Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Supplies
2.2. Instrumental Techniques
2.3. Synthesis of AuNPs
2.4. Modification of SPGE with CuNWs and AuNPs
3. Results and Discussion
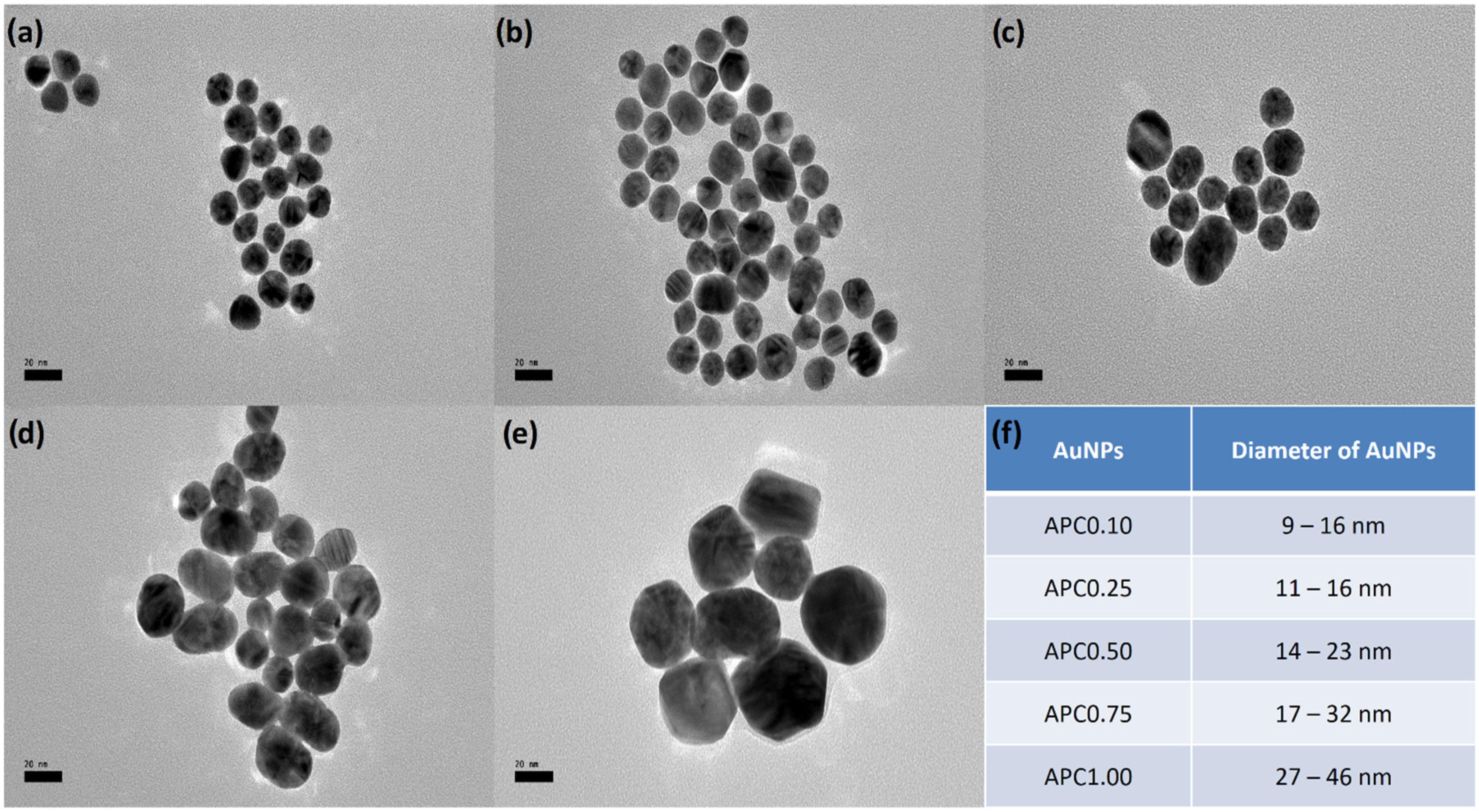
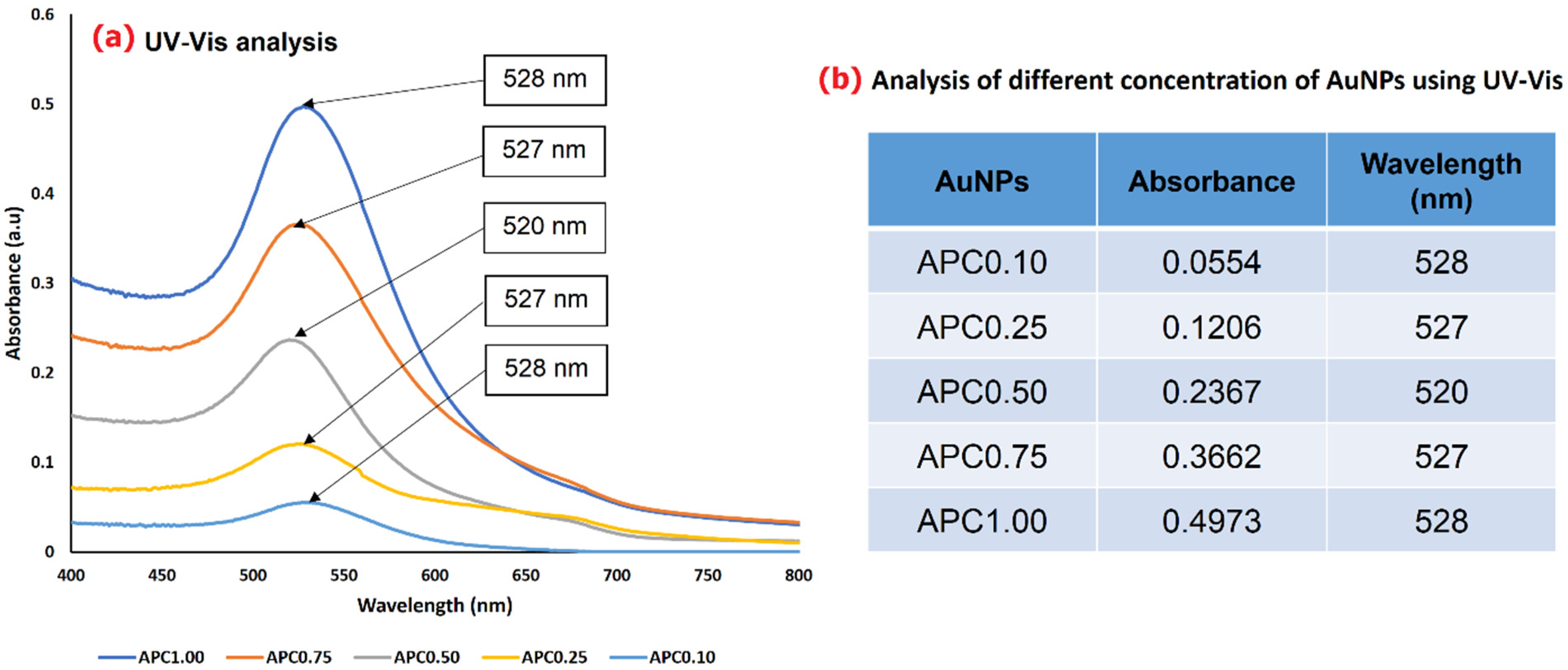
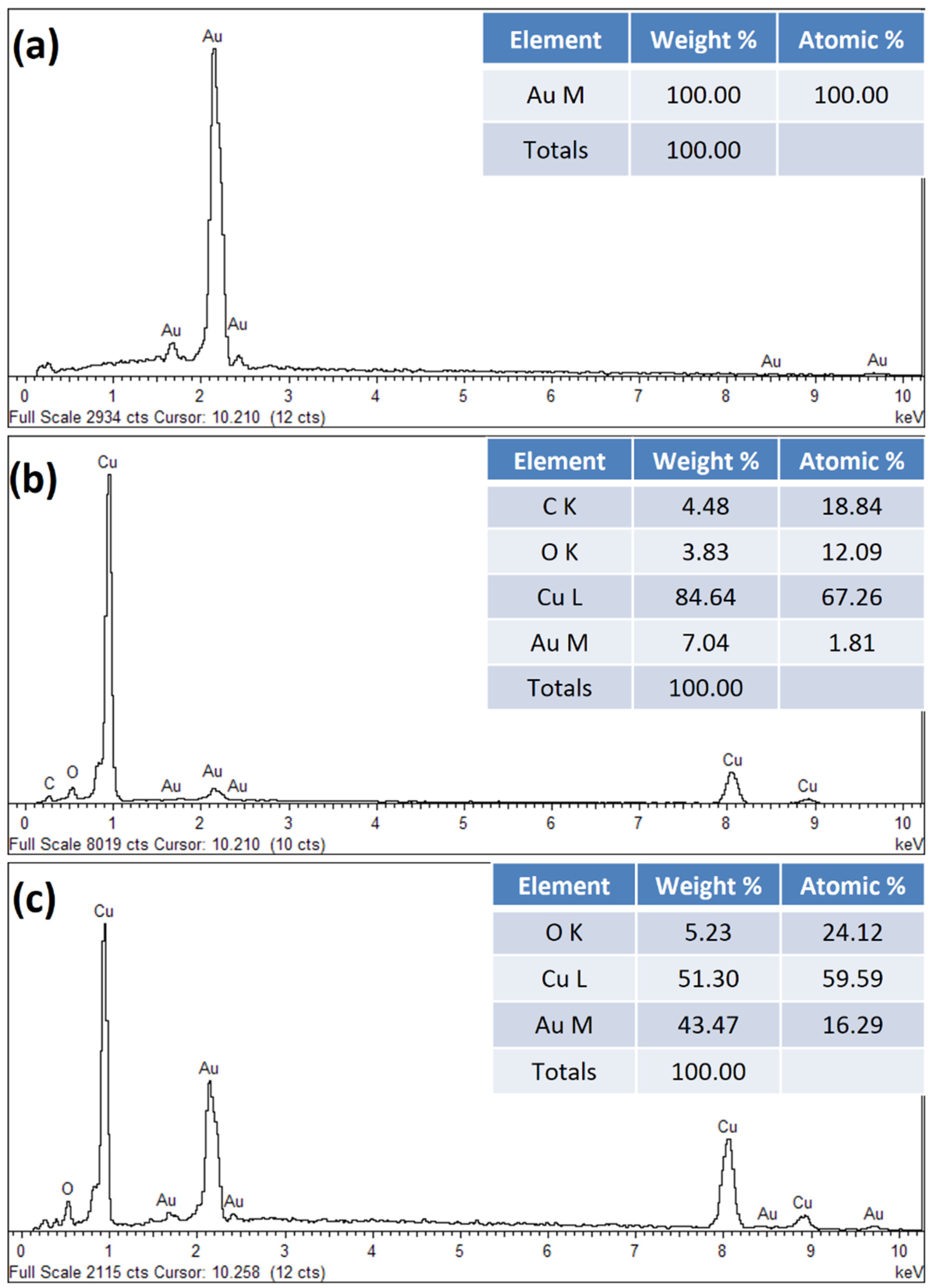
3.1. Physicochemical Characterization of Synthesized AuNPs
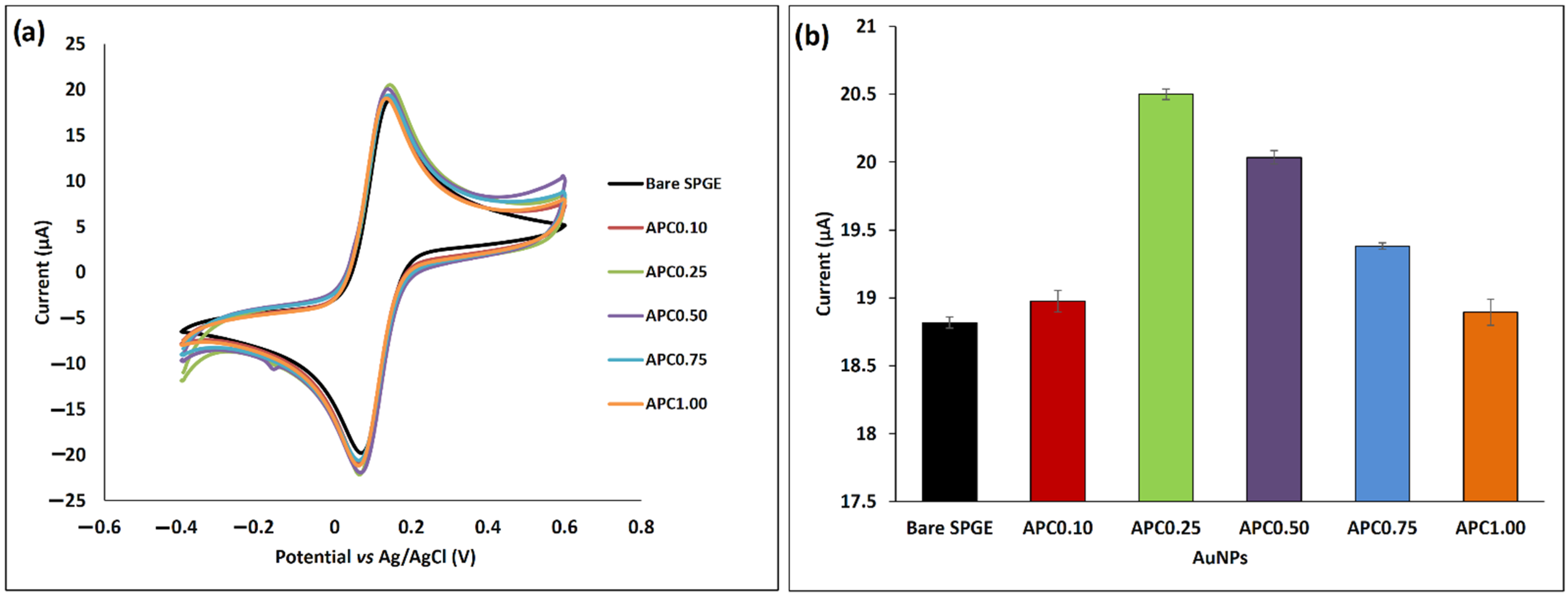
3.2. Conductivity of Bare and Crosslinked AuNPs-Modified SPGE
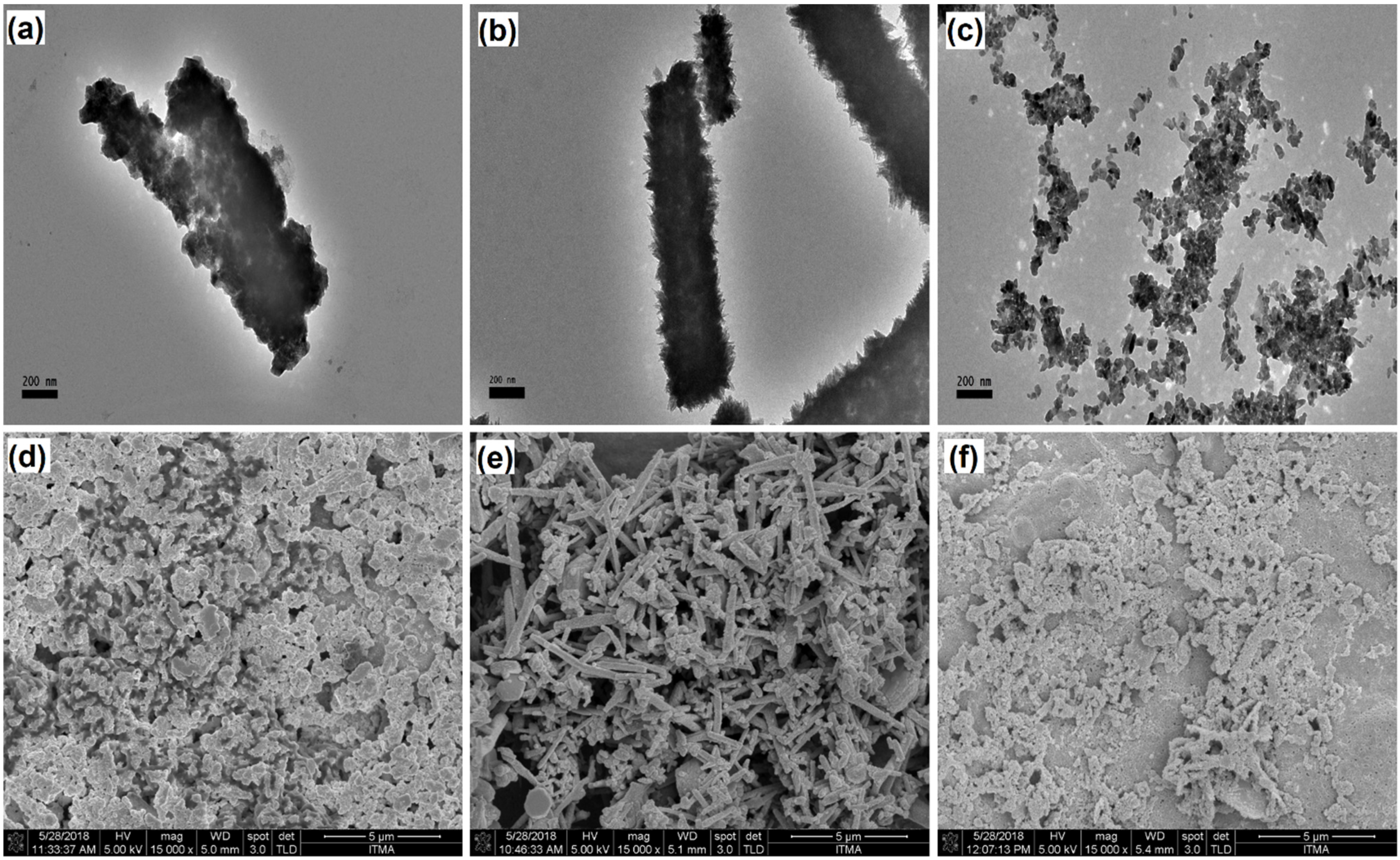
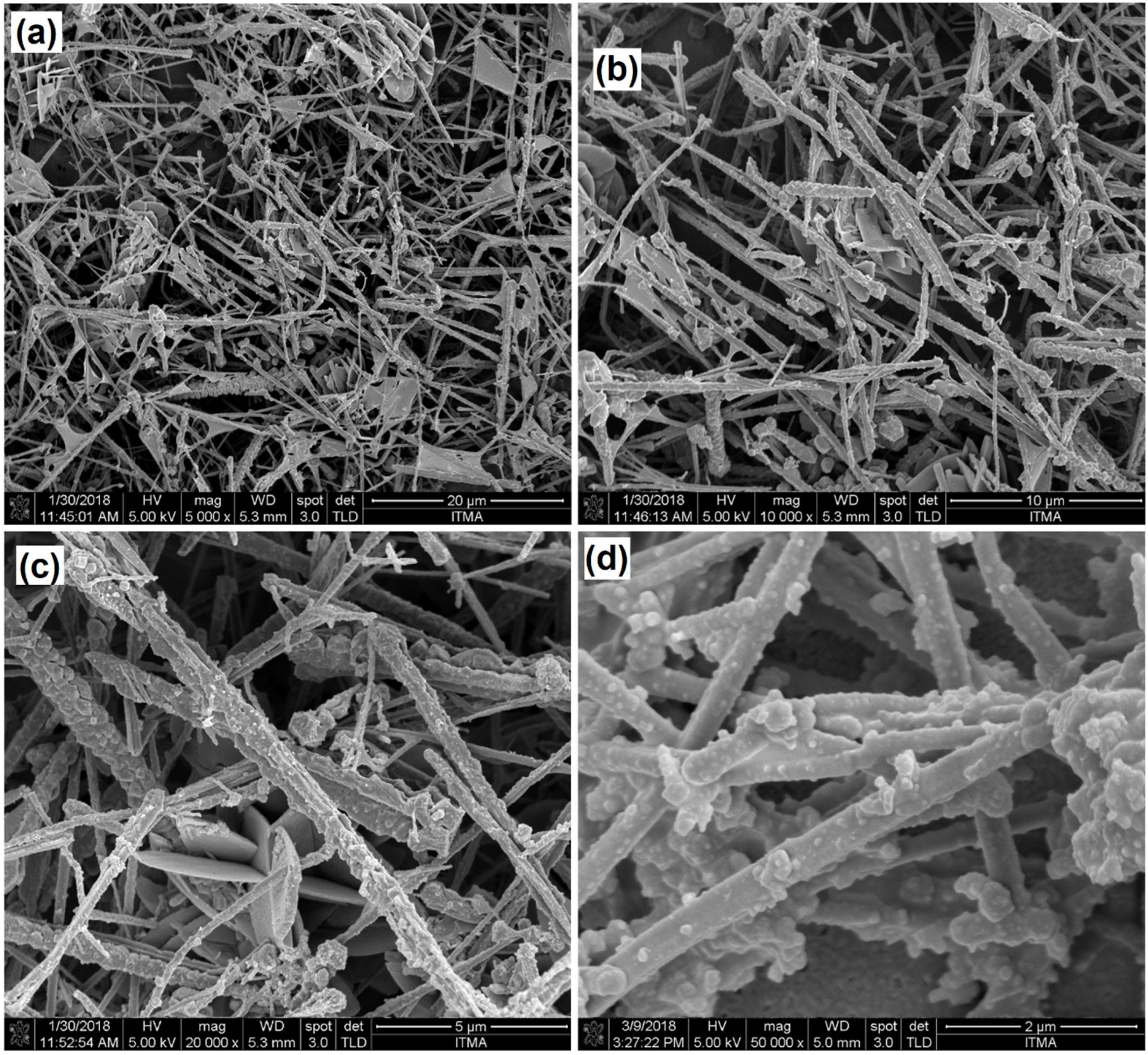
3.3. Morphological Analysis of CuNWs-Modified SPGE
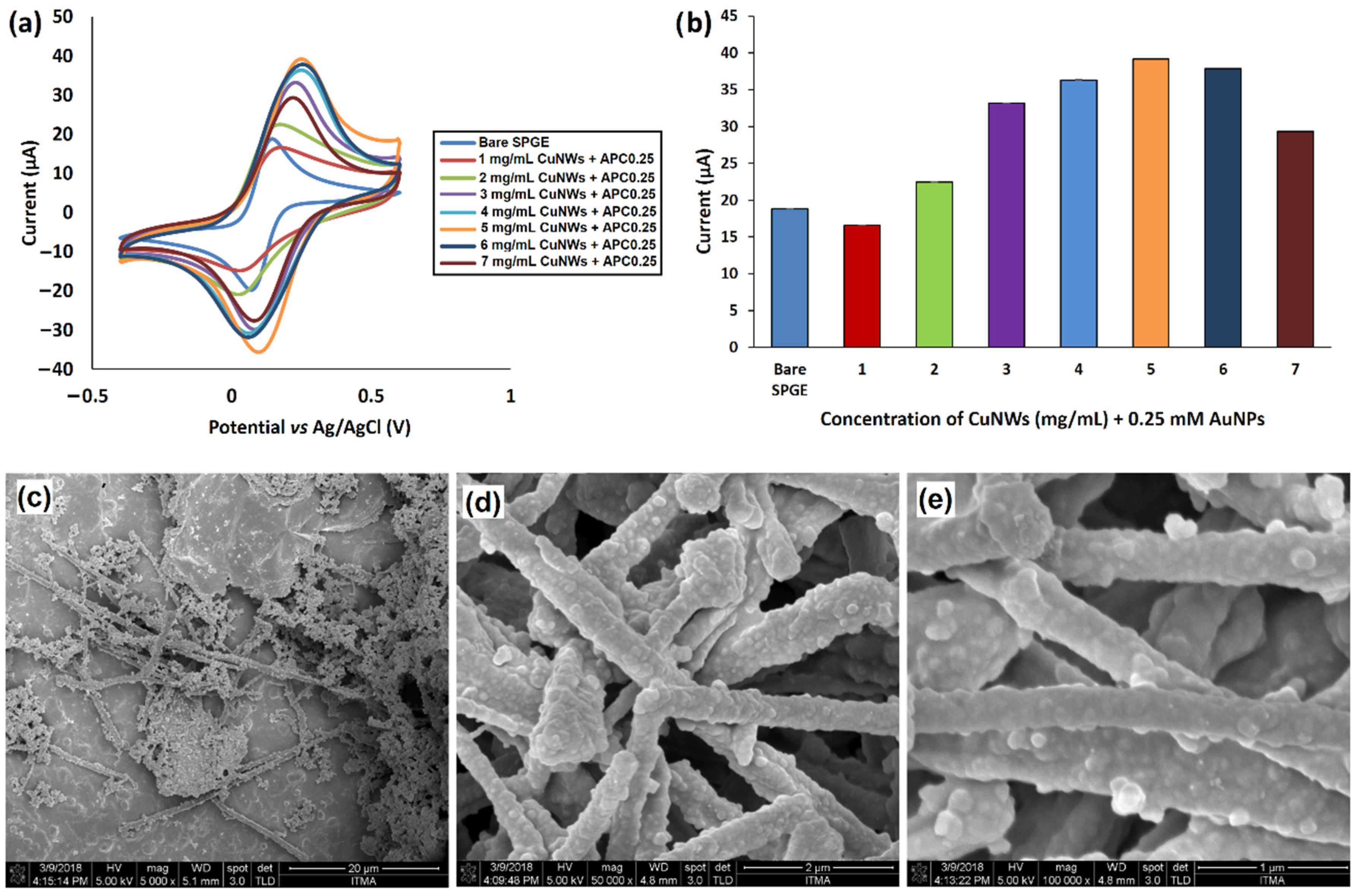
3.4. CuNWs/AuNPs Modified SPGE
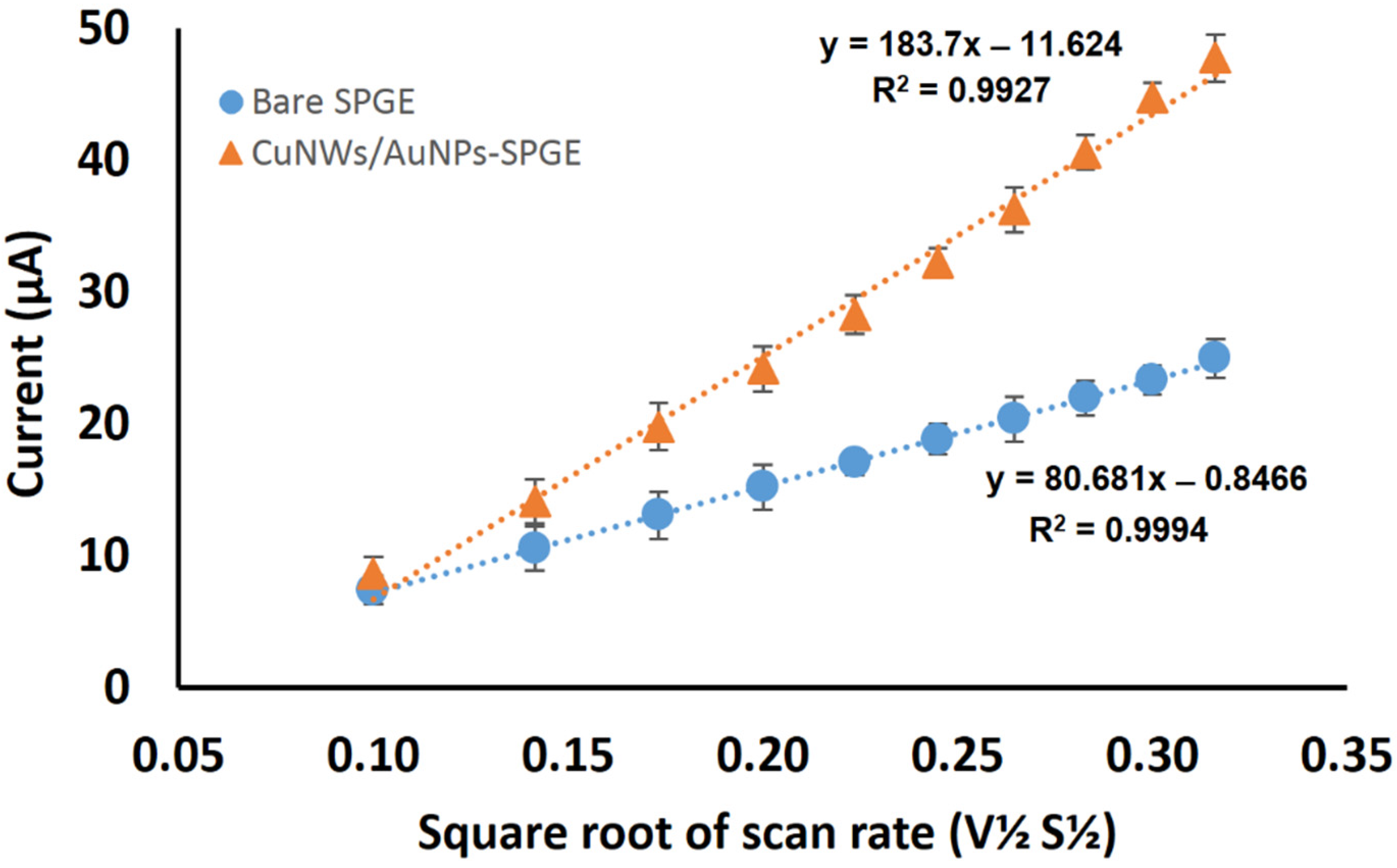
3.5. Effective Surface Area for CuNWs-AuNPs-Modified SPGE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noh, M.F.M.; Tothill, I.E. Development and characterisation of disposable gold electrodes, and their use for lead (II) analysis. Anal. Bioanal. Chem. 2006, 386, 2095–2106. [Google Scholar] [CrossRef]
- Sanllorente-Méndez, S.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Determination of arsenic (III) using platinum nanoparticle-modified screen-printed carbon-based electrodes. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 635–639. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Ogorevc, B. Bismuth-coated screen-printed electrodes for stripping voltammetric measurements of trace lead. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2001, 13, 13–16. [Google Scholar] [CrossRef]
- Yang, N.; Uetsuka, H.; Williams, O.A.; Osawa, E.; Tokuda, N.; Nebel, C.E. Vertically aligned diamond nanowires: Fabrication, characterization, and application for DNA sensing. Phys. Status Solidi (A) 2009, 206, 2048–2056. [Google Scholar] [CrossRef]
- Ramulu, T.; Venu, R.; Sinha, B.; Lim, B.; Jeon, S.; Yoon, S.; Kim, C. Nanowires array modified electrode for enhanced electrochemical detection of nucleic acid. Biosens. Bioelectron. 2013, 40, 258–264. [Google Scholar] [CrossRef]
- Ganguly, A.; Chen, C.-P.; Lai, Y.-T.; Kuo, C.-C.; Hsu, C.-W.; Chen, K.-H.; Chen, L.-C. Functionalized GaN nanowire-based electrode for direct label-free voltammetric detection of DNA hybridization. J. Mater. Chem. 2009, 19, 928–933. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, H.; Hu, Y.; Yao, T.; Huang, S. A novel electrochemical DNA biosensor based on graphene and polyaniline nanowires. Electrochim. Acta 2011, 56, 2676–2681. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, B.; Wang, F.; Li, J.; Deng, L.; Liu, Y.-N. Construction of highly ordered polyaniline nanowires and their applications in DNA sensing. Biosens. Bioelectron. 2014, 52, 422–426. [Google Scholar] [CrossRef]
- Ramulu, T.; Venu, R.; Sinha, B.; Yoon, S.; Kim, C. Electrodeposition of CoPtP/Au multisegment nanowires: Synthesis and DNA functionalization. Int. J. Electrochem. Sci. 2012, 7, 7762–7769. [Google Scholar]
- Wang, J.; Li, S.; Zhang, Y. A sensitive DNA biosensor fabricated from gold nanoparticles, carbon nanotubes, and zinc oxide nanowires on a glassy carbon electrode. Electrochim. Acta 2010, 55, 4436–4440. [Google Scholar] [CrossRef]
- Coffer, J. Overview of semiconducting silicon nanowires for biomedical applications. In Semiconducting Silicon Nanowires for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–7. [Google Scholar]
- Zhao, S.; Han, F.; Li, J.; Meng, X.; Huang, W.; Cao, D.; Zhang, G.; Sun, R.; Wong, C.P. Advancements in copper nanowires: Synthesis, purification, assemblies, surface modification, and applications. Small 2018, 14, 1800047. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, Y.; Lu, X.; Sun, M.; Liu, H.; Yu, G.; Gao, F. Palladium-copper nanowires-based biosensor for the ultrasensitive detection of organophosphate pesticides. Anal. Chim. Acta 2017, 982, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dong, Z.; Li, Y.; Li, J.; Wang, J.; Yang, H.; Li, S.; Guo, S.; Jin, J.; Li, R. High performance non-enzymatic glucose biosensor based on copper nanowires–carbon nanotubes hybrid for intracellular glucose study. Sens. Actuators B Chem. 2013, 182, 618–624. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.; Li, Y.; Li, Y.; Ma, G.; Liu, X. Construction of Poly (methylene blue)/Copper Nanowires Modified Electrode for High-Performance Luteolin Sensing. J. Electrochem. Soc. 2020, 167, 147513. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Lin, Y.; Du, D. The vital function of Fe3O4@ Au nanocomposites for hydrolase biosensor design and its application in detection of methyl parathion. Nanoscale 2013, 5, 1121–1126. [Google Scholar] [CrossRef]
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, B.; Boroujeni, M.K.; Ensafi, A.A. Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole, sol-gel, and gold nanoparticles hybrid nanocomposite modified pencil graphite electrode. Biosens. Bioelectron. 2014, 60, 77–83. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Ciaurriz, P.; Fernández, F.; Tellechea, E.; Moran, J.F.; Asensio, A.C. Comparison of four functionalization methods of gold nanoparticles for enhancing the enzyme-linked immunosorbent assay (ELISA). Beilstein J. Nanotechnol. 2017, 8, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, M.; Kaneko, K.; Hara, M.; Matsui, M.; Morita, K.; Maruyama, T. Covalent immobilization of gold nanoparticles on a plastic substrate and subsequent immobilization of biomolecules. RSC Adv. 2021, 11, 23409–23417. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. A novel disposable biosensor based on SiNWs/AuNPs modified-screen printed electrode for dengue virus DNA oligomer detection. IEEE Sens. J. 2015, 15, 4420–4427. [Google Scholar] [CrossRef] [Green Version]
- Lian, W.; Liu, S.; Yu, J.; Xing, X.; Li, J.; Cui, M.; Huang, J. Electrochemical sensor based on gold nanoparticles fabricated molecularly imprinted polymer film at chitosan–platinum nanoparticles/graphene–gold nanoparticles double nanocomposites modified electrode for detection of erythromycin. Biosens. Bioelectron. 2012, 38, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. The utilization of SiNWs/AuNPs-modified indium tin oxide (ITO) in fabrication of electrochemical DNA sensor. Mater. Sci. Eng. C 2014, 45, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Shoaie, N.; Forouzandeh, M.; Omidfar, K. Highly sensitive electrochemical biosensor based on polyaniline and gold nanoparticles for DNA detection. IEEE Sens. J. 2017, 18, 1835–1843. [Google Scholar] [CrossRef]
- Fani, M.; Rezayi, M.; Meshkat, Z.; Rezaee, S.A.; Makvandi, M.; Angali, K.A. A novel electrochemical DNA biosensor based on a gold nanoparticles-reduced graphene oxide-polypyrrole nanocomposite to detect human T-lymphotropic virus-1. IEEE Sens. J. 2020, 20, 10625–10632. [Google Scholar] [CrossRef]
- Taei, M.; Salavati, H.; Banitaba, S.H.; Shahidi, L. A novel hydrazine electrochemical sensor based on gold nanoparticles decorated redox-active 2-amino-4H-chromene-3-carbonitrile. IEEE Sens. J. 2017, 17, 7325–7331. [Google Scholar] [CrossRef]
- Nazarpour, S.; Hajian, R.; Sabzvari, M.H. A novel nanocomposite electrochemical sensor based on green synthesis of reduced graphene oxide/gold nanoparticles modified screen printed electrode for determination of tryptophan using response surface methodology approach. Microchem. J. 2020, 154, 1–7. [Google Scholar] [CrossRef]
- Ji, D.; Xu, N.; Liu, Z.; Shi, Z.; Low, S.S.; Liu, J.; Cheng, C.; Zhu, J.; Zhang, T.; Xu, H. Smartphone-based differential pulse amperometry system for real-time monitoring of levodopa with carbon nanotubes and gold nanoparticles modified screen-printing electrodes. Biosens. Bioelectron. 2019, 129, 216–223. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Chen, K.-L.; Linn, J.-J.; Yang, C.-F. Electrochemically controlling the size of gold nanoparticles. J. Electrochem. Soc. 2006, 153, D193–D198. [Google Scholar] [CrossRef]
- Nur, H.; Nasir, S.M. Gold nanoparticles embedded on the surface of polyvinyl alcohol layer. Malays. J. Fundam. Appl. Sci. 2008, 4, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.-M.; Su, W.-F.; Lin, Y.-J.; Wu, C.-S.; Chen, C.-D. Two-dimensional arrays of self-assembled gold and sulfur-containing fullerene nanoparticles. Langmuir 2002, 18, 3332–3335. [Google Scholar] [CrossRef]
- Łuczak, T. Gold electrodes modified with self-assembled layers made of sulphur compounds and gold nanoparticles used for selective electrocatalytic oxidation of catecholamine in the presence of interfering ascorbic and uric acids. Int. J. Electrochem. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Verma, H.N.; Singh, P.; Chavan, R. Gold nanoparticle: Synthesis and characterization. Vet. World 2014, 7, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Jiang, T.; Chen, X.; Jiang, D.; Xie, K.; Jiang, Y.; Wang, Y. Electrolyte additive induced fast-charge/slow-discharge process: Potassium ferricyanide and potassium persulfate for CoO-based supercapacitors. J. Colloid Interface Sci. 2020, 576, 505–513. [Google Scholar] [CrossRef]
- Portet, C.; Yushin, G.; Gogotsi, Y. Effect of carbon particle size on electrochemical performance of EDLC. J. Electrochem. Soc. 2008, 155, A531–A536. [Google Scholar] [CrossRef]
- Łuczak, T.; Bełtowska-Brzezinska, M. Gold electrodes modified with gold nanoparticles and thio compounds for electrochemical sensing of dopamine alone and in presence of potential interferents. A comparative study. Microchim. Acta 2011, 174, 19–30. [Google Scholar] [CrossRef]
- Paulose, R.; Mohan, R.; Parihar, V. Nanostructured nickel oxide and its electrochemical behaviour—A brief review. Nano-Struct. Nano-Objects 2017, 11, 102–111. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, M.; Qu, F.; Shen, G.; Yu, R. Enzyme-functionalized gold nanowires for the fabrication of biosensors. Bioelectrochemistry 2007, 71, 211–216. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. Surface modifications to boost sensitivities of electrochemical biosensors using gold nanoparticles/silicon nanowires and response surface methodology approach. J. Mater. Sci. 2016, 51, 1083–1097. [Google Scholar] [CrossRef]
- Saberi, R.S.; Shahrokhian, S.; Marrazza, G. Amplified electrochemical DNA sensor based on polyaniline film and gold nanoparticles. Electroanalysis 2013, 25, 1373–1380. [Google Scholar] [CrossRef]
- Qu, F.; Yang, M.; Shen, G.; Yu, R. Electrochemical biosensing utilizing synergic action of carbon nanotubes and platinum nanowires prepared by template synthesis. Biosens. Bioelectron. 2007, 22, 1749–1755. [Google Scholar] [CrossRef]
- Zhang, R.; Hummelgård, M.; Olin, H. Simple and efficient gold nanoparticles deposition on carbon nanotubes with controllable particle sizes. Mater. Sci. Eng. B 2009, 158, 48–52. [Google Scholar] [CrossRef]


| Nanomaterials | Detection | References |
|---|---|---|
| Silicon nanowires/AuNPs | Dengue virus | [24] |
| Polyaniline/AuNPs | DNA molecules | [25] |
| Reduced grapheme oxide/polypyrrole/AuNPs | Human T-Lymphotropic Virus-1 (HTLV-1) | [26] |
| 2-Amino-4H-chromene-3-carbonitrile/AuNPs | Hydrazine | [27] |
| Reduced grapheme oxide/AuNPs | L-tryptophan | [28] |
| Single wall carbon nanotubes/chitosan/AuNPs | Levodopa | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusnin, N.; Yusof, N.A.; Mutalib, N.A.A.; Mohammad, F.; Abdullah, J.; Sabri, S.; Mustafa, S.; Mohamad Saman, A.F.; Mohd Faudzi, F.N.; Soleiman, A.A. Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode. Coatings 2022, 12, 622. https://doi.org/10.3390/coatings12050622
Kusnin N, Yusof NA, Mutalib NAA, Mohammad F, Abdullah J, Sabri S, Mustafa S, Mohamad Saman AF, Mohd Faudzi FN, Soleiman AA. Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode. Coatings. 2022; 12(5):622. https://doi.org/10.3390/coatings12050622
Chicago/Turabian StyleKusnin, Norzila, Nor Azah Yusof, Nurul Asyikeen Ab Mutalib, Faruq Mohammad, Jaafar Abdullah, Suriana Sabri, Shuhaimi Mustafa, Ahmad Farabi Mohamad Saman, Fatin Nabilah Mohd Faudzi, and Ahmed A. Soleiman. 2022. "Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode" Coatings 12, no. 5: 622. https://doi.org/10.3390/coatings12050622
APA StyleKusnin, N., Yusof, N. A., Mutalib, N. A. A., Mohammad, F., Abdullah, J., Sabri, S., Mustafa, S., Mohamad Saman, A. F., Mohd Faudzi, F. N., & Soleiman, A. A. (2022). Enhanced Electrochemical Conductivity of Surface-Coated Gold Nanoparticles/Copper Nanowires onto Screen-Printed Gold Electrode. Coatings, 12(5), 622. https://doi.org/10.3390/coatings12050622







