Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products
Abstract
:1. Introduction
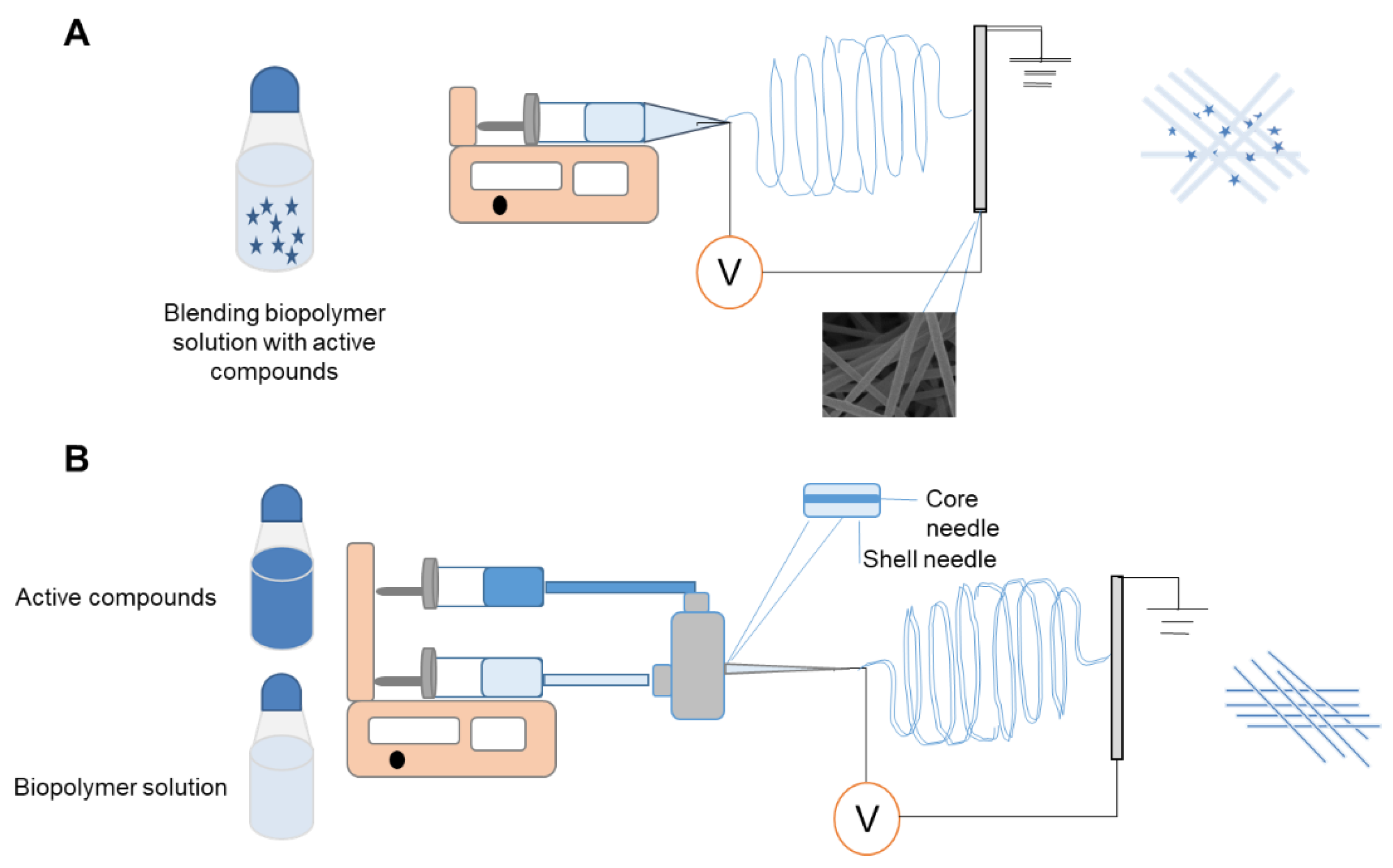
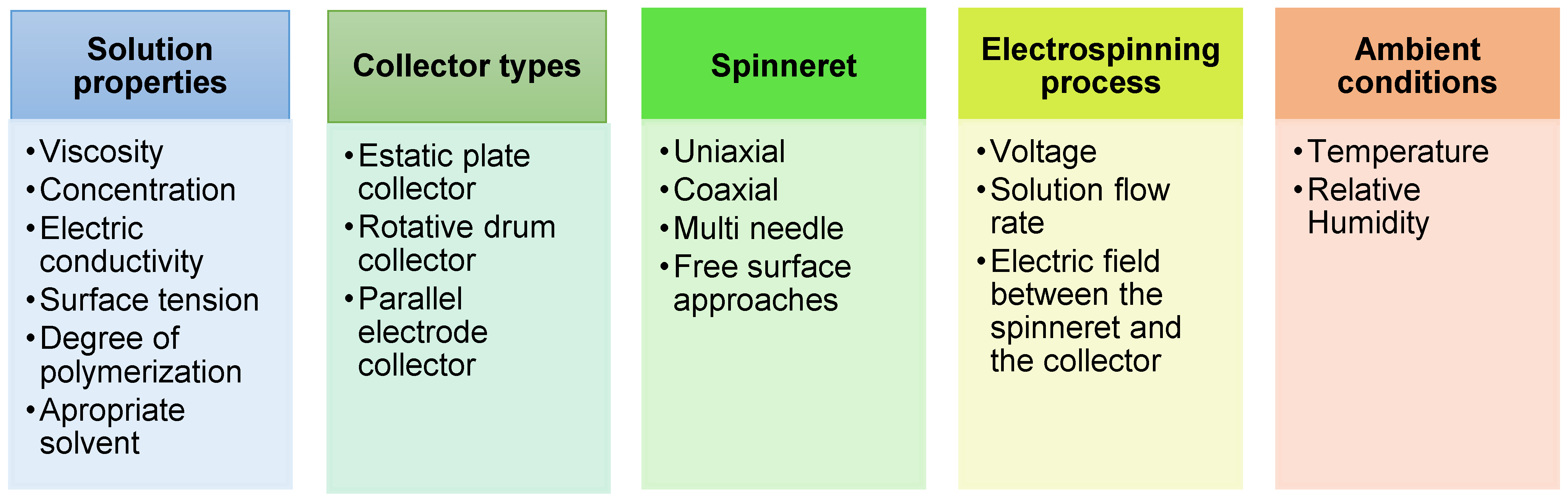
2. Strategies of Electrospinning Process
3. Overview of the Biopolymers Used for Electrospun Fibres
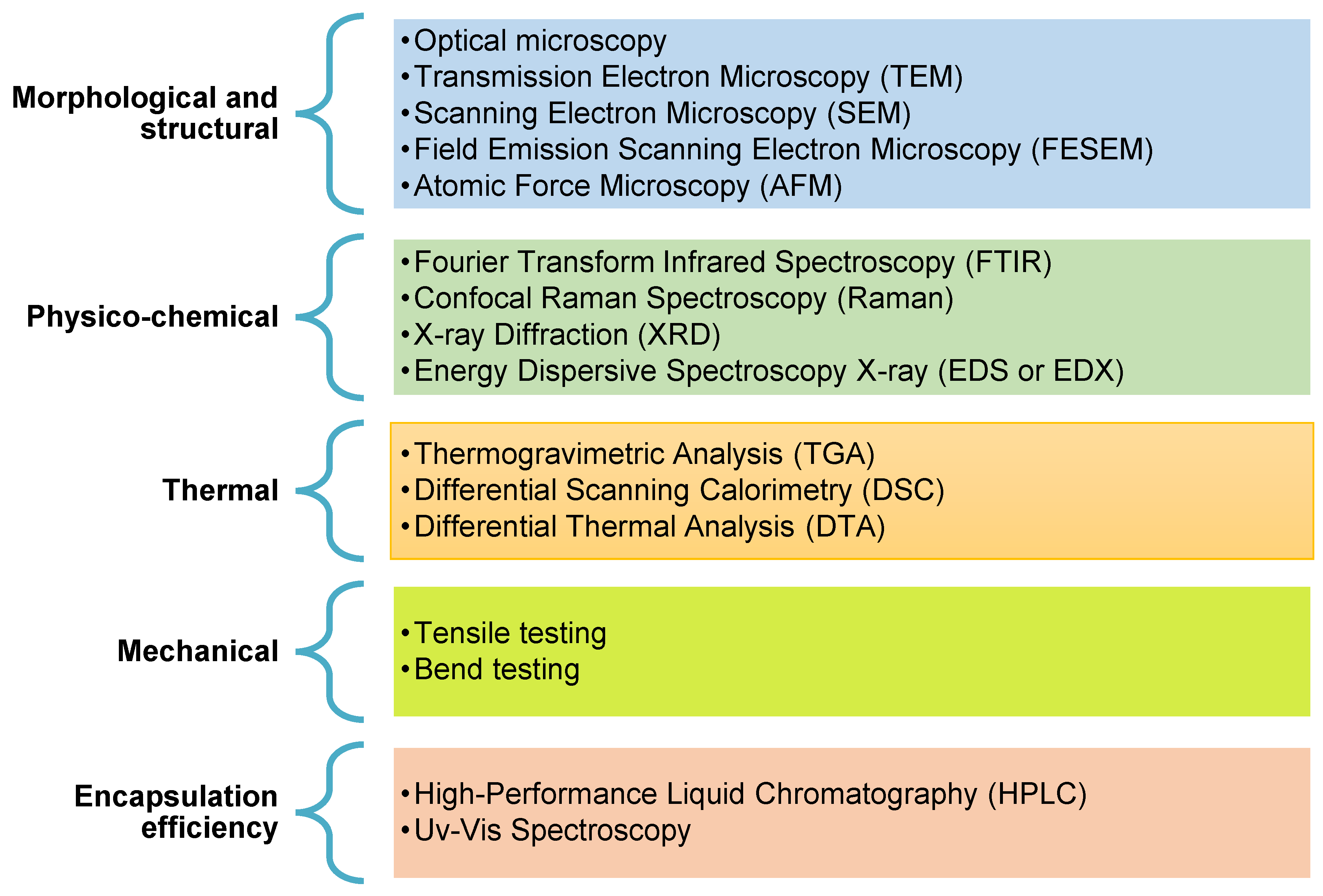
4. Characterization of Electrospun Structures
5. Active Compounds for Meat Processing and Preservation
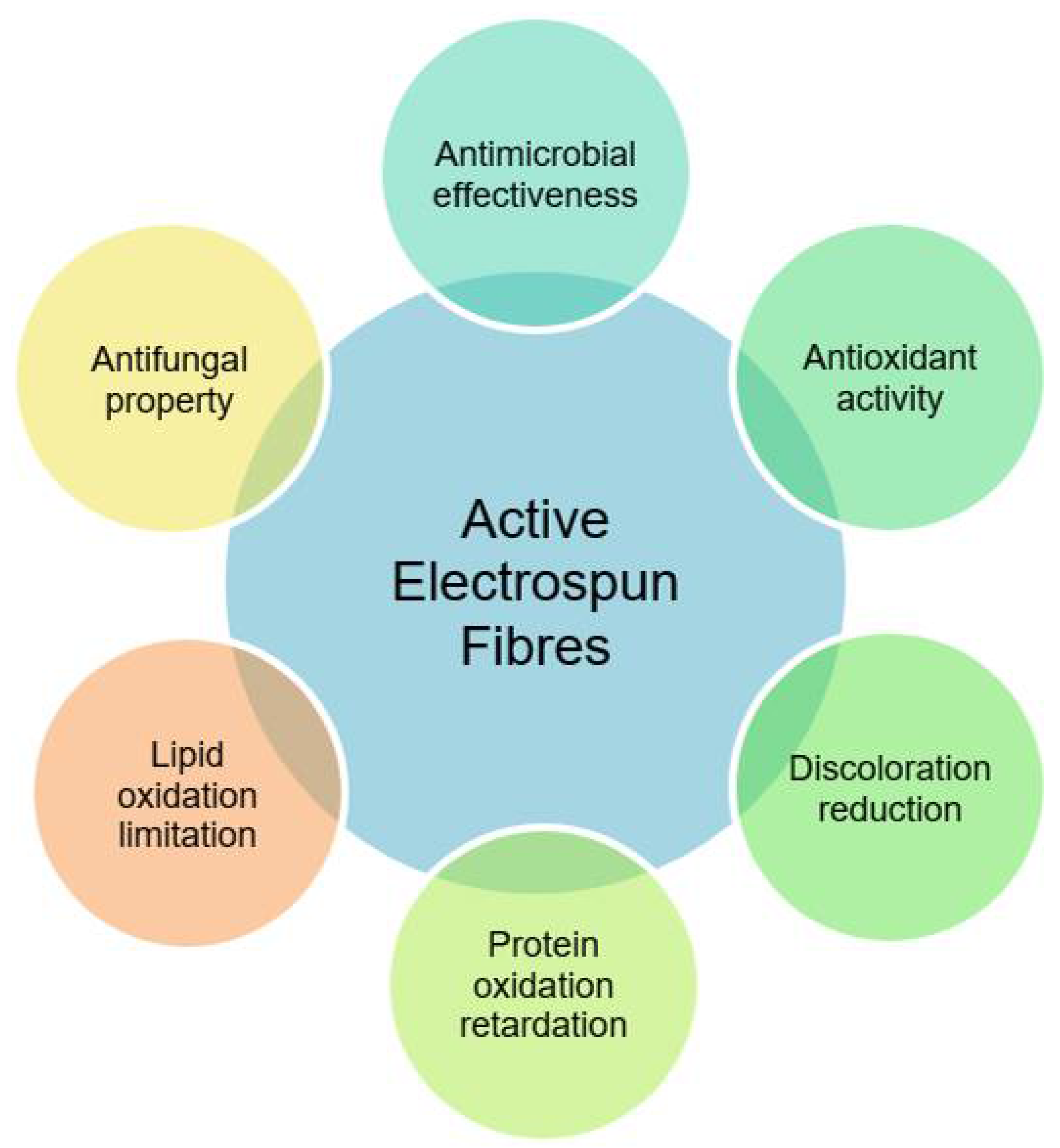
6. Role of Electrospun Fibres in Meat Processing and Preservation
6.1. Active Packaging
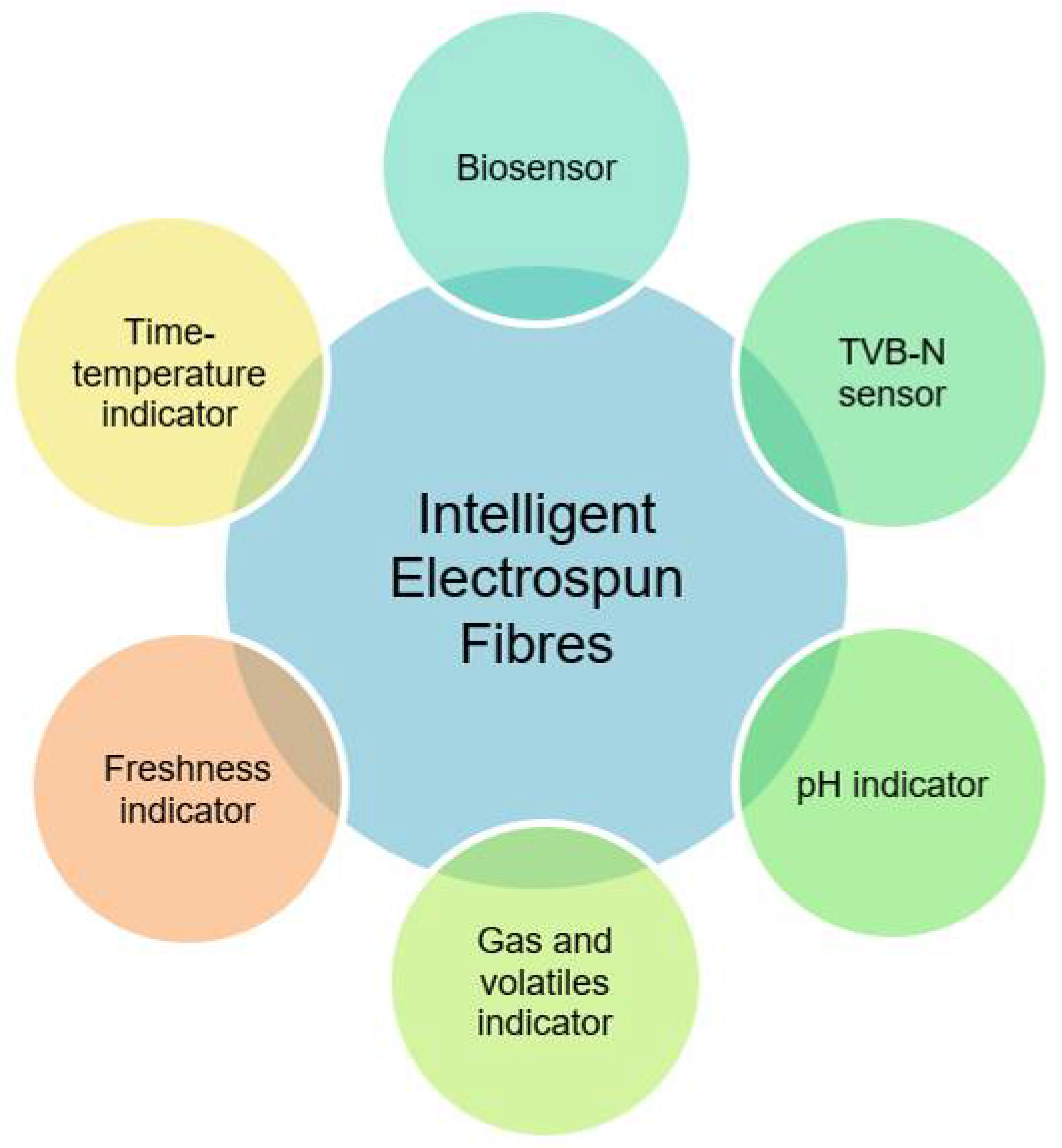
6.2. Intelligent Packaging
7. Packaging Safety and Risk Assessment
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gagaoua, M.; Bhattacharya, T.; Lamri, M.; Oz, F.; Dib, A.L.; Oz, E.; Uysal-Unalan, I.; Tomasevic, I. Green Coating Polymers in Meat Preservation. Coatings 2021, 11, 1379. [Google Scholar] [CrossRef]
- Gagaoua, M.; Duffy, G.; Álvarez García, C.; Burgess, C.; Hamill, R.; Crofton, E.C.; Botinestean, C.; Ferragina, A.; Cafferky, J.; Mullen, A.M.; et al. Current research and emerging tools to improve fresh red meat quality. Ir. J. Agric. Food Res. 2022. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B. Current Advances in Meat Nutritional, Sensory and Physical Quality Improvement. Foods 2020, 9, 321. [Google Scholar] [CrossRef] [Green Version]
- Gagaoua, M.; Monteils, V.; Picard, B. Data from the farmgate-to-meat continuum including omics-based biomarkers to better understand the variability of beef tenderness: An integromics approach. J. Agric. Food Chem. 2018, 66, 13552–13563. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Gagaoua, M.; Durand, D.; Micol, D.; Santé-Lhoutellier, V.; Terlouw, C.; Ellies-Oury, M.P.; Boudjellal, A.; Hocquette, J.F.; Picard, B. Biomarkers of meat sensory qualities of Angus beef breed: Towards the development of prediction equations. In Proceedings of the 15èmes Journées Sciences du Muscle et Technologies des Viandes, Clermont-Ferrand, France, 4–5 November 2014; pp. 137–138. [Google Scholar]
- Gagaoua, M.; Terlouw, E.M.; Micol, D.; Boudjellal, A.; Hocquette, J.F.; Picard, B. Understanding Early Post-Mortem Biochemical Processes Underlying Meat Color and pH Decline in the Longissimus thoracis Muscle of Young Blond d’Aquitaine Bulls Using Protein Biomarkers. J. Agric. Food Chem. 2015, 63, 6799–6809. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Kilic, G.; Ozturk, B. The effects of koruk products used as marination liquids against foodborne pathogens (Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Typhimurium) inoculated on poultry meat. LWT 2020, 133, 110148. [Google Scholar] [CrossRef]
- Amna, T.; Yang, J.; Ryu, K.-S.; Hwang, I.H. Electrospun antimicrobial hybrid mats: Innovative packaging material for meat and meat-products. J. Food Sci. Technol. 2015, 52, 4600–4606. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Lin, L.; Liao, X.; Cui, H. Cold plasma treated thyme essential oil/silk fibroin nanofibers against Salmonella Typhimurium in poultry meat. Food Packag. Shelf Life 2019, 21, 100337. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of Phlorotannin in Alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H.; Moradi, M. Electrospun nanofibers as food freshness and time-temperature indicators: A new approach in food intelligent packaging. Innov. Food Sci. Emerg. Technol. 2021, 73, 102804. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Madamsetty, V.S.; Kumar, A.; Varzandeh, M.; Dehshahri, A.; Zarrabi, A.; Sharififar, F.; Mohammadi, M.; Fahimipour, A.; Ramakrishna, S. Electrospun nanocarriers for delivering natural products for cancer therapy. Trends Food Sci. Technol. 2021, 118, 887–904. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef] [Green Version]
- Moreira, J.B.; Morais, M.G.d.; Morais, E.G.d.; Vaz, B.d.S.; Costa, J.A.V. Chapter 14—Electrospun Polymeric Nanofibers in Food Packaging. In Impact of Nanoscience in the Food Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 387–417. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun nanofibers food packaging: Trends and applications in food systems. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patiño Vidal, C.; López de Dicastillo, C.; Rodríguez-Mercado, F.; Guarda, A.; Galotto, M.J.; Muñoz-Shugulí, C. Electrospinning and cyclodextrin inclusion complexes: An emerging technological combination for developing novel active food packaging materials. Crit. Rev. Food Sci. Nutr. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Yuan, J. Recent advances in electrospinning supramolecular systems. J. Mater. Chem. B 2022, 10, 8–19. [Google Scholar] [CrossRef]
- Lamri, M.; Bhattacharya, T.; Boukid, F.; Chentir, I.; Dib, A.L.; Das, D.; Djenane, D.; Gagaoua, M. Nanotechnology as a Processing and Packaging Tool to Improve Meat Quality and Safety. Foods 2021, 10, 2633. [Google Scholar] [CrossRef]
- Hemmati, F.; Bahrami, A.; Esfanjani, A.F.; Hosseini, H.; McClements, D.J.; Williams, L. Electrospun antimicrobial materials: Advanced packaging materials for food applications. Trends Food Sci. Technol. 2021, 111, 520–533. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Senthil Muthu Kumar, T.; Senthil Kumar, K.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Varada Rajulu, A. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An Overview of Biopolymeric Electrospun Nanofibers Based on Polysaccharides for Wound Healing Management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem 2021, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2020, 23, 100439. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2021, 288, 110140. [Google Scholar] [CrossRef]
- Yaman, M.; Sar, M.; Ceylan, Z. A nanofiber application for thiamine stability and enhancement of bioaccessibility of raw, cooked salmon and red meat samples stored at 4 °C. Food Chem. 2022, 373, 131447. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on Electrospun Nanofiber-Applied Products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef]
- Rosell-Llompart, J.; Grifoll, J.; Loscertales, I.G. Electrosprays in the cone-jet mode: From Taylor cone formation to spray development. J. Aerosol Sci. 2018, 125, 2–31. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and electrospraying techniques: Potential food based applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.-H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Lim, L.-T. 7-Electrospinning and electrospraying technologies for food and packaging applications. In Electrospun Polymers and Composites; Dong, Y., Baji, A., Ramakrishna, S., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 217–259. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Dierings de Souza, E.J.; Kringel, D.H.; Guerra Dias, A.R.; da Rosa Zavareze, E. Polysaccharides as wall material for the encapsulation of essential oils by electrospun technique. Carbohydr. Polym. 