Preparation, Microstructure and Thermal Conductivity of Plasma-Sprayed (Y0.8Gd0.2)3Al5O12 Coatings
Abstract
:1. Introduction
2. Experimental
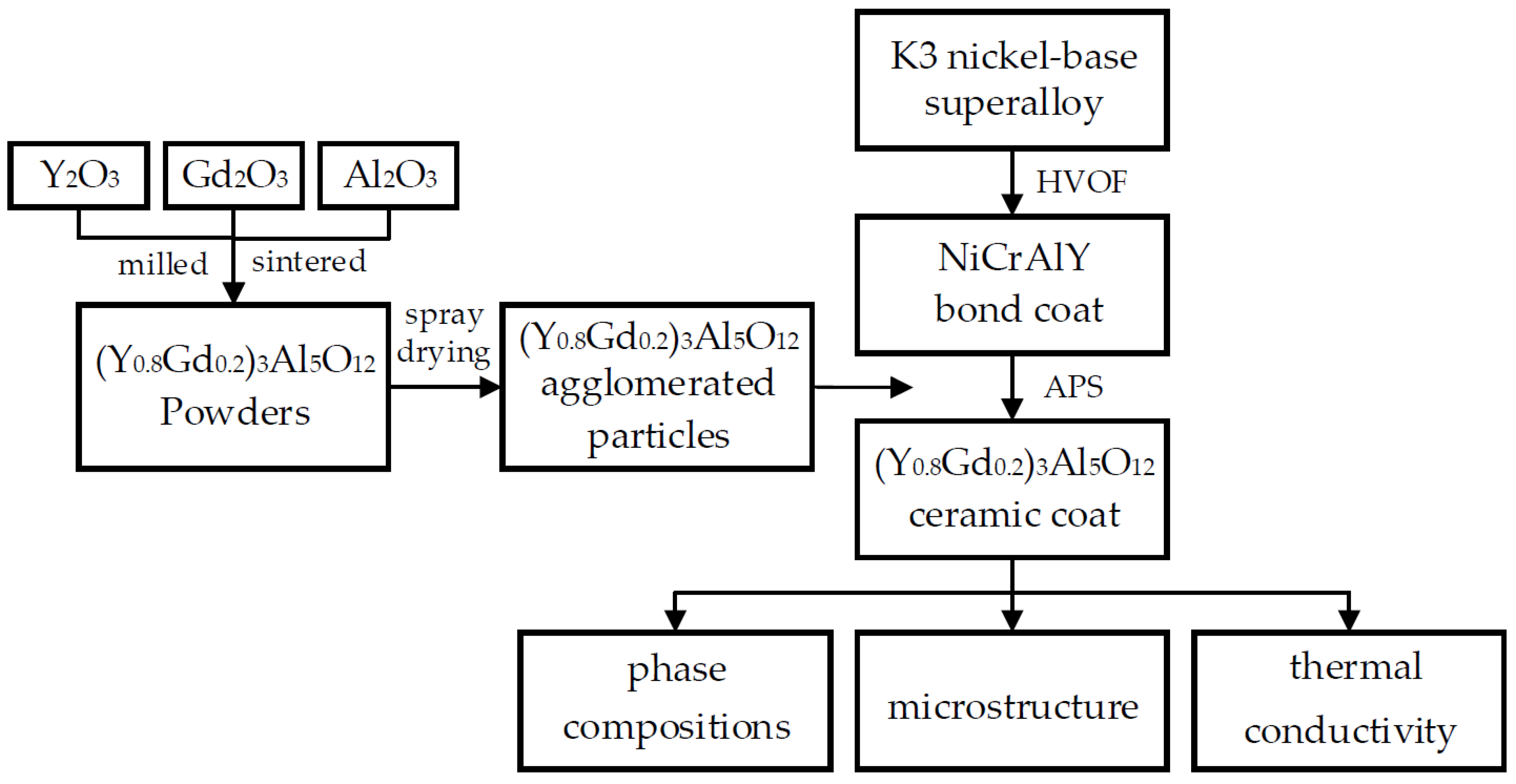
2.1. Preparation of (Y0.8Gd0.2)3Al5O12 Coating
2.2. Characterization Analysis
3. Result and Discussion
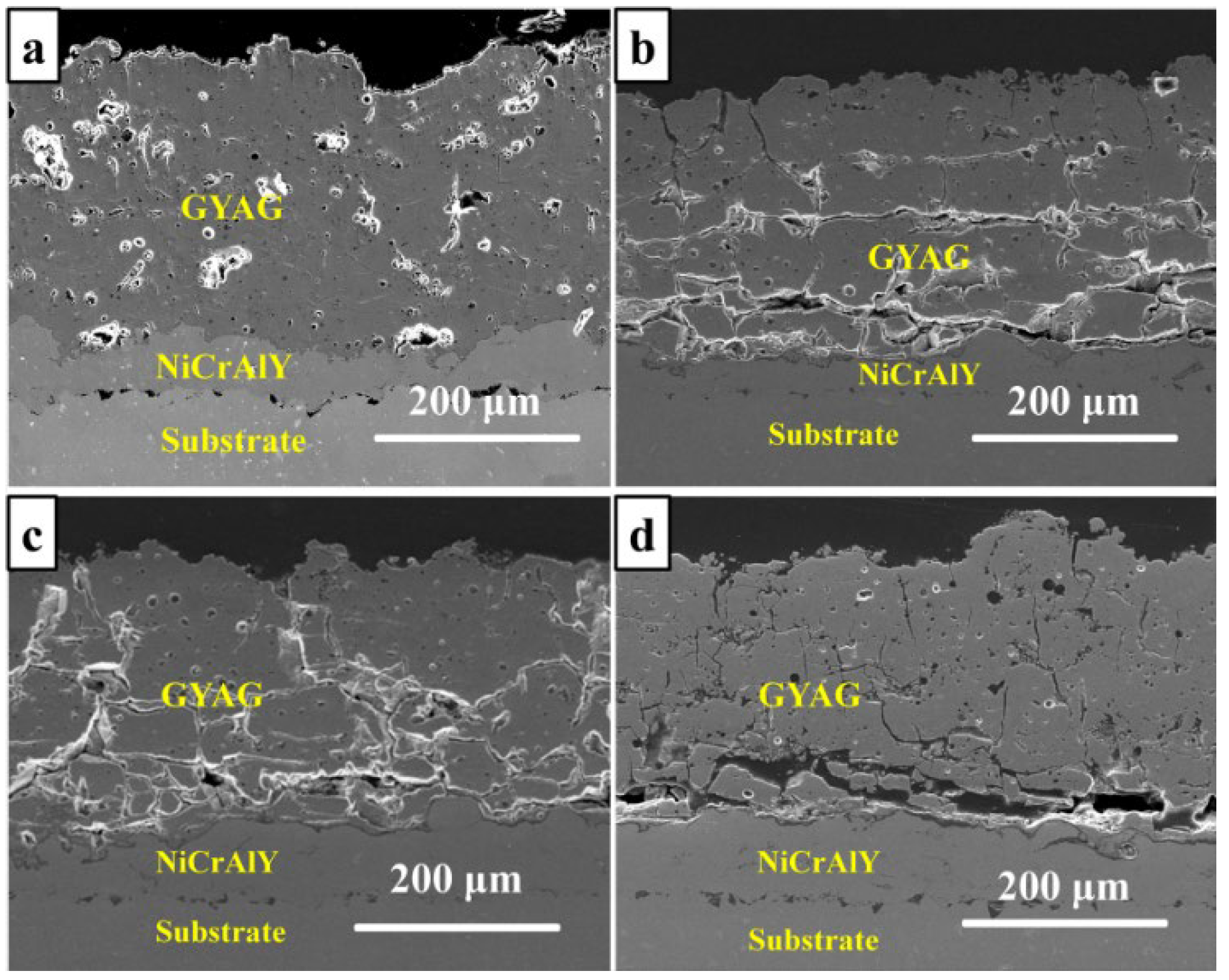
3.1. Preparation and Microstructure
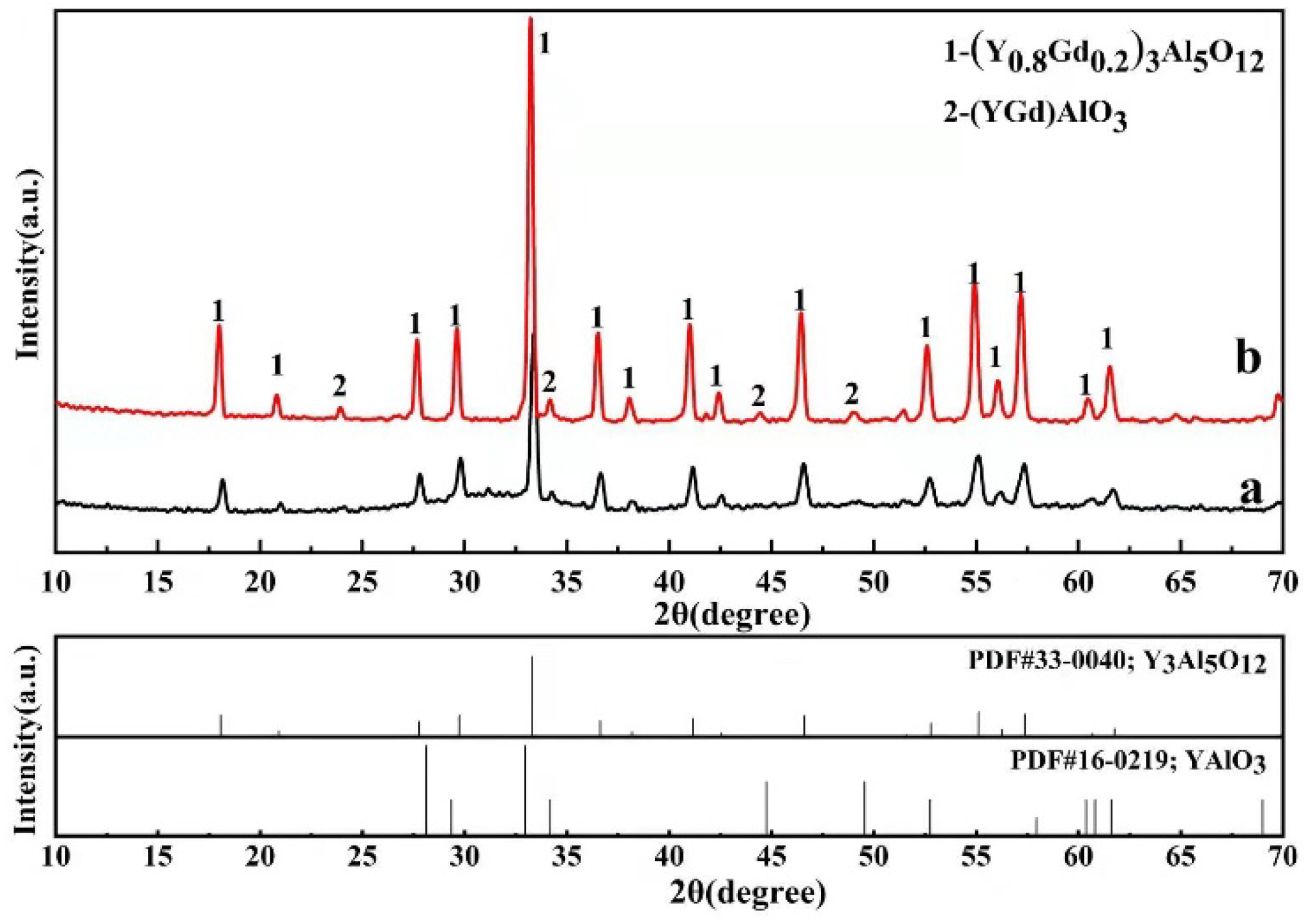
3.2. High-Temperature Phase Stability
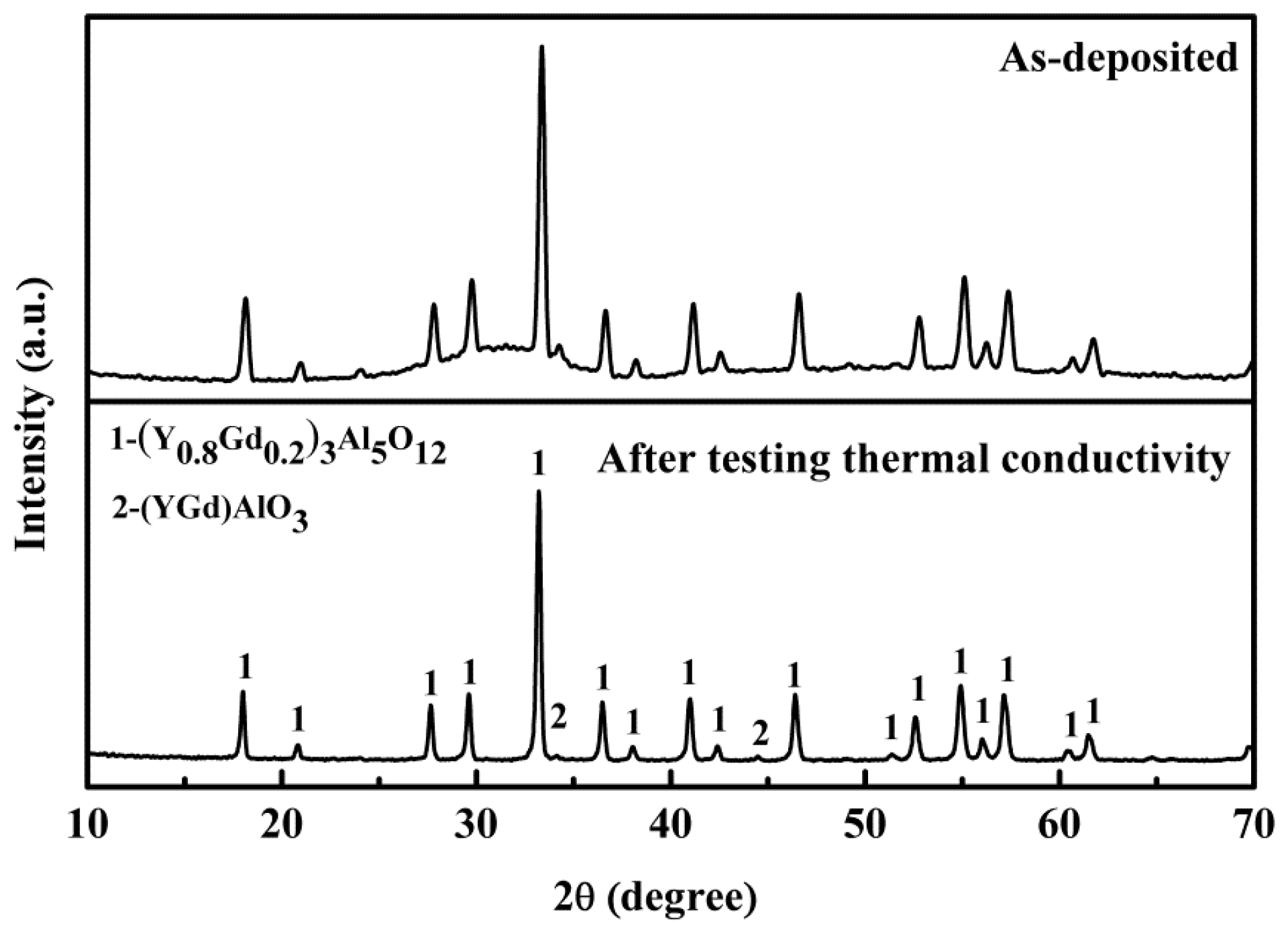
3.3. Thermal Conductivity
3.4. The Effect of Heat Treatment on the Microstructure
4. Conclusions
- The (Y0.8Gd0.2)3Al5O12 (GYAG) coating was successfully fabricated by air plasma spraying. The as-deposited GYAG coating was relatively dense, and mainly contained garnet-type (Y0.8Gd0.2)3Al5O12 phase, and a small amount of (Y,Gd)AlO3 phase and amorphous phase.
- There was only one exothermic peak at about 850 °C existing in the as-deposited GYAG ceramic coating from room temperature to 1450 °C. The crystallized GYAG coating exhibited good phase stability in the above temperature range. The thermal conductivity of the coating slowly decreased up to 800 °C, then quickly increased from 800 to 1000 °C, and subsequently decreased from 1000 to 1200 °C. The lowest thermal conductivity value of the GYAG coating was 1.17 W·m−1·K−1 at 800 ℃, around 15% lower than that of the plasma-sprayed YSZ coating. Meanwhile, the as-deposited coating had been completely crystallized after testing the thermal conductivity.
- The heat-treated coating became denser and had some fine grains precipitated. The coating had some transverse cracks and longitudinal cracks generated after the heat treatment at 1100 °C. Serious transverse cracks were produced at the bottom of the GYAG coating due to the mismatch of thermal expansion between GYAG and NiCrAlY.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darolia, R. Thermal Barrier Coatings Technology: Critical Review, Progress Update, Remaining Challenges and Prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic Materials for Thermal Barrier Coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Y.; Guo, H. Synthesis, Thermal Conductivities and Phase Stability of Gd3TaO7 and La Doped Gd3TaO7 Ceramics. J. Alloys Compd. 2018, 732, 759–764. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.; Gong, S.; Xu, H. Improvement on the Phase Stability, Mechanical Properties and Thermal Insulation of Y2O3-Stabilized ZrO2 by Gd2O3 and Yb2O3 Co-Doping. Ceram. Int. 2013, 39, 9009–9015. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on Advanced Thermal Barrier Coatings. Surf. Coatings Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Zhu, C.; Xiang, H.; Chen, H.; Sun, L.; Gao, Y.; Zhou, Y. Advances on Strategies for Searching for next Generation Thermal Barrier Coating Materials. J. Mater. Sci. Technol. 2019, 35, 833–851. [Google Scholar] [CrossRef]
- Lashmi, P.G.; Ananthapadmanabhan, P.V.; Unnikrishnan, G.; Aruna, S.T. Present Status and Future Prospects of Plasma Sprayed Multilayered Thermal Barrier Coating Systems. J. Eur. Ceram. Soc. 2020, 40, 2731–2745. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, Y. Structural Evolution of Plasma-Sprayed Nanoscale 3 Mol% and 5 Mol% Yttria-Stabilized Zirconia Coatings during Sintering. Appl. Surf. Sci. 2017, 425, 1033–1039. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.; Wang, Y.; Wang, L.; Gou, J.; Chen, W. A Promising Non-Transformable Tetragonal YSZ Nanostructured Feedstocks for Plasma Spraying-Physical Vapor Deposition. Ceram. Int. 2018, 44, 1201–1204. [Google Scholar] [CrossRef]
- Ganvir, A.; Calinas, R.F.; Markocsan, N.; Curry, N.; Joshi, S. Experimental Visualization of Microstructure Evolution during Suspension Plasma Spraying of Thermal Barrier Coatings. J. Eur. Ceram. Soc. 2019, 39, 470–481. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Zhang, H.; Dong, S.; Jiang, J.; Deng, L.; Zhou, X.; Cao, X. Effect of Spraying Power on Microstructure and Property of Nanostructured YSZ Thermal Barrier Coatings. J. Alloys Compd. 2018, 730, 471–482. [Google Scholar] [CrossRef]
