Supercritical Fluid-Assisted Fabrication of PDA-Coated Poly (l-lactic Acid)/Curcumin Microparticles for Chemo-Photothermal Therapy of Osteosarcoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of PLLA/CM Microparticles by SEDS Process
2.2.2. Preparation of PDA–PLLA/CM Microparticles
2.2.3. Characterization of PDA–PLLA/CM Microparticles
2.2.4. Drug Loading and Controlled Release Study
2.2.5. In Vitro Photothermal Performance
2.2.6. Cell Culture and Proliferation
2.2.7. In Vitro Biocompatibility and Antitumor Effect
2.2.8. In Vitro Chemo-Photothermal Therapy Evaluation
2.2.9. Statistical Analysis
3. Results and Discussion
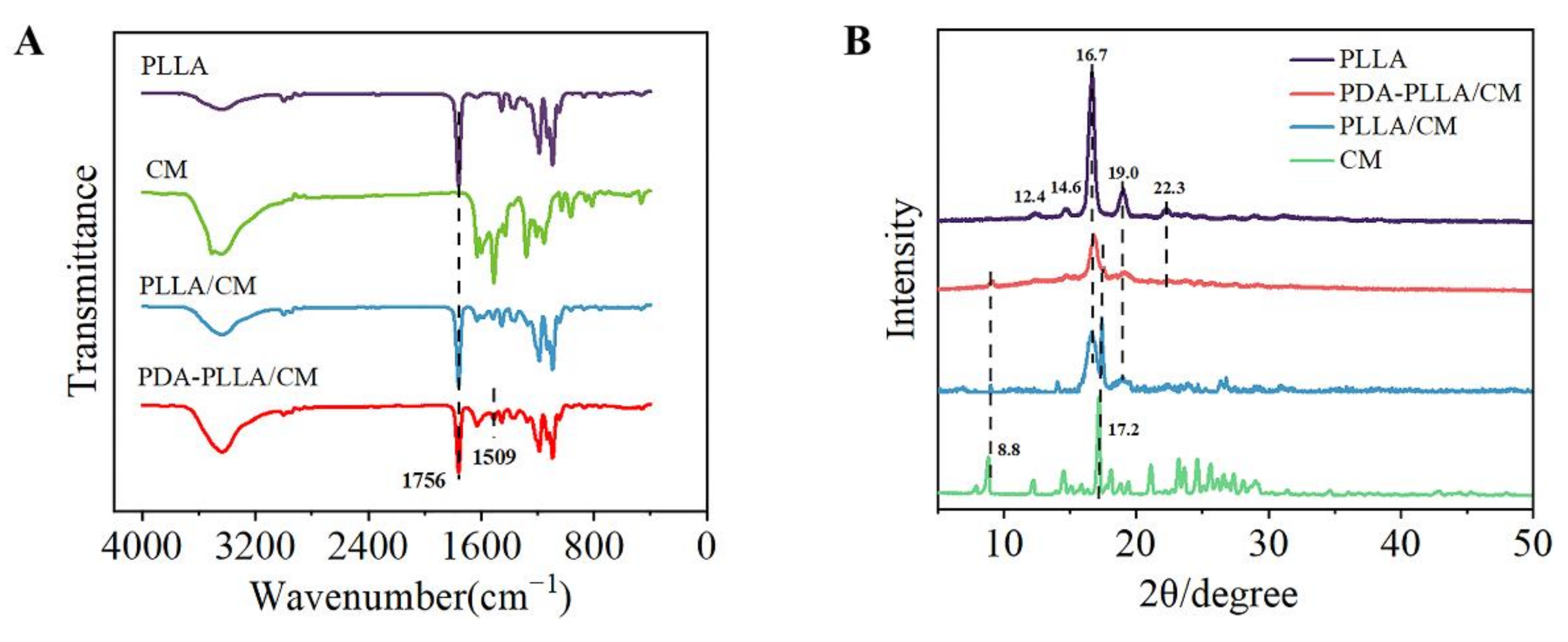
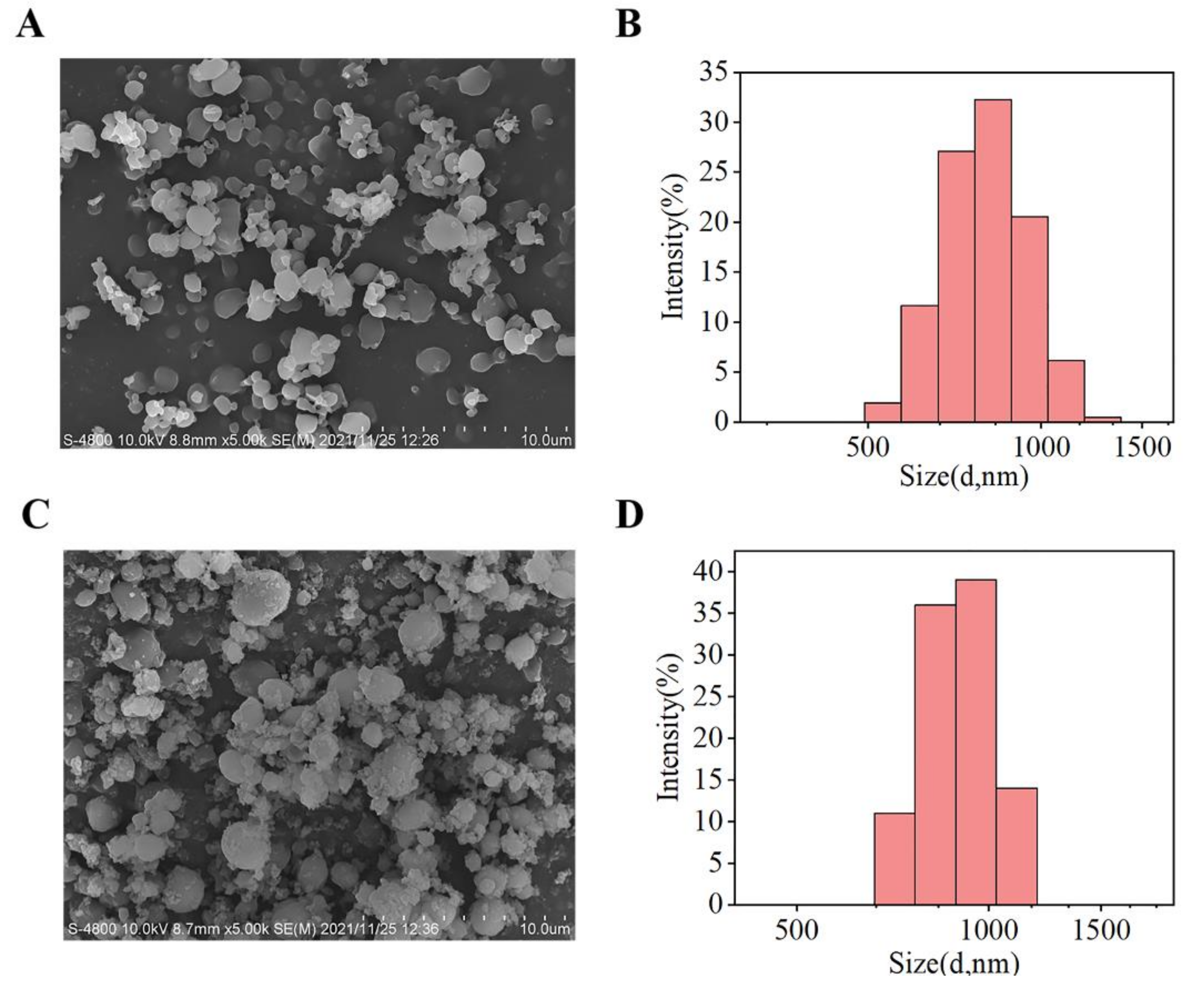
3.1. Synthesis and Characterization of PDA–PLLA/CM Microparticles
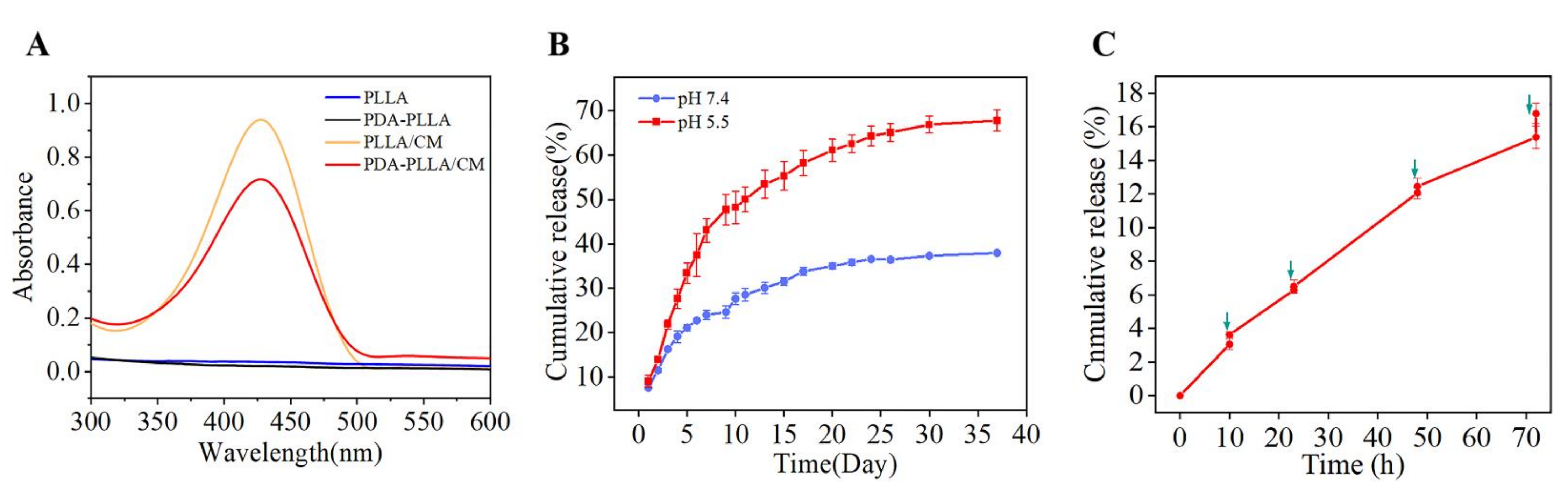
3.2. Drug Loading and Responsive Release
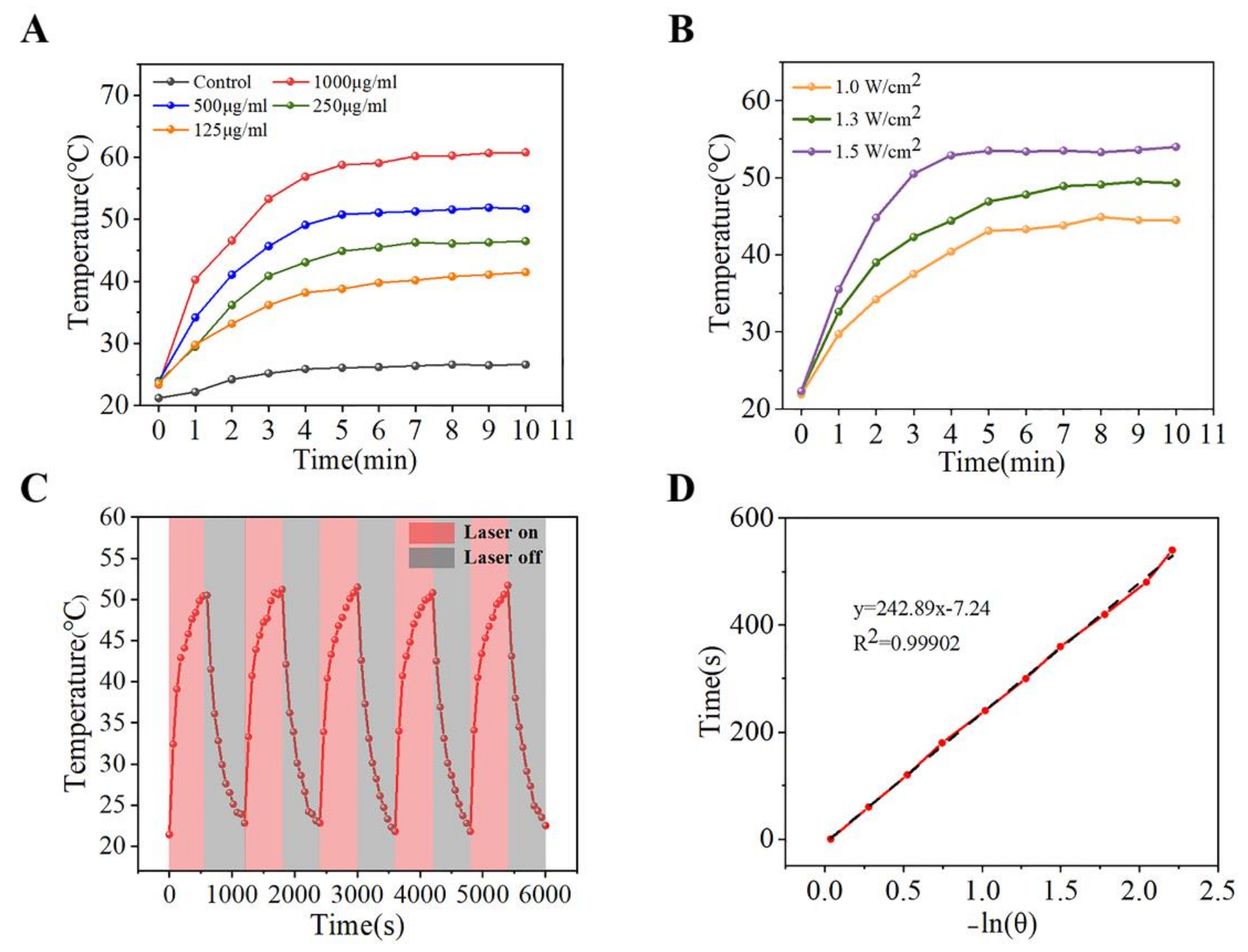
3.3. Photothermal Property Evaluation
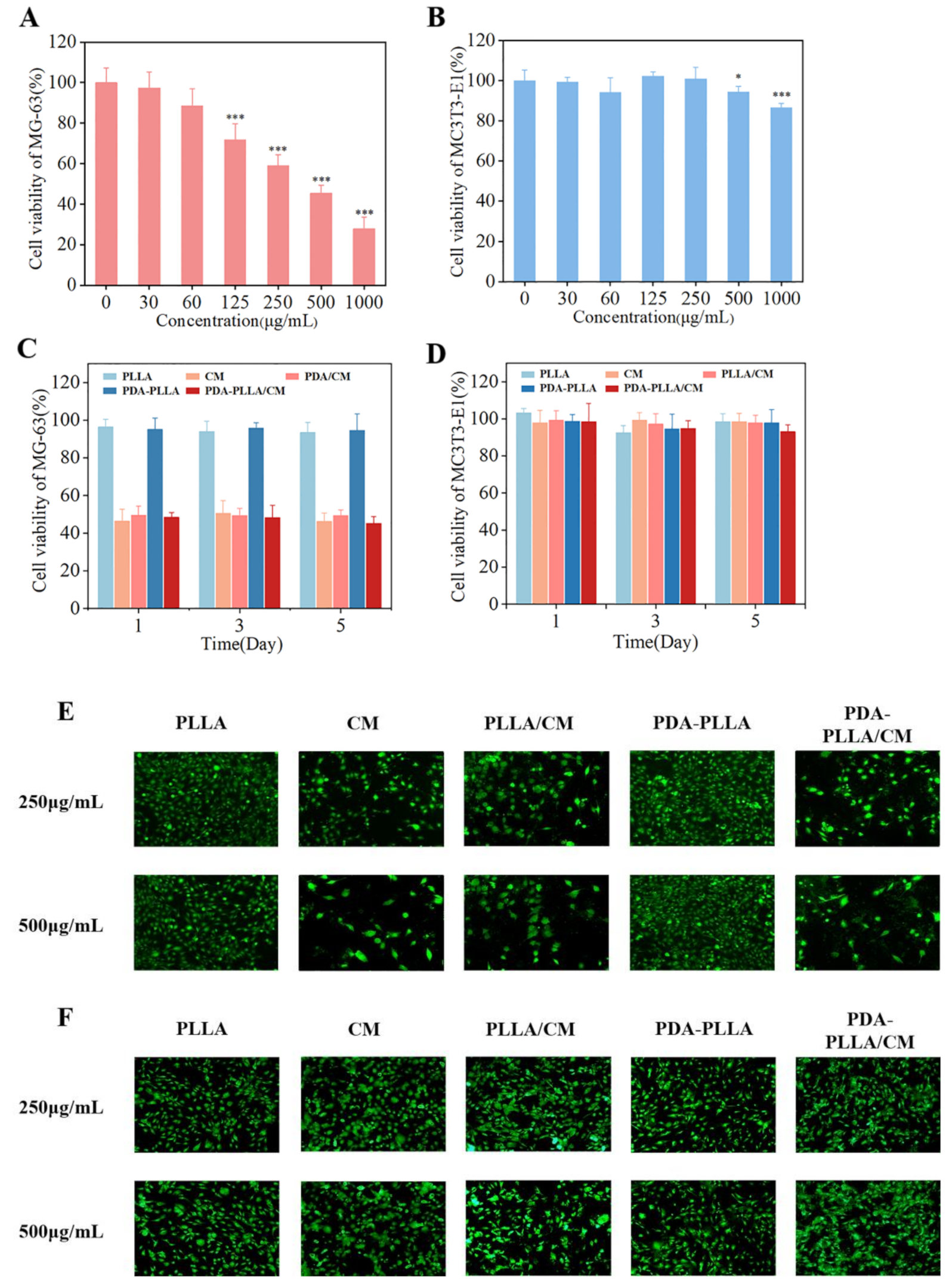
3.4. In Vitro Anti-Cancer of PDA–PLLA/CM Microparticles
3.5. In Vitro Chemo-Photothermal Therapy Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozorgi, A.; Sabouri, L. Osteosarcoma, personalized medicine, and tissue engineering; an overview of overlapping fields of research. Cancer Treat. Res. Commun. 1800, 27, 100324. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Geller, D.S.; Gill, J.D.; Lewis, V.O.; Gorlick, R. Current and future therapeutic approaches for osteosarcoma. Expert Rev. Anticancer Ther. 2018, 18, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Isakoff, M.S.; Bielack, S.S.; Meltzer, P.; Gorlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029. [Google Scholar] [CrossRef] [Green Version]
