Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peach Fruit Preparation and Experimental Design
2.2. Manufacturing Aloe Vera Gel (AVG) Extract
2.3. Making Chitosan (CH) Coating
2.4. Preparation of the Tested Coating Mixtures
2.5. Physical Features
2.5.1. Fruit Weight Loss %
2.5.2. Fruit Firmness (lb\inch2)
2.5.3. The Peach Fruit Skin Color
2.6. The Chemical Features
2.6.1. Total Soluble Solids Percentage (TSS%)
2.6.2. Total Acidity Percentage (TA%)
2.6.3. Total Phenolic Contents (TPC)
2.6.4. Antioxidant Enzymes Activities (AEAs)
2.6.5. Ion Leakage (IL%) and Malondialdehyde (MDA) Accumulation
2.6.6. O2•−, H2O2 Production Rate, and Antioxidant Capacity (DPPH%)
2.7. Statistical Analysis
3. Results
3.1. Weight Loss%, Firmness (lb/inch2), and Peach Skin Color (Chroma, c*, and Hue Angle, h)
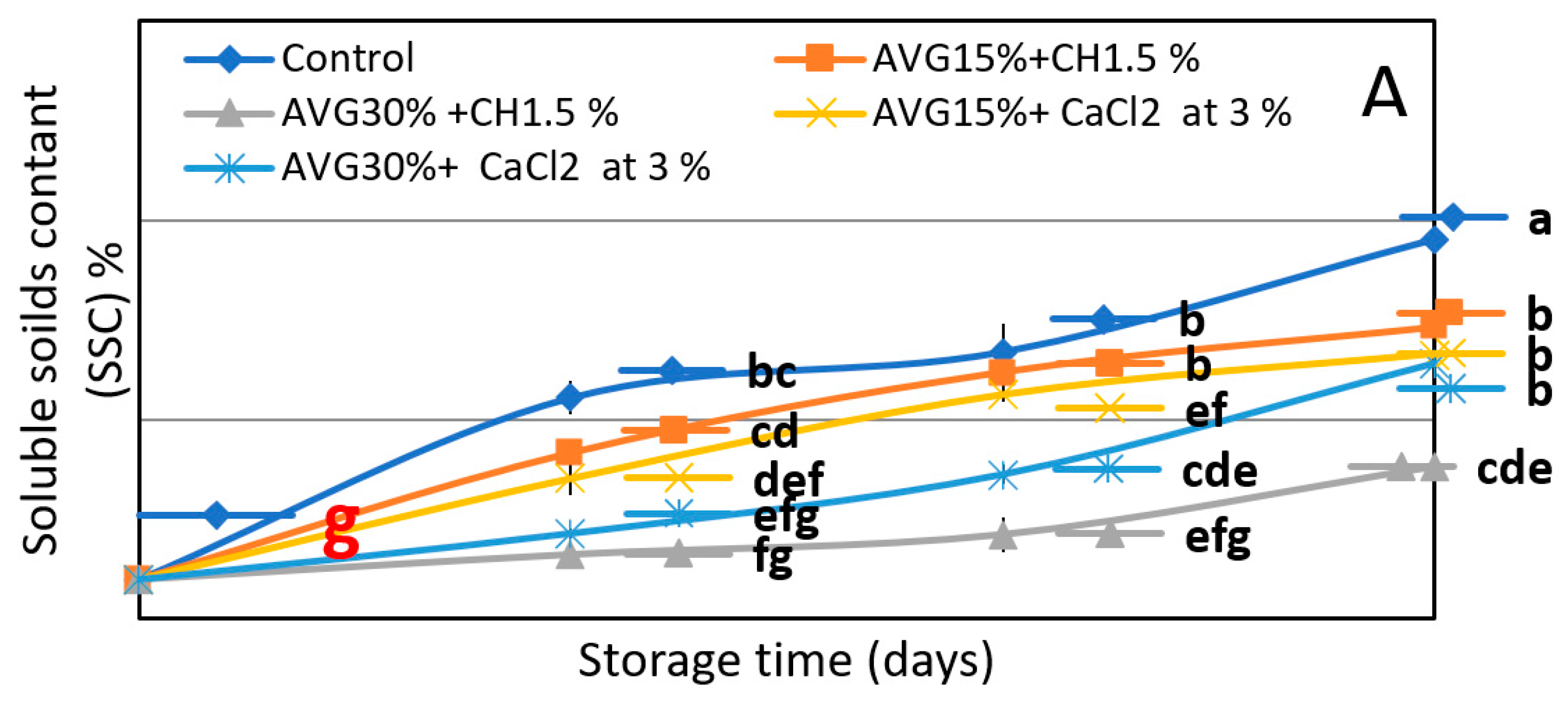
3.2. TSS% and TA%
3.3. Analysis of Ion Leakage (IL%) and Malondialdehyde (MDA) Accumulation
3.4. H2O2, O2 Production, and DPPH Reduction
3.5. Antioxidant Enzyme Activity and TPC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2019. Available online: www.fao.org/faostat/en/#data/QC (accessed on 2 March 2022).
- Mohammadi, L.; Hassanzadeh Khankahdani, H.; Tanaka, F. Effect of Aloe vera gel combined with basil (Ocimum basilicum L.) essential oil as a natural coating on maintaining post-harvest quality of peach (Prunus persica L.) during storage. IOP Conf. Ser. Earth Environ. Sci. 2020, 594, 012008. [Google Scholar] [CrossRef]
- Scattino, C.; Negrini, N.; Morgutti, S.; Cocucci, M.; Crisosto, C.H.; Tonutti, P.; Castagna, A.; Ranieri, A. Cell wall metabolism of peaches and nectarines treated with UV-B radiation: A biochemical and molecular approach. J. Sci. Food Agric. 2016, 96, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. Sucrose degradation is regulated by 1-methycyclopropene treatment and is related to chilling tolerance in two peach cultivars. Postharvest Biol. Technol. 2017, 124, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.J.; Ye, Z.W.; Su, M.S. Effects of MAP Treatment on Aroma Compounds and Enzyme Activities in Flat Peach during Storage and Shelf Life. HortScience 2018, 53, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Ochiki, S.; Gesimba, M.R.; Wolukau, J.N. Effect of Aloe vera gel coating on postharvest quality and shelf life of mango (Mangifera indica L.) fruits Var. Ngowe. J. Hortic. For. 2015, 7, 1–7. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.W. Improved electrochemical properties of Li(Ni0.6Mn0.2Co0.2)O2 by surface coating with Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2016, 307, 63–68. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- Ananou, S.; Martínez-Bueno, M.; Valdivia, E.; Ananou, S.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Biopreservation, an ecological approach to improve the safety and shelf-life of foods. Commun. Curr. Res. Educ. Top. Trends Appl. Microbiol. 2007, 1, 475–487. [Google Scholar]
- Tripathi, P.; Dubey, N.K. Exploitation of natural products as an alternative strategy to control postharvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol. 2004, 32, 235–245. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 46, 849–875. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Burns, J.K.; Kazokas, W.; Brecht, J.K.; Hagenmaier, R.D.; Bender, R.J.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biol. Technol. 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Hazrati, S.; Beyraghdar Kashkooli, A.; Habibzadeh, F.; Tahmasebi-Sarvestani, Z.; Sadeghi, A.R. Beurteilung von Aloe-vera-Gel als alternative essbare Beschichtung für Pfirsichfrüchte während der kalten Lagerphase. Gesunde Pflanz. 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ejaz, S.; Sardar, H. Effect of pre-storage ascorbic acid and Aloe vera gel coating application on enzymatic browning and quality of lotus root slices. J. Food Biochem. 2020, 44, e13136. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.; Koushesh Saba, M.; Ramezanian, A. Inhibitory effect of salicylic acid and Aloe vera gel edible coating on microbial load and chilling injury of orange fruit. Sci. Hortic. 2019, 247, 27–34. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, C.; Chen, M.; Chen, C.; Chen, Y.; Fu, Y.; Wan, C.; Chen, J. Effects of chitosan-based coatings enriched with cinnamaldehyde on Mandarin fruit cv. Ponkan during room-temperature storage. Coatings 2018, 8, 372. [Google Scholar] [CrossRef]