- Shen, Y.; Leckie, R.M.; Levi, C.G.; Clarke, D.R. Low Thermal Conductivity without Oxygen Vacancies in Equimolar YO1.5 + TaO2.5- and YbO1.5 + TaO2.5-Stabilized Tetragonal Zirconia Ceramics. Acta Mater. 2010, 58, 4424–4431. [Google Scholar] [CrossRef]
- Masó, N.; West, A.R. Electronic Conductivity in Yttria-Stabilized Zirconia under a Small Dc Bias. Chem. Mater. 2015, 27, 1552–1558. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Vaßen, R.; Traeger, F.; Stöver, D. New Thermal Barrier Coatings Based on Pyrochlore/YSZ Double-Layer Systems. Int. J. Appl. Ceram. Technol. 2005, 1, 351–361. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Wang, L.; Gong, S. Thermo-Physical Properties and Thermal Shock Resistance of Segmented La2Ce2O7/YSZ Thermal Barrier Coatings. J. Therm. Spray Technol. 2009, 18, 665–671. [Google Scholar] [CrossRef]
- Padture, N.P.; Klemens, P.G. Low Thermal Conductivity in Garnets. J. Am. Ceram. Soc. 1997, 80, 1018–1020. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Feng, Z. Theoretical Investigation on Mechanical and Thermal Properties of a Promising Thermal Barrier Material: Yb3Al5O12. J. Mater. Sci. Technol. 2014, 30, 631–638. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Mah, T.-I.; Keller, K. Creep Mechanism of Polycrystalline Yttrium Aluminum Garnet. J. Am. Ceram. Soc. 1992, 75, 1756–1759. [Google Scholar] [CrossRef]
- Li, J.Y.; Dai, H.; Zhong, X.H.; Zhang, Y.F.; Ma, X.F.; Meng, J.; Cao, X.Q. Effect of the Addition of YAG (Y3Al5O12) Nanopowder on the Mechanical Properties of Lanthanum Zirconate. Mater. Sci. Eng. A 2007, 460–461, 504–508. [Google Scholar] [CrossRef]
- Su, Y.J.; Trice, R.W.; Faber, K.T.; Wang, H.; Porter, W.D. Thermal Conductivity, Phase Stability, and Oxidation Resistance of Y3Al5O12 (YAG)/Y2O3–ZrO2 (YSZ) Thermal-Barrier Coatings. Oxid. Met. 2004, 61, 253–271. [Google Scholar] [CrossRef]
- Weyant, C.M.; Faber, K.T. Processing-Microstructure Relationships for Plasma-Sprayed Yttrium Aluminum Garnet. Surf. Coatings Technol. 2008, 202, 6081–6089. [Google Scholar] [CrossRef]
- Ravi, B.G.; Gandhi, A.S.; Guo, X.Z.; Margolies, J.; Sampath, S. Liquid Precursor Plasma Spraying of Functional Materials: A Case Study for Yttrium Aluminum Garnet (YAG). J. Therm. Spray Technol. 2008, 17, 82–90. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Wheatley, R.J.; Neate, N.; Hou, X. Yb3+ Doping Effects on Thermal Conductivity and Thermal Expansion of Yttrium Aluminium Garnet. Ceram. Int. 2016, 42, 14228–14235. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Y.; Gong, S.; Guo, H. Influence of Yb3+ Doping on Phase Stability and Thermophysical Properties of (Y1-XYbx)3Al5O12 under High Temperature. Ceram. Int. 2017, 43, 7153–7158. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, Y.; Guo, H. The Influence of Gd Doping on Thermophysical Properties, Elasticity Modulus and Phase Stability of Garnet-Type (Y1-XGdx)3Al5O12 Ceramics. J. Eur. Ceram. Soc. 2017, 37, 4171–4177. [Google Scholar] [CrossRef]
- Ilavsky, J.; Allen, A.J.; Long, G.G.; Krueger, S.; Berndt, C.C.; Herman, H. Influence of Spray Angle on the Pore and Crack Microstructure of Plasma-Sprayed Deposits. J. Am. Ceram. Soc. 1997, 80, 733–742. [Google Scholar] [CrossRef]
- ASTM International ASTM C373-17; Standard Test Methods for Determination of Water Absorption and Associated Properties by Vacuum Method for Pressed Ceramic Tiles and Glass Tiles and Boil Method for Extruded Ceramic Tiles and Non-Tile Fired Ceramic Whiteware Products. ASTM International: West Conshohocken, PA, USA, 2016.
- Cao, X.Q.; Vassen, R.; Schwartz, S.; Jungen, W.; Tietz, F.; Stöever, D. Spray-Drying of Ceramics for Plasma-Spray Coating. J. Eur. Ceram. Soc. 2000, 20, 2433–2439. [Google Scholar] [CrossRef]
- Loghman-Estarki, M.R.; Edris, H.; Razavi, R.S.; Jamali, H.; Ghasemi, R.; Pourbafrany, M.; Erfanmanesh, M.; Ramezani, M. Spray Drying of Nanometric SYSZ Powders to Obtain Plasma Sprayable Nanostructured Granules. Ceram. Int. 2013, 39, 9447–9457. [Google Scholar] [CrossRef]
- Wan, C.; Qu, Z.; Du, A.; Pan, W. Influence of B Site Substituent Ti on the Structure and Thermophysical Properties of A2B2O7-Type Pyrochlore Gd2Zr2O7. Acta Mater. 2009, 57, 4782–4789. [Google Scholar] [CrossRef]
- Haoming, Z.; Yan, F.; Xiaoge, C.; Hongsong, Z.; Yanxu, L.; An, T.; Bo, R. Thermal Properties of La3TaO7 and La2AlTaO7 Oxides. Ceram. Int. 2017, 43, 755–759. [Google Scholar] [CrossRef]
- Abrams, H. Grain Size Measurement by the Intercept Method. Metallography 1971, 4, 59–78. [Google Scholar] [CrossRef]
- Guo, H.B.; Murakami, H.; Kuroda, S. Effect of Hollow Spherical Powder Size Distribution on Porosity and Segmentation Cracks in Thermal Barrier Coatings. J. Am. Ceram. Soc. 2006, 89, 3797–3804. [Google Scholar] [CrossRef]
- Dehkharghani, A.M.F.; Rahimipour, M.R.; Zakeri, M. Improving the Thermal Shock Resistance and Fracture Toughness of Synthesized La2Ce2O7 Thermal Barrier Coatings through Formation of La2Ce2O7/YSZ Composite Coating via Air Plasma Spraying. Surf. Coatings Technol. 2020, 399, 126174. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Krishnasamy, J.; Ponnusami, S.A.; Turteltaub, S.; van der Zwaag, S. Thermal Cyclic Behavior and Lifetime Prediction of Self-Healing Thermal Barrier Coatings. Int. J. Solids Struct. 2021, 222–223, 111034. [Google Scholar] [CrossRef]
- Wang, L.; Ming, C.; Zhong, X.H.; Ni, J.X.; Yang, J.S.; Tao, S.Y.; Zhou, F.F.; Wang, Y. Microstructure and Self-Healing Properties of Multi-Layered NiCoCrAlY/TAZ/YSZ Thermal Barrier Coatings Fabricated by Atmospheric Plasma Spraying. Appl. Surf. Sci. 2019, 488, 246–260. [Google Scholar] [CrossRef]
- Ouyang, T.; Suo, J. TiC-Self-Healing Thermal Barrier Coating Structures and Oxidation Resistance. Surf. Coatings Technol. 2021, 412, 127065. [Google Scholar] [CrossRef]





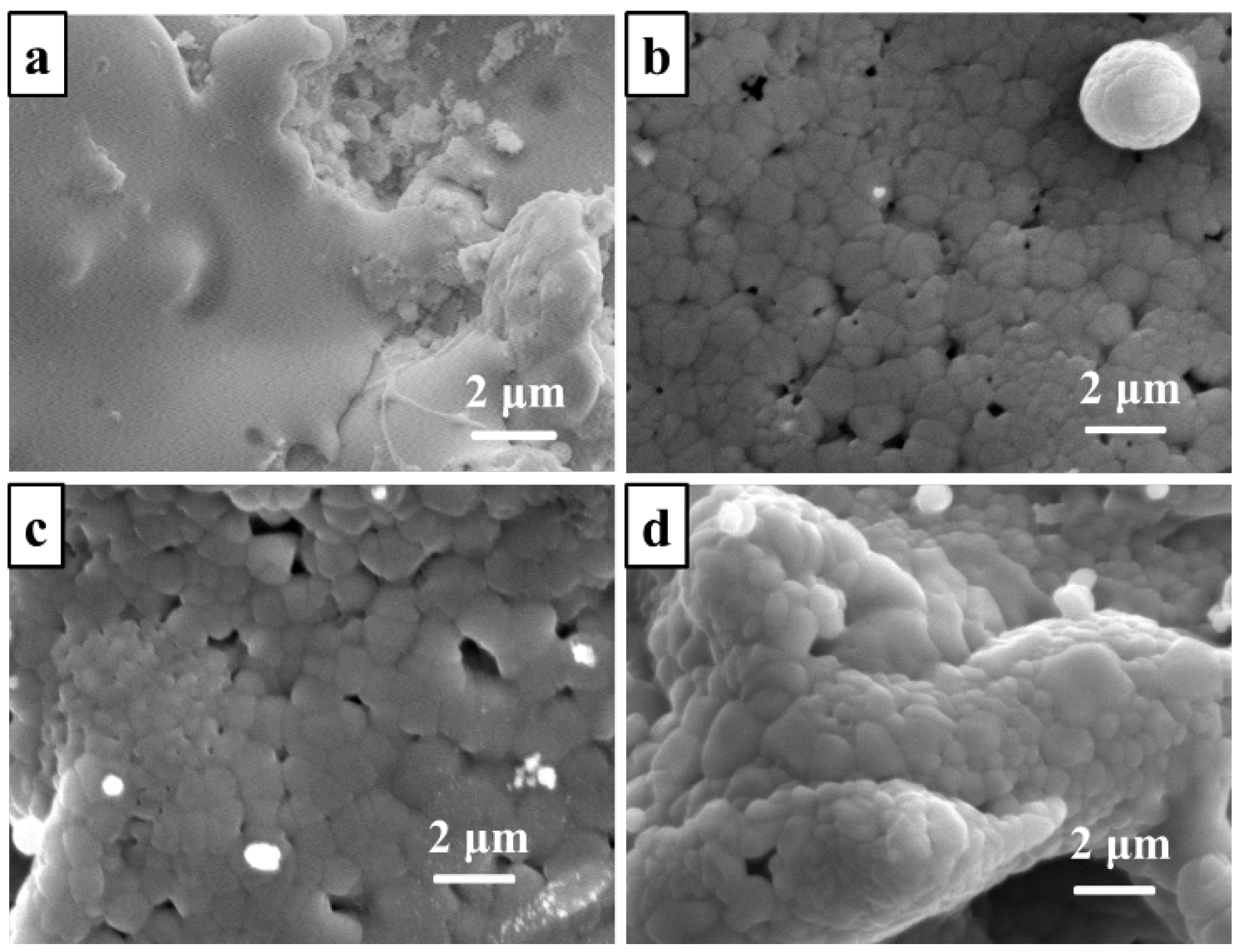

| Eelement | C | Ni | Cr | Co | Al | Ti | Mo | W | Fe | Mn | Si | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.1~ 0.2 | Bal. | 10.0~ 12.0 | 4.5~ 6.0 | 5.3~ 5.9 | 2.3~ 2.9 | 3.8~ 4.5 | 4.8~ 5.5 | ≤2.0 | ≤0.50 | ≤0.50 | ≤0.01 | ≤0.02 |
| Spraying Parameters | NiCrAlY |
|---|---|
| Oxygen L/h | 40 |
| O2 working pressure/MPa | 1.00 |
| Fuel L/h | 17 |
| Combustion chamber pressure/MPa | 0.70 |
| Working pressure/MPa | 0.76 |
| Spray distance/(mm) | 300 |
| Feed rate/(g·min−1) | 50~60 |
| Carrier gas/Nitrogen (MPa) | 0.3 |
| Spraying Parameters | GYAG | YSZ |
|---|---|---|
| Current/(A) | 600 | 600 |
| Voltage/(V) | 68 | 75.5 |
| Flow rate of plasma gas (Ar, H2)/(L/min) | 26/1.42 | 27/1.75 |
| Spray distance/(mm) | 85 | 85 |
| Powder feed rate/(g·min−1) | 21 | 22 |
| Gun traverse speed/(mm·s−1) | 300 | 300 |
| T/°C | 25 | 200 | 400 | 600 | 800 | 1000 | 1200 |
|---|---|---|---|---|---|---|---|
| GYAG/J·g−1·K−1 | 0.56 | 0.68 | 0.74 | 0.78 | 0.80 | 0.82 | 0.83 |
| YSZ/J·g−1·K−1 | 0.45 | 0.54 | 0.58 | 0.60 | 0.62 | 0.63 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; He, J.; Xue, Z. Preparation, Microstructure and Thermal Conductivity of Plasma-Sprayed (Y0.8Gd0.2)3Al5O12 Coatings. Coatings 2022, 12, 510. https://doi.org/10.3390/coatings12040510
Wang S, He J, Xue Z. Preparation, Microstructure and Thermal Conductivity of Plasma-Sprayed (Y0.8Gd0.2)3Al5O12 Coatings. Coatings. 2022; 12(4):510. https://doi.org/10.3390/coatings12040510
Chicago/Turabian StyleWang, Shixing, Jian He, and Zhaolu Xue. 2022. "Preparation, Microstructure and Thermal Conductivity of Plasma-Sprayed (Y0.8Gd0.2)3Al5O12 Coatings" Coatings 12, no. 4: 510. https://doi.org/10.3390/coatings12040510
APA StyleWang, S., He, J., & Xue, Z. (2022). Preparation, Microstructure and Thermal Conductivity of Plasma-Sprayed (Y0.8Gd0.2)3Al5O12 Coatings. Coatings, 12(4), 510. https://doi.org/10.3390/coatings12040510