2021, 265, 118068. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Qin, X. 3-Coaxial electrospinning of nanofibers. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–71. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. ChemPlusChem 2019, 84, 1453–1497. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.F.; Nuge, T.; Andriyana, A.; Ang, B.C.; Muhamad, F. Core–Shell Fibers: Design, Roles, and Controllable Release Strategies in Tissue Engineering and Drug Delivery. Polymers 2019, 11, 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Yang, H.-S.; Lee, B.-S.; Yu, W.-R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, 1704765. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Hosseini, S.M.; Yousefi, M. Application of electrospinning technique in development of intelligent food packaging: A short review of recent trends. Food Sci. Nutr. 2020, 8, 4656–4665. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian, A.; Shafiee, A.; Aliahmad, N.; Agarwal, M. Overview of Nano-Fiber Mats Fabrication via Electrospinning and Morphology Analysis. Textiles 2021, 1, 206–226. [Google Scholar] [CrossRef]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewińska, D. Effect of electrospinning process variables on the size of polymer fibers and bead-on-string structures established with a 23 factorial design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkoyun, S.; Öktem, N. Effect of viscoelasticity in polymer nanofiber electrospinning: Simulation using FENE-CR model. Eng. Sci. Technol. Int. J. 2021, 24, 620–630. [Google Scholar] [CrossRef]
