Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Film-Forming Solutions
2.3. Determination of Flow Curves of Film-Forming Solutions
2.4. Examination of the Gelling Process
2.5. Calculation Methods
2.6. Statistical Analysis
3. Results
3.1. Flow Curves of Tested Film-Forming Solutions
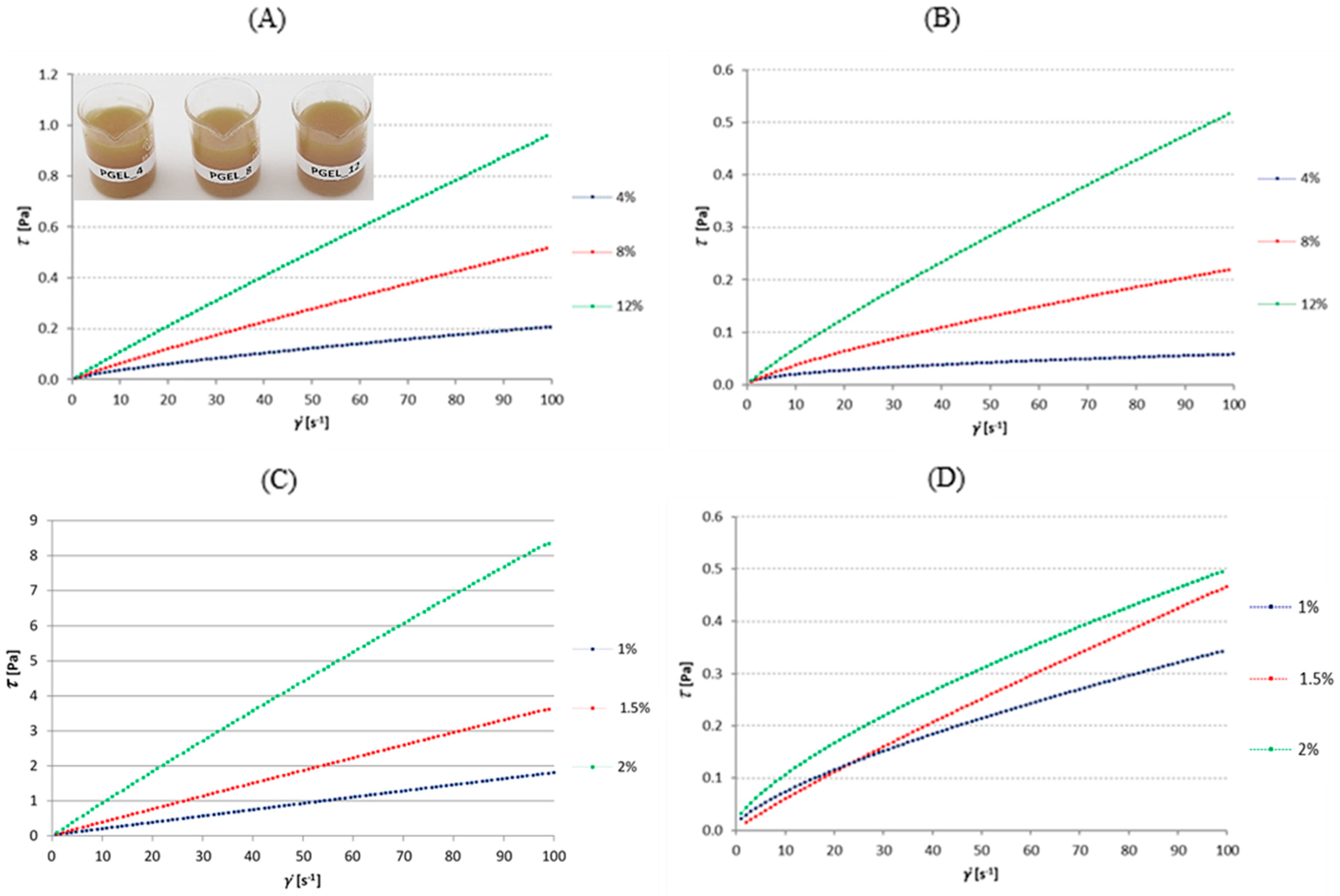
3.1.1. Gelatin Solutions
3.1.2. Soy Protein Isolate Solutions
3.1.3. Sodium Alginate Solutions
3.1.4. High Methylated Apple Pectin Solution
3.2. Influence of Hydrocolloid Concentration and Cooling Rate on the Gelling
3.2.1. Gelatin Solutions
3.2.2. Soy Protein Isolate Solutions
3.2.3. High Methylated Apple Pectin Solution
3.2.4. Sodium Alginate Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Domian, E.; Kowalska, J.; Ciurzynska, A.; Galus, S. Edible coatings as osmotic dehydration pretreatment in nutrient-enhanced fruit or vegetable snacks development: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5641–5674. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible packaging: Sustainable solutions and novel trends in food packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Lenart, A. Effect of protein concentration on kinetics of water vapour adsorption by coatings prepared on the basis of whey protein isolate. Food Sci. Technol. Qual. 2011, 4, 66–73. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S. Powlekanie żywności–materiały, metody i zastosowanie w przemyśle spożywczym. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2020, 125, 5–24. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The Effect of Whey Protein-Based Edible Coatings Incorporated with Lemon and Lemongrass Essential Oils on the Quality Attributes of Fresh-Cut Pears during Storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. J. Food Process Eng. 2019, 42, e13245. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Garcia-Henao, C.E.; Valderrama-Sanchez, V.; Arboleda-Murillo, J.A.; Pinzon, M.I.; Sanchez, L.T.; Villa, C.C. Bioactive food coating: A review. Packag. Technol. Sci. 2022. [Google Scholar] [CrossRef]
- Lim, J.W.; Lim, W.S.; Lee, M.H.; Park, H.J. Barrier and structural properties of polyethylene terephthalate film coated with poly(acrylic acid)/montmorillonite nanocomposites. Packag. Technol. Sci. 2021, 34, 141–150. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzynska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montes, E.; Castro-Munoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, J.; Dong, M.; Zhang, H.; Li, L.; Wang, L. Food spoilage, bioactive food fresh-keeping films and functional edible coatings: Research status, existing problems and development trend. Trends Food Sci. Technol. 2022, 119, 122–132. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D. Novel Techniques for Extrusion, Agglomeration, Encapsulation, Gelation, and Coating of Foods. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 379–392. [Google Scholar]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An overview of fruit and vegetable edible packaging materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Petkoska, A.T.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Taktak, W.; Hamdi, M.; Chentir, I.; Boughriba, S.; Ben Azaza, Y.; Li, S.; Nasri, M.; Karra-Chaâbouni, M.; Nasri, R. Development of emulsion gelatin gels for food application: Physicochemical, rheological, structural and thermal characterization. Int. J. Biol. Macromol. 2021, 182, 1–10. [Google Scholar] [CrossRef]
- Winter, H.H. Can the gel point of a cross-linking polymer be detected by the G′–G″ crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Tosh, S.M.; Marangoni, A.G. Determination of the maximum gelation temperature in gelatin gels. Appl. Phys. Lett. 2004, 84, 4242–4244. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Harkous, A.; Colomines, G.; Leroy, E.; Mousseau, P.; Deterre, R. The kinetic behavior of Liquid Silicone Rubber: A comparison between thermal and rheological approaches based on gel point determination. React. Funct. Polym. 2016, 101, 20–27. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.-C.; Wang, H.; Shangguan, X.; Zhang, L.; Zhang, N.-H.; Bansal, N. Pectin and enzyme complex modified fish scales gelatin: Rheological behavior, gel properties and nanostructure. Carbohydr. Polym. 2017, 156, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Sun, Q.; Xu, X.; Li, M.; Xie, F. Hydroxypropyl methylcellulose hydrocolloid systems: Effect of hydroxypropy group content on the phase structure, rheological properties and film characteristics. Food Chem. 2022, 379, 132075. [Google Scholar] [CrossRef]
- Pałacha, Z.; Sitkiewicz, I. Physical Properties of Food; WNT: Warsaw, Poland, 2010; p. 340. [Google Scholar]
- Duan, R.; Zhang, J.; Liu, L.; Cui, W.; Regenstein, J.M. The functional properties and application of gelatin derived from the skin of channel catfish (Ictalurus punctatus). Food Chem. 2018, 239, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, M.; Taherian Hoshahili, A.R.; Ramaswamy, H.S. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhao, Y.; Yang, Y. Rheological properties of soy protein isolate solution for fibers and films. Food Hydrocoll. 2017, 64, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT-Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Cevoli, C.; Balestra, F.; Ragni, L.; Fabbri, A. Rheological characterisation of selected food hydrocolloids by traditional and simplified techniques. Food Hydrocoll. 2013, 33, 142–150. [Google Scholar] [CrossRef]
- Huang, X.; Li, D.; Wang, L.-J. Characterization of pectin extracted from sugar beet pulp under different drying conditions. J. Food Eng. 2017, 211, 1–6. [Google Scholar] [CrossRef]
- Anvari, M.; Chung, D. Effect of cooling–heating rate on sol-gel transformation of fish gelatin–gum arabic complex coacervate phase. Int. J. Biol. Macromol. 2016, 91, 450–456. [Google Scholar] [CrossRef]
- van Otterloo, J.; Cruden, A.R. Rheology of pig skin gelatine: Defining the elastic domain and its thermal and mechanical properties for geological analogue experiment applications. Tectonophysics 2016, 683, 86–97. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A.; Voilley, A.; Debeaufort, F. Effect of oxidized potato starch on the physicochemical properties of soy protein isolate-based edible films. Food Technol. Biotechnol. 2013, 51, 403–409. [Google Scholar]
- Yuan, Y.; Kong, Z.-Y.; Sun, Y.-E.; Zeng, Q.-Z.; Yang, X.-Q. Complex coacervation of soy protein with chitosan: Constructing antioxidant microcapsule for algal oil delivery. LWT 2017, 75, 171–179. [Google Scholar] [CrossRef]
- Caillard, R.; Remondetto, G.E.; Subirade, M. Rheological investigation of soy protein hydrogels induced by Maillard-type reaction. Food Hydrocoll. 2010, 24, 81–87. [Google Scholar] [CrossRef]
- Rutkowski, A.G.S.; Dabrowski, K. Compendium of Food Additives; Hortimex: Konin, Poland, 2003; pp. 362–363. [Google Scholar]
- Kastner, H.; Kern, K.; Wilde, R.; Berthold, A.; Einhorn-Stoll, U.; Drusch, S. Structure formation in sugar containing pectin gels–Influence of tartaric acid content (pH) and cooling rate on the gelation of high-methoxylated pectin. Food Chem. 2014, 144, 44–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, Y.; Li, L. Enhanced stability and mechanical strength of sodium alginate composite films. Carbohydr. Polym. 2017, 160, 62–70. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Germann, N. Phase transition kinetics and rheology of gelatin-alginate mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Yang, Y.; Campanella, O.H.; Hamaker, B.R.; Zhang, G.; Gu, Z. Rheological investigation of alginate chain interactions induced by concentrating calcium cations. Food Hydrocoll. 2013, 30, 26–32. [Google Scholar] [CrossRef]



| Type of Hydrocolloid | Concentration (%) | Glycerol (%) | Initial Temperature (°C) | Final Temperature (°C) |
|---|---|---|---|---|
| Gelatin | 4 | 50 | 22 | 10 |
| 8 | 50 | 30 | 10 | |
| 12 | 50 | 40 | 10 | |
| Soy protein isolate | 4 | 50 | 20 | 0 |
| 8 | 50 | 20 | 0 | |
| 12 | 50 | 20 | 0 | |
| Sodium alginate | 1 | 50 | 20 | 0 |
| 1.5 | 50 | 20 | 0 | |
| 2 | 50 | 20 | 0 | |
| High-methylated apple pectin | 1 | 50 | 25 | 0 |
| 1.5 | 50 | 25 | 0 | |
| 2 | 50 | 25 | 0 |
| Gelatin | ||||
|---|---|---|---|---|
| Concentration (%) | k | n | R2 | Apparent Viscosity (Pa·s) |
| 4 | 0.999 | 0.0024 ± 0.0001 | ||
| 8 | 0.999 | 0.0055 ± 0.0005 | ||
| 12 | 0.993 | 0.0100 ± 0.0011 | ||
| The correlation coefficient between the concentration of gelatin solutions and the apparent viscosity of the subject solutions | ||||
| 0.964901 | ||||
| Soy Protein Isolate | ||||
| Concentration (%) | k | n | R2 | Apparent Viscosity (Pa·s) |
| 4 | 0.007129 | 0.6496 | 0.9832 | 0.0018 ± 0.0000 |
| 8 | 0.006449 | 0.7661 | 0.9915 | 0.0026 ± 0.0000 |
| 12 | 0.009233 | 0.8737 | 0.9985 | 0.0056 ± 0.0000 |
| The correlation coefficient between the concentration of soy protein isolate solutions and the apparent viscosity of the subject solutions | ||||
| 0.986394 | ||||
| Sodium Alginate | ||||
| Concentration (%) | k | n | R2 | Apparent Viscosity (Pa·s) |
| 1 | 0.999 | 0.0187 ± 0.0001 | ||
| 1.5 | 0.999 | 0.0374 ± 0.0002 | ||
| 2 | 0.999 | 0.0884 ± 0.0002 | ||
| The correlation coefficient between the concentration of sodium alginate solutions and the apparent viscosity of the subject solutions | ||||
| 0.960769 | ||||
| High-Methylated Apple Pectin | ||||
| Concentration (%) | k | n | R2 | Apparent Viscosity (Pa·s) |
| 1 | 0.00776 | 0.697 | 0.997 | 0.0042 ± 0.0001 |
| 1.5 | 0.01385 | 0.889 | 1 | 0.0050 ± 0.0000 |
| 2 | 0.02006 | 0.696 | 0.997 | 0.0061 ± 0.0001 |
| The correlation coefficient between the concentration of high-methylated apple pectin solutions and the apparent viscosity of the subject solutions | ||||
| 0.948683 | ||||
| Concentration (%) | Cooling Rate (K/min) | Gelation Temperature (°C) | |||
|---|---|---|---|---|---|
| 4 | 1 | 21.69 ± 0.18 a | |||
| 3 | 19.74 ± 0.30 e | ||||
| 5 | 20.74 ± 0.12 f | ||||
| 8 | 1 | 25.57 ± 0.23 d | |||
| 3 | 23.21 ± 0.31 c | ||||
| 5 | 22.40 ± 0.14 b | ||||
| 12 | 1 | 25.36 ± 0.05 d | |||
| 3 | 23.00 ± 0.26 c | ||||
| 5 | 22.26 ± 0.02 ab | ||||
| Statistical analysis of the effect of concentration and cooling rate on the gelatinization temperature of gelatin solutions | |||||
| SS | Degrees of Freedom | MS | F | p-Value | |
| Cooling rate (K/min) | 32.32 | 2 | 16.16 | 395.7 | 0.0·10−6 |
| Concentration (%) | 51.01 | 2 | 25.51 | 624.5 | 0.0·10−6 |
| Cooling rate * Concentration | 5.38 | 4 | 1.35 | 32.9 | 0.0·10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowicz, M.; Sitkiewicz, I.; Ciurzyńska, A.; Galus, S. Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating. Coatings 2022, 12, 1704. https://doi.org/10.3390/coatings12111704
Janowicz M, Sitkiewicz I, Ciurzyńska A, Galus S. Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating. Coatings. 2022; 12(11):1704. https://doi.org/10.3390/coatings12111704
Chicago/Turabian StyleJanowicz, Monika, Iwona Sitkiewicz, Agnieszka Ciurzyńska, and Sabina Galus. 2022. "Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating" Coatings 12, no. 11: 1704. https://doi.org/10.3390/coatings12111704
APA StyleJanowicz, M., Sitkiewicz, I., Ciurzyńska, A., & Galus, S. (2022). Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating. Coatings, 12(11), 1704. https://doi.org/10.3390/coatings12111704









