 5a illustrates the interface structure and atomic diffusion at the Ni/Ti/Al interface. The original flat interface between Al and Ti layers was replaced by a transition region with severe intermixing of Al and Ti. Some nanosized ordered clusters formed at both the Al and Ti interface and inner Ti layer, as shown in the enlarged Figure 5b. Similar to the observation in the as-deposited foil in Figure 2 and Figure 3, a vague Al/Ti/Ni interface still existed in Figure 5c. More intensive interdiffusion between Al and Ti happened, the original interface between Al and Ti layers can hardly be found as displayed in Figure 5c,d. This result means that the interface structure can affect atomic diffusion during phase transition, coinciding with the results in past studies [21,29,30]. Interdiffusion between Ni and Ti layers also occurs, but is weaker than that between Al and Ti (seen in Figure 5a,c).
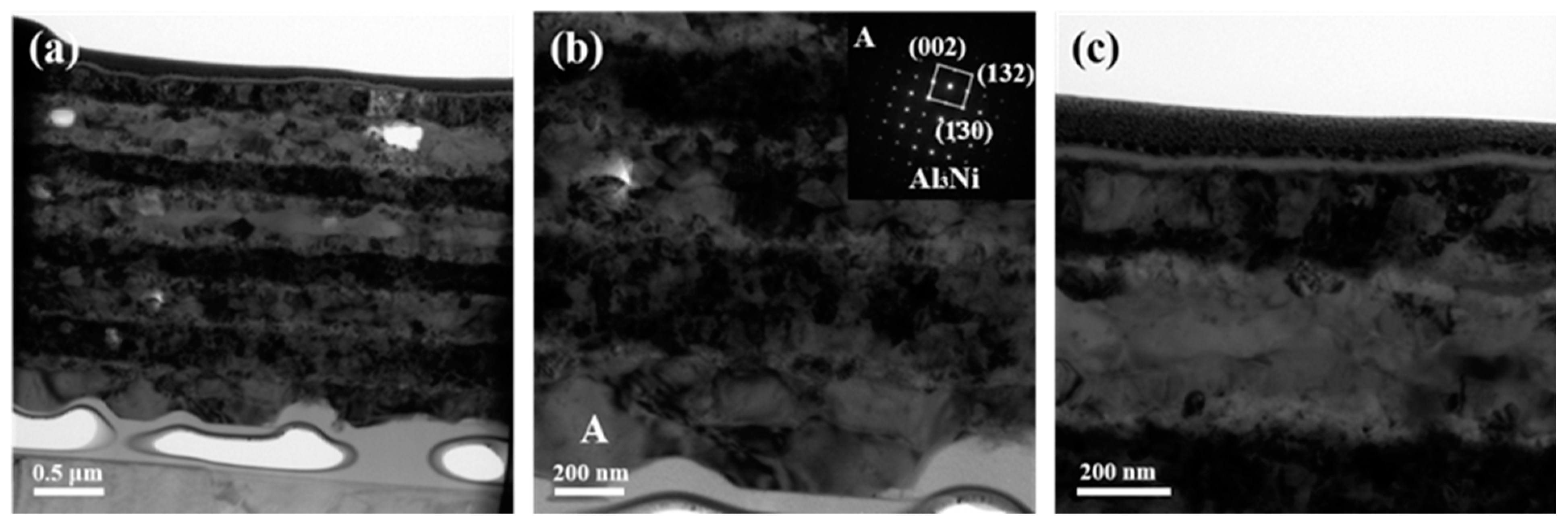
The blocking effect of inserted Ti layers on the interdiffusion between Al and Ni was significantly eliminated as the annealing temperature reached 673 K as shown in Figure 6. The initial Ti layers have totally merged with Al layers, many small grains precipitated inside the initial Al layers, which is consistent with the XRD results with the determination of Al3Ni2 in Figure 1. One thing that should be emphasized is the difference in thickness of the Ni layers. The thickness of the two middle Ni layers is apparently less than that of the initially deposited Ni layer as evident in Figure 6a. This observation was previously reported in the Al/Ni foils deposited on the same substrate, which was related to the interface roughness caused by rougher substrate surface morphology [21]. This further explains the presence of Al3Ni at the bottom Al layer in Figure 6b. The inserted SADPs obtained from grain A in the bottom Al layer were indexed to be Al3Ni, although which one is not clearly detected in XRD patterns. The slower diffusion rate across the bottom Ni/Ti/Al interface postponed the transformation from Al3Ni to Al3Ni2. In addition, the top Ti layer could still be clearly observed without mixing the Ni layer as displayed in Figure 6c. The obtained result manifests that the added Ti layers have less impact on the phase composition at this temperature even though they hindered atomic diffusion at a lower temperature. It needs to be stressed that the foil is easily delaminated from the substrate when treated at high temperatures, which then conglutinates by adhesive resulting in the gap between the foil and substrate, as well as the presence of adhesive under the foil.