- Zhang, S.; Campagne, C.; Salaün, F. Influence of Solvent Selection in the Electrospraying Process of Polycaprolactone. Appl. Sci. 2019, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.J. The stretching of an electrified non-Newtonian jet: A model for electrospinning. Phys. Fluids 2002, 14, 3912–3926. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of Apparatus Orientation and Gravity in Electrospinning—A Review of Empirical Evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Bona, N.P.; Crizel, R.L.; Pedra, N.S.; Stefanello, F.M.; Lim, L.-T.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.d.R. Electrospun Starch Nanofibers as a Delivery Carrier for Carvacrol as Anti-Glioma Agent. Starch-Stärke 2022, 74, 2100115. [Google Scholar] [CrossRef]
- Beikzadeh, S.; Akbarinejad, A.; Swift, S.; Perera, J.; Kilmartin, P.A.; Travas-Sejdic, J. Cellulose acetate electrospun nanofibers encapsulating Lemon Myrtle essential oil as active agent with potent and sustainable antimicrobial activity. React. Funct. Polym. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Pérez-Nava, A.; Reyes-Mercado, E.; González-Campos, J.B. Production of chitosan nanofibers using the HFIP/acetic acid mixture as electrospinning solvent. Chem. Eng. Processing Process Intensif. 2022, 173, 108849. [Google Scholar] [CrossRef]
- Moomand, K.; Lim, L.-T. Properties of Encapsulated Fish Oil in Electrospun Zein Fibres Under Simulated In Vitro Conditions. Food Bioprocess Technol. 2015, 8, 431–444. [Google Scholar] [CrossRef]
- Pinheiro Bruni, G.; dos Santos Acunha, T.; de Oliveira, J.P.; Martins Fonseca, L.; Tavares da Silva, F.; Martins Guimarães, V.; da Rosa Zavareze, E. Electrospun protein fibers loaded with yerba mate extract for bioactive release in food packaging. J. Sci. Food Agric. 