- Djunic, I.; Elezovic, I.; Marinkovic, M.; Suvajdzic-Vukovic, N.; Tomin, D.; Jankovic, G.; Bila, J.; Antic, D.; Vidovic, A.; Neskovic, B.; et al. Osteolytic lesions marker in multiple myeloma. Med. Oncol. 2011, 28, 237–240. [Google Scholar] [CrossRef]
- Tennant, D.A.; Duran, R.V.; Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, Y.; Xu, K.; Sun, X.; Yu, Q.; Wu, Z.; Zhao, Z. Biomimetic Polydopamine-Modified Silk Fibroin/Curcumin Nanofibrous Scaffolds for Chemo-photothermal Therapy of Bone Tumor. ACS Omega 2021, 6, 22213–22223. [Google Scholar] [CrossRef]
- Hu, C.-W.; Sheng, Y.; Zhang, Q.; Liu, H.-B.; Xie, X.; Ma, W.-C.; Huo, R.; Dong, D.-L. Curcumin inhibits hERG potassium channels in vitro. Toxicol. Lett. 2012, 208, 192–196. [Google Scholar] [CrossRef]
- Komal, K.; Chaudhary, S.; Yadav, P.; Parmanik, R.; Singh, M. The Therapeutic and Preventive Efficacy of Curcumin and Its Derivatives in Esophageal Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Piper, J.T.; Singhal, S.S.; Salameh, M.S.; Torman, R.T.; Awasthi, Y.C.; Awasthi, S. Mechanisms of anticarcinogenic properties of curcumin: The effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int. J. Biochem. Cell Biol. 1998, 30, 445–456. [Google Scholar] [CrossRef]
- Oetari, S.; Sudibyo, M.; Commandeur, J.N.; Samhoedi, R.; Vermeulen, N.P. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem. Pharmacol. 1996, 51, 39–45. [Google Scholar] [CrossRef]
