Development of Liquid Phase Sintering Silicon Carbide Composites for Light Water Reactor
Abstract
1. Introduction
2. Materials and Methods
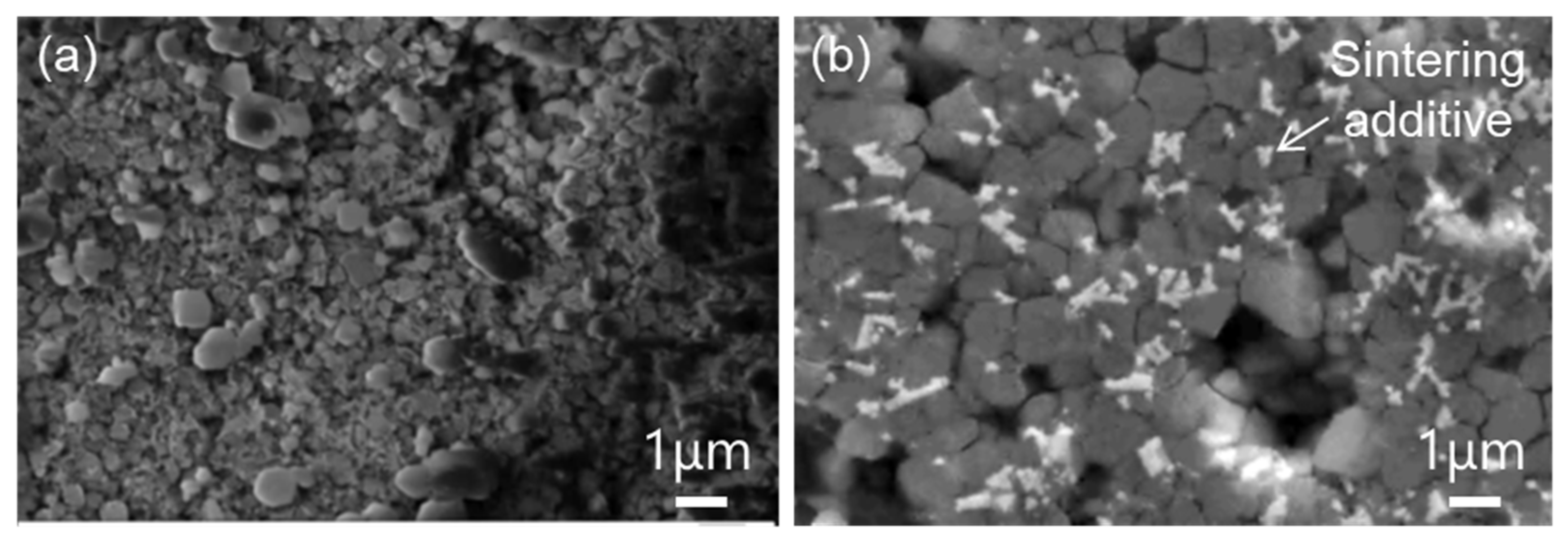
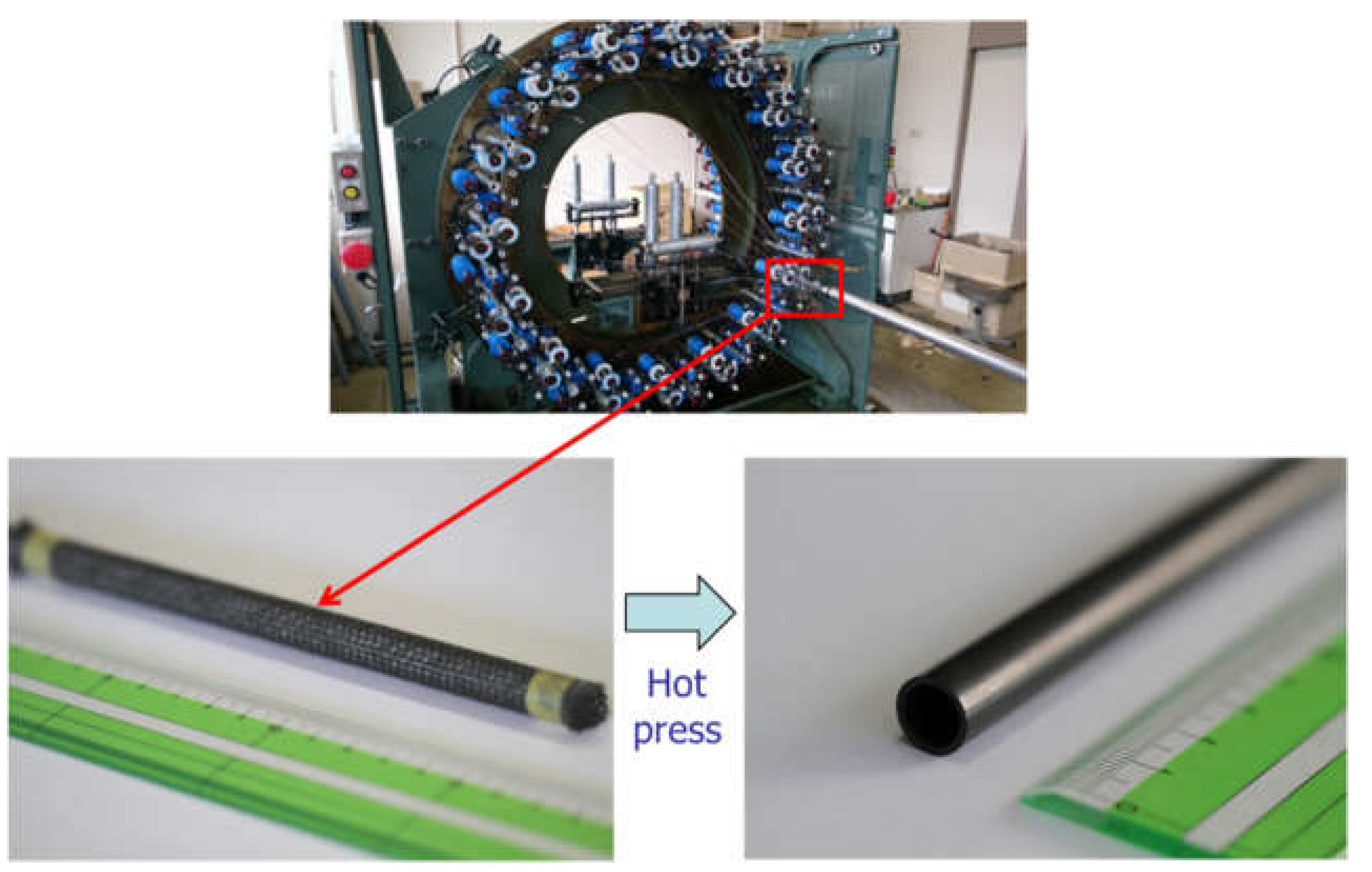
2.1. Materials
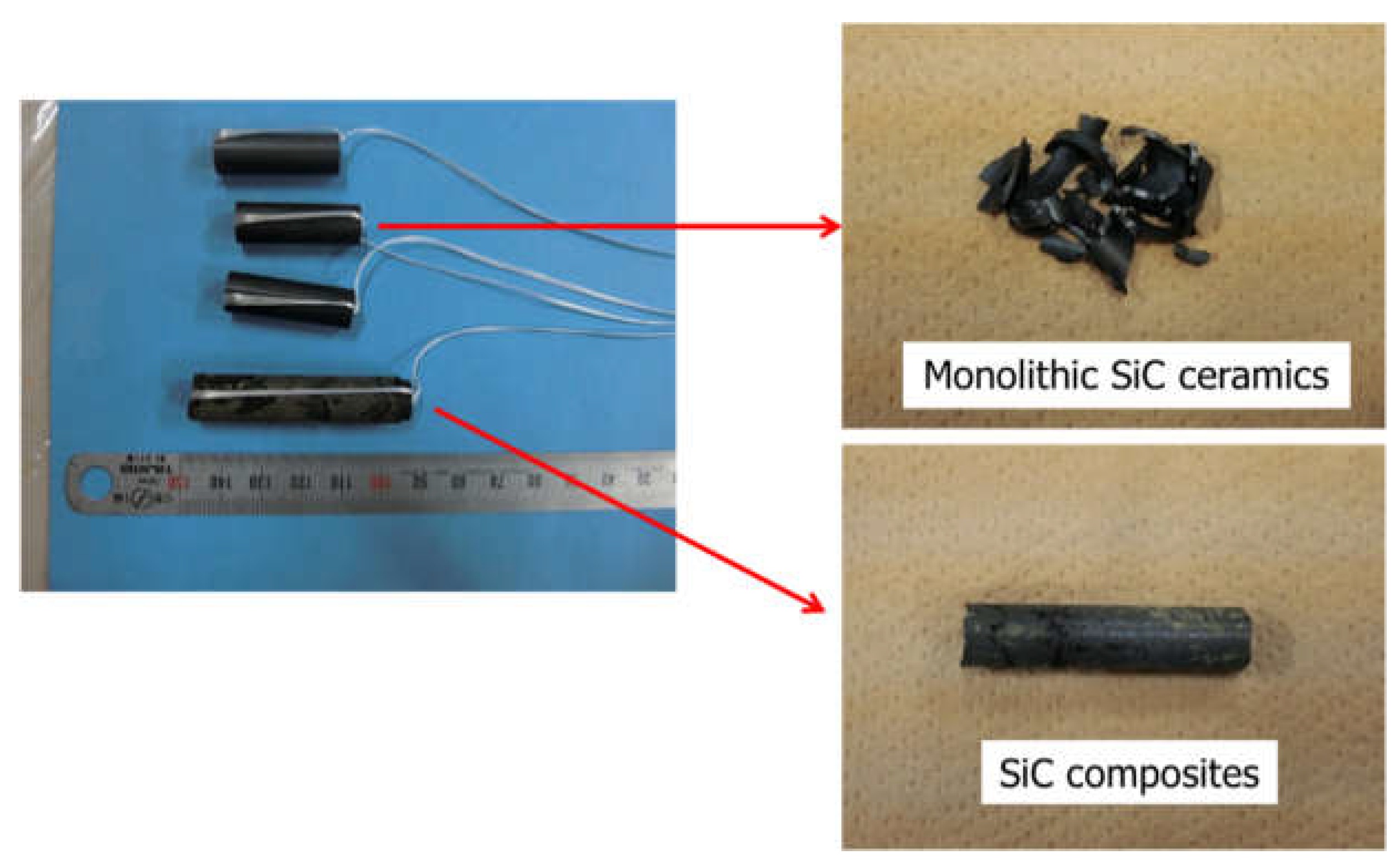
2.2. Methods
3. Results and Discussion
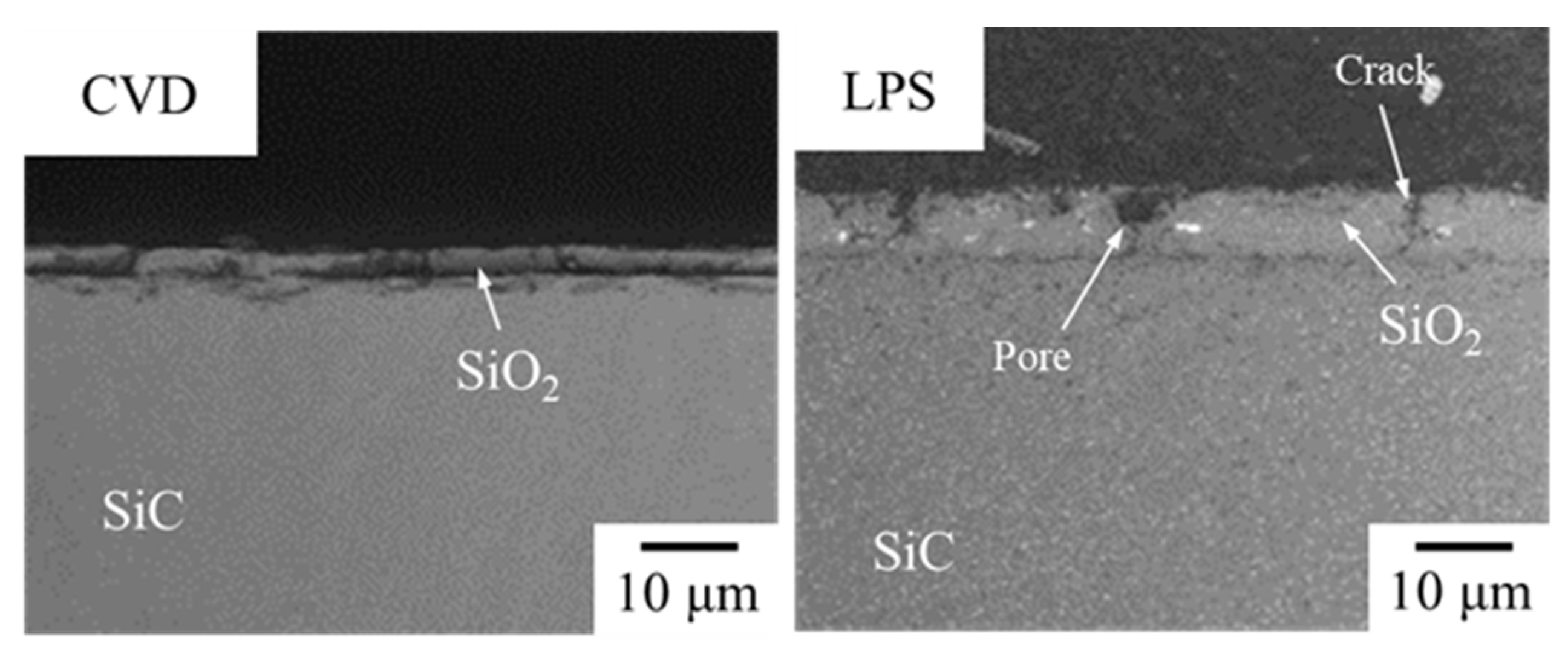
3.1. Steam Oxidation
- SiC + 3H2O(g) → SiO2 + 3H2(g) + CO(g) (Weight gain)
- SiO2 + 2H2O(g) → Si(OH)4(g) (Weight loss)
- Al2O3(s) + 3H2O(g) → 2Al(OH)3(g) (Weight loss)
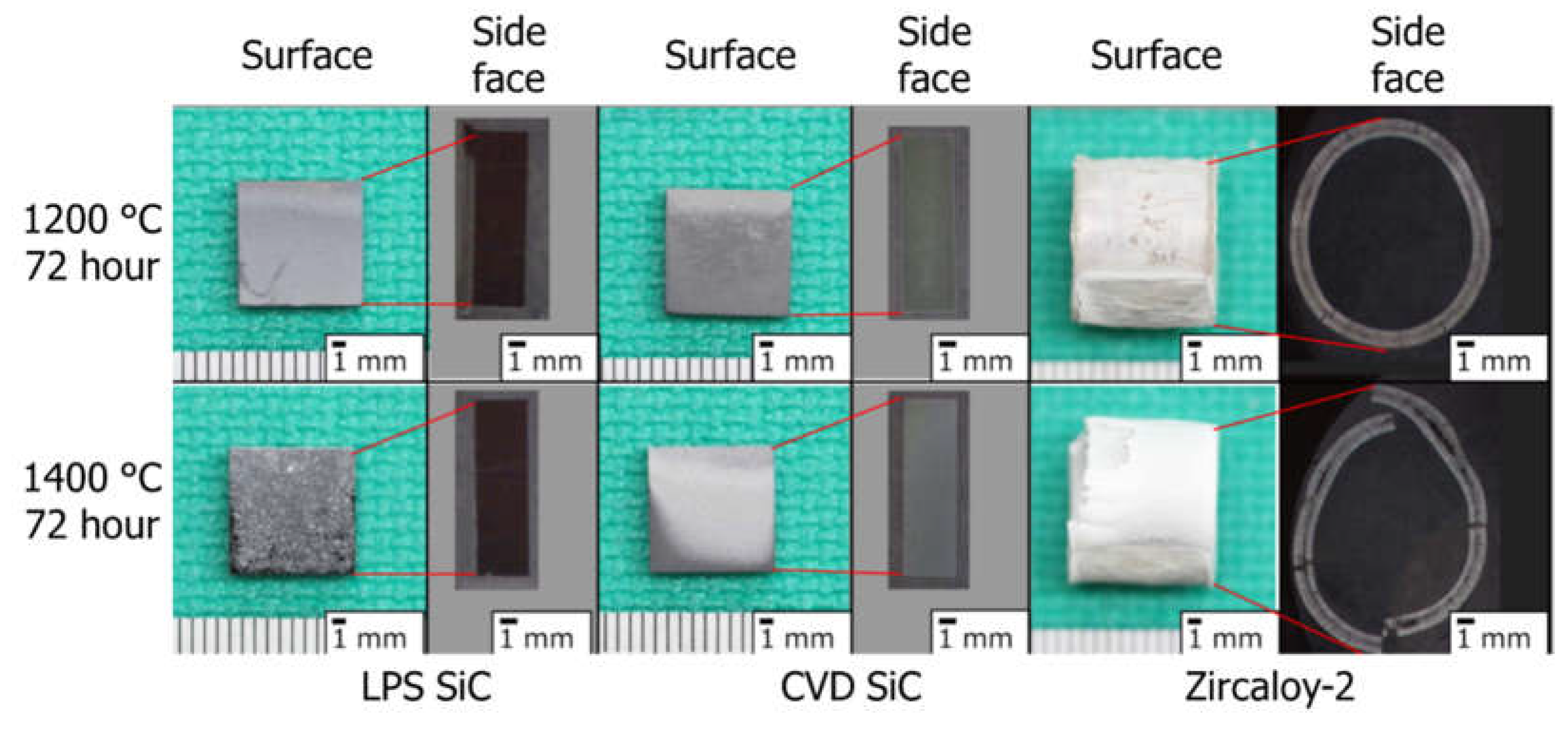
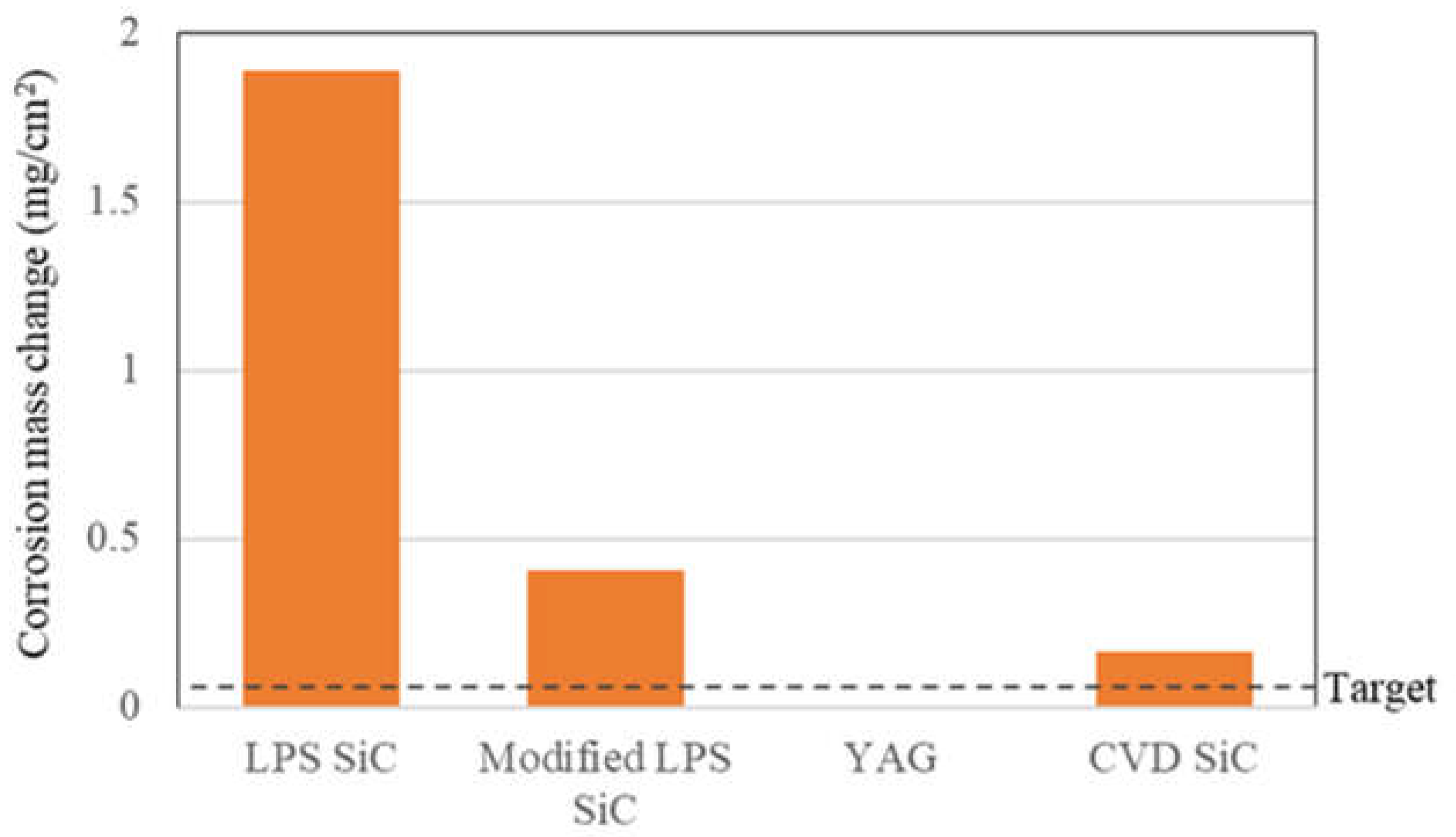
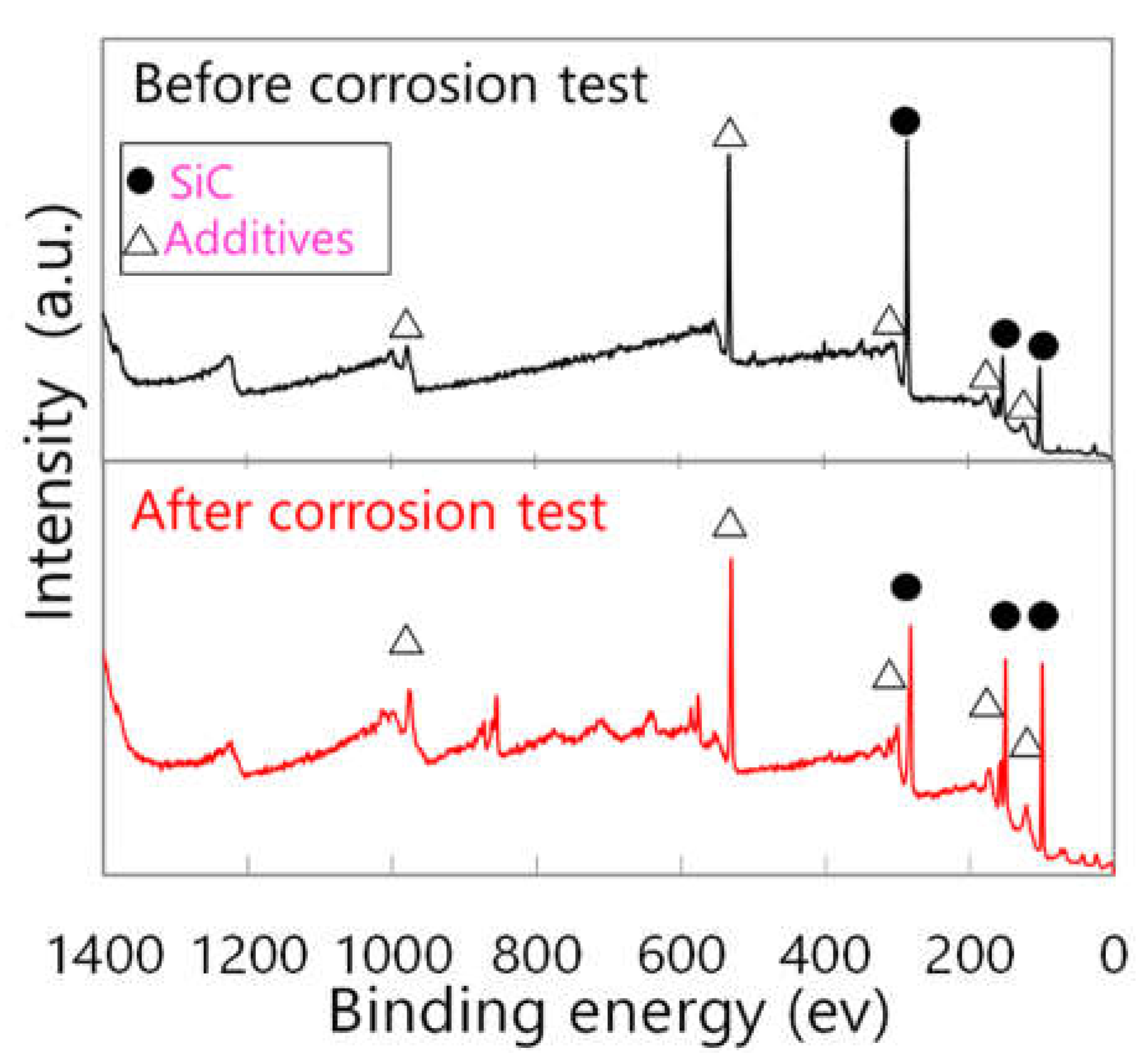
3.2. High-Temperature Water Corrosion
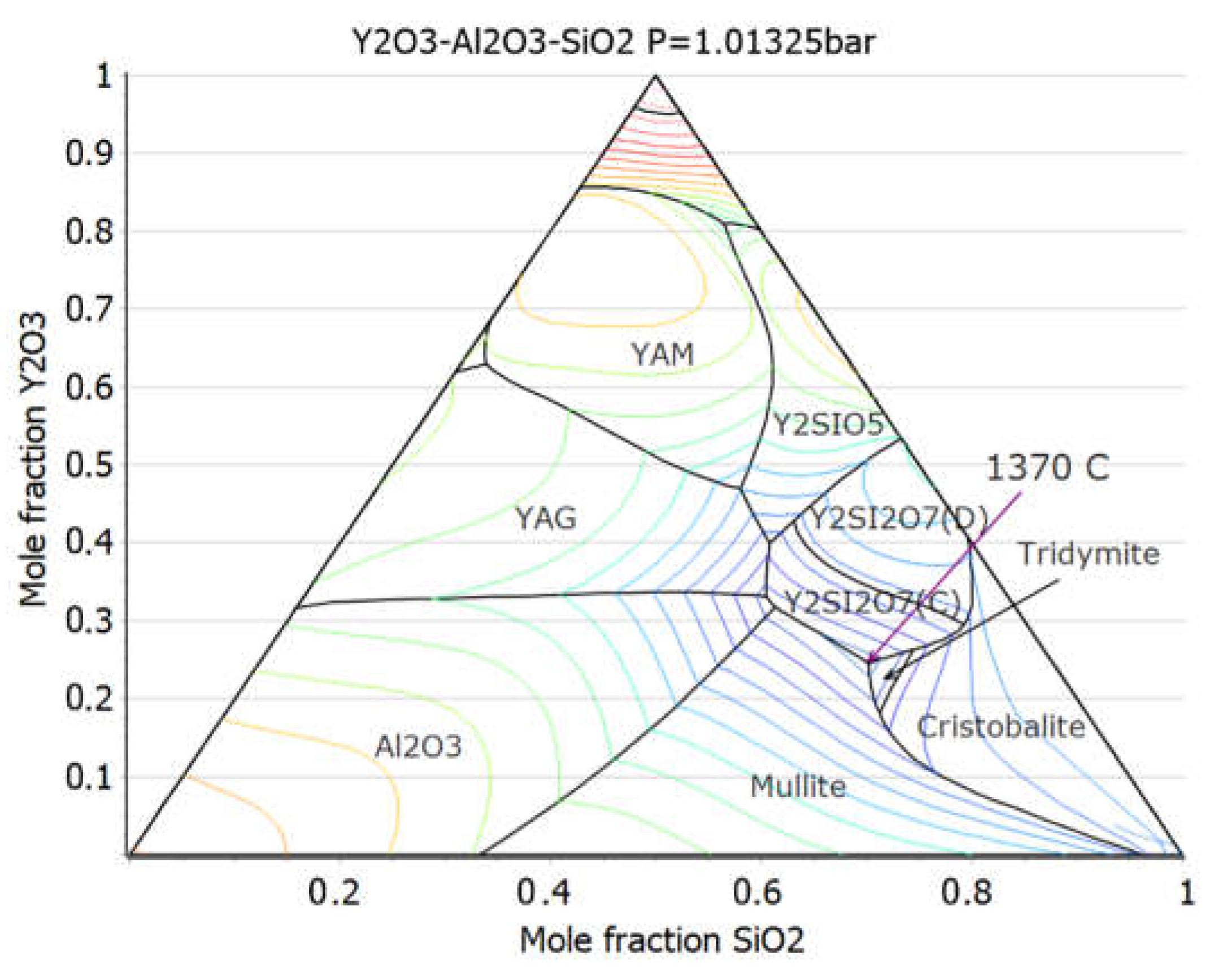
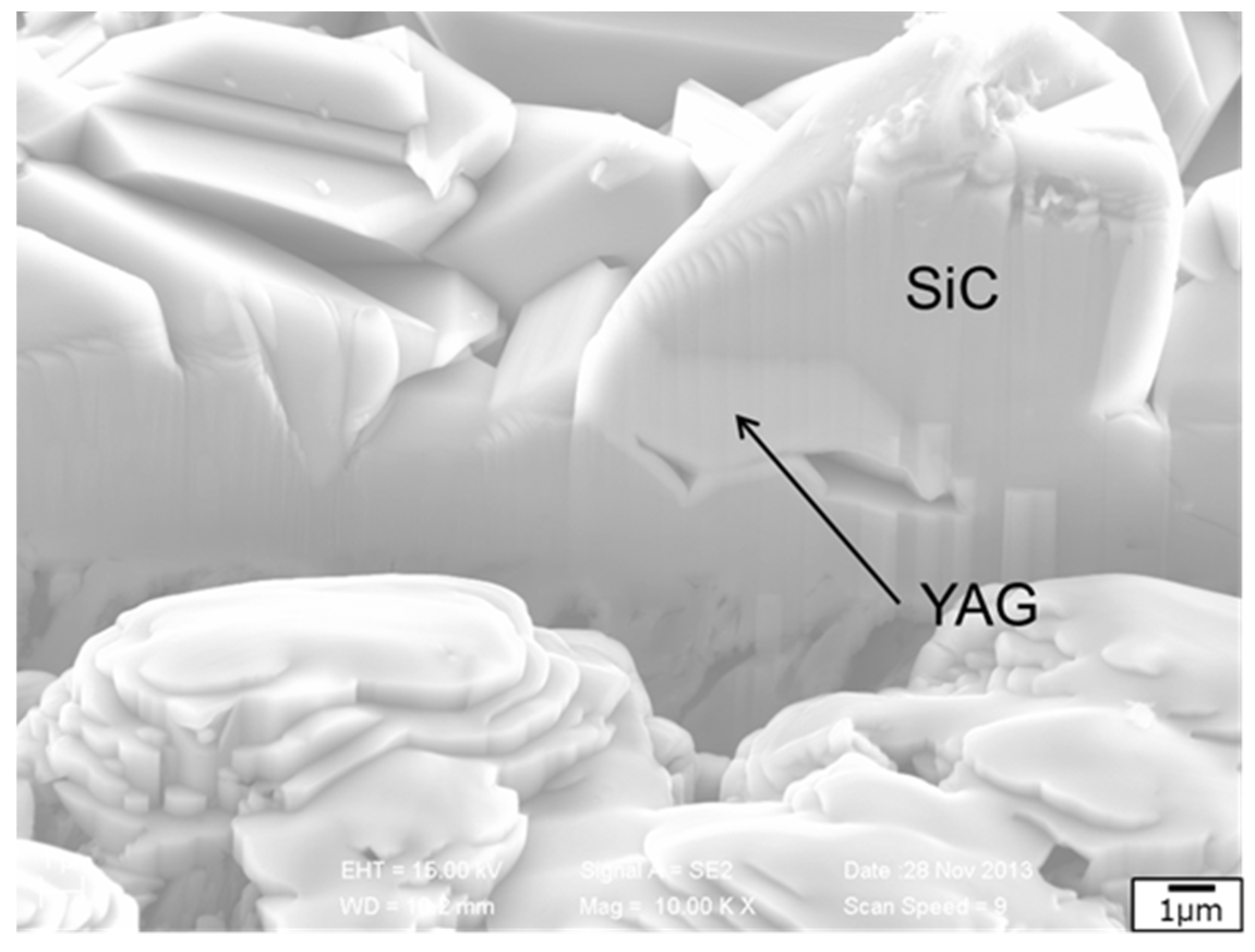
3.3. Liquid Phase Sintering SiC Composites for Light Water Reactor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Čížek, J.; Kalivodov, J.; Janeček, M.; Stráský, J.; Srba, O.; Macková, A. Advanced structural materials for gas-cooled fast reactors—A review. Metals. 2021, 11, 76. [Google Scholar] [CrossRef]
- Norajitra, P.; Bühler, L.; Fischer, U.; Gordeev, S.; Malang, S.; Reimann, G. Reimann, Conceptual design of the dual-coolant blanket in the frame of the EU power plant conceptual study. Fusion Eng. Des. 2003, 69, 669–673. [Google Scholar] [CrossRef]
- Najmabadi, F.; The Aries Team. Spherical torus concept as power plants-the ARIES-ST study. Fusion Eng. Des. 2003, 65, 143–164. [Google Scholar] [CrossRef]
- Steibel, J. Ceramic matrix composites taking flight at GE Aviation. Am. Ceram. Soc. Bull. 2019, 98, 30–33. [Google Scholar]
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Pint, B.A.; Terrani, K.A.; Brady, M.P.; Cheng, T.; Keiser, J.R. High temperature oxidation of fuel cladding candidate materials in steam–hydrogen environments. J. Nucl. Mater. 2013, 440, 420–427. [Google Scholar] [CrossRef]
- Johnson, S.C.; Henry, R.E.; Paik, C.Y. Severe Accident Modeling of a PWR Core with Different Cladding Materials. In Proceedings of the 2012 International Congress on Advances in Nuclear Power Plants-ICAPP’12, Chicago, IL, USA, 24–28 June 2012; p. 12175. [Google Scholar]
- Ishibashi, R.; Ikegawa, T.; Noshita, K.; Kitou, K.; Kamoshida, M. Hydrogen explosion prevention system using SiC fuel cladding for large scale BWRs with inherently safe technologies. Mech. Eng. J. 2016, 3, 15–00215. [Google Scholar] [CrossRef][Green Version]
- Hinoki, T.; Katoh, Y.; Kohyama, A. Effect of Fiber Properties on Neutron Irradiated SiC/SiC Composites Materials. Transactions 2002, 43, 617–621. [Google Scholar]
- Koyanagi, T.; Katoh, Y.; Nozawa, T.; Snead, L.L.; Kondo, S.; Henager, C.H., Jr.; Ferrarisf, M.; Hinokig, T.; Huangh, Q. Recent progress in the development of SiC composites for nuclear fusion applications. J. Nucl. Mater. 2018, 511, 544–555. [Google Scholar] [CrossRef]
- Koyanagi, T.; Ozawa, K.; Hinoki, T.; Shimoda, K.; Katoh, Y. Effects of neutron irradiation on mechanical properties of silicon carbide composites fabricated by nano-infiltration and transient eutectic-phase precess. J. Nucl. Mater. 2014, 448, 478–486. [Google Scholar] [CrossRef]
- Avincola, V.A.; Grosse, M.; Stegmaier, U.; Steinbrueck, M.; Seifert, H.J. Oxidation at high temperatures in steam atmosphere and quench of silicon carbide composites for nuclear application. Nucl. Eng. Des. 2015, 295, 468–478. [Google Scholar] [CrossRef]
- Cheng, T.; Keiser, J.R.; Brady, M.P.; Terrani, K.A.; Pint, B.A. Oxidation of fuel cladding candidate materials in steam environments at high temperature and pressure. J. Nucl. Mater. 2012, 427, 396–400. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, I.H.; Jung, Y.I.; Kim, H.G.; Park, D.J.; Kim, W.J. Kim. Long-term corrosion behavior of CVD SiC in 360 C water and 400 C. J. Nucl. Mater. 2013, 443, 603–607. [Google Scholar] [CrossRef]
- Kondo, S.; Lee, M.; Hinoki, T.; Hyodo, Y.; Kano, F. Effect of irradiation damage on hydrothermal corrosion of SiC. J. Nucl. Mater. 2015, 464, 36–42. [Google Scholar] [CrossRef]
- Lee, K.N.; Fox, D.S.; Bansal, N.P. Rare earth silicate environmental barrier coatings for SiC/SiC composites and Si3N4 ceramics. J. Eur. Ceram. Soc. 2005, 25, 1705–1715. [Google Scholar] [CrossRef]
- Shimoda, K.; Hinoki, T.; Park, Y.H. Development of non-brittle fracture in SiCf/SiC composites without a fiber/matrix interface due to the porous structure of the matrix. Compos. Part A Appl. Sci. Manuf. 2018, 115, 397–404. [Google Scholar] [CrossRef]
- Hinoki, T.; Kanayama, T.; Ogitani, S.; Fukuhara, S.; Kamoshida, K.; Shimoda, K.; Tsurui, N.; Hokari, S. Development of oxidation resistant BN particle dispersion SiC composites. Bull. Ceram. Soc. Jpn. 2020, 55, 423–426. [Google Scholar]
- Rebillat, F.; Lamon, J.; Naslain, R.; Lara-Curzio, E.; Ferber, M.K.; Besmann, T.M. Interfacial Bond Strength in SiC/C/SiC Composite Materials, as Studied by Single-Fiber Push-Out Tests. J. Am. Ceram. Soc. 1998, 81, 965–978. [Google Scholar] [CrossRef]
- Shimoda, K.; Park, J.S.; Hinoki, T.; Kohyama, A. Influence of pyrolytic carbon interface thickness on microstructure and mechanical properties of SiC/SiC composites by NITE process. Compos. Sci. Technol. 2008, 68, 98–105. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinoki, T.; Kano, F.; Kondo, S.; Kawaharada, Y.; Tsuchiya, Y.; Lee, M.; Sakai, H. Development of Liquid Phase Sintering Silicon Carbide Composites for Light Water Reactor. Coatings 2022, 12, 623. https://doi.org/10.3390/coatings12050623
Hinoki T, Kano F, Kondo S, Kawaharada Y, Tsuchiya Y, Lee M, Sakai H. Development of Liquid Phase Sintering Silicon Carbide Composites for Light Water Reactor. Coatings. 2022; 12(5):623. https://doi.org/10.3390/coatings12050623
Chicago/Turabian StyleHinoki, Tatsuya, Fumihisa Kano, Sosuke Kondo, Yoshiyuki Kawaharada, Yumiko Tsuchiya, Moonhee Lee, and Hiroyuki Sakai. 2022. "Development of Liquid Phase Sintering Silicon Carbide Composites for Light Water Reactor" Coatings 12, no. 5: 623. https://doi.org/10.3390/coatings12050623
APA StyleHinoki, T., Kano, F., Kondo, S., Kawaharada, Y., Tsuchiya, Y., Lee, M., & Sakai, H. (2022). Development of Liquid Phase Sintering Silicon Carbide Composites for Light Water Reactor. Coatings, 12(5), 623. https://doi.org/10.3390/coatings12050623