2020, 100, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Prietto, L.; Pinto, V.Z.; El Halal, S.L.M.; de Morais, M.G.; Costa, J.A.V.; Lim, L.-T.; Dias, A.R.G.; Zavareze, E.d.R. Ultrafine fibers of zein and anthocyanins as natural pH indicator. J. Sci. Food Agric. 2018, 98, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Liu, Y.; Ritzoulis, C.; Niu, B. Preparation of zein nanofibers with cinnamaldehyde encapsulated in surfactants at critical micelle concentration for active food packaging. Food Packag. Shelf Life 2019, 22, 100385. [Google Scholar] [CrossRef]
- Maria Leena, M.; Yoha, K.S.; Moses, J.A.; Anandharamakrishnan, C. Edible coating with resveratrol loaded electrospun zein nanofibers with enhanced bioaccessibility. Food Biosci. 2020, 36, 100669. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Syahariza, Z.A.; Norziah, M.H.; Wan, A.K.M.; Juliano, P. Encapsulation of polyphenolic antioxidants obtained from Momordica charantia fruit within zein/gelatin shell core fibers via coaxial electrospinning. Food Biosci. 2018, 21, 60–71. [Google Scholar] [CrossRef]
- Kwak, H.W.; Shin, M.; Lee, J.Y.; Yun, H.; Song, D.W.; Yang, Y.; Shin, B.-S.; Park, Y.H.; Lee, K.H. Fabrication of an ultrafine fish gelatin nanofibrous web from an aqueous solution by electrospinning. Int. J. Biol. Macromol. 2017, 102, 1092–1103. [Google Scholar] [CrossRef]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; de la Caba, K.; Focarete, M.L. Electrospinning of Fish Gelatin Solution Containing Citric Acid: An Environmentally Friendly Approach to Prepare Crosslinked Gelatin Fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Ramalingam, S.; Parida, S.; Rao, J.R.; Nishter, N.F. Chromium containing leather trimmings valorization: Sustainable sound absorber from collagen hydrolysate intercalated electrospun nanofibers. J. Hazard. Mater. 2021, 405, 124231. [Google Scholar] [CrossRef]