- Sun, X.; Meng, Z.; Yu, Q.; Wang, X.; Zhao, Z. Engineering PDA-coated CM-CS nanoparticles for photothermo-chemotherapy of osteosarcoma and bone regeneration. Biochem. Eng. J. 2021, 175, 108138. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Dai, H.-L.; Li, Z.-H.; Meng, Z.-Y.; Xiao, Y.; Zhao, Z. Mesoporous polydopamine-coated hydroxyapatite nano-composites for ROS-triggered nitric oxide-enhanced photothermal therapy of osteosarcoma. J. Mater. Chem. B 2021, 9, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complementary Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Liu, Y.; Jia, L.; Zhou, H.-M.; Kong, Y.; Yang, G.; Jiang, L.-P.; Li, Q.-J.; Zhong, L.-F. Curcurnin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free. Radic. Biol. Med. 2007, 43, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef]
- Lelli, D.; Sahebkar, A.; Johnston, T.P.; Pedone, C. Curcumin use in pulmonary diseases: State of the art and future perspectives. Pharmacol. Res. 2017, 115, 133–148. [Google Scholar] [CrossRef]
- Liu, F.; Lin, L.; Zhang, Y.; Sheng, S.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Two-dimensional nanosheets with high curcumin loading content for multimodal imaging-guided combined chemo-photothermal therapy. Biomaterials 2019, 223, 119470. [Google Scholar] [CrossRef]
- Song, Y.; Cai, L.; Tian, Z.; Wu, Y.; Chen, J. Phytochemical Curcumin-Coformulated, Silver-Decorated Melanin-like Polydopamine/Mesoporous Silica Composites with Improved Antibacterial and Chemotherapeutic Effects against Drug-Resistant Cancer Cells. ACS Omega 2020, 5, 15083–15094. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, P.; Xu, Z.; Chen, J. Dual-Responsive Dual-Drug-Loaded Bioinspired Polydopamine Nanospheres as an Efficient Therapeutic Nanoplatform against Drug-Resistant Cancer Cells. ACS Appl. Bio Mater. 2020, 3, 5730–5740. [Google Scholar] [CrossRef] [PubMed]
- Bale, S.; Khurana, A.; Reddy, A.S.S.; Singh, M.; Godugu, C. Overview on Therapeutic Applications of Microparticulate Drug Delivery Systems. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 309–361. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, S.; Zhu, X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gaba, B.; Fazil, M.; Ali, A.; Baboota, S.; Sahni, J.K.; Ali, J. Nanostructured lipid (NLCs) carriers as a bioavailability enhancement tool for oral administration. Drug Deliv. 2015, 22, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Gu, Z.; Gu, W.; Liu, J.; Xu, Z.P. Efficient drug delivery using SiO2-layered double hydroxide nanocomposites. J. Colloid Interface Sci. 2016, 470, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Ding, A.; Teng, L.; Zhou, Y.; Chen, P.; Nie, W. Synthesis and characterization of bovine serum albumin-loaded microspheres based on star-shaped PLLA with a xylitol core and their drug release behaviors. Polym. Bull. 2018, 75, 2917–2931. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Tao, F.; Ma, S.; Tao, H.; Jin, L.; Luo, Y.; Zheng, J.; Xiang, W.; Deng, H. Chitosan-based drug delivery systems: From synthesis strategy to osteomyelitis treatment-A review. Carbohydr. Polym. 2021, 251, 117063. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Lin, X.-F.; Kankala, R.K.; Tang, N.; Xu, P.-Y.; Hao, L.-Z.; Yang, D.-Y.; Wang, S.-B.; Zhang, Y.S.; Chen, A.-Z. Supercritical Fluid-Assisted Porous Microspheres for Efficient Delivery of Insulin and Inhalation Therapy of Diabetes. Adv. Healthc. Mater. 2019, 8, 23546–23557. [Google Scholar] [CrossRef]
- Yang, F.; Niu, X.; Gu, X.; Xu, C.; Wang, W.; Fan, Y. Biodegradable Magnesium-Incorporated Poly(L-lactic acid) Microspheres for Manipulation of Drug Release and Alleviation of Inflammatory Response. ACS Appl. Mater. Interfaces 2019, 11, 23546–23557. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, S.; Li, W.; Du, Z.; Li, Y. In vitro drug controlled-release behavior of an electrospun modified poly(lactic acid)/bacitracin drug delivery system. RSC Adv. 2016, 6, 515–521. [Google Scholar] [CrossRef]
- Margulis, K.; Magdassi, S.; Lee, H.S.; Macosko, C.W. Formation of curcumin nanoparticles by flash nanoprecipitation from emulsions. J. Colloid Interface Sci. 2014, 434, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pinon-Segundo, E.; Nava-Arzaluz, M.G.; Lechuga-Ballesteros, D. Pharmaceutical polymeric nanoparticles prepared by the double emulsion- solvent evaporation technique. Recent Pat. Drug Deliv. Formul. 2012, 6, 224–235. [Google Scholar] [CrossRef]
- Zheng, W. A water-in-oil-in-oil-in-water (W/O/O/W) method for producing drug-releasing, double-walled microspheres. Int. J. Pharm. 2009, 374, 90–95. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Pathak, P.; Meziam, M.J.; Desai, T.; Sun, Y.-P. Formation and stabilization of ibuprofen nanoparticles in supercritical fluid processing. J. Supercrit. Fluids 2006, 37, 279–286. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martin, A.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, Z.; Meng, Z.; Wang, Y.; Li, Z.; Zhao, Z. Synthesis of curcumin and indocyanine green co-loaded PLLA microparticles via solution-enhanced dispersion using supercritical CO2 for chemo-photothermal therapy of osteosarcoma. J. Supercrit. Fluids 2022, 180, 105462. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jerome, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G.; De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 2008, 47, 70–84. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef] [PubMed]
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Poinard, B.; Neo, S.Z.Y.; Yeo, E.L.L.; Heng, H.P.S.; Neoh, K.G.; Kah, J.C.Y. Polydopamine Nanoparticles Enhance Drug Release for Combined Photodynamic and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 21125–21136. [Google Scholar] [CrossRef]
- Peng, Y.; Nie, J.; Cheng, W.; Liu, G.; Zhu, D.; Zhang, L.; Liang, C.; Mei, L.; Huang, L.; Zeng, X. A multifunctional nanoplatform for cancer chemo-photothermal synergistic therapy and overcoming multidrug resistance. Biomater. Sci. 2018, 6, 1084–1098. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Zheng, K.; Ren, Y.; Wang, M.; Wu, Q.; Zhou, F.; Liu, C.; Liu, L.; Song, J.; et al. Degradable mesoporous semimetal antimony nanospheres for near-infrared II multimodal theranostics. Nat. Commun. 2022, 13, 539. [Google Scholar] [CrossRef]
- Zheng, Q.; Lin, T.; Wu, H.; Guo, L.; Ye, P.; Hao, Y.; Guo, Q.; Jiang, J.; Fu, F.; Chen, G. Mussel-inspired polydopamine coated mesoporous silica nanoparticles as pH-sensitive nanocarriers for controlled release. Int. J. Pharm. 2014, 463, 22–26. [Google Scholar] [CrossRef]
- Pierini, F.; Nakielski, P.; Urbanek, O.; Pawlowska, S.; Lanzi, M.; De Sio, L.; Kowalewski, T.A. Polymer-Based Nanomaterials for Photothermal Therapy: From Light-Responsive to Multifunctional Nanoplatforms for Synergistically Combined Technologies. Biomacromolecules 2018, 19, 4147–4167. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, H.; Yang, F.; Chen, H.; He, W.; Wang, J. Curcumin Promotes Osteosarcoma Cell Death by Activating miR-125a/ERR Signal Pathway. J. Cell. Biochem. 2017, 118, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, K.; Zhu, Y.; Wang, D.; Shao, Y.; Zhang, J. Curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 osteosarcoma cells via downregulating Notch1. Mol. Med. Rep. 2017, 15, 1747–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaque, D.; Maestro, L.M.; Rosal, B.D.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.; Nanoscale, J.S.J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Chen, S.; Xiao, Y.; Xie, M.; Yu, W. Supercritical Fluid-Assisted Fabrication of PDA-Coated Poly (l-lactic Acid)/Curcumin Microparticles for Chemo-Photothermal Therapy of Osteosarcoma. Coatings 2022, 12, 524. https://doi.org/10.3390/coatings12040524
Zhao Z, Chen S, Xiao Y, Xie M, Yu W. Supercritical Fluid-Assisted Fabrication of PDA-Coated Poly (l-lactic Acid)/Curcumin Microparticles for Chemo-Photothermal Therapy of Osteosarcoma. Coatings. 2022; 12(4):524. https://doi.org/10.3390/coatings12040524
Chicago/Turabian StyleZhao, Zheng, Shilu Chen, Yao Xiao, Maobin Xie, and Wen Yu. 2022. "Supercritical Fluid-Assisted Fabrication of PDA-Coated Poly (l-lactic Acid)/Curcumin Microparticles for Chemo-Photothermal Therapy of Osteosarcoma" Coatings 12, no. 4: 524. https://doi.org/10.3390/coatings12040524
APA StyleZhao, Z., Chen, S., Xiao, Y., Xie, M., & Yu, W. (2022). Supercritical Fluid-Assisted Fabrication of PDA-Coated Poly (l-lactic Acid)/Curcumin Microparticles for Chemo-Photothermal Therapy of Osteosarcoma. Coatings, 12(4), 524. https://doi.org/10.3390/coatings12040524




