Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nylon 66 Sample Preparation
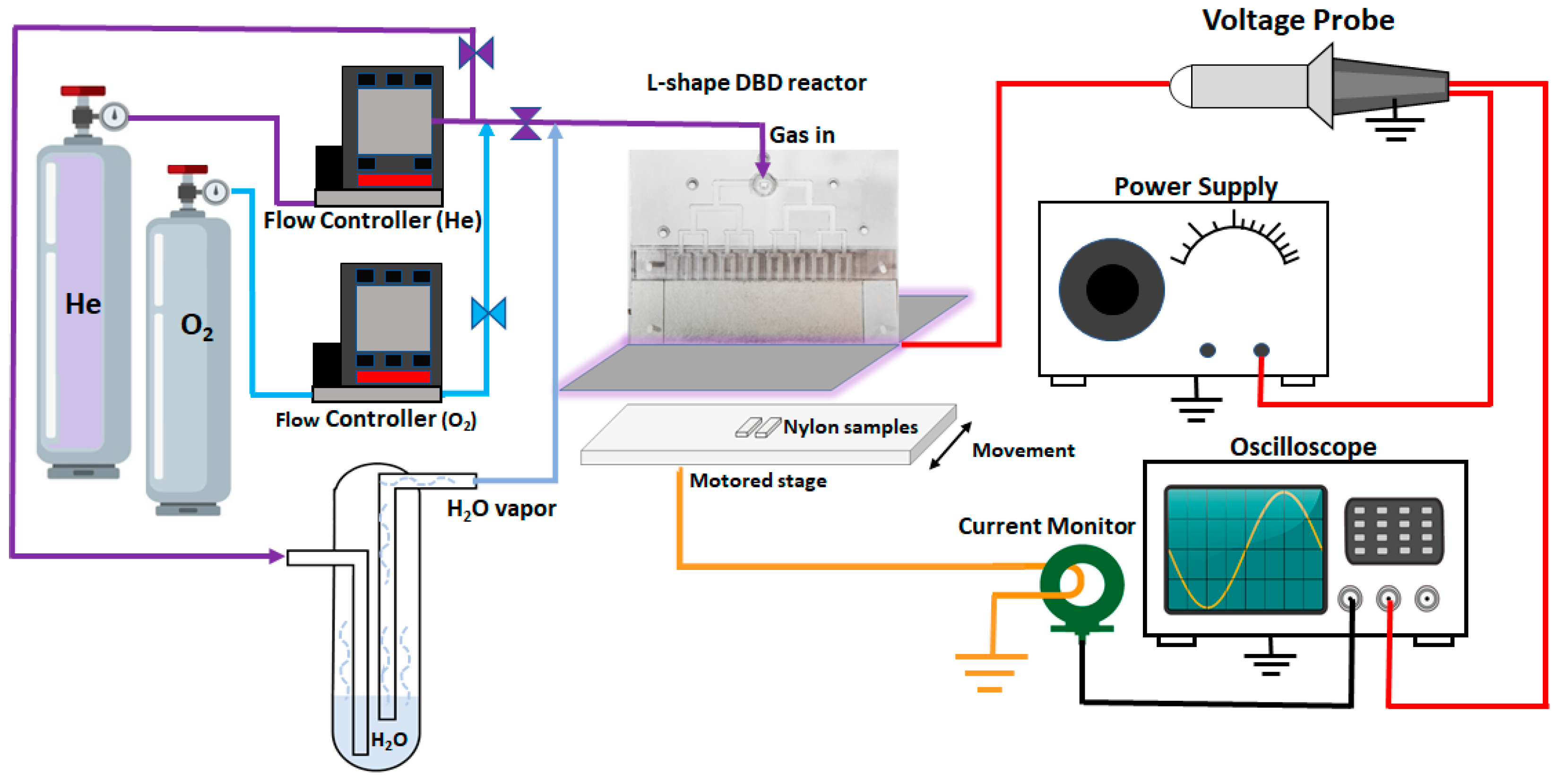
2.2. Plasma Setup and Surface Treatments
2.3. Characterization of Surface Functionality before and after Plasma Treatments
2.4. Silane Treatment
2.5. Adhesive Preparation
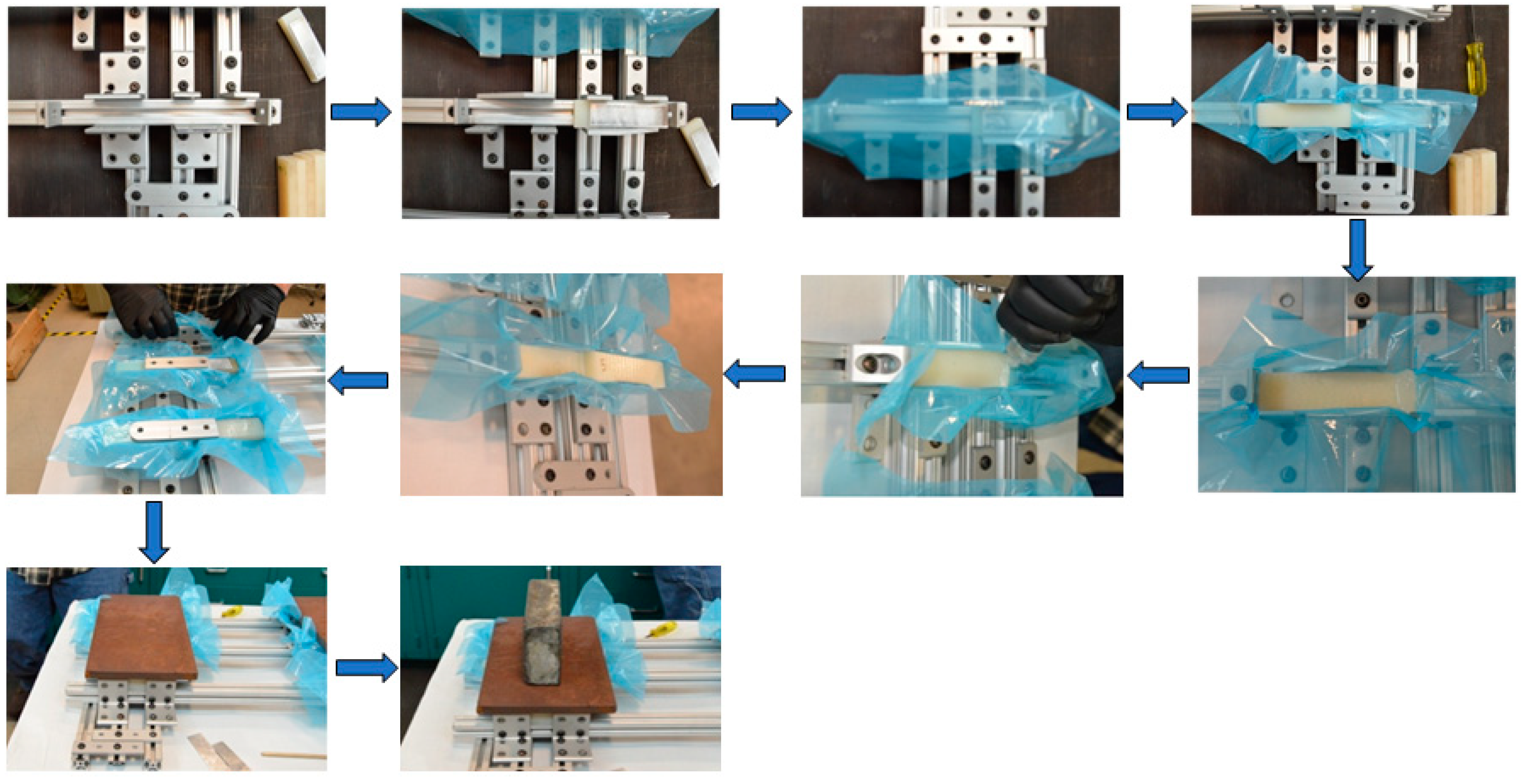
2.6. Lap Shear Assembly Procedure
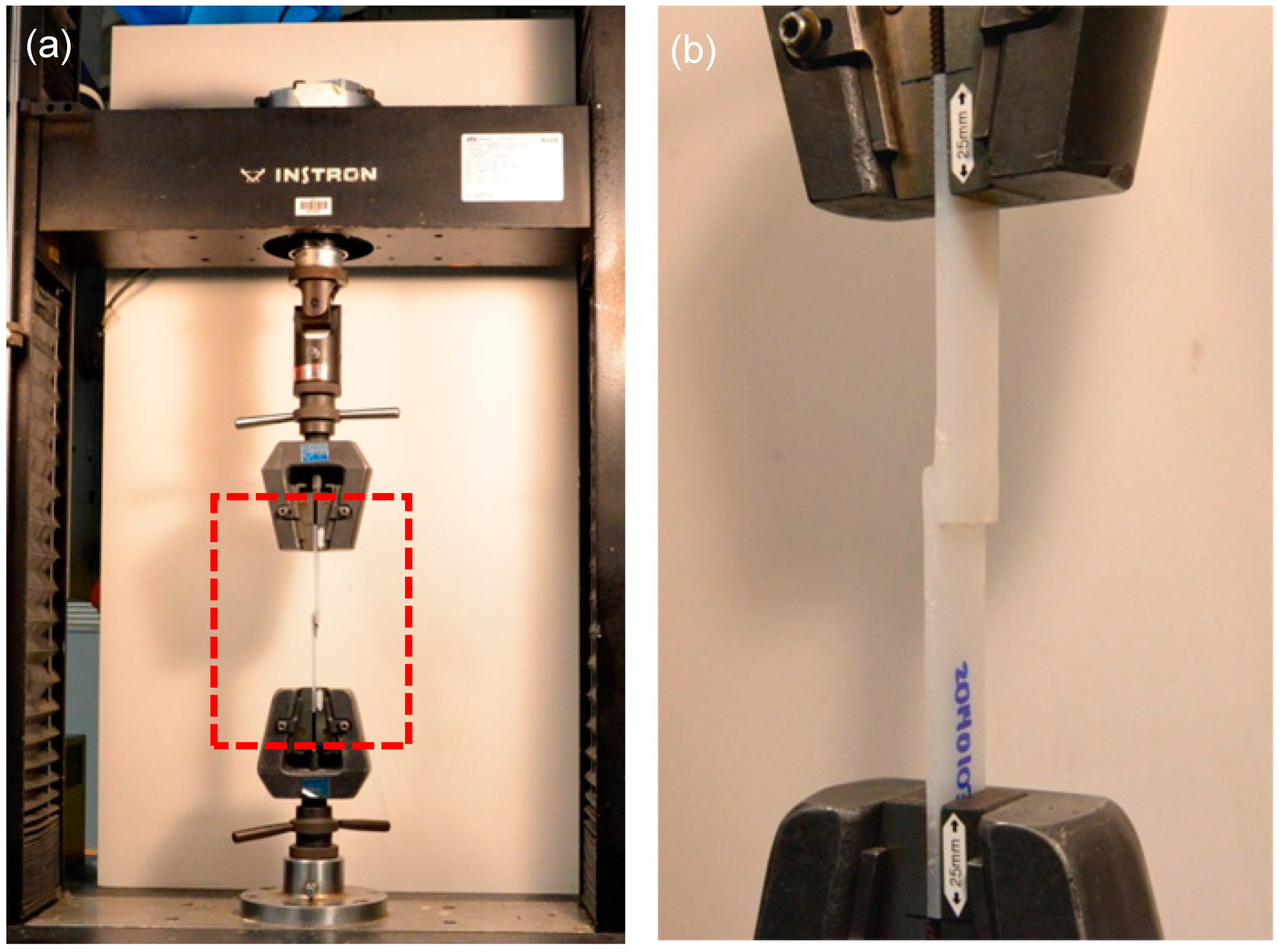
2.7. Uniaxial Tensile Test with an Instron
3. Results and Discussion
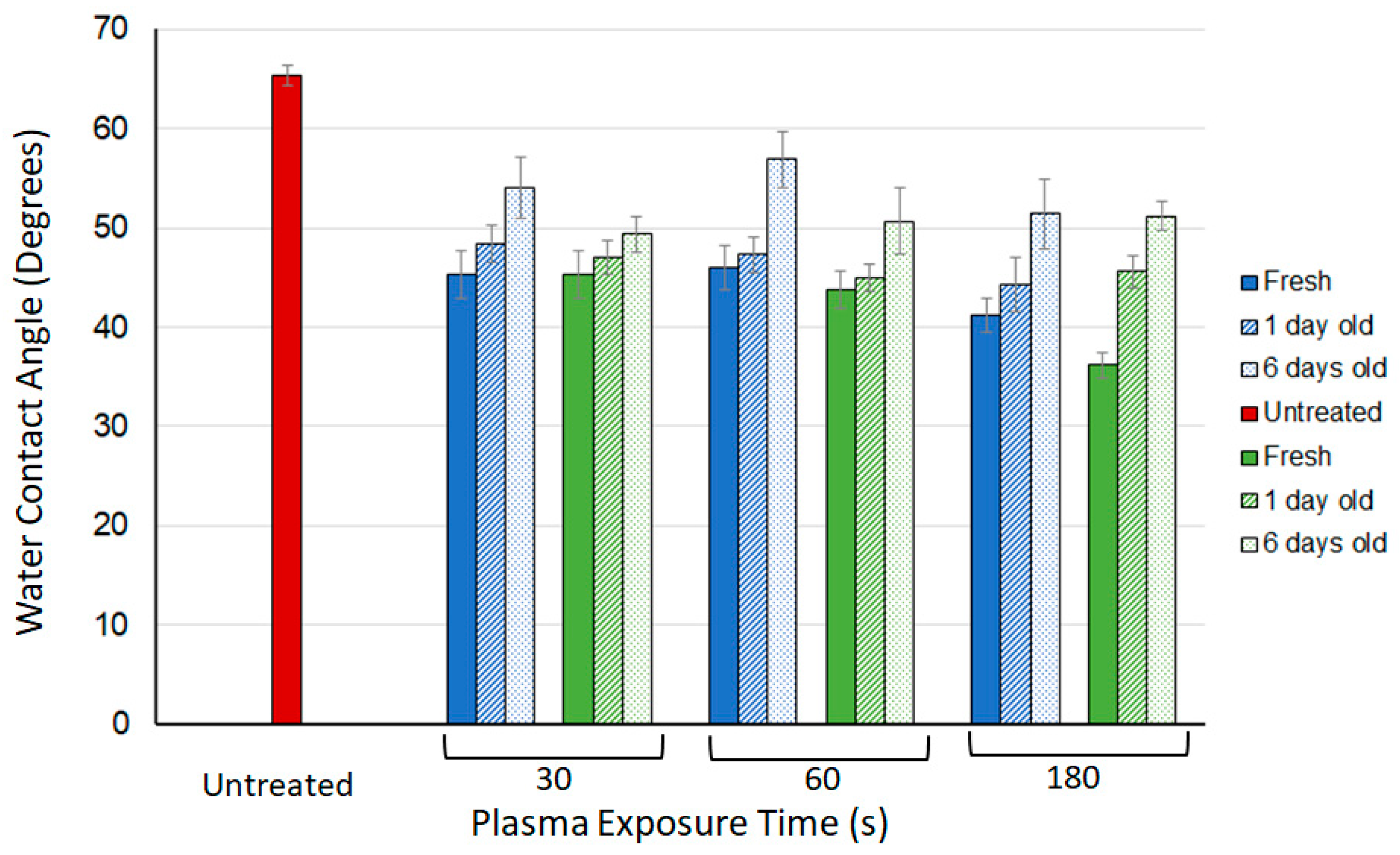
3.1. Water Contact Angle (WCA) Measurements
3.2. Surface Functionality via X-ray Photoemission Spectroscopy (XPS)
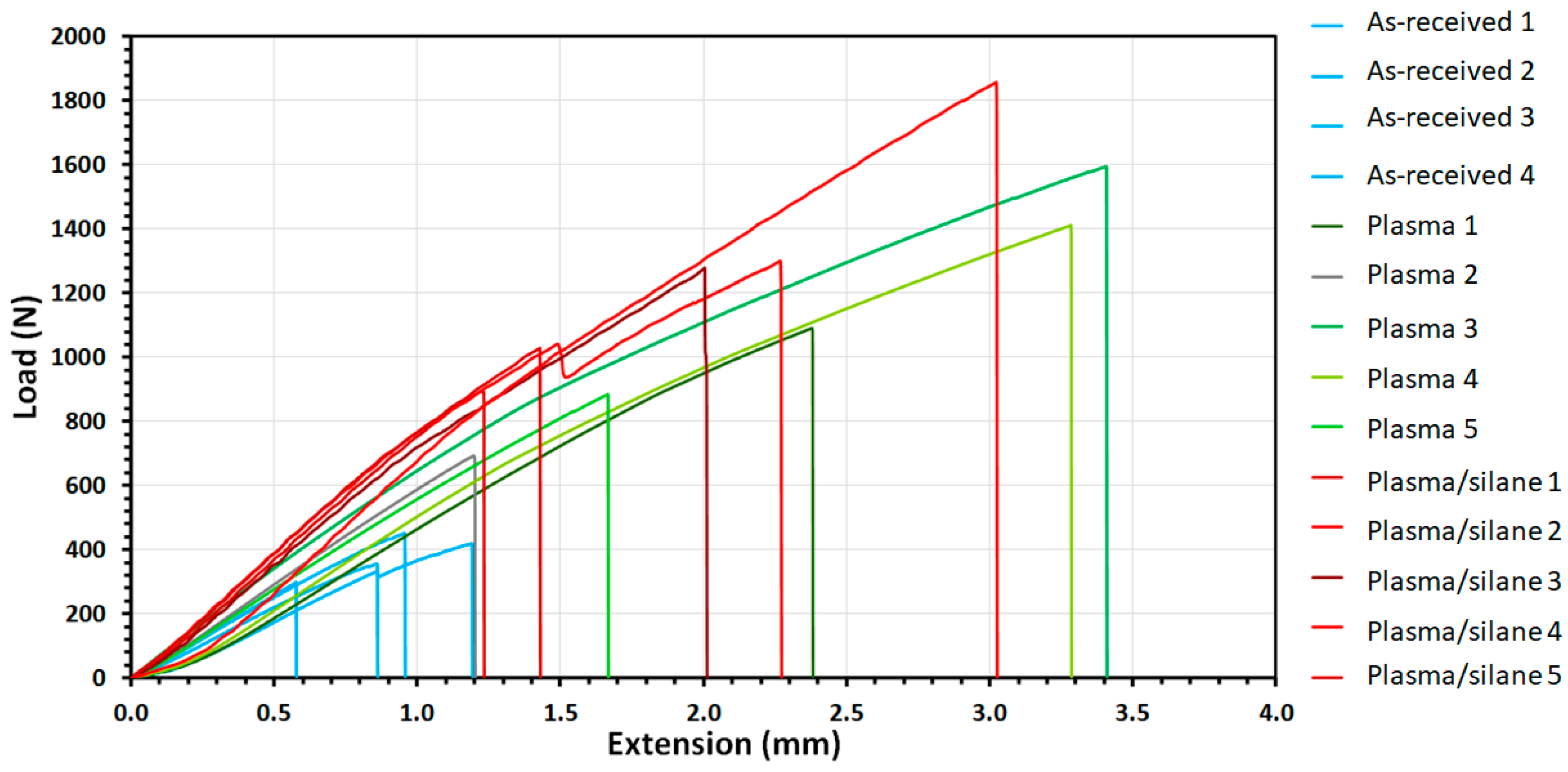
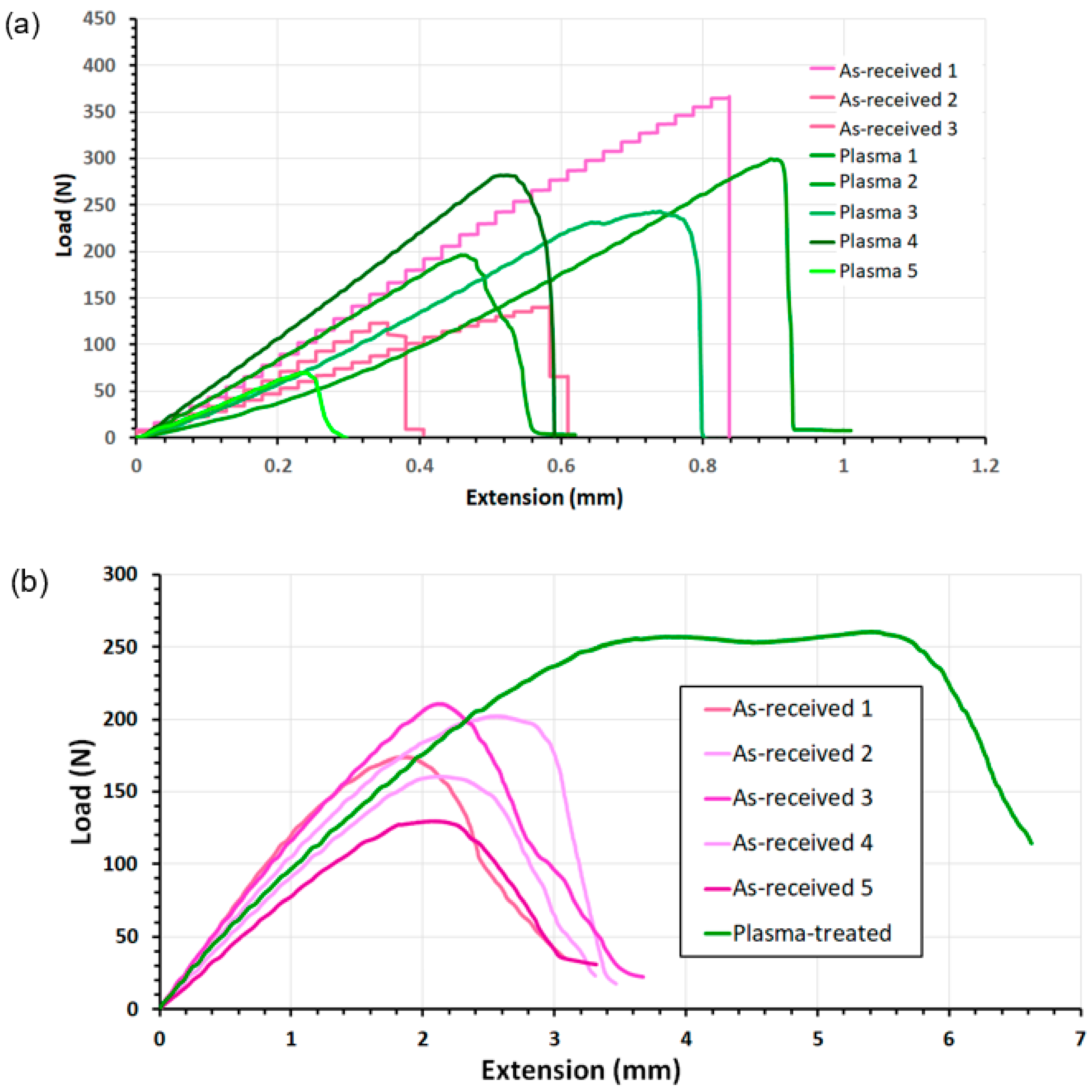
3.3. Bond Strengths of Nylon Single-Joint Lap Shears via Uniaxial Tensile Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mark, H.F. Polyamides, plastics. In Encyclopedia of Polymer Science and Technology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 978-1-118-63389-2. [Google Scholar]
- Lee, Y.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J. Renewable routes to monomeric precursors of nylon 66 and nylon 6 from food waste. J. Clean. Prod. 2019, 227, 624–633. [Google Scholar] [CrossRef]
- Vagholkar, P.K. Nylon (Chemistry, Properties and Uses). Int. J. Sci. Res. 2016, 5, 349–351. [Google Scholar]
- Hsiung, H.M.; Ren, H.J.; Ning, W.P.; Chi, K.S. Study on Tensile Properties of Nylon 66 Reinforced Composites. In Proceedings of the 2016 International Conference on Education, Management, Computer and Society (EMCS), Shenyang, China, 1–3 January 2016; Volume 37, pp. 1660–1663. [Google Scholar]
- Tate, M.L.; Kamath, Y.K.; Wesson, S.P.; Ruetsch, S.B. Surface energetics of nylon 66 fibers. J. Colloid Interface Sci. 1996, 177, 579–588. [Google Scholar] [CrossRef]
- Petrie, E.M. Handbook of Adhesives and Sealants, 2nd ed.; McGraw-Hill Books: New York, NY, USA, 2007; ISBN 9780071479165. [Google Scholar]
- Burton, B.; Alexander, D.; Klein, H.; Garibay-Vasquez, A.; Pekarik, A.; Henkee, C. Epoxy Formulations using Jeffamine Polyetheramines; EFB-0307; Huntsman Corp: Salt Lake City, UT, USA, 2010. [Google Scholar]
- Lee, I.; Wool, R.P. Thermodynamic analysis of polymer-solid adhesion sticker and receptor group effects. J. Polym. Sci. B 2002, 40, 2343–2353. [Google Scholar] [CrossRef]
- Singh, V.R.; John, N. Blends of nylon-66 with chemically modified ABS (m-ABS) using a novel method of UV irradiation. Polym. Plastics Technol. Eng. 2011, 50, 312–319. [Google Scholar] [CrossRef]
- Pramanik, N.K.; Haldar, R.S.; Bhardwaj, Y.K.; Sabharwal, S.; Niyogi, U.K.; Khndal, R.K. Modification of nylon 66 by electron beam irradiation for improved properties and superior performances. J. Appl. Polym. Sci. 2011, 122, 193–202. [Google Scholar] [CrossRef]
- Du, J.M.; Zhang, L.L.; Dong, J.; Li, Y.; Xu, C.H.; Gao, W.D. Preparation of hydrophobic nylon fabric. J. Eng. Fibers Fabr. 2016, 11, 31–37. [Google Scholar] [CrossRef]
- Hao, W.; Shao, Z.Z. Superhydrophobic and highly oleophobic nylon surface. Acta Chim. Sin. 2014, 72, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Teli, M.; Annaldewar, B.N. Towards superhydrophobic and ultraviolet protective nylon fabrics. Int. J. Cloth. Sci. Technol. 2017, 29, 696–705. [Google Scholar] [CrossRef]
- Gao, Z.Q.; Qiu, Y.P. The Surface Modification of Nylon 6 Films Treated with Atmospheric Pressure Plasma. In Proceedings of the Fiber Society 2009 Spring Conference, Shanghai, China, 27–29 May 2009; Volume 1–2, pp. 310–312. [Google Scholar]
- Oh, K.W.; Seong, J.H.; Kim, S.H. Conductivity improvement of polyaniline/nylon 6 fabrics. Polym. Korea 2000, 24, 673–681. [Google Scholar]
- Deogaonkar-Baride, S.; Palaskar, S.S. Atmospheric pressure plasma treatment for enhancing the conducting properties of polypyrrole coated nylon fabric. J. Appl. Polym. Sci. 2022, 139, e52443. [Google Scholar] [CrossRef]
- Pappas, D.; Andres, A.; Demaree, J.D.; Hirvonen, J.K.; Kosik, W.; Jensen, R.; McKnight, S. Surface modification of polyamide fibers and films using atmospheric plasmas. Surf. Coat. Technol. 2006, 201, 4384–4388. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment—an SEM investigation. Sens. Actuators B 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Tokoro, T.; Hackam, R. Loss and recovery of hydrophobicity, surface energies, diffusion coefficients and activation energy of nylon. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 754–762. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, J.L.; Peng, S.; Yao, L.; Qiu, Y. Surface modification of nylon 6 films treated with an He/O2 atmospheric pressure plasma jet. J. Appl. Polym. Sci. 2011, 120, 2201–2206. [Google Scholar] [CrossRef]
- Michielsen, S.; Lee, H.J. Design of a superhydrophobic surface using woven structures. Langmuir 2007, 23, 6004–6010. [Google Scholar] [CrossRef]
- Guo, Y.; Lia, Y.; Wanga, S.; Liua, Z.-X.; Caia, B.; Wang, P.-C. Effect of silane treatment on adhesion of adhesive-bonded carbon fiber reinforced nylon 6 composite. Int. J. Adhes. Adhes. 2019, 91, 102–115. [Google Scholar] [CrossRef]
- Hillborg, H.C. Loss and Recovery of Hydrophobicity of Polydimethylsiloxane after Exposure to Electrical Discharge; Royal Institute of Technology: Stockholm, Sweden, 2001. [Google Scholar]
- Boulares-Pender, A.; Thomas, I.; Prager, A.; Schulze, A. Surface modification of polyamide and poly(vinylidene fluoride) membranes. J. Appl. Polym. Sci. 2013, 128, 322–330. [Google Scholar] [CrossRef]
- Nourbakhsh, S. Comparison between laser application and atmospheric air plasma treatment on nanocellulose coating of polyester and nylon 66 fabrics. J. Laser Appl. 2015, 27, 012005. [Google Scholar] [CrossRef]
- Bujanda, A.A.; Wu, C.C.; Demaree, J.D.; Robinette, E.J.; Weerasooriya, A.; Flanagan, D. Atmospheric Plasma Treatment of Nylon 6,6 for Improved Interfacial Adhesion in Thermoplastic Composites. In Advanced Composites for Aerospace, Marine and Land Applications; Sano, T., II, Srivatsan, T.S., Eds.; The Minerals, Metals & Materials Society (TMS): Warrendale, PA, USA, 2015; pp. 259–271. [Google Scholar]
- Pleşa, I.; Noţingher, P.V.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of polymer composites used in high-voltage applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Louette, P.; Bodino, F.; Pireaux, J.-J. Nylon 6 (N6) Reference XPS reference core level and energy loss spectra. Surf. Sci. Spectra 2005, 12, 11–17. [Google Scholar] [CrossRef]
- McGrath, L.M.; Parnas, R.S.; King, S.H.; Schroeder, J.L.; Fischer, D.A.; Lenhart, J.L. Investigation of the thermal, mechanical, and fracture properties of alumina-epoxy composites. Polymer 2008, 49, 999–1014. [Google Scholar] [CrossRef]
- Copeman, K. Evaluating Epoxy Cure and Adhesion Strength through Single-Sided NMR Measurements of Molecular Mobility. Master’s Thesis, William & Mary College, Williamsburg, VA, USA, 2021. [Google Scholar]



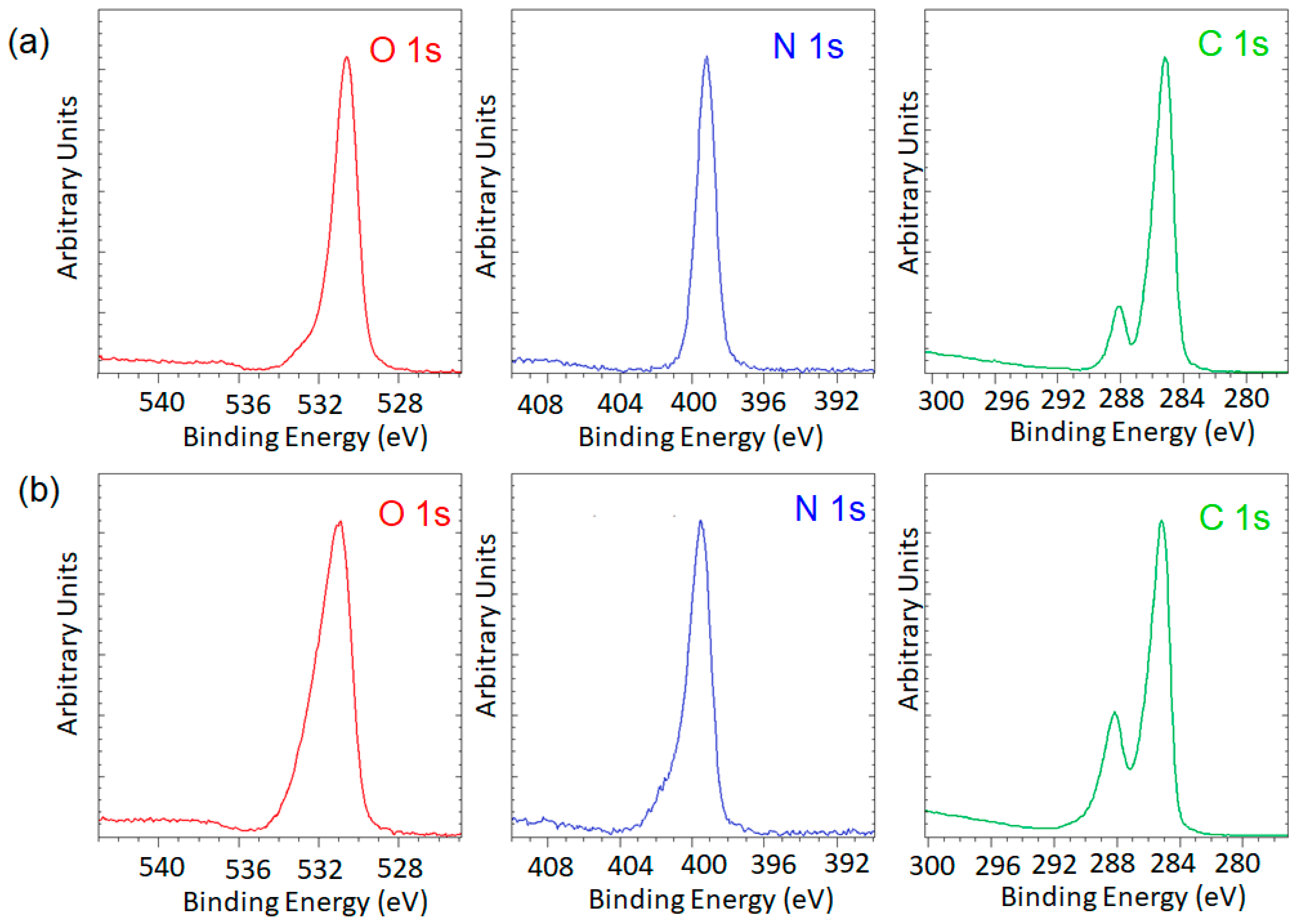
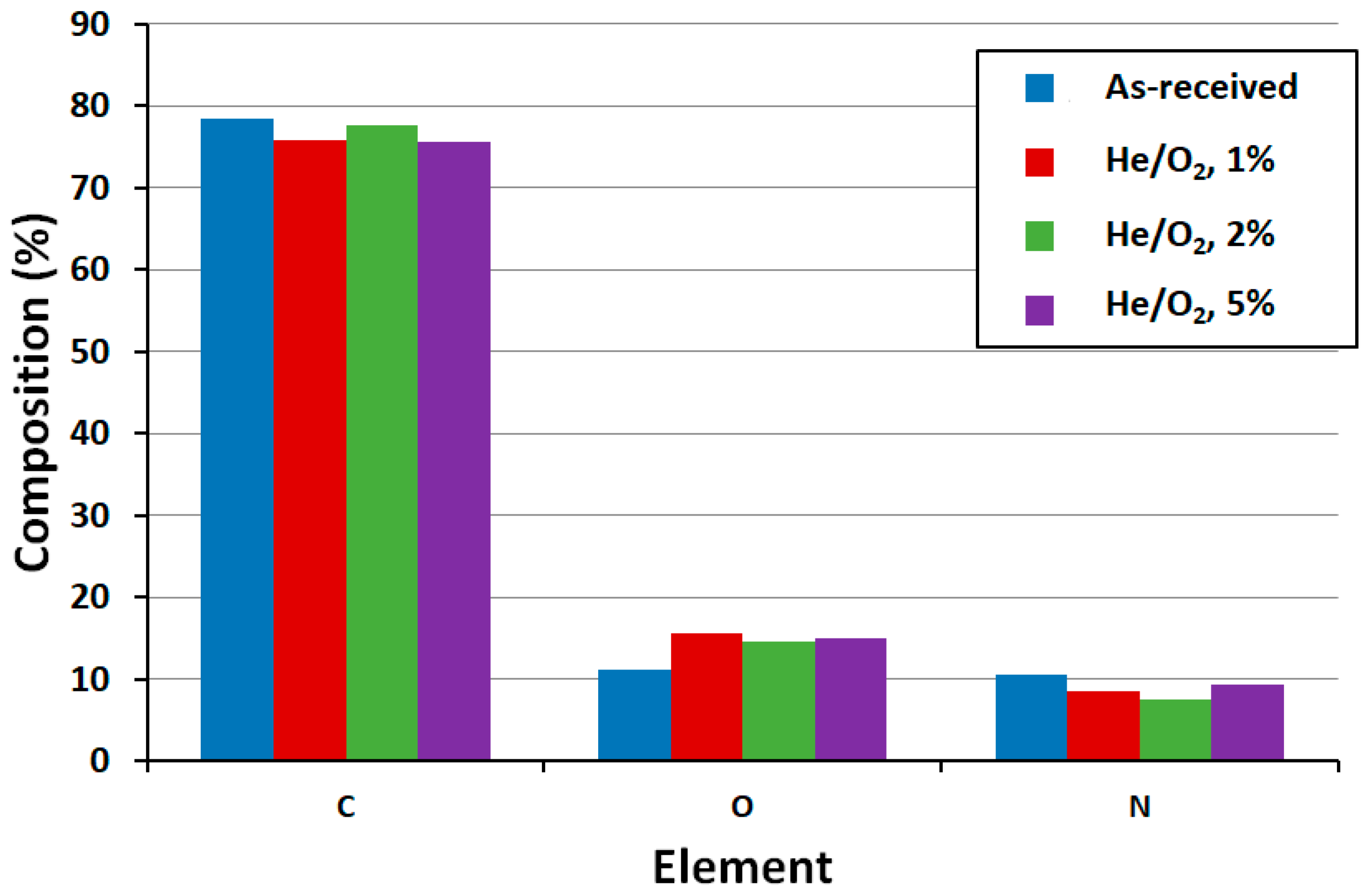
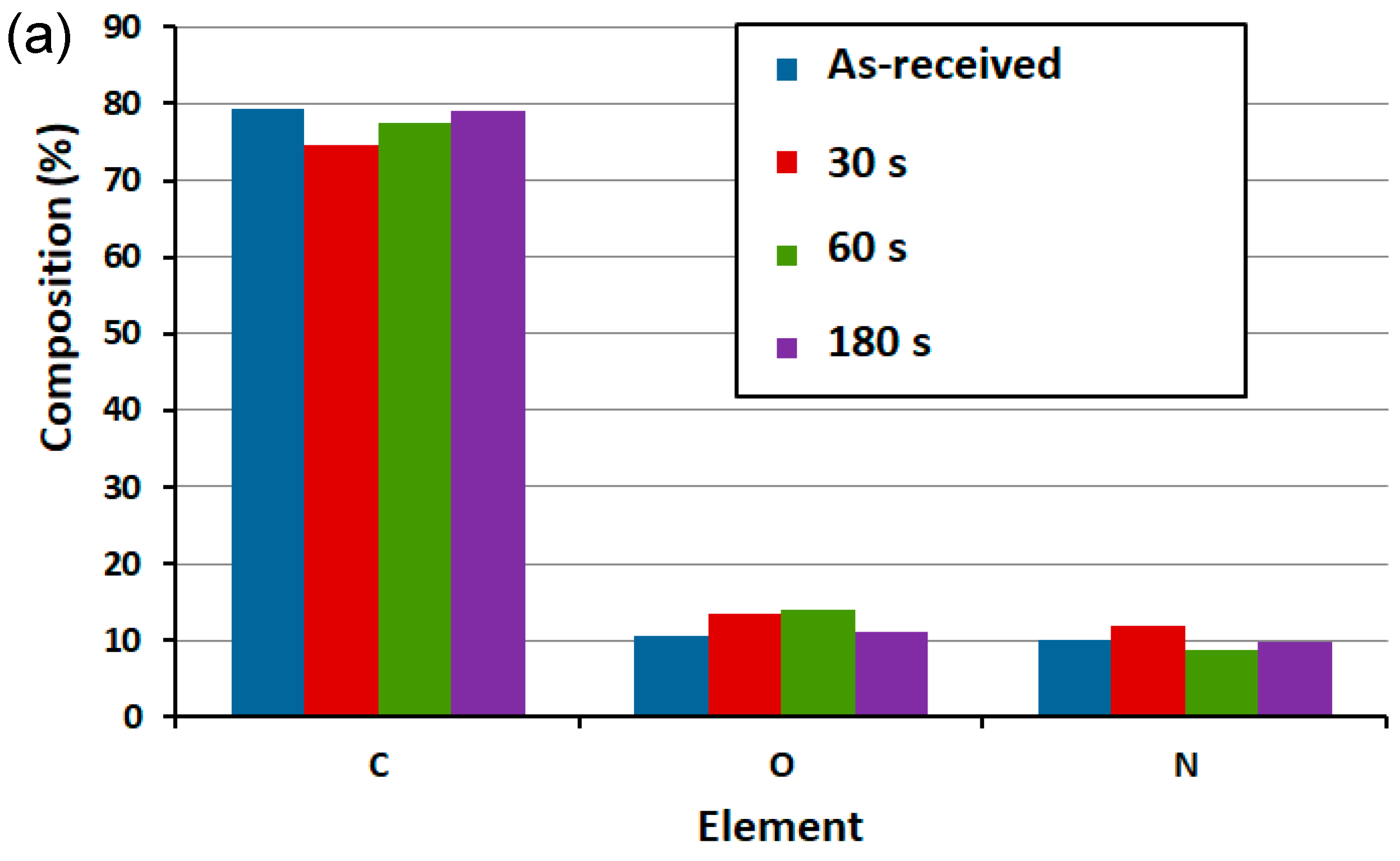
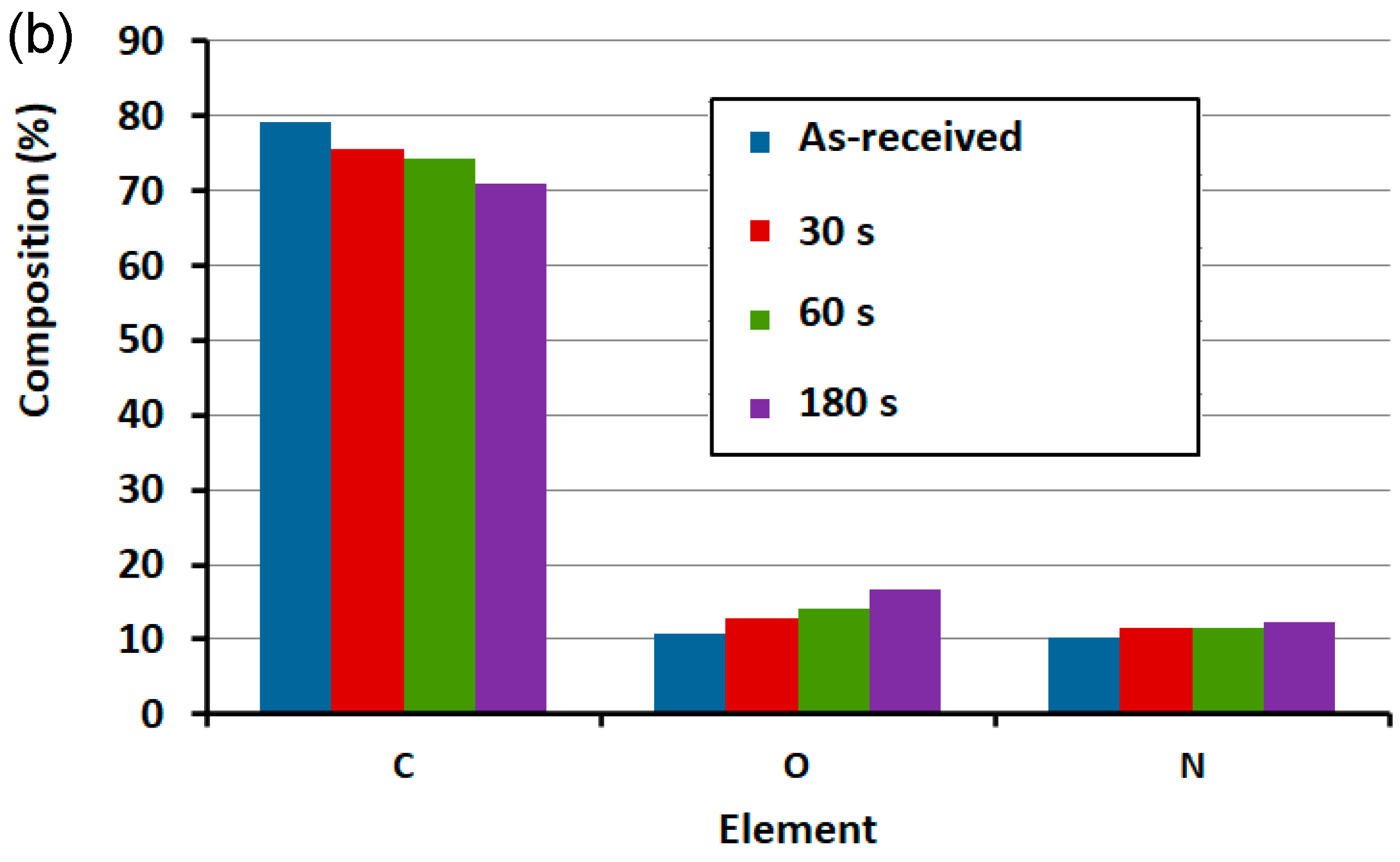
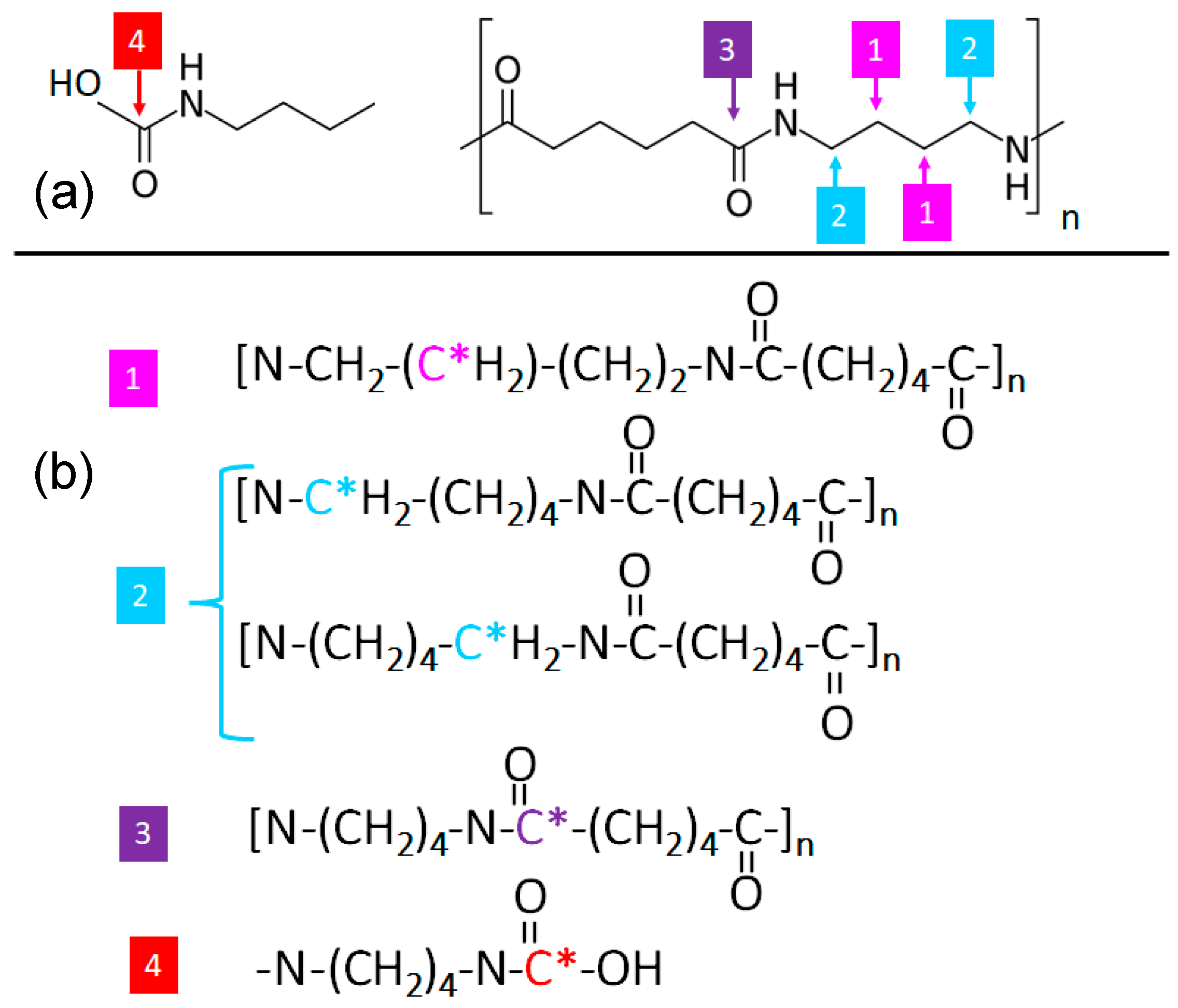
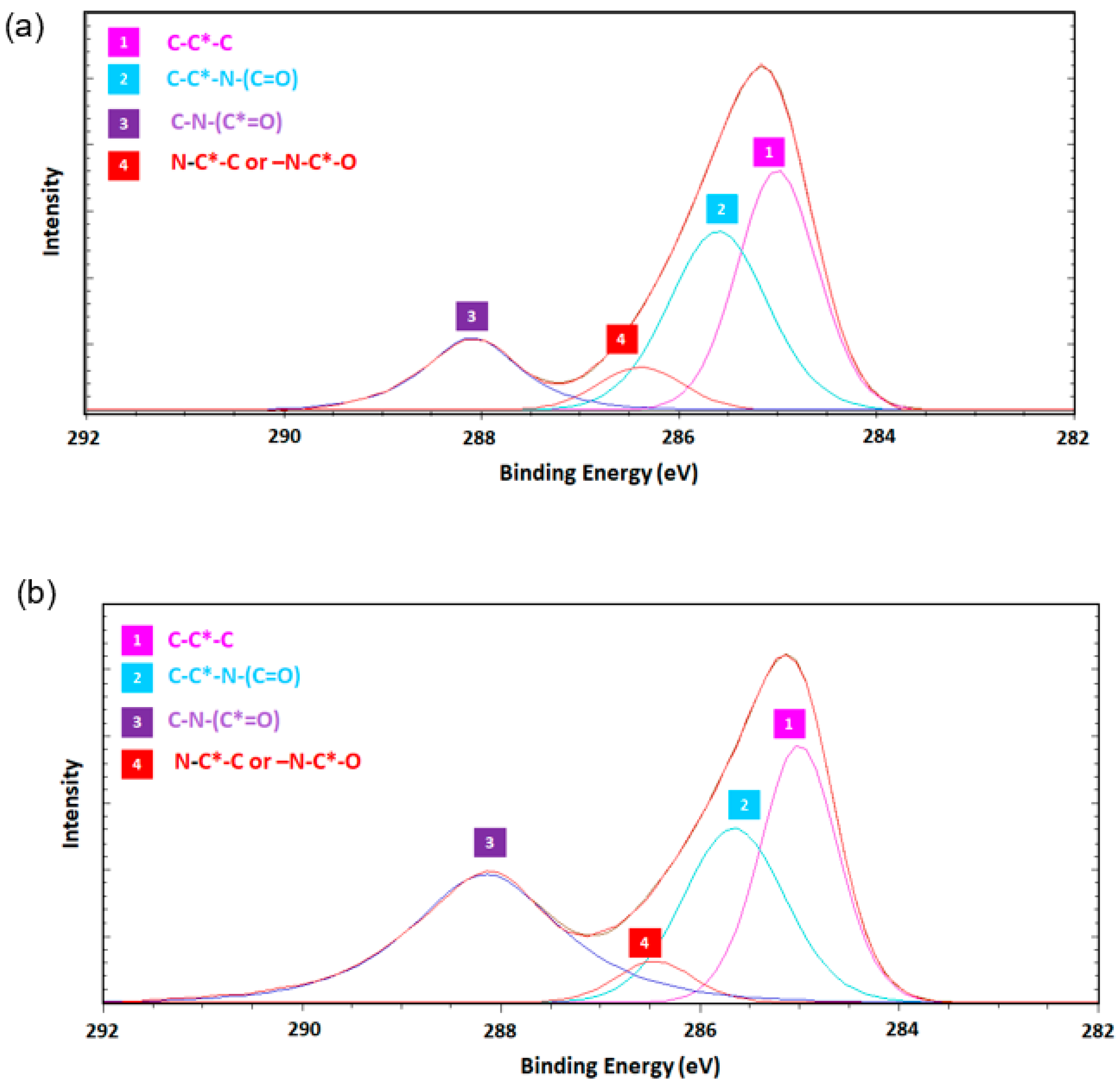
| Sample | C (%) | O (%) | N (%) | C/O | C/N | O/N |
|---|---|---|---|---|---|---|
| As-received | 78.50 | 11.10 | 10.60 | 7.07 | 7.41 | 1.05 |
| He/1% O2 plasma 180 s | 75.90 | 15.60 | 8.50 | 4.87 | 8.93 | 1.84 |
| He/2% O2 plasma 180 s | 77.70 | 14.70 | 7.60 | 5.29 | 10.22 | 1.93 |
| He/5% O2 plasma 180 s | 75.70 | 15.10 | 9.30 | 5.01 | 8.15 | 1.63 |
| He/H2O plasma 30 s | 75.48 | 12.94 | 11.59 | 5.83 | 6.51 | 2.34 |
| He/H2O plasma 60 s | 74.26 | 14.15 | 11.60 | 5.25 | 6.40 | 2.92 |
| He/H2O plasma 180 s | 70.80 | 16.78 | 12.42 | 4.22 | 5.70 | 1.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Demaree, J.D.; Weerasooriya, A.; Bujanda, A.; Robinette, E.J. Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas. Coatings 2022, 12, 919. https://doi.org/10.3390/coatings12070919
Wu C-C, Demaree JD, Weerasooriya A, Bujanda A, Robinette EJ. Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas. Coatings. 2022; 12(7):919. https://doi.org/10.3390/coatings12070919
Chicago/Turabian StyleWu, Chi-Chin, John Derek Demaree, Amanda Weerasooriya, Andres Bujanda, and Eric Jason Robinette. 2022. "Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas" Coatings 12, no. 7: 919. https://doi.org/10.3390/coatings12070919
APA StyleWu, C.-C., Demaree, J. D., Weerasooriya, A., Bujanda, A., & Robinette, E. J. (2022). Enhanced Interfacial Adhesion of Nylon 66 to Epoxy Resin EPON 825 by Non-thermal Atmospheric Pressure Dielectric Barrier Discharge Plasmas. Coatings, 12(7), 919. https://doi.org/10.3390/coatings12070919






