Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Prepacyclen
2.3. Surface Characterization
3. Results and Discussion
3.1. Orthogonal Test
3.2. Effect of Electrical Parameters
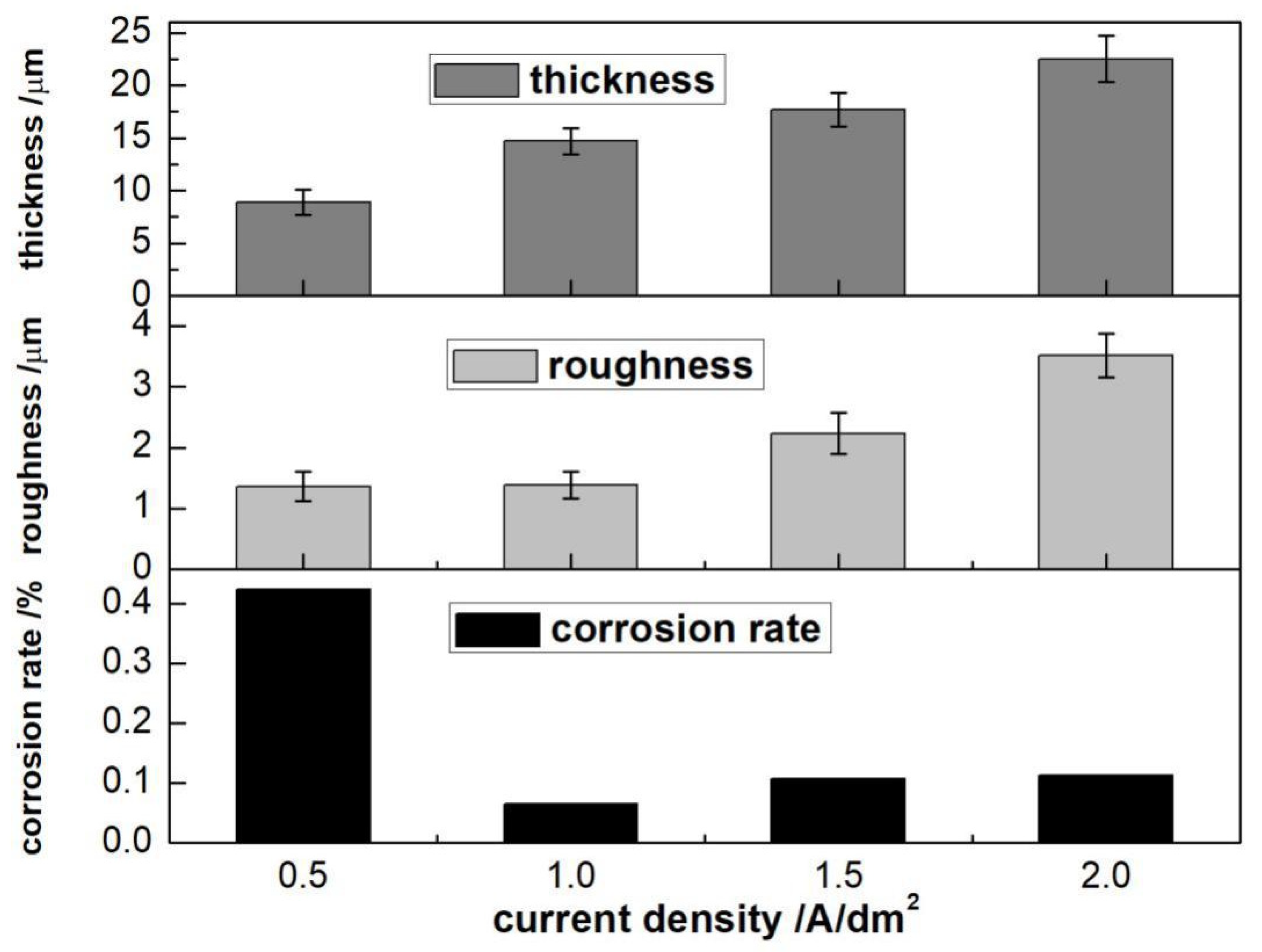
