Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- The oxidic coating prepared by plasma electrolytic oxidation on magnesium alloy AZ91 represents a typical porous structure with variable pore and micro-crack size.
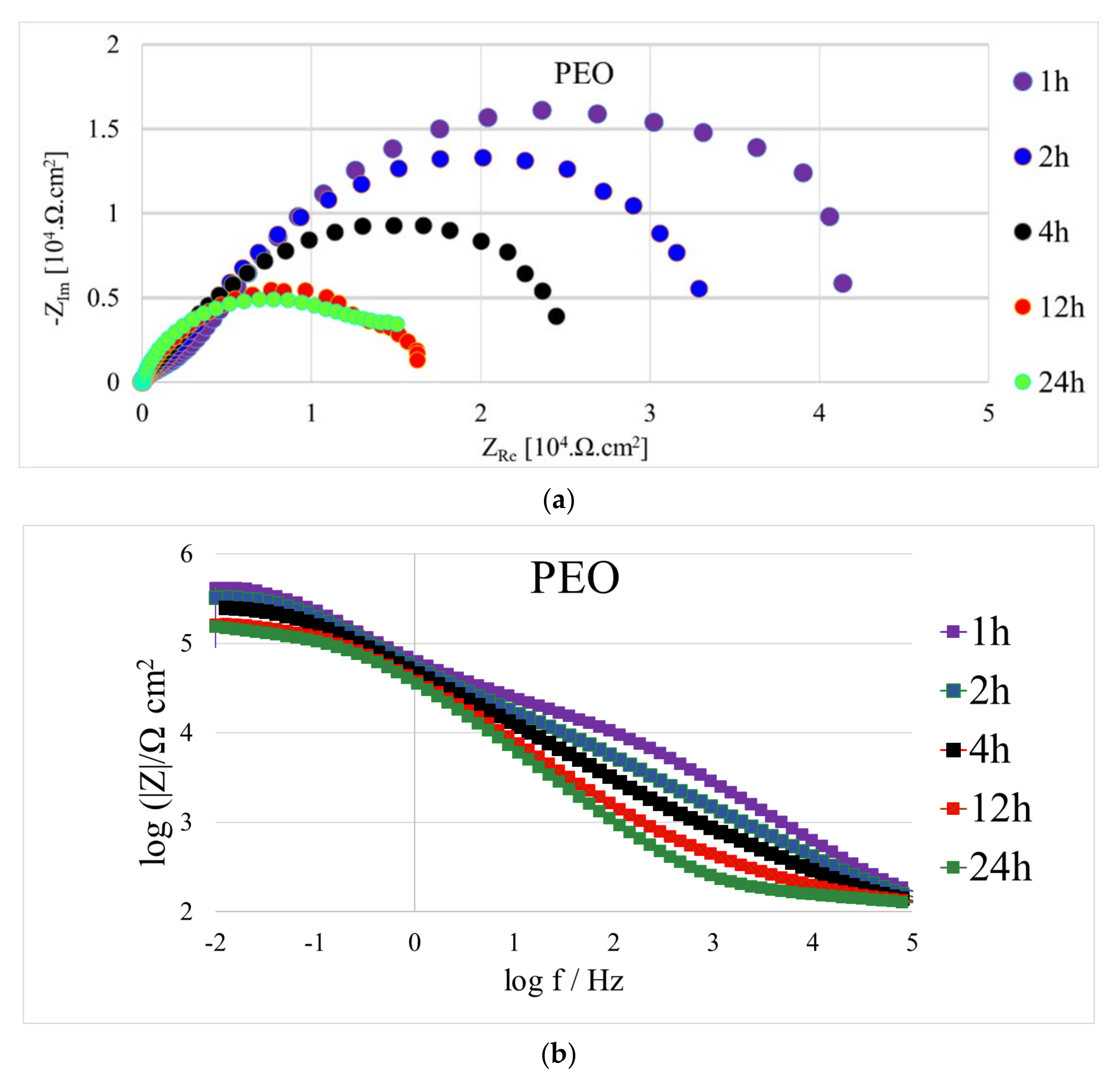
- In terms of increasing corrosion resistance, the prepared PEO coating increased the polarisation resistance value Rp after 24 h of exposure in 0.1 M NaCl more than 73 times to 207,515 Ω·cm2, while the as-cast surface reached only 2825 Ω·cm2.
- When comparing the Rp values from the EIS measurements from our previous work [22], the obtained value of Rp 29,297 Ω·cm2 from our previous work on PEO coated AZ31 magnesium alloy represented only 14% of the Rp value obtained on PEO coated AZ91 in this work after 24 h of exposure in 0.1 M NaCl.
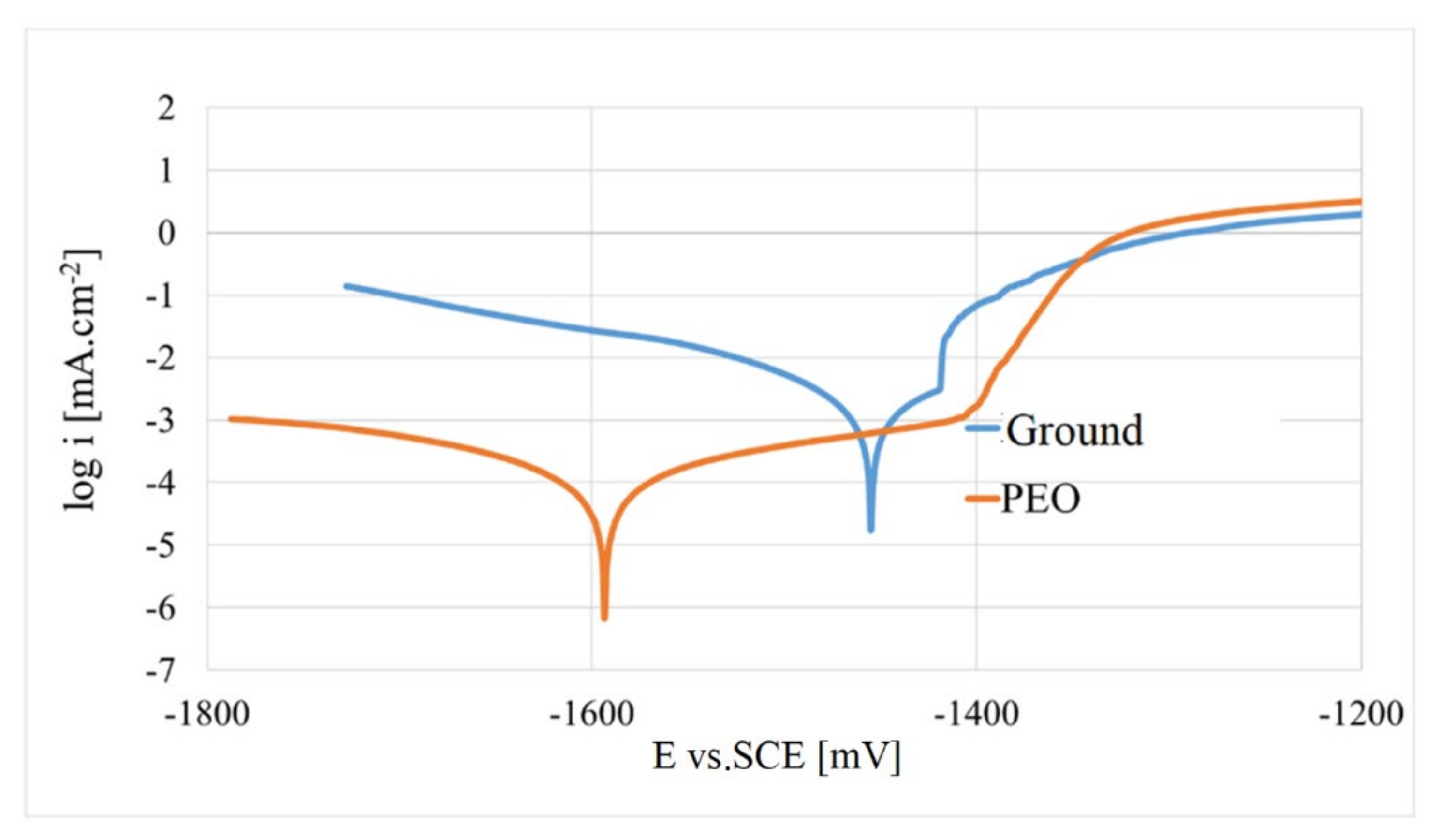
- A significant increase in corrosion resistance was also demonstrated by results obtained from potentiodynamic polarisation in 0.1 M NaCl as follows: The corrosion current density of icorr for the PEO coating decreased by more than 27 times to 0.14 μA·cm−2, compared to the as-cast surface (3.86 μA·cm−2).
- From a thermodynamic point of view, the corrosion potential Ecorr shifted towards a more negative value (−1625 mV) for the PEO surface compared to the Ecorr value at the level (−1465 mV) measured for the as-cast surface.
- However, when comparing the results of Ecorr values between as-cast surfaces of AZ31 from our previous work [23] and AZ91 from this study, the AZ91 magnesium alloy in this work was characterised by a shift of Ecorr towards more positive values by the difference of +114 mV.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaily, M.; Shahabi-Navid, M.; Svensson, J.E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.G. Influence of Temperature on the Atmospheric Corrosion of the Mg-Al Alloy AM50. Corros. Sci. 2015, 90, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Barati Darband, G.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma Electrolytic Oxidation of Magnesium and Its Alloys: Mechanism, Properties and Applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Monteiro, W.A.; Buso, S.J.; Silva, L.V. Application of Magnesium Alloys in Transport. In New Features on Magnesium Alloys; InTech: London, UK, 2012. [Google Scholar]
- Westengen, H.; Aune, T.K. 5 Magnesium Casting Alloys 5.1 Casting Alloys; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Mondet, M.; Barraud, E.; Lemonnier, S.; Guyon, J.; Allain, N.; Grosdidier, T. Microstructure and Mechanical Properties of AZ91 Magnesium Alloy Developed by Spark Plasma Sintering. Acta Mater. 2016, 119, 55–67. [Google Scholar] [CrossRef]
- Hadzima, B.; Liptáková, T. Základy Elektrochemickej Korózie Kovov; EDIS-Žilinská Univerzita: Žilina, Slovak, 2008. [Google Scholar]
- Clyne, T.W.; Troughton, S.C. A Review of Recent Work on Discharge Characteristics during Plasma Electrolytic Oxidation of Various Metals. Int. Mater. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.U.; Blawert, C.; Wei, K. Science Review of Studies on Corrosion of Magnesium Alloys. Trans. Nonferrous Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Amirudin, A.; Thierry, D. Progress in Organic Coatings Application of Electrochemical Impedance Spectroscopy to Study the Degradation of Polymer-Coated Metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Bagherifard, S.; Hickey, D.J.; Fintová, S.; Pastorek, F.; Fernandez-Pariente, I.; Bandini, M.; Webster, T.J.; Guagliano, M. Effects of Nanofeatures Induced by Severe Shot Peening (SSP) on Mechanical, Corrosion and Cytocompatibility Properties of Magnesium Alloy AZ31. Acta Biomater. 2018, 66, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Czerwinski, F. Controlling the Ignition and Flammability of Magnesium for Aerospace Applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Wang, B.J.; Xu, D.K.; Cai, X.; Qiao, Y.X.; Sheng, L.Y. Effect of Rolling Ratios on the Microstructural Evolution and Corrosion Performance of an As-Rolled Mg-8 wt.% Li Alloy. J. Magnes. Alloy. 2021, 9, 560–568. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Plasma Electrolytic Oxidation Coatings on Mg-Alloys for Improved Wear and Corrosion Resistance. Corros. Mater. Perform. Cathodic Prot. 2017, 99, 133–147. [Google Scholar]
- Chen, Y.; Lu, X.; Lamaka, S.V.; Ju, P.; Blawert, C.; Zhang, T.; Wang, F.; Zheludkevich, M.L. Active Protection of Mg Alloy by Composite PEO Coating Loaded with Corrosion Inhibitors. Appl. Surf. Sci. 2020, 504, 144462. [Google Scholar] [CrossRef]
- Zhu, L.; Qiu, J.; Chen, J.; Zhang, W.; Chen, Z.; Zhang, T.; Wang, F. Microstructure and Corrosion Resistance of the PEO Coating on Extruded Al6Cu Alloy. Surf. Coat. Technol. 2019, 369, 116–126. [Google Scholar] [CrossRef]
- Feliu, S. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Blawert, C.; Dietzel, W.; Ghali, E.; Song, G. Anodizing Treatments for Magnesium Alloys and Their Effect on Corrosion Resistance in Various Environments. Adv. Eng. Mater. 2006, 8, 511–533. [Google Scholar] [CrossRef]
- Curran, J.A.; Clyne, T.W. The Thermal Conductivity of Plasma Electrolytic Oxide Coatings on Aluminium and Magnesium. Surf. Coat. Technol. 2005, 199, 177–183. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Yang, Y.; Wang, Y.; Sheng, L.; Li, Y.; Huo, M.; Zhang, K.; Xing, L.; Zhang, G. Study on the Microstructure and Mechanical Properties of a Ti/Mg Alloy Clad Plate Produced by Explosive Welding. Metals 2022, 12, 399. [Google Scholar] [CrossRef]
- Zhang, P.; Nie, X.; Hu, H. Wear and Galvanic Corrosion Protection of Mg Alloy via Plasma Electrolytic Oxidation Process for Mg Engine Application. SAE Tech. Pap. 2009, 1, 0790. [Google Scholar]
- Hadzima, B.; Kajánek, D.; Jambor, M.; Drábiková, J.; Březina, M.; Buhagiar, J.; Pastorková, J.; Jacková, M. Peo of Az31 Mg Alloy: Effect of Electrolyte Phosphate Content and Current Density. Metals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Kajánek, D.; Hadzima, B.; Tkacz, J.; Pastorková, J.; Jacková, M.; Wasserbauer, J. Corrosion Resistance of AZ31 Magnesium Alloy Treated by Plasma Electrolytic Oxidation. Koroze A Ochr. Mater. 2019, 63, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Pastorek, F.; Štrbák, M.; Kajánek, D.; Jacková, M.; Pastorková, J.; Florková, Z. Corrosion Behaviour of Preserved Peo Coating on Az31 Magnesium Alloy. Commun. Sci. Lett. Univ. Zilina 2021, 23, B76–B88. [Google Scholar] [CrossRef]
- Yang, C.; Cai, H.; Cui, S.; Huang, J.; Zhu, J.; Wu, Z.; Ma, Z.; Fu, R.K.Y.; Sheng, L.; Tian, X.; et al. A Zinc-Doped Coating Prepared on the Magnesium Alloy by Plasma Electrolytic Oxidation for Corrosion Protection. Surf. Coat. Technol. 2022, 433, 128148. [Google Scholar] [CrossRef]
- Kang, L.; Shi, L.; Zeng, Q.; Liao, B.; Wang, B.; Guo, X. Melamine Resin-Coated Lignocellulose Fibers with Robust Superhydrophobicity for Highly Effective Oil/Water Separation. Sep. Purif. Technol. 2021, 279, 119793. [Google Scholar] [CrossRef]
- Guo, Y.; Rogov, A.; Hird, A.; Mingo, B.; Matthews, A.; Yerokhin, A. Plasma Electrolytic Oxidation of Magnesium by Sawtooth Pulse Current. Surf. Coat. Technol. 2022, 429, 127938. [Google Scholar] [CrossRef]
- Lein, J.E.; Kr Aune, T.; Ni sancioglu, K. The Role of Mg17AI12 Phase in the Corrosion of Mg AIIoy AZ91. Corrosion 1989, 45, 741–748. [Google Scholar]
- Zeng, G.; Xian, J.W.; Gourlay, C.M. Nucleation and Growth Crystallography of Al8Mn5 on B2-Al(Mn,Fe) in AZ91 Magnesium Alloys. Acta Mater. 2018, 153, 364–376. [Google Scholar] [CrossRef] [Green Version]
- Lun Sin, S.; Dubé, D.; Tremblay, R. An Investigation on Microstructural and Mechanical Properties of Solid Mould Investment Casting of AZ91D Magnesium Alloy. Mater. Charact. 2008, 59, 178–187. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion Mechanisms of Magnesium Alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Kiełbus, A.; Moskal, G. The Influence of Mg17Al12 Phase Volume Fraction on the Corrosion Behaviour of AZ91 Magnesium Alloy. Int. J. Microstruct. Mater. Prop. 2009, 4, 196–206. [Google Scholar]
- Martin, J.; Nominé, A.V.; Stef, J.; Nominé, A.; Zou, J.X.; Henrion, G.; Grosdidier, T. The Influence of Metallurgical State of Substrate on the Efficiency of Plasma Electrolytic Oxidation (PEO) Process on Magnesium Alloy. Mater. Des. 2019, 178, 107859. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Störmer, M.; Dietzel, W. Electrochemical Corrosion Behaviour of Plasma Electrolytic Oxidation Coatings on AM50 Magnesium Alloy Formed in Silicate and Phosphate Based Electrolytes. Electrochim. Acta 2009, 54, 3842–3850. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Monakhova, E.P.; Khabibullina, Z.V.; Serdechnova, M.; Blawert, C.; Zheludkevich, M.L.; Gladkova, A.A. Plasma Electrolytic Oxidation of AZ31 and AZ91 Magnesium Alloys: Comparison of Coatings Formation Mechanism. J. Magnes. Alloy. 2020, 8, 587–600. [Google Scholar] [CrossRef]
- Vladimirov, B.V.; Krit, B.L.; Lyudin, V.B.; Morozova, N.V.; Rossiiskaya, A.D.; Suminov, I.V.; Epel’feld, A.V. Microarc Oxidation of Magnesium Alloys: A Review. Surf. Eng. Appl. Electrochem. 2014, 50, 195–232. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Laleh, M.; Rouhaghdam, A.S.; Shahrabi, T.; Shanghi, A. Effect of Alumina Sol Addition to Micro-Arc Oxidation Electrolyte on the Properties of MAO Coatings Formed on Magnesium Alloy AZ91D. J. Alloys Compd. 2010, 496, 548–552. [Google Scholar] [CrossRef]
- Hadzima, B.; Mhaede, M.; Pastorek, F. Electrochemical Characteristics of Calcium-Phosphatized AZ31 Magnesium Alloy in 0.9 % NaCl Solution. J. Mater. Sci. Mater. Med. 2014, 25, 1227–1237. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg Corrosion and Research Suggestions. J. Magnes. Alloy. 2013, 1, 177–200. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Atrens, A. Recent insights into the mechanism of magnesium corrosion and research suggestions. Adv. Eng. Mater. 2007, 9, 177–183. [Google Scholar] [CrossRef]
- Kiełbus, A.; Jarosz, R.; Gryc, A. Effect of Modification on Microstructure and Properties of AZ91 Magnesium Alloy. Crystals 2020, 10, 536. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.F. Formation of Micro-Arc Oxidation Coatings on AZ91HP Magnesium Alloys. Corros. Sci. 2009, 51, 2820–2825. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G.E. Corrosion Resistance of WE43 and AZ91D Magnesium Alloys with Phosphate PEO Coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Kajánek, D.; Hadzima, B.; Buhagiar, J.; Wasserbauer, J.; Jacková, M. Corrosion Degradation of AZ31 Magnesium Alloy Coated by Plasma Electrolytic Oxidation. Transp. Res. Procedia 2019, 40, 51–58. [Google Scholar] [CrossRef]
- Merino, M.C.; Pardo, A.; Arrabal, R.; Merino, S.; Casajús, P.; Mohedano, M. Influence of Chloride Ion Concentration and Temperature on the Corrosion of Mg-Al Alloys in Salt Fog. Corros. Sci. 2010, 52, 1696–1704. [Google Scholar] [CrossRef]
- Arrabal, R.; Mingo, B.; Pardo, A.; Matykina, E.; Mohedano, M.; Merino, M.C.; Rivas, A.; Maroto, A. Role of Alloyed Nd in the Microstructure and Atmospheric Corrosion of As-Cast Magnesium Alloy AZ91. Corros. Sci. 2015, 97, 38–48. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Kazemi, A.; Molaei, M.; Fattah-alhosseini, A. Multipurpose Surface Modification of PEO Coatings Using Tricalcium Phosphate Addition to Improve the Bedding for Apatite Compounds. J. Alloys Compd. 2021, 877, 160275. [Google Scholar] [CrossRef]
- Nordlien, J.H.; One, S.; Masuko, N.; Nianciow, K. Morphology and Structure of Oxide Films Formed on Magnesium by Exposure to Air and Water. J. Electrochem. Soc. 1995, 142, 1330. [Google Scholar] [CrossRef]
- Singh, I.B.; Singh, M.; Das, S. A Comparative Corrosion Behavior of Mg, AZ31 and AZ91 Alloys in 3.5% NaCl Solution. J. Magnes. Alloy. 2015, 3, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liang, J.; Zhou, J.; Li, Q.; Peng, Z.; Wang, L. Characterization and Corrosion Behavior of Plasma Electrolytic Oxidation Coated AZ91-T6 Magnesium Alloy. Surf. Coat. Technol. 2016, 304, 179–187. [Google Scholar] [CrossRef]
- Khaselev, O.; Weiss, D.; Yahalom, J. Structure and Composition of Anodic® lms Formed on Binary Mg±Al Alloys in KOH±aluminate Solutions under Continuous Sparking. Corros. Sci. 2001, 43, 1295–1307. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Bhattacharyya, U.; Biswas, A.; Manna, I. Studies on Thermal Oxidation of Mg-Alloy (AZ91) for Improving Corrosion and Wear Resistance. Surf. Coat. Technol. 2008, 202, 3638–3642. [Google Scholar] [CrossRef]
- Gunduz, K.O.; Oter, Z.C.; Tarakci, M.; Gencer, Y. Plasma Electrolytic Oxidation of Binary Mg-Al and Mg-Zn Alloys. Surf. Coat. Technol. 2017, 323, 72–81. [Google Scholar] [CrossRef]
- Kerner, Z.; Pajkossy, T. Impedance of Rough Capacitive Electrodes: The Role of Surface Disorder. J. Electroanal. Chem. 1998, 448, 139–142. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Influence of Chloride Ion Concentration on the Electrochemical Corrosion Behaviour of Plasma Electrolytic Oxidation Coated AM50 Magnesium Alloy. Electrochim. Acta 2010, 55, 6802–6811. [Google Scholar] [CrossRef] [Green Version]
- Ruhi, G.; Modi, O.P.; Singh, I.B. Corrosion Behaviour of Nano Structured Sol-Gel Alumina Coated 9Cr-1Mo Ferritic Steel in Chloride Bearing Environments. Surf. Coat. Technol. 2009, 204, 359–365. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An Investigation of Ceramic Coating Growth Mechanisms in Plasma Electrolytic Oxidation (PEO) Processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Olivier, M.-G.; Poelman, M. Use of Electrochemical Impedance Spectroscopy (EIS) for the Evaluation of Electrocoatings Performances; Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Rahman, S.U.; Atta Ogwu, A. Corrosion and Mott-Schottky Probe of Chromium Nitride Coatings Exposed to Saline Solution for Engineering and Biomedical Applications. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 239–265. [Google Scholar]
- M.Rúa, J.; Zuleta, A.A.; Ramírez, J.; Fernández-Morales, P. Micro-Arc Oxidation Coating on Porous Magnesium Foam and Its Potential Biomedical Applications. Surf. Coat. Technol. 2019, 360, 213–221. [Google Scholar] [CrossRef]

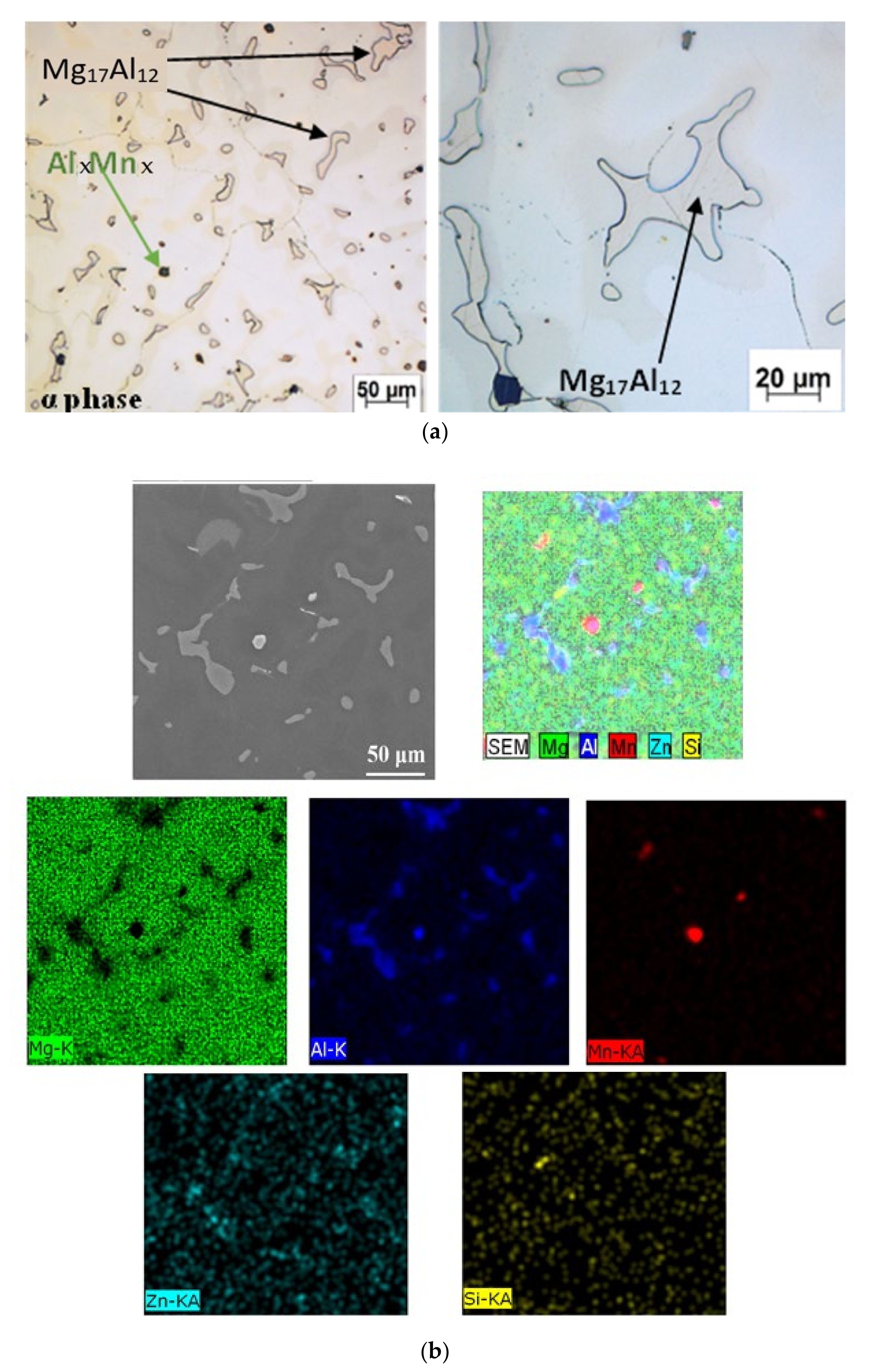
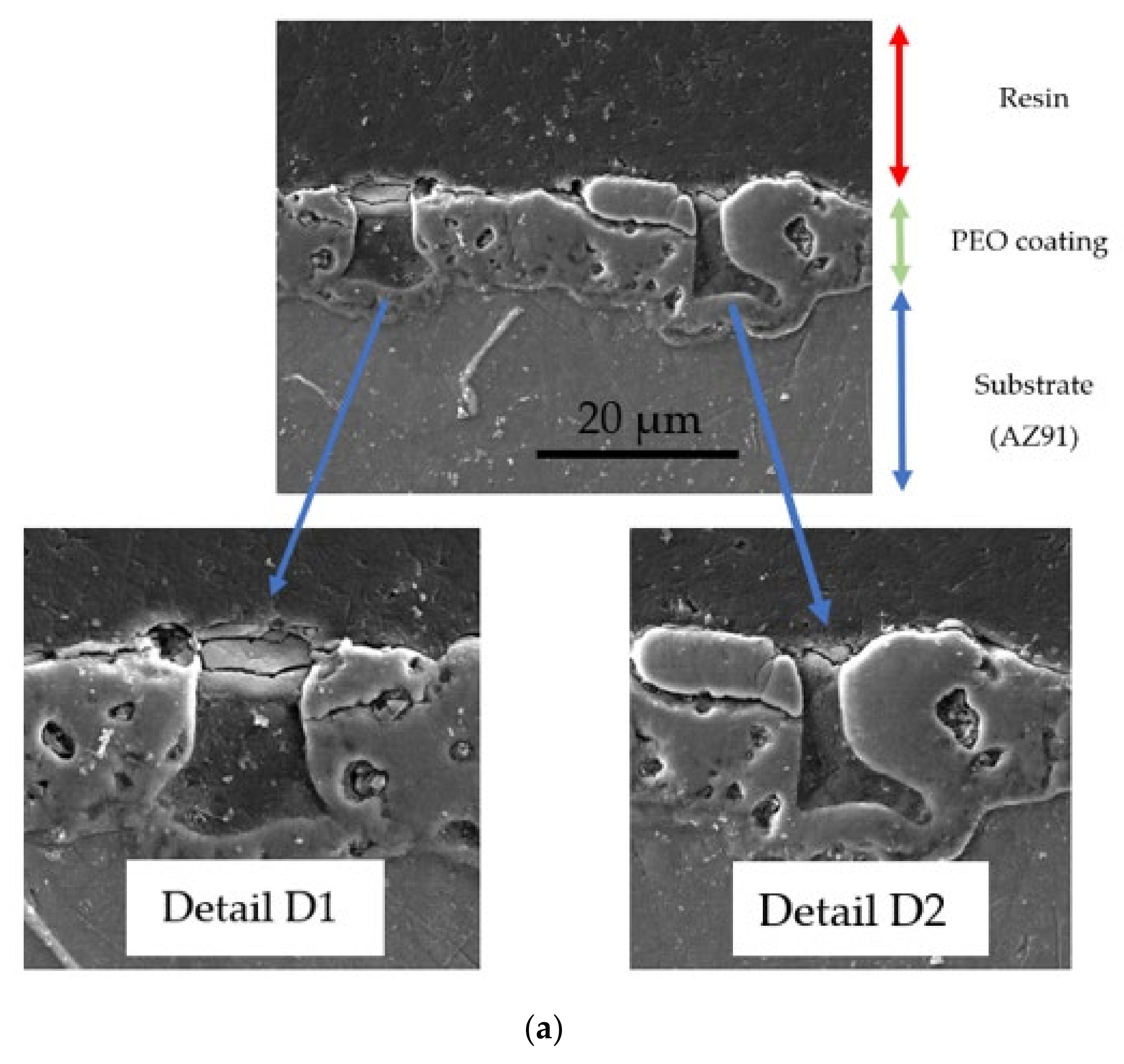
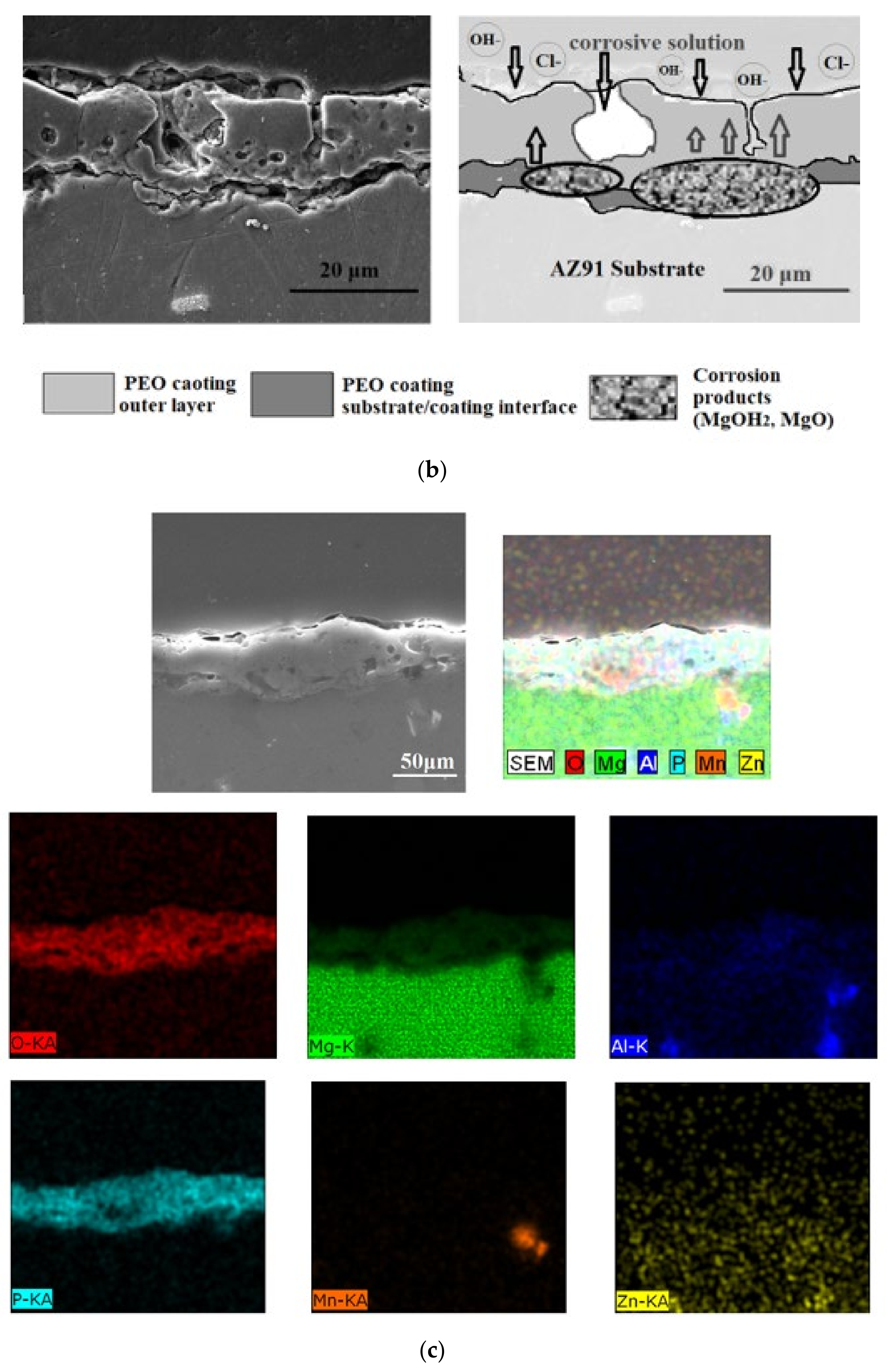
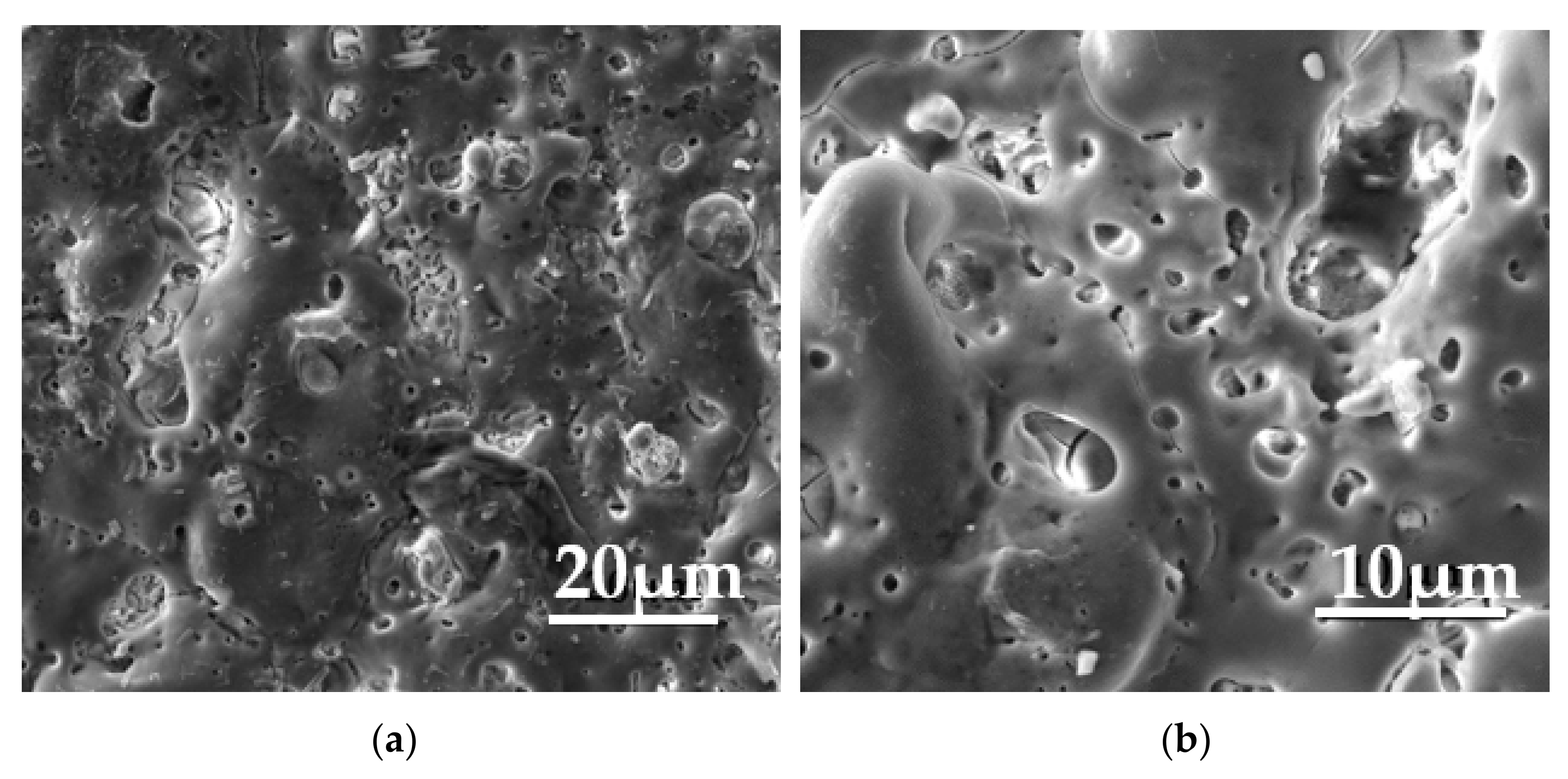
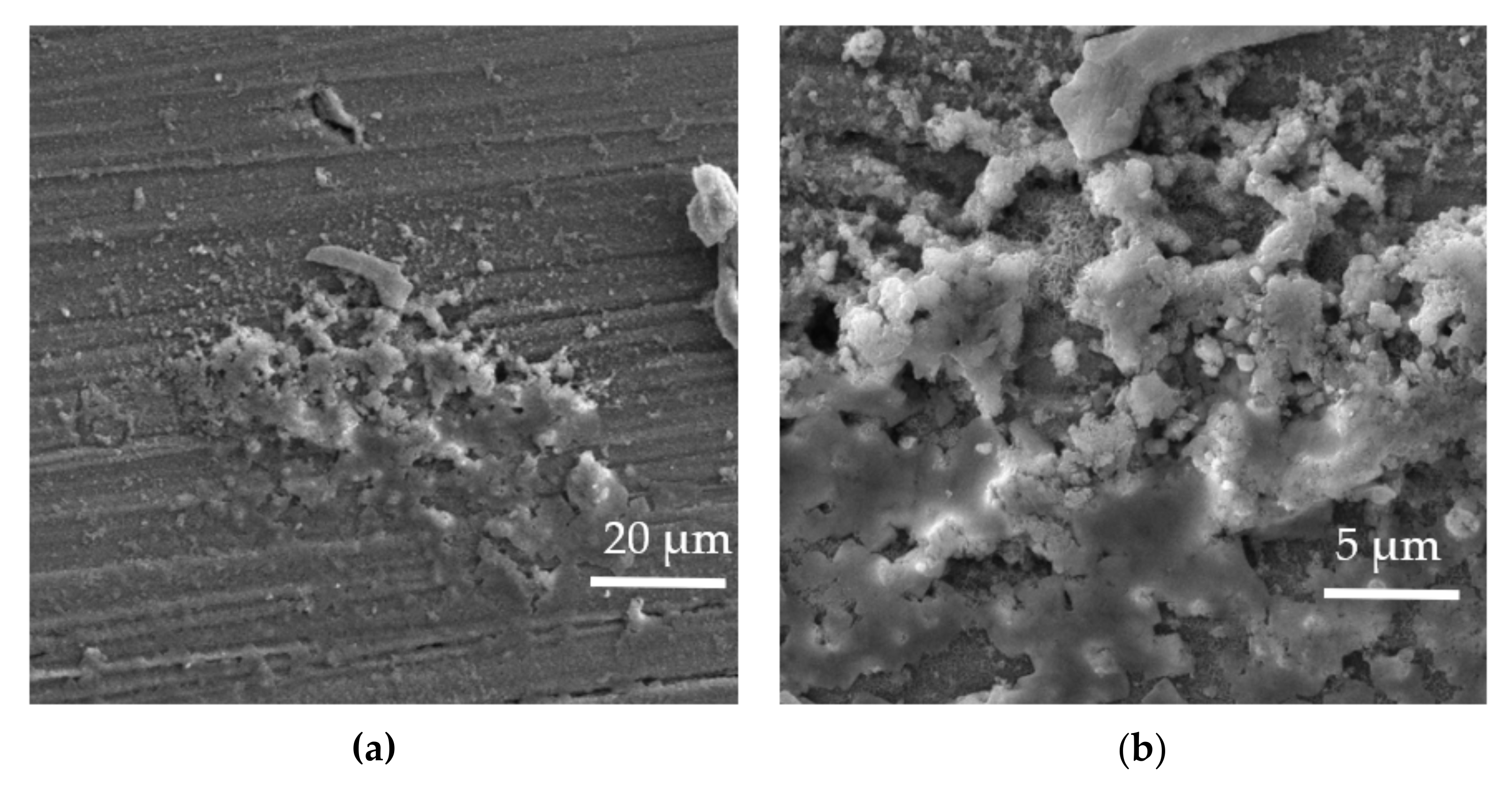

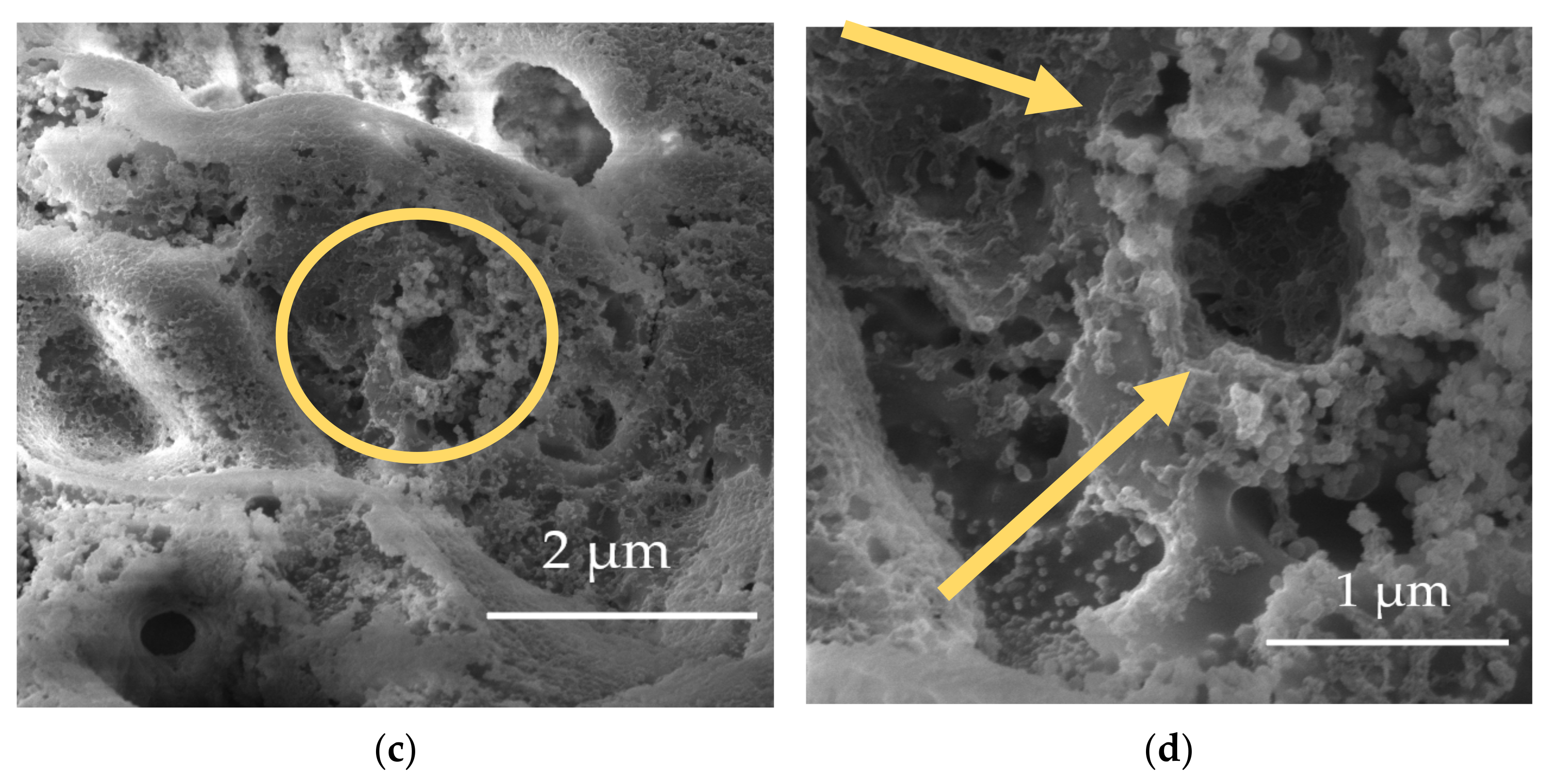
| Element | Al | Zn | Mn | Si | Ca | Fe | Mg |
| wt.% | 8.77 | 0.68 | 0.218 | 0.168 | 0.001 | 0.005 | 90.20 |
| Surface | Coating Thickness (μm) | Roughness (μm) | ||
|---|---|---|---|---|
| PEO | 16 ± 2 | 1.27 ± 0.6 | 4.92 ± 1.8 | −0.63 ± 0.4 |
| As-cast | 0.165 ± 0.1 | 1.176 ± 0.1 | −1.018 ± 0.1 | |
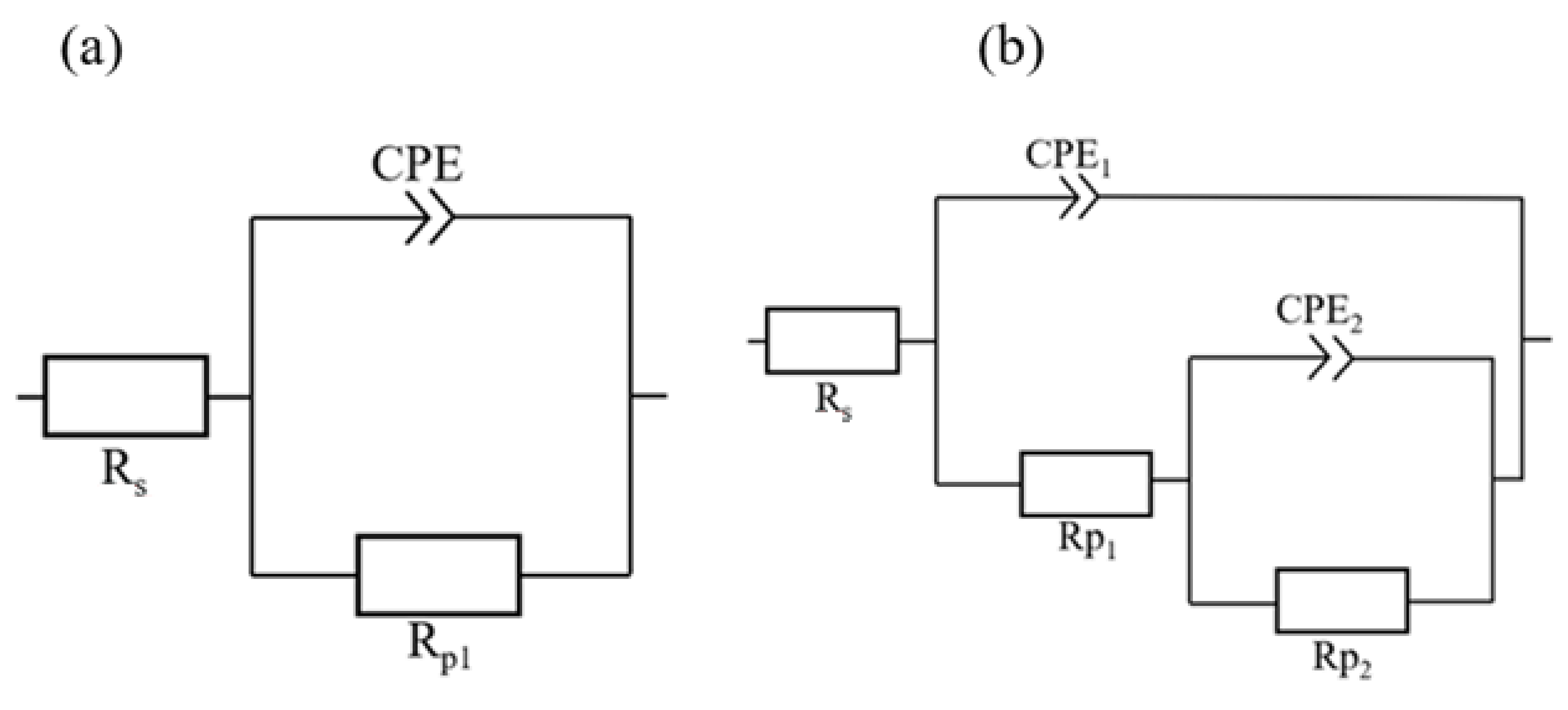
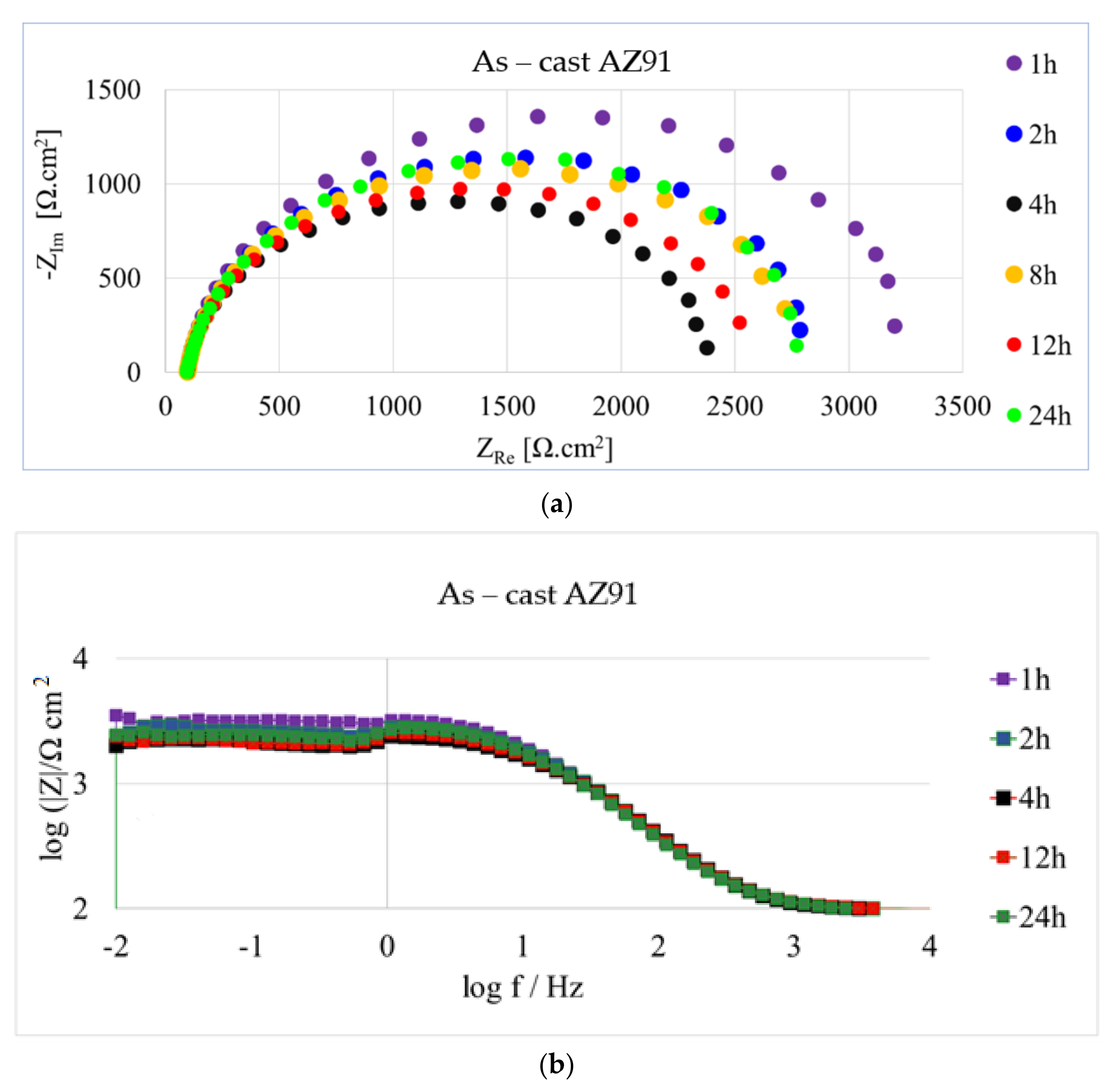
| Time | Rs (Ω·cm2) | Rp1 (Ω·cm2) | Rp (Ω·cm2) | CPE1 (F·sn−1·10−6) | n1 |
|---|---|---|---|---|---|
| 1 h | 100 | 3180 | 3280 | 8.05 | 0.9 |
| 2 h | 100 | 2710 | 2710 | 8.10 | 0.9 |
| 4 h | 95 | 2240 | 2240 | 8.65 | 0.9 |
| 12 h | 95 | 2410 | 2410 | 8.95 | 0.89 |
| 24 h | 95 | 2730 | 2730 | 9.50 | 0.89 |
| Time | Rs (Ω·cm2) | Rp1 (Ω·cm2) | Rp2 (Ω·cm2) | Rp (Ω·cm2) | CPE1 (F·sn−1·10−6) | CPE2 (F·sn−1·10−6) | n1 | n2 |
|---|---|---|---|---|---|---|---|---|
| 1 h | 95 | 26,130 | 403,840 | 429,970 | 0.80 | 3.90 | 0.7 | 0.7 |
| 2 h | 90 | 18,250 | 330,450 | 348,700 | 0.68 | 6.60 | 0.7 | 0.6 |
| 4 h | 90 | 4435 | 257,850 | 262,285 | 0.87 | 5.70 | 0.6 | 0.9 |
| 12 h | 100 | 1855 | 189,950 | 191,805 | 0.68 | 5.70 | 0.9 | 0.8 |
| 24 h | 95 | 2170 | 205,345 | 207,515 | 8.05 | 4.30 | 0.8 | 0.6 |
| Surface | Ecorr (mV) | Epitt (mV) | icorr (µA·cm−2) | βa (mV/dec.) | βc (mV/dec.) | rcorr (mm·year−1·10−3) |
|---|---|---|---|---|---|---|
| As-cast | −1465 | −1410 | 3.86 | 70 | 150 | 87.5 |
| PEO | −1625 | −1445 | 0.14 | 240 | 165 | 3.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štrbák, M.; Kajánek, D.; Knap, V.; Florková, Z.; Pastorková, J.; Hadzima, B.; Goraus, M. Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment. Coatings 2022, 12, 566. https://doi.org/10.3390/coatings12050566
Štrbák M, Kajánek D, Knap V, Florková Z, Pastorková J, Hadzima B, Goraus M. Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment. Coatings. 2022; 12(5):566. https://doi.org/10.3390/coatings12050566
Chicago/Turabian StyleŠtrbák, Milan, Daniel Kajánek, Vidžaja Knap, Zuzana Florková, Jana Pastorková, Branislav Hadzima, and Matej Goraus. 2022. "Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment" Coatings 12, no. 5: 566. https://doi.org/10.3390/coatings12050566
APA StyleŠtrbák, M., Kajánek, D., Knap, V., Florková, Z., Pastorková, J., Hadzima, B., & Goraus, M. (2022). Effect of Plasma Electrolytic Oxidation on the Short-Term Corrosion Behaviour of AZ91 Magnesium Alloy in Aggressive Chloride Environment. Coatings, 12(5), 566. https://doi.org/10.3390/coatings12050566