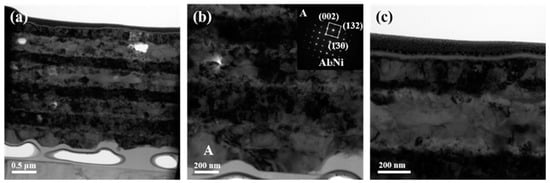
Figure 6.
TEM images together with selected area electron diffraction patterns of the ATNT foil after annealing at 673 K for 3 h: (a) the whole morphology; (b) the bottom region; (c) the top region.
The microstructure of 773 K-annealed ATNT foil was further investigated. The original layers merged with each other as seen in Figure 7a. The grain size in the initial Al layers is obviously larger than that in the Ni and Ti layers, which is clearly observed in Figure 7b,c. Here, the SADPS of both grains, A and B, individually chosen from the initial Al and Ni layers were identified to be AlNi, agreeing well with the XRD results in Figure 1. Figure 7d further shows that the pure Ti layer still existed at the top surface accompanied by some tiny particles precipitated at the interface between Ni and Ti layers.
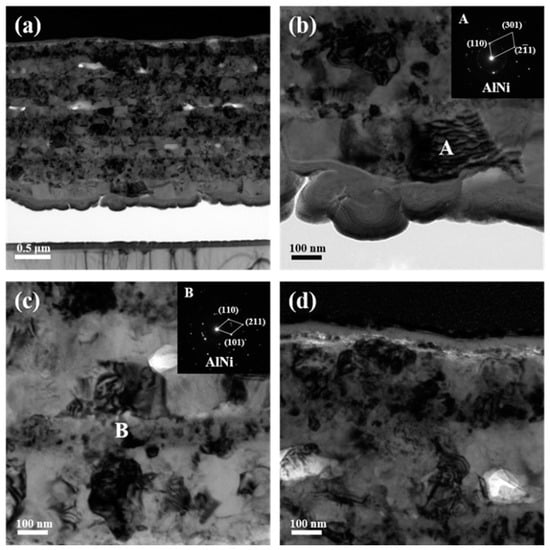
Figure 7.
TEM images together with selected area electron diffraction patterns of the ATNT foil after annealing at 773 K for 3 h: (a) the whole morphology; (b) the bottom region; (c) the middle region; (d) the top region.
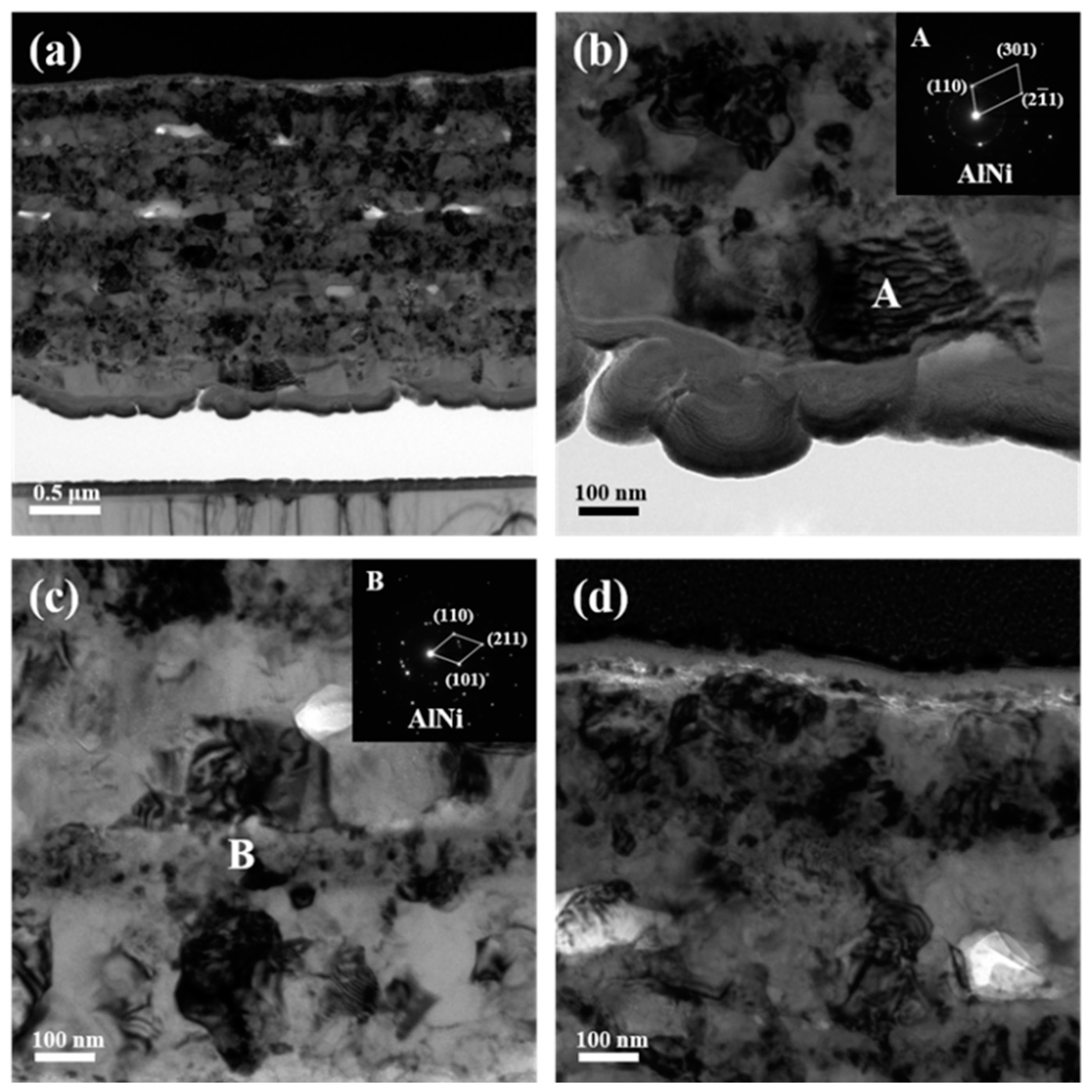
Figure 8 presents the cross-sectional images of the ATNT foils after isothermal annealing at 873 K for 3 h. It can be clearly found that the initial layered structure almost vanished after heating treatment, as shown in Figure 8a, while the grain size is apparently larger than that in the sample after annealing at 773 K (Figure 8a–c). Similar to the results in Figure 7b,c, all the grains marked as A–E selected from the bottom to upper region of the annealed foil were detected to be AlNi. Interestingly, the Ni3(AlTi) phase was determined to be located in the position below the initial top Ti layer, which is due to the unilateral atomic diffusion for the layer close to the surface. Simultaneously, many fine particles formed in the initial pure top Ti layer when annealing at this temperature, which explains the presence of diffraction peaks of NiTi in Figure 1. Combined with the TEM observation in Figure 7 and Figure 8 and the XRD results in Figure 1, it is concluded that, apart from the top Ti and Ni layers, the pure Al, Ni, and Ti layers in other regions of the foils have almost transformed into the AlNi phase despite the existence of a few Al3Ti. However, the peak intensity of the Al3Ti becomes much weaker in comparison to that at low annealing temperature, which means that the Al3Ti dissolved during phase transformation.