3.2.1. Current Density
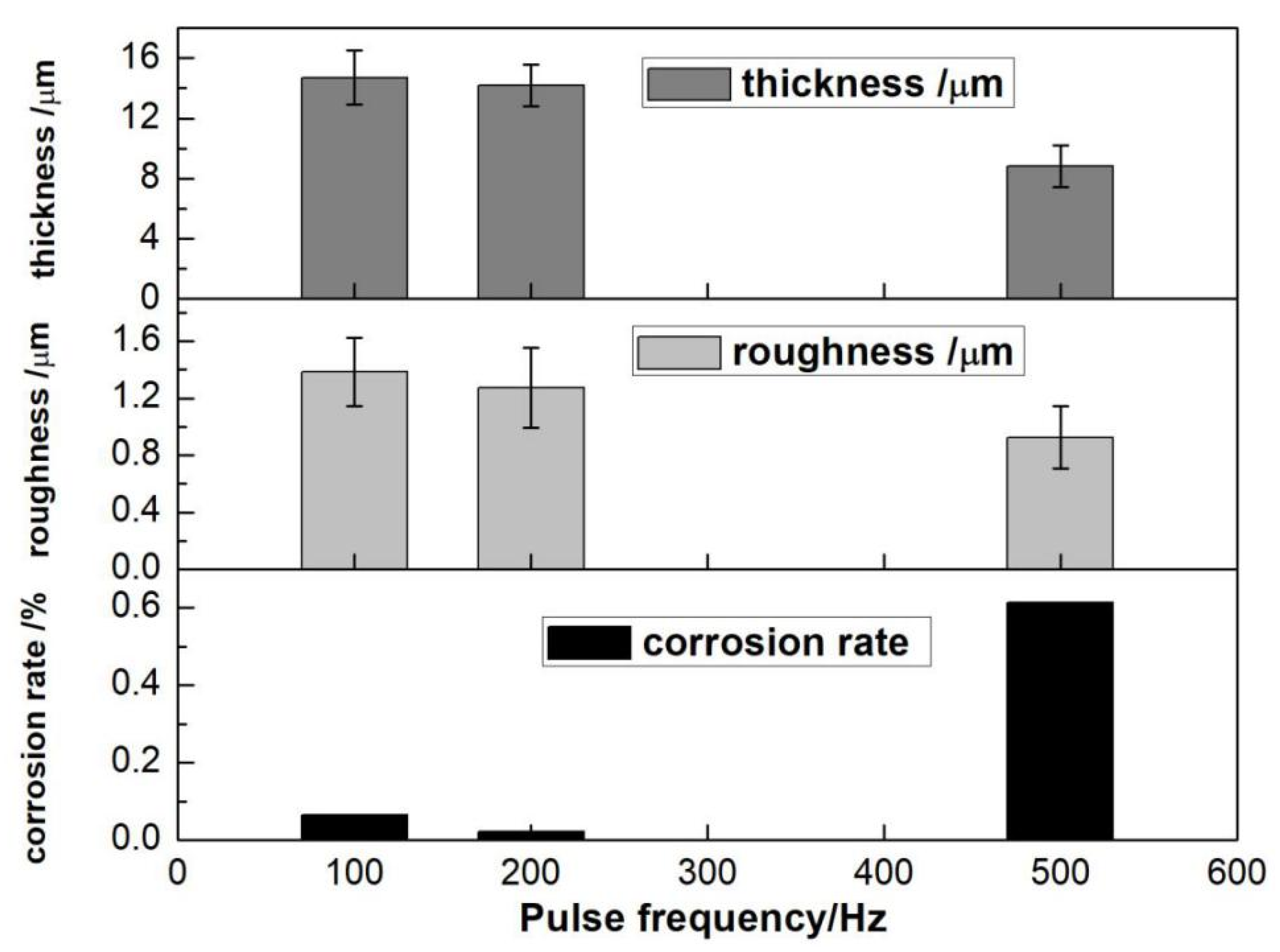
3.2.2. Pulse Frequency
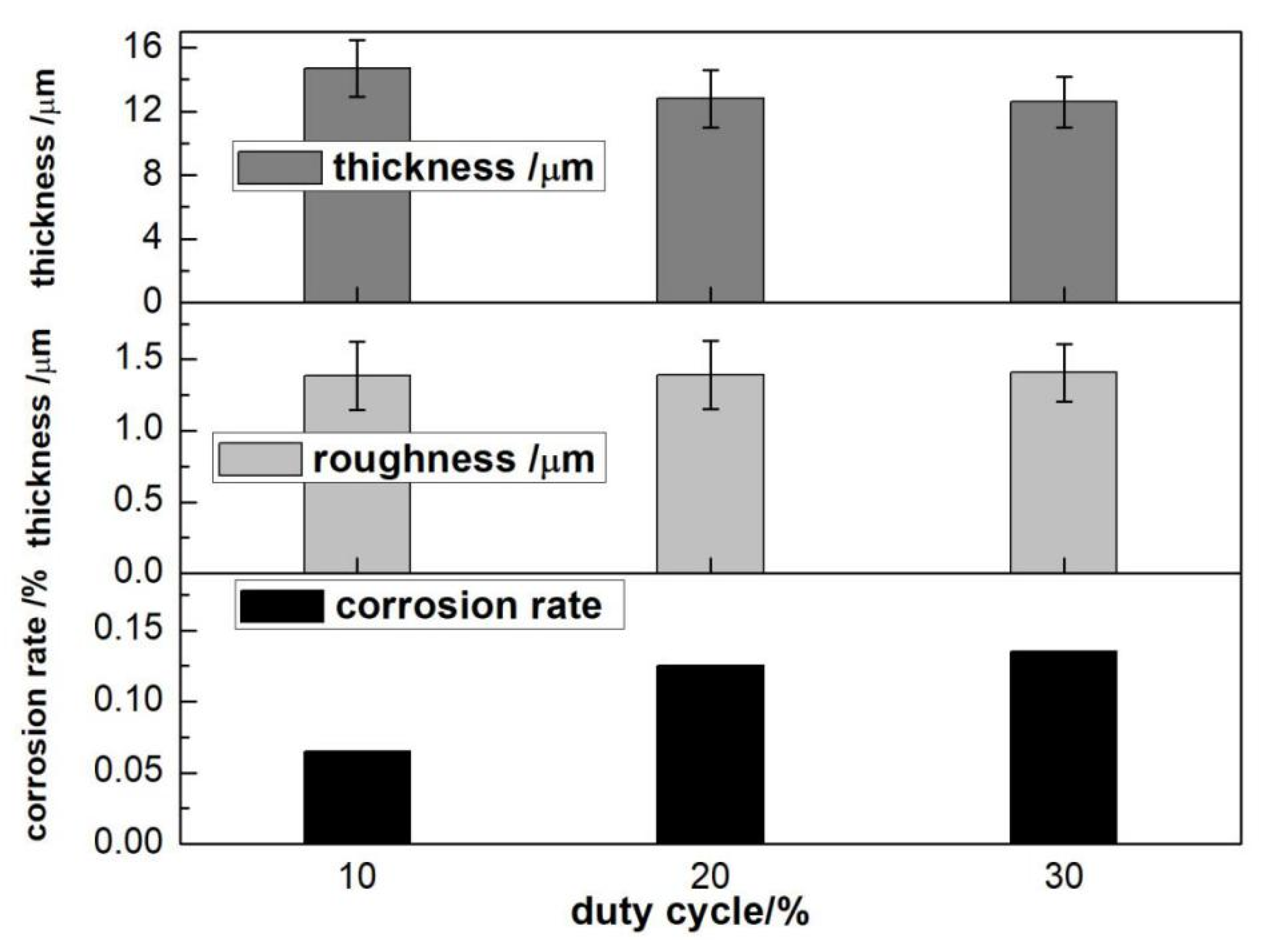
3.2.3. Duty Cycle
3.2.4. Oxidation Time
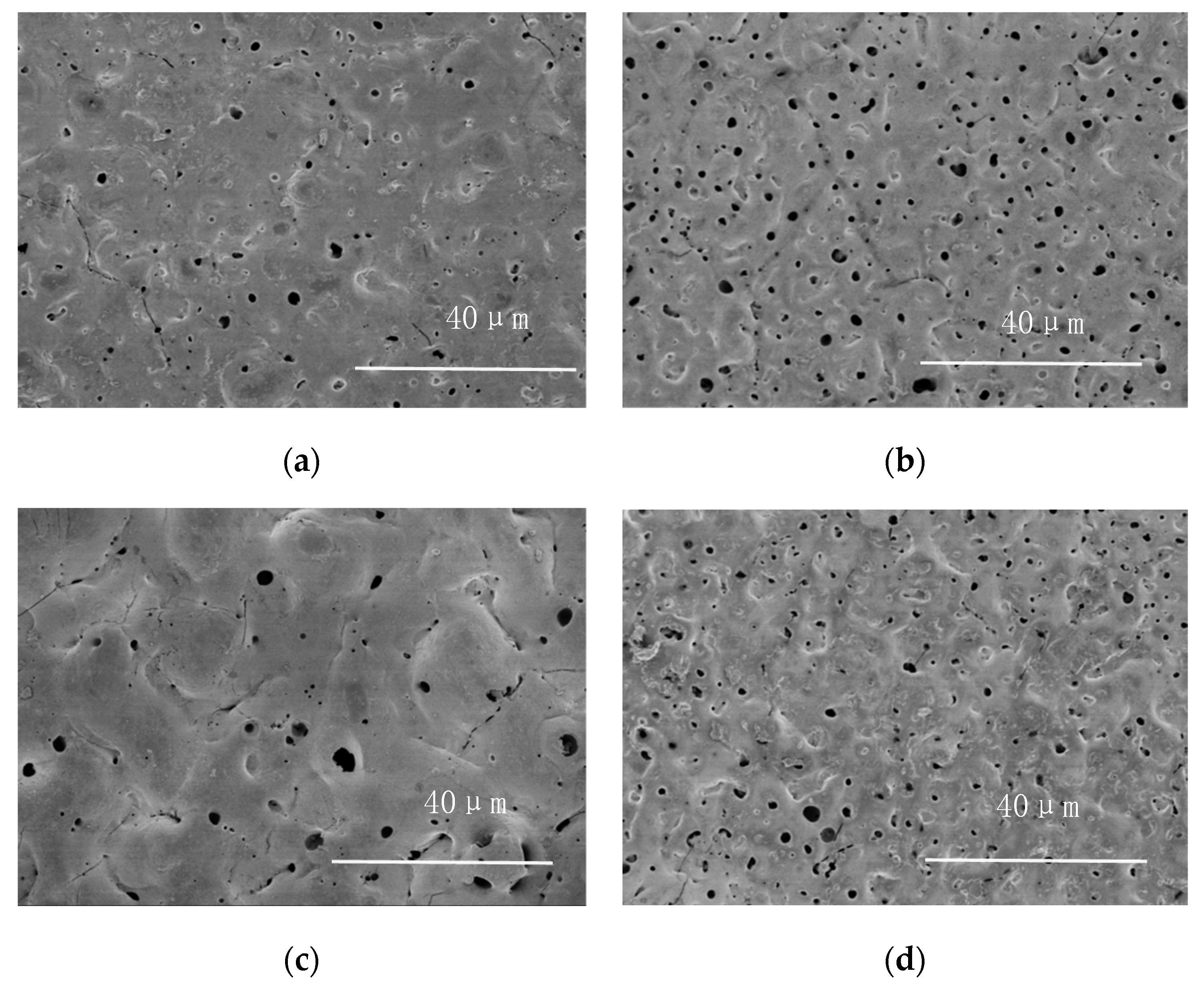
3.3. Morphology and Phase Analysis
3.4. Electrochemical Performance
4. Conclusions
- The four-factor and three-level orthogonal experiments were applied. It was observed that the coating thickness was significantly affected by NaOH, while Na2SiO3 had the greatest impact on corrosion resistance of the oxide coating. The orthogonal tests showed that the optimal formulation of the electrolyte was NaOH 45 g/L, Na2SiO3 50 g/L and Na2B4O7 90 g/L.
- The optimal electrical parameter process was represented by current density 1 A/dm2, oxidation time 15 min, pulse frequency 200 Hz, and duty cycle 10%.
- The surface of the PEO coating obtained by the optimal condition is uniform and compact. The XRD results revealed that the phase compositions of PEO coatings were almost the same including MgO and Mg2SiO4.
- Potentiodynamic polarization behavior showed that the corrosion resistance of Mg alloy was greatly improved by PEO, and the electrochemical performances of the PEO coatings were the same as that of the orthogonal tests.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamil, M.; Kaseem, M.; Lee, Y.; Ko, Y. Microstructural characteristics of oxide layer formed by plasma electrolytic oxidation: Nanocrystalline and amorphous structures. J. Alloys Compd. 2017, 707, 167–171. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, P.B.; Knorchild, G. Anodization of Mg-alloy AZ91 in NaOH solutions. Surf. Coat. Technol. 2009, 203, 1629–1636. [Google Scholar] [CrossRef]
- Gray, X.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Li, R.; Feng, X.; Pang, X.; Rao, J.; Cong, D.; Yin, C.; Zhang, Y. Active corrosion protection of Mg–Al layered double hydroxide for magnesium alloys: A Short Review. Coatings 2021, 11, 1316. [Google Scholar] [CrossRef]
- Dziková, J.; Fintová, S.; Kajánek, D.; Florková, Z.; Wasserbauer, J.; Doležal, P. Characterization and Corrosion Properties of Fluoride Conversion Coating Prepared on AZ31 Magnesium Alloy. Coatings 2021, 11, 675. [Google Scholar] [CrossRef]
- Baloch, A.; Kannan, M.B. Electropolymerisation of aniline on AZ91 magnesium alloy: The effect of coating electrolyte corrosiveness. Metals 2017, 7, 533. [Google Scholar] [CrossRef] [Green Version]
- Song, G. An irreversible dipping sealing technique for anodized ZE41 Mg alloy. Surf. Coat. Technol. 2009, 203, 3618–3625. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Li, H.; Lu, Y.; Yu, H.; Chen, C. Enhanced corrosion resistance of magnesium alloy by plasma electrolytic oxidation plus hydrothermal treatment. Surf. Coat. Technol. 2022, 424, 127662. [Google Scholar] [CrossRef]
- Evangelides, H.A. Method of Electrolytically Coating Magnesium and Electrolyte Therefore. U.S. Patent 2723952, 15 November 1955. [Google Scholar]
- Dow Chemical Co. Bath for and Method of Producing a Corrosion Resistant Coating upon Light Metals. GB Patent 762195, 28 November 1956. [Google Scholar]
- Shi, Z.; Song, G.; Atrens, A. Influence of anodising current on the corrosion resistance of anodised AZ91D magnesium alloy. Corros. Sci. 2006, 48, 1939–1959. [Google Scholar] [CrossRef]
- Wu, X.; Su, P.; Jiang, Z.; Meng, S. Influences of current density on tribological characteristics of ceramic coatings on ZK60 Mg alloy by plasma electrolytic oxidation. ACS Appl. Mater. Interfaces 2010, 3, 808–812. [Google Scholar]
- Rapheal, G.; Kumar, S.; Scharnagl, N.; Blawert, C. Effect of current density on the microstructure and corrosion properties of plasma electrolytic oxidation (PEO) coatings on AM50 Mg alloy produced in an electrolyte containing clay additives. Surf. Coat. Technol. 2016, 289, 150–164. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, W.; Chen, J.; Lu, S. Characterization of ceramic coating on ZK60 magnesium alloyprepared in a dual electrolyte system by micro-arc oxidation. Rare. Met. 2013, 32, 459–464. [Google Scholar] [CrossRef]
- Choi, Y.; Salman, S.; Kuroda, K.; Okido, M. Improvement in corrosion characteristics of AZ31 Mg alloy by square pulse anodizing between transpassive and active regions. Corros. Sci. 2012, 63, 5–11. [Google Scholar] [CrossRef]
- Keyvani, A.; Zamani, M.; Bahamirian, M.; Nikoomanzari, E.; Fattah-alhosseini, A.; Sinad, H. Role of incorporation of ZnO nanoparticles on corrosion behavior of ceramic coatings developed on AZ31 magnesium alloy by plasma electrolytic oxidation technique. Surf. Interfaces 2021, 22, 100728. [Google Scholar] [CrossRef]
- Guo, Y.; Rogov, A.; Hird, A.; Mingo, B.; Matthews, A.; Yerokhin, A. Plasma electrolytic oxidation of magnesium by sawtooth pulse current. Surf. Coat. Technol. 2022, 429, 127938. [Google Scholar] [CrossRef]
- Baghdadabad, D.; Baghdadabad, A.; Khoei, S. Characterization of bioactive ceramic coatings synthesized by plasma electrolyte oxidation on AZ31 magnesium alloy having different Na2SiO3·9H2O concentrations. Mater. Today Commun. 2020, 25, 101642. [Google Scholar] [CrossRef]
- Ballam, L.R.; Arab, H.; Bestetti, M.; Franz, S.; Masi, G.; Sola, R.; Donati, L.; Martini, C. Improving the corrosion resistance of wrought ZM21 magnesium alloys by plasma electrolytic oxidation and powder coating. Materials 2021, 14, 2268. [Google Scholar] [CrossRef]
- Hwang, D.; Kim, Y.; Park, D.; Yoo, B.; Shin, D. Corrosion resistance of oxide layers formed on AZ91 Mg alloy in KMnO4 electrolyte by plasma electrolytic oxidation. Electrochim. Acta 2009, 54, 5479–5485. [Google Scholar] [CrossRef]
- Barchiche, C.; Rocca, E.; Juers, C.; Hazan, J.; Steinmetz, J. Corrosion resistance of plasma-anodized AZ91D magnesium alloy by electrochemical methods. Electrochim. Acta 2007, 53, 417–425. [Google Scholar] [CrossRef]
- Barchiche, C.; Veys-Renaux, D.; Rocca, E. A better understanding of PEO on Mg alloys by using a simple galvanostatic electrical regime in a KOH-KF-Na3PO4 electrolyte. Surf. Coat. Technol. 2011, 205, 4243–4248. [Google Scholar] [CrossRef]
- Lamaka, S.; Knorchild, G.; Snihirova, D.; Taryba, M.; Zheludkevich, M.; Ferreira, M. Complex anticorrosion coating for ZK30 magnesium alloy. Electrochim. Acta 2009, 55, 131–141. [Google Scholar] [CrossRef]
- Say, W.; Chen, C.; Hsieh, S. Electrochemical characterization of non-chromate surface treatments on AZ80 magnesium. Mater. Charact. 2008, 59, 1400–1406. [Google Scholar] [CrossRef]
- Yoo, B.; Shin, K.; Hwang, D.; Dong, H.; Dong, H. Effect of surface roughness on leakage current and corrosion resistance of oxide layer on AZ91 Mg alloy prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2010, 256, 6667–6672. [Google Scholar] [CrossRef]
- Guo, H.; An, M.; Huo, H.; Xu, S.; Wu, L. Microstructure characteristic of ceramic coatings fabricated on magnesium alloys by micro-arc oxidation in alkaline silicate solutions. Appl. Surf. Sci. 2006, 252, 7911–7916. [Google Scholar] [CrossRef]
- Hsiao, H.; Tsung, H.; Tsai, W. Anodization of AZ91D magnesium alloy in silicate-containing electrolytes. Surf. Coat. Technol. 2005, 199, 127–134. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Li, L.; Chen, Z.; Wang, H.; Zhang, Z. The anodization of ZK60 magnesium alloy in alkaline solution containing silicate and the corrosion properties of the anodized films. Appl. Surf. Sci. 2007, 253, 9387–9394. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, Y.; Jing, X. Comparison of plasma electrolytic oxidation coatings on Mg-Li alloy formed in molybdate/silicate and aluminates/silicate composite electrolytes. Mater. Corros. 2014, 65, 493–501. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Skeldon, P. Incorpocyclen of zirconia particles into coatings formed on magnesium by plasma electrolytic oxidation. J. Mater. Sci. 2008, 43, 1532–1538. [Google Scholar] [CrossRef]
- Lee, K.; Shin, K.; Namgung, S.; Yoo, B.; Shin, D. Electrochemical response of ZrO2-incorporated oxide layer on AZ91 Mg alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2011, 205, 3779–3784. [Google Scholar] [CrossRef]
- Mashtalyar, D.; Gnedenkov, S.; Sinebryukhov, S.; Imshinetskiy, I.; Puz, A. Plasma electrolytic oxidation of the magnesium alloy MA8 in electrolytes containing TiN nanoparticles. J. Mater. Sci. Technol. 2017, 33, 461–468. [Google Scholar] [CrossRef]
- Tu, X.; Miao, C.; Zhang, Y.; Xu, Y.; Li, J. Plasma electrolytic oxidation of magnesium alloy AZ31B in electrolyte containing Al2O3 sol as additives. Materials 2018, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, X.; Chen, L.; Wu, J. Effect of glucose on properties of anodizing film on AZ31B magnesium alloy. Chin J. Nonferr. Met. 2013, 23, 727–734. [Google Scholar]
- Fukuda, H.; Matsumoto, Y. Effects of Na2SiO3 on anodization of Mg-Al-Zn alloy in 3 M KOH solution. Corros. Sci. 2004, 46, 2135–2142. [Google Scholar] [CrossRef]
- Chai, L.; Yu, X.; Yang, Z.; Wang, Y.; Okido, M. Anodizing of magnesium alloy AZ31 in alkaline solutions with silicate under continuous sparking. Corros. Sci. 2008, 50, 3274–3279. [Google Scholar] [CrossRef]
- Qian, J.; Li, D.; Wang, X.; Guo, B. Effects of concentcyclen of sodium borate on anodizing for magnesium alloy. J. Chin. Soc. Corros. Prot. 2005, 25, 275–279. [Google Scholar]
- Gao, X.; Zhang, Y.; Zhang, H.; Wu, Q. Effects of machine tool configucyclen on its dynamics based on orthogonal experiment method. Chin. J. Aeronaut. 2012, 25, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; An, M.; Yang, P.; Su, C. Effects of benzotriazole on anodized film formed on AZ31B magnesium alloy in environmental-friendly electrolyte. J. Alloys Compd. 2009, 482, 487–497. [Google Scholar] [CrossRef]
- Zou, B.; Lv, G.; Zhang, G.; Tian, Y. Effect of current frequency on properties of coating formed by microarc oxidation on AZ91D magnesium alloy. Trans. Nonferr. Met. Soc. China 2015, 25, 1500–1505. [Google Scholar] [CrossRef]





| Levels | Factors | ||
|---|---|---|---|
| A NaOH/g/L | B Na2SiO3/g/L | C Na2B4O7/g/L | |
| 1 | 30 | 30 | 50 |
| 2 | 45 | 50 | 70 |
| 3 | 60 | 70 | 90 |
| Current Density/A·dm−2 | Oxidation Time/min | Pulse Frequency/Hz | Duty Cycle/% |
|---|---|---|---|
| 0.5 | 5 | 100 | 10 |
| 1.0 | 10 | 200 | 20 |
| 1.5 | 15 | 500 | 30 |
| 2.0 | 20 | -- | -- |
| Evaluation Index | H1 | H2 | H3 | h1 | h2 | h3 | R | Influence Degree | Optimal Formulation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness/μm | A | 48.2 | 44.1 | 33.5 | 16.1 | 14.7 | 11.2 | 4.9 | A > C > B | A1B2C2 |
| B | 41.4 | 43.3 | 41.1 | 13.8 | 14.4 | 13.7 | 0.7 | |||
| C | 36.9 | 44.7 | 44.2 | 12.3 | 14.9 | 14.7 | 2.6 | |||
| Evaluation Index | H1 | H2 | H3 | h1 | h2 | h3 | R | Influence Degree | Optimal Formulation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Corrosion rate | A | 0.89 | 0.67 | 1.41 | 0.30 | 0.22 | 0.47 | 0.25 | B> C > A | A2B2C3 |
| B | 1.38 | 0.57 | 1.02 | 0.46 | 0.19 | 0.34 | 0.27 | |||
| C | 1.43 | 0.88 | 0.66 | 0.48 | 0.29 | 0.22 | 0.26 | |||
| Samples | Ecorr (V) | jcorr (A/cm2) | βa (V/Decade) | βc (V/Decade) | Rp (Ω·cm2) |
|---|---|---|---|---|---|
| Bare Mg alloy substrate | −1.55 | 2.88 × 10−5 | 0.123 | 0.112 | 883 |
| PEO coating of optimal condition | −1.44 | 1.98 × 10−7 | 0.184 | 0.175 | 196,699 |
| PEO coating of test 5 | −1.45 | 2.46 × 10−7 | 0.189 | 0.225 | 181,307 |
| PEO coating of test 4 | −1.48 | 1.91 × 10−6 | 0.126 | 0.046 | 7161 |
| PEO coating of test 3 | −1.50 | 7.57 × 10−7 | 0.133 | 0.041 | 17,976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Tu, X.; Yu, J.; Zhang, Y.; Miao, C.; Xu, Y.; Fu, R.; Li, J. Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte. Coatings 2022, 12, 578. https://doi.org/10.3390/coatings12050578
Pan S, Tu X, Yu J, Zhang Y, Miao C, Xu Y, Fu R, Li J. Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte. Coatings. 2022; 12(5):578. https://doi.org/10.3390/coatings12050578
Chicago/Turabian StylePan, Su, Xiaohua Tu, Jianxing Yu, Yang Zhang, Chengping Miao, Yaling Xu, Rui Fu, and Jiayou Li. 2022. "Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte" Coatings 12, no. 5: 578. https://doi.org/10.3390/coatings12050578
APA StylePan, S., Tu, X., Yu, J., Zhang, Y., Miao, C., Xu, Y., Fu, R., & Li, J. (2022). Optimization of AZ31B Magnesium Alloy Anodizing Process in NaOH-Na2SiO3-Na2B4O7 Environmental-Friendly Electrolyte. Coatings, 12(5), 578. https://doi.org/10.3390/coatings12050578