- Varasteh, F.; Arzani, K.; Barzegar, M.; Zamani, Z. Changes in anthocyanins in arils of chitosan-coated pomegranate (Punica granatum L. cv. Rabbab-e-Neyriz) fruit during cold storage. Food Chem. 2012, 130, 267–272. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Jongsri, P.; Rojsitthisak, P.; Wangsomboondee, T.; Seraypheap, K. Influence of chitosan coating combined with spermidine on anthracnose disease and qualities of ‘Nam Dok Mai’ mango after harvest. Sci. Hortic. 2017, 224, 180–187. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-López, M.L.; de Rodríguez, D.J.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Effect of chitosan–Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, E.; Conway, W.; Hickey, K.; HortScience, C.S. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. HortScience 1997, 32, 831–835. [Google Scholar] [CrossRef] [Green Version]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohail, M.; Ayub, M.; Khalil, S.A.; Zeb, A.; Ullah, F.; Afridi, S.R.; Ullah, R. Effect of calcium chloride treatment on post harvest quality of peach fruit during cold storage. Int. Food Res. J. 2015, 22, 2225–2229. [Google Scholar]
- Lieberman, M.; Physiology, S.W.-P. Influence of calcium and magnesium on ethylene production by apple tissue slices. Plant Physiol. 1982, 69, 1150–1155. [Google Scholar] [CrossRef] [Green Version]
- Huo, L.; Guo, Z.; Zhang, Z.; Jia, X.; Sun, Y.; Sun, X.; Wang, P.; Gong, X.; Ma, F. The Apple Autophagy-Related Gene MdATG9 Confers Tolerance to Low Nitrogen in Transgenic Apple Callus. Front. Plant Sci. 2020, 11, 423. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Abbasi, N.A.; Hafiz, I. Application of Calcium Chloride at Different Phenological Stages Alleviates Chilling Injury and Delays Climacteric Ripening in Peach Fruit during Low-Temperature Storage. Int. J. Fruit Sci. 2021, 21, 1040–1058. [Google Scholar] [CrossRef]
- Hassanpour, H. Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT Food Sci. Technol. 2015, 60, 495–501. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Dawood, H.D. Active chitosan/PVA with ascorbic acid and berry quality of ‘Superior seedless’ grapes. Sci. Hortic. 2017, 224, 286–292. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Tian, S.P.; Li, B.Q.; Xu, Y. Effects of O2 and CO2 concentrations on physiology and quality of litchi fruit in storage. Food Chem. 2005, 91, 659–663. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hakim, A.; Purvis, A.C.; Mullinix, B.G. Differences in chilling sensitivity of cucumber varieties depends on storage temperature and the physiological dysfunction evaluated. Postharvest Biol. Technol. 1999, 17, 97–104. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative Damage in Pea Plants Exposed to Water Deficit or Paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Cao, S.; Bian, K.; Shi, L.; Chung, H.H.; Chen, W.; Yang, Z. Role of Melatonin in Cell-Wall Disassembly and Chilling Tolerance in Cold-Stored Peach Fruit. J. Agric. Food Chem. 2018, 66, 5663–5670. [Google Scholar] [CrossRef]
- Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martínez-Romero, D. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol. Technol. 2013, 83, 54–57. [Google Scholar] [CrossRef]
- Allegra, A.; Farina, V.; Inglese, P.; Gallotta, A.; Sortino, G. Qualitative traits and shelf life of fig fruit (‘Melanzana’) treated with Aloe vera gel coating. Acta Hortic. 2021, 1310, 87–92. [Google Scholar] [CrossRef]
- Banks, N.H.; Dadzie, B.K.; Cleland, D.J. Reducing gas exchange of fruits with surface coatings. Postharvest Biol. Technol. 1993, 3, 269–284. [Google Scholar] [CrossRef]
- Carrillo-Lopez, A.; Ramirez-Bustamante, F.; Valdez-Torres, J.B.; Rojas-Villegas, R.; Yahia, E.M. Ripening and quality changes in mango fruit as affected by coating with an edible film. J. Food Qual. 2000, 23, 479–486. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh Saba, M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Kittur, F.S.; Saroja, N.; Tharanathan, R.N. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Nanjappa, C.; Ashok, N.; Ravi, N.; Roopa, N.; Raju, P.S. Shellac and Aloe vera gel based surface coating for shelf life extension of tomatoes. J. Food Sci. Technol. 2015, 52, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Chien, P.J.; Sheu, F.; Yang, F.H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Han, C.; Zuo, J.; Wang, Q.; Xu, L.; Zhai, B.; Wang, Z.; Dong, H.; Gao, L. Effects of chitosan coating on postharvest quality and shelf life of sponge gourd (Luffa cylindrica) during storage. Sci. Hortic. 2014, 166, 1–8. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible Coatings for Fresh-Cut Fruits. Crit. Rev. Food Sci. Nutr. 2007, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Ö.; Bayoindirli, L. Effects of an Edible Coating and Cold Storage on Shelf-life and Quality of Cherries. LWT Food Sci. Technol. 2002, 35, 146–150. [Google Scholar] [CrossRef]
- Xu, W.T.; Peng, X.L.; Luo, Y.B.; Wang, J.A.; Guo, X.; Huang, K.L. Physiological and biochemical responses of grapefruit seed extract dip on ‘Redglobe’ grape. LWT Food Sci. Technol. 2009, 42, 471–476. [Google Scholar] [CrossRef]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. HortScience 2000, 35, 575–579. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Khatri, D.; Panigrahi, J.; Prajapati, A.; Bariya, H. Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of post-harvest tomatoes. Sci. Hortic. 2020, 259, 108837. [Google Scholar] [CrossRef]
- Shah, S.; Hashmi, M.S. Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020, 61, 279–289. [Google Scholar] [CrossRef]
- Aboryia, M.S.; Omar, A.S.M.; Mark, C. Effectiveness of Some Edible Coatings on Storage Ability of Zaghloul Date Palm Fruits. J. Plant Prod. 2020, 11, 1477–1485. [Google Scholar] [CrossRef]
- El-gioushy, S.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; Abou, H.M.; Ghit, E.; Fikry, M.; Bahloul, A.M.E.; Morsy, A.R.; Lo, A.A.; Abdelaziz, A.M.R.A. The Effects of a Gum Arabic-Based Edible Coating on Guava Fruit Characteristics during Storage. Coatings 2022, 12, 90. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, N.; Manickam, S.; Siddiqui, Y.; Alderson, P.G.; Maqbool, M. Effectiveness of submicron chitosan dispersions in controlling anthracnose and maintaining quality of dragon fruit. Postharvest Biol. Technol. 2013, 86, 147–153. [Google Scholar] [CrossRef]
- Li, Y.; Wills, R.B.H.; Golding, J.B. Interaction of ethylene concentration and storage temperature on postharvest life of the green vegetables pak choi, broccoli, mint, and green bean. J. Hortic. Sci. Biotechnol. 2017, 92, 288–293. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Adejumo, I.O.; Afolabi, I.S.; Adetunji, J.B.; Ajisejiri, E.S. Prolonging the shelf life of ‘Agege Sweet’ orange with chitosan–rhamnolipid coating. Hortic. Environ. Biotechnol. 2018, 59, 687–697. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The Oxidative Burst in Plant Disease Resistance. Annu. Rev. Plant Biol. 2003, 48, 251–275. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Dang, Q.F.; Yan, J.Q.; Li, Y.; Cheng, X.J.; Liu, C.S.; Chen, X.G. Chitosan Acetate as an Active Coating Material and Its Effects on the Storing of Prunus avium L. J. Food Sci. 2010, 75, S125–S131. [Google Scholar] [CrossRef]
- Zeng, K.; Deng, Y.; Ming, J.; Deng, L. Induction of disease resistance and ROS metabolism in navel oranges by chitosan. Sci. Hortic. 2010, 126, 223–228. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Rakwal, R.; Tamogami, S.; Yonekura, M.; Kubo, A.; Saji, H. Chitosan activates defense/stress response(s) in the leaves of Oryza sativa seedlings. Plant Physiol. Biochem. 2002, 40, 1061–1069. [Google Scholar] [CrossRef]
- Serrano, M.; Valverde, J.M.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D. Use of Aloe vera Gel Coating Preserves the Functional Properties of Table Grapes. J. Agric. Food Chem. 2006, 54, 3882–3886. [Google Scholar] [CrossRef]
- Reyes, L.F.; Cisneros-Zevallos, L. Wounding Stress Increases the Phenolic Content and Antioxidant Capacity of Purple-Flesh Potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar] [CrossRef] [PubMed]






| Coating Treatments (F2) | Cold Storage Duration (Days) (F1) | |||
|---|---|---|---|---|
| 0 | 12 | 24 | 36 | |
| weight loss % | ||||
| Control | 0.00 | 10.59 ± 0.061 hi | 22.2 ± 0.538 de | 40.3 ± 3.078 a |
| AVG 15% + CH 1.5% | 0.00 | 8.62 ± 1.085 ij | 19.9 ± 2.083 ef | 30.1 ± 2.085 b |
| AVG 30% + CH 1.5% | 0.00 | 3.53 ± 2.213 kl | 13.7 ± 1.226 gh | 23.8 ± 2.442 cd |
| AVG 15% + CaCl2 at 3% | 0.00 | 5.25 ± 0.494 jk | 18.4 ± 0.776 ef | 27.9 ± 2.026 bc |
| AVG 30% + CaCl2 at 3% | 0.00 | 4.56 ± 0.683 jk | 16.1 ± 1.496 fg | 26.4 ± 1.709 bcd |
| p-value | F1 = 1.755 | F2 = 1.963 | F1 × F2 = 4.01 | |
| firmness (lb/inch2) | ||||
| Control | 11.85 | 7.75 ± 0.304 g | 7.01 ± 0.341 h | 6.1 ± 0.264 i |
| AVG 15% + CH 1.5% | 11.85 | 10.23 ± 0.233 d | 9.41 ± 0.088 ef | 7.7 ± 0.076 g |
| AVG 30% + CH 1.5% | 11.85 | 11.36 ± 0.044 ab | 11 ± 0.02 bc | 10.6 ± 0.133 cd |
| AVG 15% + CaCl2 at 3% | 11.85 | 10.43 ± 0.092 d | 9.71 ± 0.148 e | 9.18 ± 0.092 f |
| AVG 30% + CaCl2 at 3% | 11.85 | 11 ± 0.086 a | 10.6 ± 0.175 cd | 9.71 ± 0.072 e |
| p-value | F1 = 0.191 | F2 = 0.214 | F1 × F2 = 0.439 | |
| hue angle (h) | ||||
| Control | 79.02 | 59.62 ± 0.291 h | 54.3 ± 0.003 j | 49.4 ± 0.881 k |
| AVG 15% + CH 1.5% | 79.02 | 61.62 ± 0.58 fg | 57.1 ± 0.000 i | 55.6 ± 0.003 j |
| AVG 30% + CH 1.5% | 79.02 | 70.65 ± 0.003 b | 65.1 ± 0.000 d | 62.9 ± 0.000 ef |
| AVG 15% + CaCl2 at 3% | 79.02 | 63.75 ± 0.577 ef | 61.3 ± 0.437 g | 57.2 ± 1.151 i |
| AVG 30% + CaCl2 at 3% | 79.02 | 66.92 ± 1.166 c | 62.5 ± 0.176 efg | 59.4 ± 0.577 h |
| p-value | F1 = 0.624 | F2 = 0.698 | F1 × F2 = 1.429 | |
| chroma (c*) | ||||
| Control | 32.49 | 36.84 ± 0.288 def | 38.7 ± 0.28 c | 48.7 ± 0.291 a |
| AVG 15% + CH 1.5% | 32.49 | 33.34 ± 1.166 jk | 36.6 ± 0.271 efg | 42.2 ± 0.285 b |
| AVG 30% +CH 1.5% | 32.49 | 32.27 ± 0.000 ij | 34.5 ± 0.294 fg | 35.6 ± 0.003 cd |
| AVG 15% + CaCl2 at 3% | 32.49 | 34.27 ± 0.606 k | 36.2 ± 0.291 hi | 39.9 ± 0.291 gh |
| AVG 30% + CaCl2 at 3% | 32.49 | 35.02 ± 0.005 hi | 36.4 ± 0.005 efg | 37.5 ± 0.28 dc |
| p-value | F1 = 0.448 | F2 = 0.501 | F1 × F2 = 1.027 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboryia, M.S.; El-Gioushy, S.F.; Sami, R.; Aljumayi, H.; Alyamani, A.; Almasoudi, A.; Gawish, M.S. Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits. Coatings 2022, 12, 498. https://doi.org/10.3390/coatings12040498
Aboryia MS, El-Gioushy SF, Sami R, Aljumayi H, Alyamani A, Almasoudi A, Gawish MS. Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits. Coatings. 2022; 12(4):498. https://doi.org/10.3390/coatings12040498
Chicago/Turabian StyleAboryia, M. S., Sherif Fathy El-Gioushy, Rokayya Sami, Huda Aljumayi, Amal Alyamani, A. Almasoudi, and Mohamed S. Gawish. 2022. "Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits" Coatings 12, no. 4: 498. https://doi.org/10.3390/coatings12040498
APA StyleAboryia, M. S., El-Gioushy, S. F., Sami, R., Aljumayi, H., Alyamani, A., Almasoudi, A., & Gawish, M. S. (2022). Synergistic Effect of Dipping in Aloe Vera Gel and Mixing with Chitosan or Calcium Chloride on the Activities of Antioxidant Enzymes and Cold Storage Potential of Peach (Prunus persica L.) Fruits. Coatings, 12(4), 498. https://doi.org/10.3390/coatings12040498