Figure 8.
TEM images together with selected area electron diffraction patterns of the ATNT foil after annealing at 873 K for 3 h: (a) the whole morphology; (b) the bottom region; (c) the middle region; (d) the top region.
4. Discussion
The phase transformation of Al/Ni foil has been intensively analyzed in previous studies. A general mechanism based on both the effective heat of formation and atomic diffusion was proposed to interpret phase sequence. Since the diffusion of Ni into Al layers is much faster than that of Al into Ni layers, Al3Ni preferentially forms at the interfaces between Al and Ni layers due to the lower strain and shorter diffusion path and gradually grows into the inner Al layers, leading to the phase composition changing from Al + Ni to Al3Ni + Ni, which is in accordance with the prediction of the calculated effective heat of formation [28]. At a higher temperature, the interfaces of Al3Ni and Ni serve as nucleation sites for the formation of Al3Ni2, then phase composition transforms into Al3Ni2 + Ni. Further raising the annealing temperature results in the formation of stable AlNi. As the modulation period increases, the temperature at which AlNi forms increases due to longer diffusion distance, as well as the blocking effect of intermetallic compounds on Ni diffusion [10,29]. From the above aspect, Al3Ni acts as the precursor for the subsequent phase transition.
However, the formation of the Al3Ni phase is not obviously observed in XRD patterns after adding Ti layers in Figure 1. Additionally, the phase compositions of the ATNT foils have changed to be Al3Ni2, Ni as well as a few Ni3(AlTi) and Al3Ti when annealing at 673 K, which is consistent with the phases for the foil without inserting the Ti layer [21]. Then, the Al3Ni2 transformed into a stable AlNi as the annealing temperature increased. This result indicates that the formation of transitional Al3Ni phase is not essential for phase transformation, which further suggested that the kinetics factor related to atomic diffusion rather than thermodynamics factor plays a key role in determining phase transformation. As the annealing temperature rose, the atomic diffusion rate increased accordingly, resulting in a sufficient mixing of the Al and Ni elements. Thus, the Al3Ni2 was directly detected in XRD results despite the absence of Al3Ni on a large scale. From this viewpoint, although the inserted Ti layers impeded the interdiffusion of Al and Ni to form the Al3Ni phase at a temperature below 573 K, the transition to more stable Al3Ni2 and AlNi was not changed at higher temperatures.
The above result provides a possible approach to solve the problem of the long-term stability of vapor-deposited Al/Ni foils. The large and negative mixing heats between Al and Ni benefit a self-propagating reaction, but, in turn, make the multilayers, especially for those with smaller modulation periods, thermodynamically unstable. An increase in the average thickness of the intermixing region was reported in the as-deposited Al/Ni foils after three years of room temperature storage, leading to the decrement of stored energy associated with reaction velocity [13]. Since the solubility of Ti in both solid and molten Al is relatively low [31,32], adding an appropriate Ti layer in the Al and Ni interfaces could hinder the interdiffusion of Al and Ni, further avoiding the loss of stored energy caused by the interfacial mixing of Al and Ni. Nevertheless, the adding strategy needs to be carefully studied with regard to the reaction velocity. As seen in Figure 7 and Figure 8, the initial pure Ti layer reacted with Ni to form NiTi at 873 K, while the Ni3(AlTi) was also detected underneath the NiTi layer, which was not observed in XRD patterns in Figure 1. This means that the top Ti layer should be removed during application. Another aspect that should be clarified is the thickness of the added Ti layer with respect to the original modulation periods. In this study, inserting Ti layers with an average thickness of around 20 nm resulted in the appearance of Al3Ti. The pre-formed Al-Ni and Al-Ti intermetallic phases, such as Al3Ni and Al3Ti, were always considered as diffusion barriers for further phase transformation [33,34], because of the diffusion rate of pure Al, Ni, and Ti, in intermetallic phases is much lower than that in pure metals [35,36]. Therefore, controlling the thickness of added Ti layers to ensure the storage stability but avoid the formation of Al-Ti or Ni-Ti intermetallic phase needs to be detailed in future studies for the Al/Ni multilayer foil, while much thicker Ti layers should be avoided because the self-propagating reaction might be terminated [22].
5. Conclusions
The thin Ti layers were inserted in the interfaces of base Al/Ni multilayer foils to form the Al/Ti/Ni/Ti (ATNT) foils through magnetron deposition. Peaks from Al, Ni, and AlN substrates were detected in the as-deposited samples, the absence of diffraction peaks of Ti-(101) plane was due to the strongly textured polycrystalline structure of the as-deposited foil. TEM results indicated that the thickness of single Al and Ni layers was 380 and 250 nm, respectively. The transition Ti layers were clearly observed between the Al and Ni layers. The feature of asymmetric interfaces structure existed between the Ni/Ti/Al interfaces and Al/Ti/Ni interfaces.
After annealing at 473 K and 573 K for 3 h, the ATNT foils had the same phase composition in comparison to the initial sample, yet TEM analysis implied apparently atomic interdiffusion. When the annealing temperature was raised to 673 K, the phase composition transformed into Al3Ni2, Ni3(AlTi), Ni and a small amount of Al3Ti. Further increasing the annealing temperature to 773 K and 873 K leads to the appearance of stable AlNi in XRD patterns and the emergence of an original layered structure as shown in TEM images. The obtained results implied that the inserted Ti layers impeded atomic interdiffusion and the formation of Al3Ni during the early stage, but had less impact on the final products. This further indicated that adding the inserted transition layer provides a reference to balance the storage stability and reaction performance of Al/Ni foils with regard to the applications.
Author Contributions
Conceptualization, B.L. and Y.W.; methodology, Z.Z. and C.L.; data curation, Z.Z. and C.L.; writing—original draft preparation, B.L.; writing—review and editing, F.Y.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 11902299).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ongoing follow-up studies.
Acknowledgments
The authors also thank Yi Qiao, Huan Tong and Li You for the assistance in sample preparation and TEM analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, J.; Besnoin, E.; Duckham, A.; Spey, S.J.; Reiss, M.E.; Knio, O.M.; Weihs, T.P. Joining of stainless-steel specimens with nanostructured Al/Ni foils. J. Appl. Phys. 2004, 95, 248–256. [Google Scholar] [CrossRef]
- Swiston, A.J.; Besnoin, E.; Duckham, A.; Knio, O.M.; Weihs, T.P.; Hufnagel, T.C. Thermal and microstructural effects of welding metallic glasses by self-propagating reactions in multilayer foils. Acta Mater. 2005, 53, 3713–3719. [Google Scholar] [CrossRef]
- Michaelsen, C.; Barmak, K.; Weihs, T.P. Investigating the thermodynamics and kinetics of thin film reactions by differential scanning calorimetry. J. Phys. D Appl. Phys. 1997, 30, 3167–3186. [Google Scholar] [CrossRef] [Green Version]
- Ustinov, A.I.; Demchenkov, S.A. Influence of metastable Al9Ni2 phase on the sequence of phase transformations initiated by heating of Al/Ni multilayer foils produced by EBPVD method. Intermetallics 2017, 84, 82–91. [Google Scholar] [CrossRef]
- Morris, C.J.; Mary, B.; Zakar, E.; Barron, S.C.; Fritz, G.M.; Knio, O.M.; Weihs, T.P.; Hodgin, R.; Wilkins, P.; May, C. Rapid initiation of reactions in Al/Ni multilayers with nanoscale layering. J. Phys. Chem. Solids 2010, 71, 84–89. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Geng, J.; Wang, F.; Yan, S.; Zhao, P.L.; Meng, Q.F.; Wang, J.Y.; Wu, Q.B. Preparation of Al/Ni reactive multilayer foils and its application in thermal battery. Z. Anorg. Allg. Chem. 2020, 646, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Trenkle, J.C.; Koerner, L.J.; Tate, M.W.; Walker, N.; Gruner, S.M.; Weihs, T.P.; Hufnagel, T.C. Time-resolved x-ray microdiffraction studies of phase transformations during rapidly propagating reactions in Al/Ni and Zr/Ni multilayer foils. J. Appl. Phys. 2010, 107, 113511. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Wang, J. Experimental evidence of two-stage formation of Al3Ni in reactive Ni/Al multilayer foils. Scr. Mater. 2007, 56, 1055–1058. [Google Scholar] [CrossRef]
- Maj, Ł.; Morgiel, J.; Szlezynger, M.; Bała, P.; Cios, G. Effect of low and high heating rates on reaction path of Ni(V)/Al multilayer. Mater. Chem. Phys. 2017, 193, 244–252. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Anisothermal solid-state reactions of Ni/Al nanometric multilayers. Intermetallics 2011, 19, 350–356. [Google Scholar] [CrossRef]
- Edelstein, A.S.; Everett, R.K.; Richardson, G.Y.; Qadri, S.B.; Altman, E.I.; Foley, J.C.; Perepezko, J.H. Intermetallic phase formation during annealing of Al/Ni multilayers. J. Appl. Phys. 1994, 76, 7850–7859. [Google Scholar] [CrossRef] [Green Version]
- Blobaum, K.J.; Van Heerden, D.; Gavens, A.J.; Weihs, T.P. Al/Ni formation reactions: Characterization of the metastable Al9Ni2 phase and analysis of its formation. Acta Mater. 2003, 51, 3871–3884. [Google Scholar] [CrossRef]
- Nathani, H.; Wang, J.; Weihs, T.P. Long-term stability of nanostructured systems with negative heats of mixing. J. Appl. Phys. 2007, 101, 104315. [Google Scholar] [CrossRef]
- Alawieh, L.L.; Weihs, T.P.; Knio, O.M. A generalized reduced model of uniform and self-propagating reactions in reactive nanolaminates. Combust. Flame 2013, 160, 1857–1869. [Google Scholar] [CrossRef]
- Ma, E.; Thompson, C.V.; Clevenger, L.A. Nucleation and growth during reactions in multilayer Al/Ni films: The early stage of Al3Ni formation. J. Appl. Phys. 1991, 69, 2211–2218. [Google Scholar] [CrossRef]
- Jeske, T.; Seibt, M.; Schmitz, G. Microstructural influence on the early stages of interreaction of Al/ Ni-investigated by TAP and HREM. Mater. Sci. Eng. A 2003, 353, 105–111. [Google Scholar] [CrossRef]
- Gavens, A.J.; Van Heerden, D.; Mann, A.B.; Reiss, M.E.; Weihs, T.P. Effect of intermixing on self-propagating exothermic reactions in Al/Ni nanolaminate foils. J. Appl. Phys. 2000, 87, 1255–1263. [Google Scholar] [CrossRef]
- Ohring, M. Interdiffusion, Reactions and Transformations in Thin Films, Materials Science of Thin Films, 2nd ed.; Academic Press: San Diego, CA, USA, 2002; pp. 641–710. [Google Scholar]
- Pauly, C.; Woll, K.; Bax, B.; Mucklich, F. The role of transitional phase formation during ignition of reactive multilayers. J. Appl. Phys. 2015, 107, 113104. [Google Scholar] [CrossRef]
- Rogachev, A.S.; Grigoryan, A.E.; Illarionova, E.V.; Kanel, I.G.; Merzhanov, A.G.; Nosyrev, A.N.; Sachkova, N.V.; Khvesyuk, V.I.; Tsygankov, P.A. Gasless combustion of Ti-Al bimetallic multilayer nanofoils. Combust. Explos. Shock. Waves 2004, 40, 166–171. [Google Scholar] [CrossRef]
- Liu, B.B.; Yu, X.J.; Jiang, X.; Qiao, Y.; You, L.; Wang, Y.; Ye, F. Effect of deposition substrates on surface topography, interface roughness and phase transformation of the Al/Ni multilayers. Appl. Surf. Sci. 2021, 546, 149098. [Google Scholar] [CrossRef]
- Grapes, M.D.; Weihs, T.P. Exploring the reaction mechanism in self-propagating Al/Ni multilayers by adding inert material. Combust. Flame 2016, 172, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Li, H.; Yang, L.P.; Hu, A.M. Microstructures and reaction properties of Ti/Ni, Ti/Al and Ni/Al multilayer films. J. Nano Res. 2018, 54, 22–34. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Xu, B.Z.; Bridges, D.; Zhang, L.Y.; Feng, Z.L.; Hu, A.M. Laser welding of Ti6Al4V assisted with nanostructured Ni/Al reactive multilayer films. Mater. Des. 2019, 181, 108097. [Google Scholar] [CrossRef]
- Ma, Y.; Li, H.; Yang, L.P.; Hu, A.M. Reaction-assisted diffusion bonding of Ti6Al4V alloys with Ti/Ni nanostructured multilayers. J. Mater. Proc. Tech. 2018, 262, 204–209. [Google Scholar] [CrossRef]
- Gachon, J.C.; Rogachev, A.S.; Grigoryan, H.E.; Illarionova, E.V.; Kuntz, J.J.; Kovalev, D.Y.; Nosyrev, A.N.; Sachkova, N.V.; Tsygankov, P.A. On the mechanism of heterogeneous reaction and phase formation in Ti/Al multilayer nanofilms. Acta Mater. 2005, 53, 1225–1231. [Google Scholar] [CrossRef]
- Weihs, T.P. Metallic Films for Electronic, Optical and Magnetic Applications: Structure, Processing and Properties; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Pretorius, R.; Vredenberg, A.M.; Saris, F.W.; de Reus, R. Prediction of phase formation sequence and phase stability in binary metal aluminum thin film systems using the effective heat of formation rule. J. Appl. Phys. 1991, 70, 3636–3646. [Google Scholar] [CrossRef]
- Swain, M.; Singh, S.; Basu, S.; Gupta, M. Effect of interface morphology on intermetallics formation upon annealing of Al-Ni multilayer. J. Alloy. Compd. 2013, 576, 257–261. [Google Scholar] [CrossRef]
- Singh, S.; Basu, S.; Gupta, M.; Majkrzak, C.F.; Kienzle, P.A. Growth kinetics of intermetallic alloy phase at the interfaces of a Ni/Al multilayer using polarized neutron and X-ray reflectometry. Phys. Rev. B 2010, 81, 235413. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al-B-Nb-Ti system III. Thermodynamic re-evaluation of the constituent binary system Al–Ti. J. Alloy. Compd. 2008, 465, 64–77. [Google Scholar] [CrossRef]
- Witusiewicz, V.T.; Hallstedt, B.; Bondar, A.A.; Hecht, U.; Sleptsov, S.V.; Velikanova, T.Y. Thermodynamic description of the Al-C-Ti system. J. Alloy. Compd. 2015, 623, 480–496. [Google Scholar] [CrossRef]
- Morgiel, J.; Marszałek, K.; Pomorska, M.; Maj, Ł.; Mania, R.; Kanak, J.; Rutkowski, P. In situ TEM observation of reaction of Ti/Al multilayers. Arch. Civ. Mech. Eng. 2017, 17, 188–198. [Google Scholar] [CrossRef]
- Ramos, A.S.; Vieira, M.T.; Morgiel, J.; Grzonkab, J.; Simoes, S.; Vieira, M.F. Production of intermetallic compounds from Ti/Al and Ni/Al multilayer thin films-A comparative study. J. Alloy. Compd. 2009, 484, 335–340. [Google Scholar] [CrossRef]
- Gupta, D.; Ho, P.S. Diffusion processes in thin films. Thin Solid Film. 1980, 72, 399–418. [Google Scholar] [CrossRef]
- Balluffi, R.W.; Blakely, J.M. Special aspects of diffusion in thin films. Thin Solid Film. 1975, 25, 363–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).