- Zhou, W.; Deng, H.; Xiong, S.; Huang, Q. Fabrication and characterization of electrospun nanofibers of Hypophthalmichthys molitrix sarcoplasmic protein recovered by acid-chitosan flocculation coupling treatment. J. Appl. Polym. Sci. 2021, 138, 51472. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Ferreira, J.C.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly(hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2014, 54, 1608–1617. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Wang, S.; Qin, W.; Zhang, Q. Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications. Polymers 2018, 10, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Keskin, N.O.S.; Tekinay, T.; Uyar, T. Antioxidant α-tocopherol/γ-cyclodextrin–inclusion complex encapsulated poly(lactic acid) electrospun nanofibrous web for food packaging. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Kriegel, C.; Kit, K.M.; McClements, D.J.; Weiss, J. Nanofibers as Carrier Systems for Antimicrobial Microemulsions. Part I: Fabrication and Characterization. Langmuir 2009, 25, 1154–1161. [Google Scholar] [CrossRef]
- Pan, J.; Ai, F.; Shao, P.; Chen, H.; Gao, H. Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem. 2019, 300, 125249. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Cacciotti, I.; Garrigós, M.C. Encapsulation of Bioactive Compounds from Aloe Vera Agrowastes in Electrospun Poly (Ethylene Oxide) Nanofibers. Polymers 2020, 12, 1323. [Google Scholar] [CrossRef]
- Qin, X.; Wu, D. Effect of different solvents on poly(caprolactone) (PCL) electrospun nonwoven membranes. J. