Microstructure and Corrosion Behavior of Laser Cladding FeCoNiCrBSi Based High-Entropy Alloy Coatings
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Preparation of Gas Atomized Powders
2.2. Preparation of Laser Cladding Coatings
2.3. Microstructural Characterization of Powders and Coatings
2.4. Corrosion Behavior of Laser Cladding Coatings
2.5. Characterization of the Passivation Film
3. Results
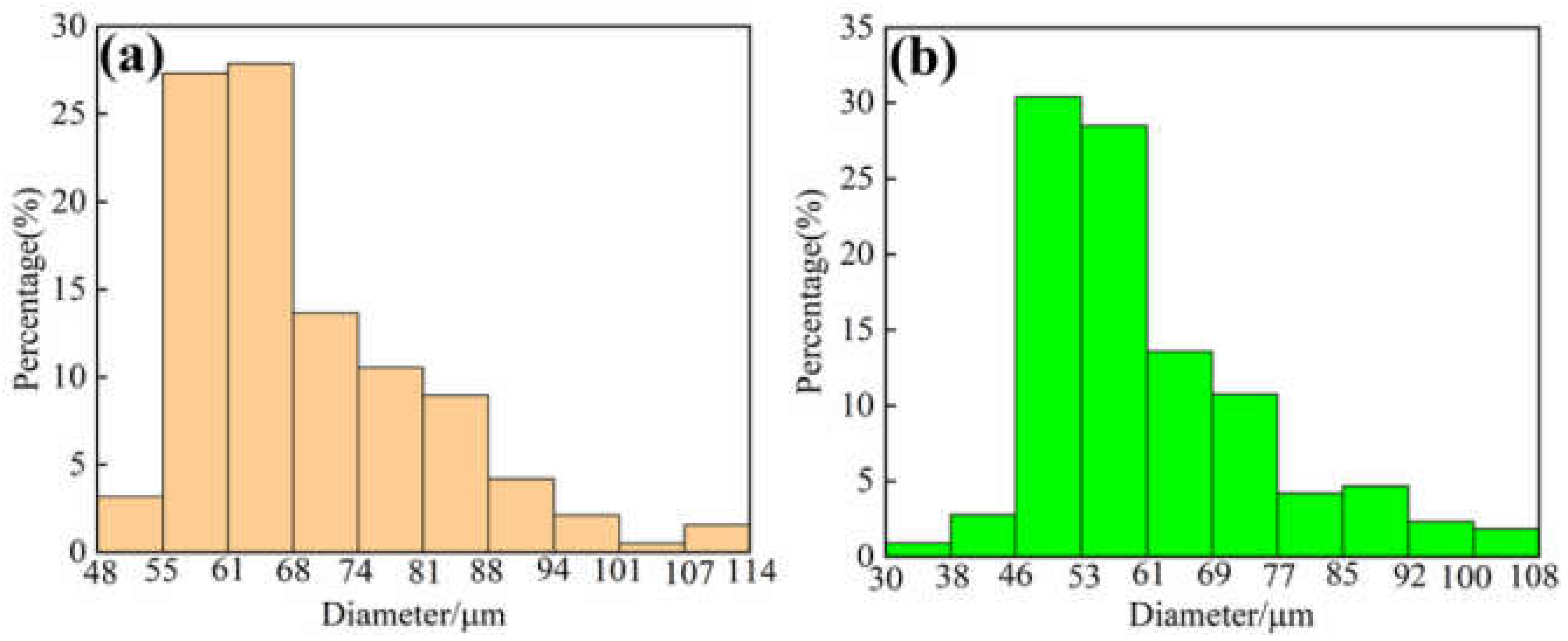
3.1. Microstructure of the Gas Atomized Powders
3.2. Phase Structure of Powders and Laser Cladding Coatings
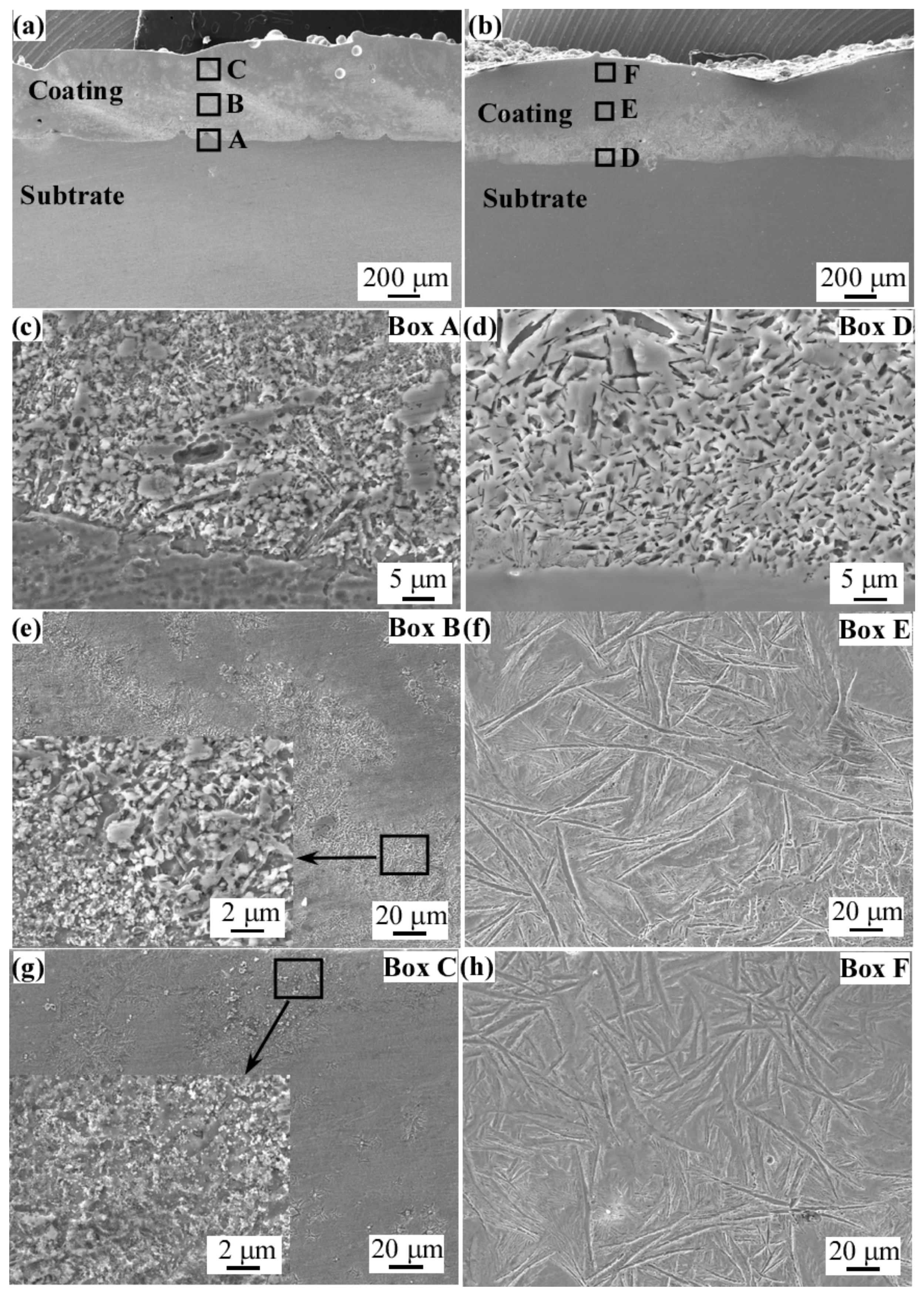
3.3. Microstructure Characterization of Laser Cladding Coatings
3.4. Corrosion Behavior of Laser Cladding Coatings
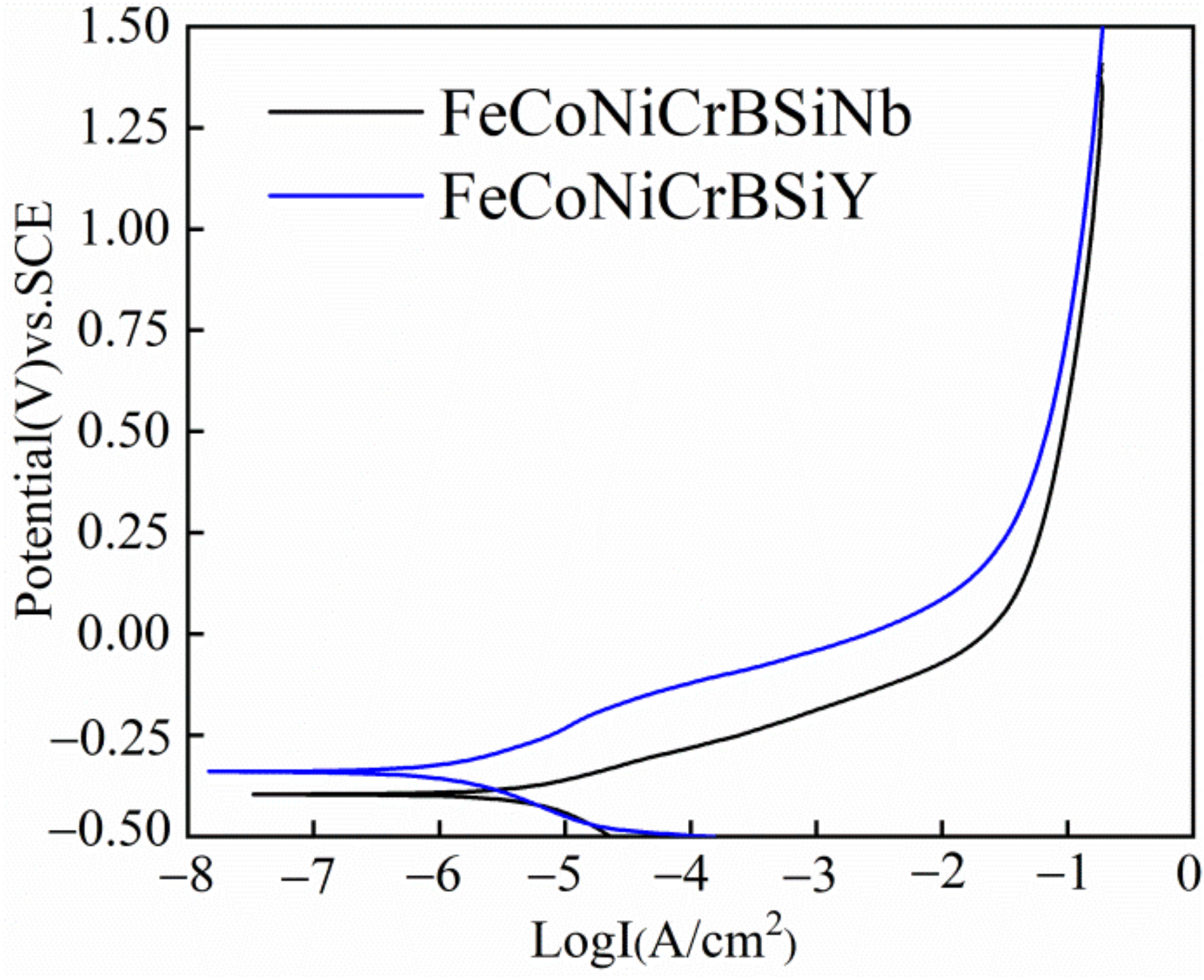
3.4.1. PDP Curves
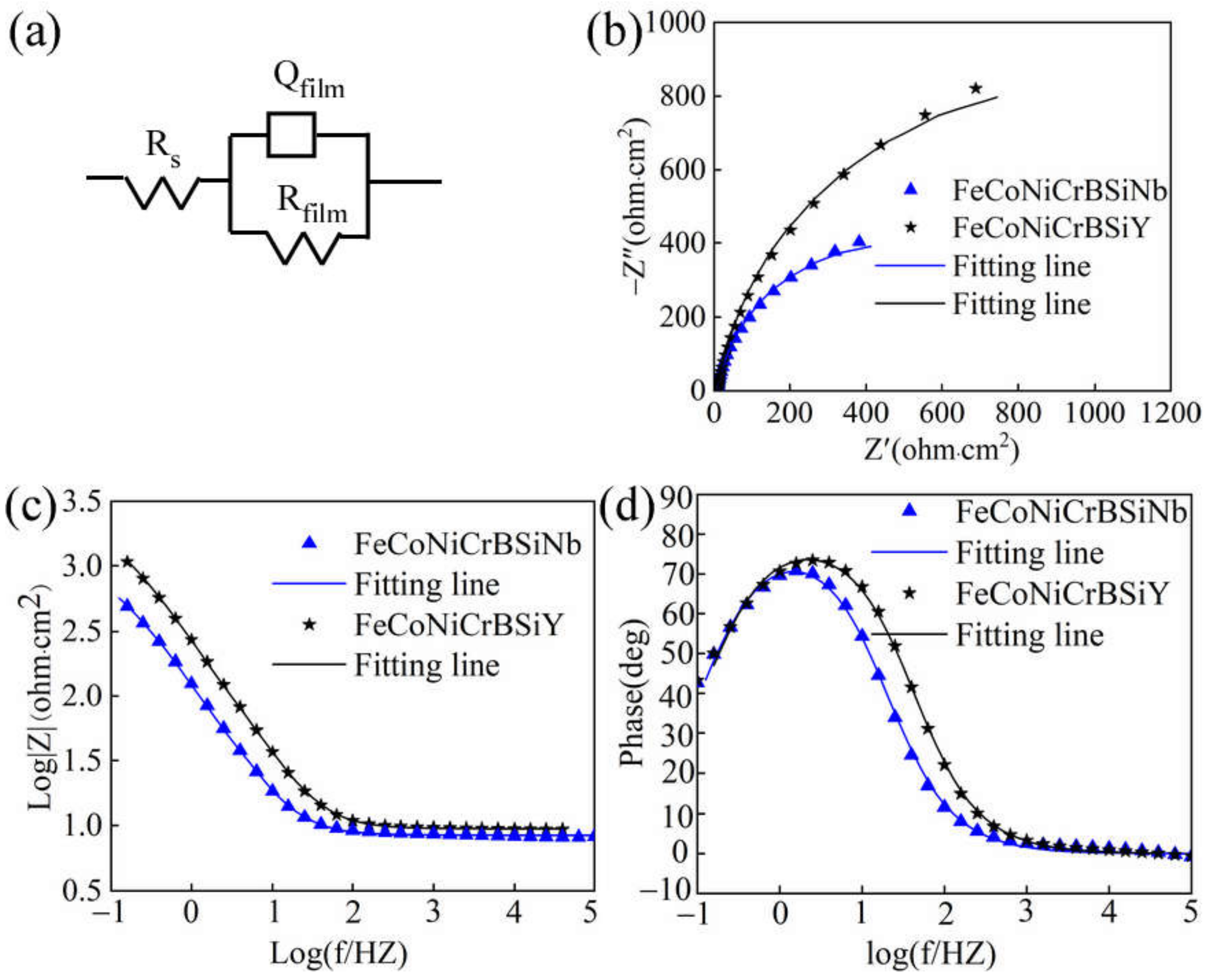
3.4.2. Electrochemical Impedance Spectroscopy
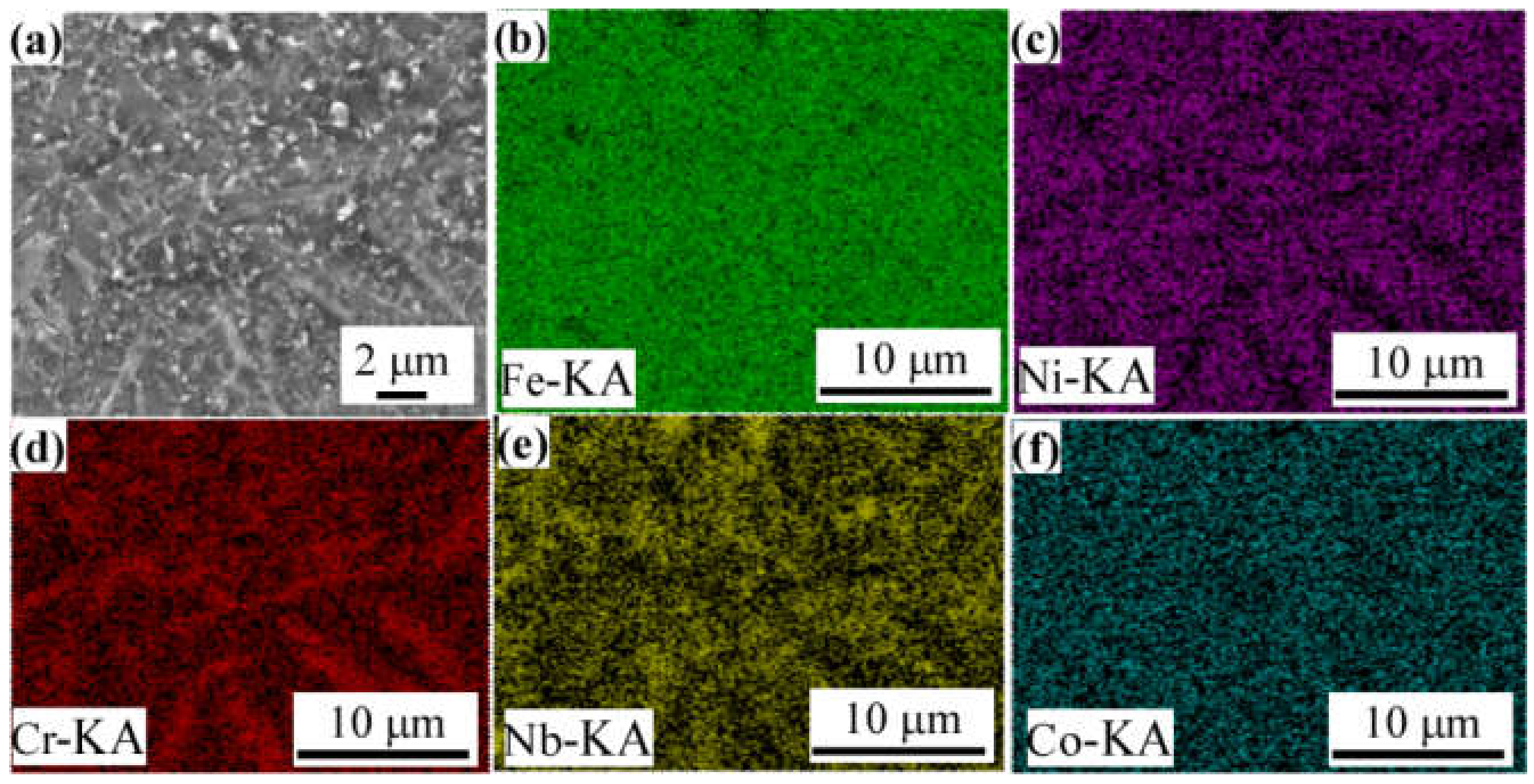
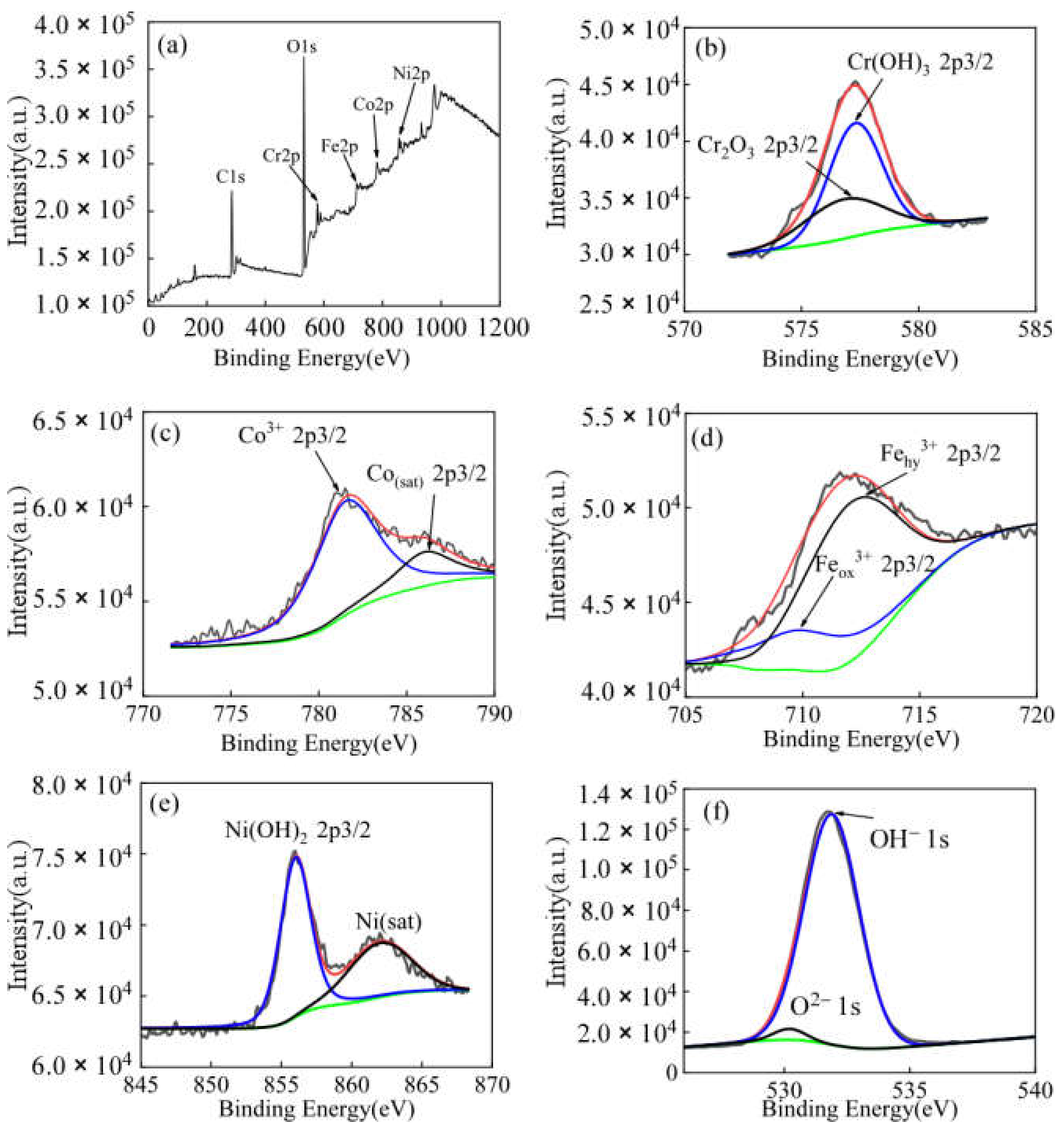
3.5. Surface Analysis of the Passive Film
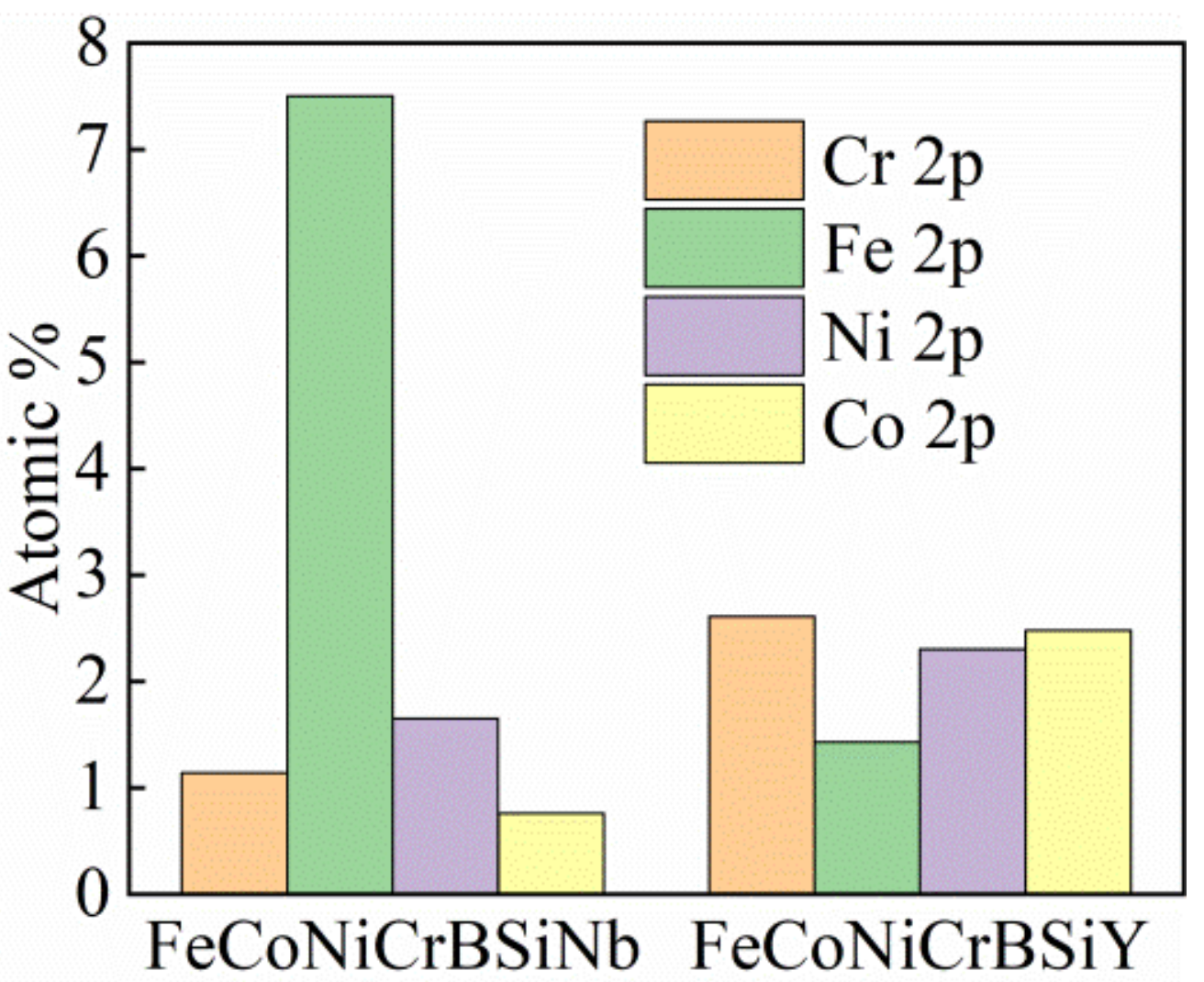
3.5.1. XPS Analysis
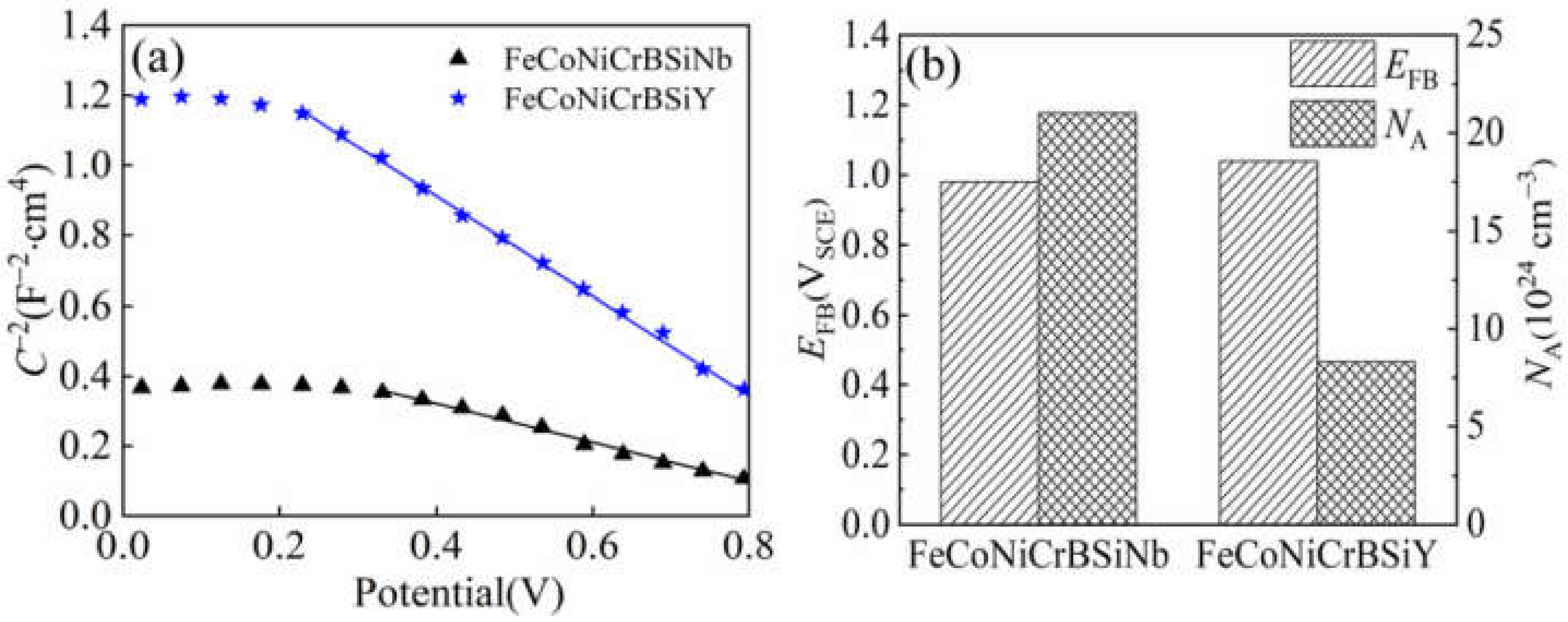
3.5.2. Mott-Schottky Curves
4. Discussion
4.1. Phase Structure of the Coating
4.2. Influence of Passive Film on Corrosion Resistance of Coatings
4.2.1. The Composition of the Passivation Film
4.2.2. The Semiconducting Properties of the Passivation Film
5. Conclusions
- (1)
- FeCoNiCrBSiNb powder is composed of face-centered cubic solid solution (FCC), Laves and Cr2B. FeCoNiCrBSiY powder is composed of face-centered cubic solid solution (FCC) and Cr2B. Due to the remelting and multiple heat treatments during the coating preparation, various borides such as Cr2B, Fe2B, Fe3B, CoB and Ni4B3 phases were precipitated in the coating. The amorphous or nanocrystalline content of the top layer of the coatings increases significantly due to the faster cooling rate.
- (2)
- Both coatings exhibited dendritic structures in the bonding area with the substrate. In the FeCoNiCrBSiNb coating, the size of the dendrites on top of the coating is significantly reduced. A large number of elongated borides precipitated in the FeCoNiCrBSiY coating. Due to the faster cooling rate of the top layer of the coating, the content of the borides decreases and the size becomes smaller.
- (3)
- FeCoNiCrBSiY coating exhibits high corrosion potential (Ecorr = −340 mV), low corrosion current density (icorr = 1.2 × 10−6 A/cm2), large polarization resistance (Rp = 20,000 Ohm·cm2) and high passivation film resistance (Rfilm = 1977 Ohm·cm2) in 3.5 wt.% NaCl solution, showing better corrosion resistance. The passivation film formed on the FeCoNiCrBSiY coating contains higher Cr/Fe ratio, indicating that the passivation film has better compactness.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, M.X.; Melchers, R.; Chaves, L. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Chen, M.D.; Pang, K.; Liu, Z.Y.; Wu, J.S.; Li, X.G. Influence of rust permeability on corrosion of E690 steel in industrial and non-industrial marine splash zones. Mater. Eng. Perform. 2018, 27, 3742–3749. [Google Scholar] [CrossRef]
- Si, C.R.; Duan, B.B. Microstructure, corrosion-resistance, and wear-resistance properties of subsonic flame sprayed amorphous Fe-Mo-Cr-Co coating with extremely high amorphous rate. J. Mater. Res. Technol. 2020, 9, 3292–3303. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, Q.; Li, M. New ferromagnetic (Fe1/3Co1/3Ni1/3)80(P1/2B1/2)20 high entropy bulk metallic glass with superior magnetic and mechanical properties. J. Alloys Compd. 2019, 791, 947–951. [Google Scholar] [CrossRef]
- Ding, J.; Inoue, A.; Han, Y.; Kong, F.; Zhu, S.; Wang, Z.; Shalaan, E.-S.; Al-Marzouki, F. High entropy effect on structure and properties of (Fe,Co,Ni,Cr)-B amorphous alloys. J. Alloys Compd. 2017, 696, 345–352. [Google Scholar] [CrossRef]
- Shu, F.Y.; Zhang, B.L.; Liu, T.; Sui, S.H.; Liu, Y.X.; He, P.; Liu, B.; Xu, B.S. Effects of laser power on microstructure and properties of laser cladded CoCrBFeNiSi high-entropy alloy amorphous coatings. Surf. Coat. Technol. 2019, 358, 667–675. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 34, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Cui, R.; Cheng, Y.H.; Meng, X.L.; Feng, S.Z.; Han, Z.T. Microstructure and properties of heat treated 1Cr17Ni4MoB steel fabricated by laser melting deposition. Mater. Process. Technol. 2017, 108, 59–68. [Google Scholar]
- Olakanmi, E.O.; Sepako, M.; Morake, J.; Hoosain, S.E.; Pityana, S.L. Microstructural characteristics, crack frequency and diffusion kinetics of functionally graded Ti-Al composite coatings: Effects of laser energy density (LED). Met. Mater. Soc. 2018, 71, 900–911. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sun, B.; Ge, Y.Y.; Hu, X.L. Nb doping in laser-cladded Fe25Co25Ni25(B0.7Si0.3)25 high entropy alloy coatings: Microstructure evolution and wear behavior. Surf. Coat Technol. 2020, 402, 126321. [Google Scholar] [CrossRef]
- Shu, F.Y.; Yang, B.; Dong, S.Y.; Zhao, H.Y.; Xu, B.S.; Xu, F.J.; Liu, B.; He, P.; Feng, J.C. Effects of Fe-to-Co ratio on microstructure and mechanical properties of laser cladded FeCoCrBNiSi high-entropy alloy coatings. Appl. Surf. Sci. 2018, 450, 538–544. [Google Scholar] [CrossRef]
- Cheng, J.B.; Sun, B.; Ge, Y.; Hu, X.; Zhang, L.; Liang, X.; Zhang, X. Effect of B/Si ratio on structure and properties of high-entropy glassy Fe25Co25Ni25(BxSi1-x)25 coating prepared by laser cladding. Surf. Coat. Technol. 2020, 402, 126320. [Google Scholar] [CrossRef]
- Shu, F.Y.; Wu, L.; Zhao, H.Y.; Sui, S.H.; Zhou, L.; Zhang, J.; He, W.X.; He, P.; Xu, B.S. Microstructure and high-temperature wear mechanism of laser cladded CoCrBFeNiSi high-entropy alloy amorphous coating. Mater. Lett. 2018, 211, 235–238. [Google Scholar] [CrossRef]
- Chen, Y.; Dai, Z.W.; Jiang, J.Z. High entropy metallic glasses: Glass formation, crystallization and properties. J. Alloys Compd. 2021, 866, 158852. [Google Scholar] [CrossRef]
- Shu, F.Y.; Liu, S.; Zhao, H.Y.; He, W.X.; Sui, S.H.; Zhang, J.; He, P.; Xu, B.S. Structure and high-temperature property of amorphous composite coating synthesized by laser cladding FeCrCoNiSiB high-entropy alloy powder. J. Alloys Compd. 2018, 731, 662–666. [Google Scholar] [CrossRef]
- Koga, G.; Otani, L.; Silva, A.; Roche, V.; Nogueira, R.; Jorge, A.; Bolfarini, C.; Kiminami, C.; Botta, W. Characterization and corrosion resistance of boron-containing-austenitic stainless steels produced by rapid solidification techniques. Materials 2018, 11, 2189. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.L.; Zhang, Z.Y.; Gong, Y.B.; Nie, G.M. Microstructures and corrosion resistance of Fe-based amorphous/nanocrystalline coating fabricated by laser cladding. J. Alloys Compd. 2017, 728, 1116–1123. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Xu, B. Microstructure and electrochemical properties of CoCrCuFeNiNb high-entropy alloys coatings. Acta. Metall. Sin.-Engl. 2014, 27, 1031–1037. [Google Scholar] [CrossRef]
- Xiao, M.Y.; Gao, H.B.; Sun, L.B.; Wang, Z. Microstructure and mechanical properties of Fe-based amorphous alloy coatings prepared by ultra-high speed laser cladding. Mater. Lett. 2021, 297, 130002. [Google Scholar] [CrossRef]
- Milanti, A.; Matikainen, V.; Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Effect of spraying parameters on the microstructural and corrosion properties of HVAF-sprayed Fe-Cr-Ni-B-C coatings. Surf. Coat. Technol. 2015, 277, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Z.; Cheng, Y.H.; Yang, J.Y.; Wang, Q.Q. Influence of laser remelting on organization, mechanical properties and corrosion resistance of Fe-based amorphous composite coating. Surf. Coat. Technol. 2021, 414, 127081. [Google Scholar] [CrossRef]
- Lin, C.H.; Duh, J.G. Corrosion behavior of (Ti-Al-Cr-Si-V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Bijalwan, P.; Kumar, A.; Nayak, S.K.; Banerjee, A.; Laha, T. Microstructure and corrosion behavior of Fe-based amorphous composite coatings developed by atmospheric plasma spraying. J. Alloys Compd. 2019, 796, 47–54. [Google Scholar] [CrossRef]
- Huang, F.; Kang, J.J.; Yue, W.; Fu, Z.Q.; Zhu, L.N.; She, D.S.; Liang, J.; Wang, C.B. Corrosion Behavior of FeCrMoCBY Amorphous Coating Fabricated by High-Velocity Air Fuel Spraying. Therm. Spray Technol. 2019, 28, 842–850. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Hong, S.; Chen, L.; Qin, Y. A Comparative study of cyclic oxidation and sulfates-Induced hot corrosion behavior of arc-sprayed Ni-Cr-Ti coatings at moderate temperatures. Therm. Spray Technol. 2015, 24, 789–797. [Google Scholar] [CrossRef]
- Guo, W.M.; Zhang, H.L.; Zhao, S.; Ding, Z.B.; Liu, B.; Li, W.J.; Xu, H.H.; Liu, H.Y. Corrosion behavior of the CoNiCrAlY-Al2O3 composite coating based on core-shell structured powder design. Materials 2021, 14, 7093. [Google Scholar] [CrossRef]
- Berger, J.; Jorge, A.M., Jr.; Koga, G.Y.; Roche, V.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Influence of chromium concentration and partial crystallization on the corrosion resistance of FeCrNiB amorphous alloys. Mater. Charact. 2021, 179, 111369. [Google Scholar] [CrossRef]
- Liu, W.H.; Shieu, F.S.; Hsiao, W.T. Enhancement of wear and corrosion resistance of iron-based hard coatings deposited by high-velocity oxygen fuel (HVOF) thermal spraying. Surf. Coat. Technol. 2014, 249, 24–41. [Google Scholar] [CrossRef]
- Kiminami, C.S.; Souza, C.; Bonavina, L.F. Partial crystallization and corrosion resistance of amorphous Fe-Cr-M-B (M = Mo, Nb) alloys. J. Non-Cryst Solids 2010, 356, 2651–2657. [Google Scholar] [CrossRef]
- Botta, W.J.; Berger, J.E.; Kiminami, C.S.; Roche, V.; Nogueira, R.P.; Bolfarini, C. Corrosion resistance of Fe-based amorphous alloys. J. Alloys Compd. 2014, 586, 105–110. [Google Scholar] [CrossRef]
- Hao, E.K.; Liu, X.; An, Y.L.; Zhou, H.D.; Yan, F.Y. The coupling effect of immersion corrosion and cavitation erosion of NiCoCrAlYTa coatings in artificial seawater. Corros. Sci. 2020, 169, 108635. [Google Scholar] [CrossRef]
- Wang, W.R.; Qi, W.; Zhang, X.L.; Yang, X.; Xie, L.; Li, D.Y.; Xiang, Y.H. Superior corrosion resistance-dependent laser energy density in (CoCrFeNi)95Nb5 high entropy alloy coating fabricated by laser cladding. Int. J. Min. Met. Mater. 2021, 28, 888–897. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Y.; Li, Z.G.; Yao, C.W.; Yao, L.; Fan, C.Y. Corrosion properties of laser cladded CrCoNi medium entropy alloy coating. Surf. Coat. Technol. 2020, 397, 126004. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhou, Z.H.; Wang, Q.J. Role of passive film in dominating the electrochemical corrosion behavior of FeCrMoCBY amorphous coating. J. Alloys Compd. 2019, 811, 151962. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, W.H.; Yang, H.L.; Huang, H.; Ji, S.X.; Ruan, J.M.; Liu, Z.L. Corrosion behavior of CoCrNi medium-entropy alloy compared with 304 stainless steel in H2SO4 and NaOH solutions. Corros. Sci. 2020, 177, 108973. [Google Scholar] [CrossRef]
- Antou, G.; Montavon, G.; Hlawka, F.; Cornet, A.; Coddet, C. Exploring thermal spray gray alumina coating pore network architecture by combining stereological protocols and impedance electrochemical spectroscopy. Therm. Spray Technol. 2006, 15, 765–772. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Y.; Wei, D.; Liu, B.; Liu, R.; Nie, Y.; Li, Y. Building direction dependence of corrosion resistance property of Ti-6Al-4V alloy fabricated by electron beam melting. Corros. Sci. 2017, 127, 101–109. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Qian, W.X.; Ma, H.F.; Zhang, H.T.; Ying, W.Y. Highly selective production of heavy hydrocarbons over cobalt–graphene–silica nanocomposite catalysts. Rsc Adv. 2017, 7, 33441–33449. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Celik, G.; Gunduz, S.; Dogu, D.; Zhang, S.; Shan, J.; Tao, F.F.; Ozkan, U.S. Oxygen mobility in prereduced nano- and macro-ceria with Co loading: An AP-XPS, In-Situ DRIFTS and TPR Study. Catal. Lett. 2017, 147, 2863–2876. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, B.; Guo, W.; Fu, A.; Duan, H.; Li, W. Corrosion behavior and mechanism of FeCrNi medium entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2021, 867, 159094. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Jiang, L.L. Comparison of the pitting susceptibility and semiconducting properties of the passive films on carbon steel in chromate and bicarbonate solutions. Appl. Surf. Sci. 2000, 167, 113–121. [Google Scholar] [CrossRef]
- Li, G.; Gan, Y.; Liu, C.; Shi, Y.; Zhao, Y.; Kou, S. Corrosion and Wear Resistance of Fe-Based Amorphous Coatings. Coatings 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Belo, M.; Hakiki, N.E.; Ferreira, M. Semiconducting properties of passive films formed on nickel-base alloys type Alloy 600: Influence of the alloying elements. Electrochim. Acta 1999, 44, 2473–2481. [Google Scholar] [CrossRef]
- De Oliveira, M.C.L.; Pereira, V.S.M.; Correa, O.V.; de Lima, N.B.; Antunes, R.A. Correlation between the corrosion resistance and the semiconducting properties of the oxide film formed on AZ91D alloy after solution treatment. Corros. Sci. 2013, 69, 311–321. [Google Scholar] [CrossRef]
- Elzbieta, S.; Macdonald, D.D. Nature of the passive film on nickel. Electrochim. Acta 2002, 48, 69–77. [Google Scholar]
- Oje, A.M.; Ogwu, A.A.; Rahman, S.U.; Tsendzughul, N. Effect of temperature variation on the corrosion behaviour and semiconducting properties of the passive film formed on chromium oxide coatings exposed to saline solution. Corros. Sci. 2019, 154, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.D.; Wei, X.S.; Wang, Y.; Jiang, H.; Shen, J. Electrochemical behaviors and passive film properties of Fe-based bulk metallic glass in Cl-containing acetic acid solutions under high temperature. J. Alloys Compd. 2018, 766, 964–972. [Google Scholar] [CrossRef]
- Yang, G.; Lin, X.; Liu, F.; Hu, Q.; Ma, L.; Li, J.; Huang, W. Laser solid forming Zr-based bulk metallic glass. Intermetallics 2012, 22, 110–115. [Google Scholar] [CrossRef]
- Pauly, S.; Löber, L.; Petters, R.; Stoica, M.; Scudino, S.; Kühn, U.; Eckert, J. Processing metallic glasses by selective laser melting. Mater. Today 2013, 16, 37–41. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.J.; Dai, P.Q. Evolution of the microstructure and properties of laser-clad FeCrNiCoBx high-entropy alloy coatings. Mater. Sci. Technol. 2016, 32, 1666–1672. [Google Scholar] [CrossRef]
- Berger, J.E.; Schulz, R.; Savoie, S.; Gallego, J.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Wear and corrosion properties of HVOF coatings from Superduplex alloy modified with addition of boron. Surf. Coat. Technol. 2017, 309, 911–919. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, Y.; Liao, B.; Sun, Y.; Dai, X.; Yang, J.; Li, Z. Effect of laser remelting on microstructure and properties of WC reinforced Fe-based amorphous composite coatings by laser cladding. Opt. Laser Technol. 2018, 103, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Sudha, C.; Shankar, P.; Rao, R.V.; Thirumurugesan, R.; Vijayalakshmi, M.; Raj, B. Microchemical and microstructural studies in a PTA weld overlay of Ni-Cr-Si-B alloy on AISI 304L stainless steel. Surf. Coat. Technol. 2008, 202, 2103–2112. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Chen, P.J.; Hao, J.B. Microstructural characterization and corrosion behaviour of AlCoCrFeNiTix high-entropy alloy coatings fabricated by laser cladding. Surf. Coat. Technol. 2019, 361, 63–74. [Google Scholar] [CrossRef]
- Cui, Z.; Qin, Z.; Dong, P.; Mi, Y.; Gong, D.; Li, W. Microstructure and corrosion properties of FeCoNiCrMn high entropy alloy coatings prepared by high speed laser cladding and ultrasonic surface mechanical rolling treatment. Mater. Lett. 2019, 259, 126769. [Google Scholar] [CrossRef]
- Zheng, Z.B.; Zheng, Y.G. Effects of surface treatments on the corrosion and erosion-corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2016, 112, 657–668. [Google Scholar] [CrossRef]
- Rossi, A.; Elsener, B. Role of the interface oxide film/alloy composition and stability of stainless steels. Mater. Corros. 2012, 63, 1188–1193. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.D.; Chen, L.X.; Yuan, J.; Zhang, Z.H.; Wang, Q.D. The effect of partial substitution of Zr for Ti on the electrochemical properties and surface passivation film of Mg35Ti10−xZrxNi55 (x = 1, 3, 5, 7, 9) electrode alloys. J. Alloys Compd. 2002, 337, 296–302. [Google Scholar] [CrossRef]
- Amri, J.; Souier, T.; Malki, B.; Baroux, B. Effect of the final annealing of cold rolled stainless steels sheets on the electronic properties and pit nucleation resistance of passive films. Corros. Sci. 2008, 50, 431–435. [Google Scholar] [CrossRef]
- Cardoso, M.V.; Amaral, S.T.; Martini, E. Temperature effect in the corrosion resistance of Ni–Fe–Cr alloy in chloride medium. Corros. Sci. 2008, 50, 2429–2436. [Google Scholar] [CrossRef]
- Lu, W.; Wang, D.; Wang, Q.; Yang, F.; Li, T.; Shi, Y.; Zhang, S.; Yang, B. Sensitivity of corrosion behavior for Fe-based amorphous coating to temperature and chloride concentration. Coatings 2021, 11, 331. [Google Scholar] [CrossRef]
- Wang, Z.M.; Ma, Y.T.; Zhang, J.; Hou, W.; Chang, X.; Wang, J. Influence of yttrium as a minority alloying element on the corrosion behavior in Fe-based bulk metallic glasses. Electrochim. Acta 2009, 54, 261–269. [Google Scholar] [CrossRef]




| Co | Ni | Cr | Fe | B | Si | Y | Nb | |
|---|---|---|---|---|---|---|---|---|
| FeCoNiCrBSiY | 19.6 | 19.6 | 19.6 | 19.6 | 13.72 | 5.88 | 2.0 | -- |
| FeCoNiCrBSiNb | 6.0 | 17.4 | 9.0 | 43.6 | 17.5 | 1.5 | -- | 5.0 |
| Fe | Cr | Ni | Mn | P | C | Si | S |
|---|---|---|---|---|---|---|---|
| Bal | 18~20 | 8~11 | 2 | 0.045 | 0.08 | 1 | 0.03 |
| Power | Scan Speed (mm/min) | Powder Feeding Rate (g/min) | Overlap Rate | Spot Diameter (mm) | |
|---|---|---|---|---|---|
| FeCoNiCrBSiY | 2000 | 380 | 13.12 | 35% | 2 |
| FeCoNiCrBSiNb | 2000 | 350 | 14.80 | 35% | 2 |
| Co | Ni | Cr | Fe | B | Si | Y | Nb | |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.27 | 15.65 | 6.09 | 38.21 | 28.50 | 1.57 | - | 4.70 |
| 2 | 0.51 | 0.98 | 6.65 | 5.26 | 79.98 | 0.34 | - | 6.27 |
| 3 | 15.43 | 20.58 | 5.81 | 11.92 | 36.72 | 7.60 | 1.53 | - |
| 4 | 7.55 | 3.52 | 22.86 | 10.57 | 53.94 | 1.03 | 0.54 | - |
| Ecorr (mV) | icorr (A·cm−2) | βa (V·dec−1) | βc (V·dec−1) | Rp (Ω·cm2) | |
|---|---|---|---|---|---|
| FeCoNiCrBSiNb | −390 | 5.2 × 10−6 | 0.08 | −0.17 | 4548 |
| FeCoNiCrBSiY | −340 | 1.2 × 10−6 | 0.11 | −0.12 | 20,000 |
| Ti21.6Al11.3Cr19.4Si23.5V22.0O2.2 [23] | −541 | 6.14 × 10−6 | 2.76 | −3.85 | 11,360 |
| Fe87.6Cr2.5Si6.7B2.5C0.7 [24] | −640 | 1.74 × 10−5 | 0.109 | −0.195 | 1746 |
| Fe48Cr15Mo14C15B6Y2 [25] | −458 | 1.13 × 10−5 | - | - | - |
| Rs (Ohm·cm2) | Qfilm − Y0 (Ω−1·cm−2·s−n) | Qfilm−n | Rfilm (Ohm·cm2) | χ2 | |
|---|---|---|---|---|---|
| FeCoNiCrBSiNb | 8.4 | 1.5 × 10−3 | 0.9 | 919 | 7.05 × 10−4 |
| FeCoNiCrBSiY | 9.4 | 6.9 × 10−4 | 0.9 | 1977 | 3.25 × 10−4 |
| Element | Co | Cr | Ni | B |
|---|---|---|---|---|
| Fe | −1 | −1 | −2 | −26 |
| Co | - | −4 | 0 | −24 |
| Cr | −4 | - | −7 | −31 |
| Ni | 0 | - | −24 | |
| Nb | −25 | −7 | −30 | −54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, W.; Xu, H.; Chen, L.; Zeng, J.; Ding, Z.; Guo, W.; Liu, B. Microstructure and Corrosion Behavior of Laser Cladding FeCoNiCrBSi Based High-Entropy Alloy Coatings. Coatings 2022, 12, 628. https://doi.org/10.3390/coatings12050628
Zhang H, Li W, Xu H, Chen L, Zeng J, Ding Z, Guo W, Liu B. Microstructure and Corrosion Behavior of Laser Cladding FeCoNiCrBSi Based High-Entropy Alloy Coatings. Coatings. 2022; 12(5):628. https://doi.org/10.3390/coatings12050628
Chicago/Turabian StyleZhang, Hongling, Wenjuan Li, Huanhuan Xu, Liang Chen, Junshan Zeng, Zhibing Ding, Wenmin Guo, and Bin Liu. 2022. "Microstructure and Corrosion Behavior of Laser Cladding FeCoNiCrBSi Based High-Entropy Alloy Coatings" Coatings 12, no. 5: 628. https://doi.org/10.3390/coatings12050628






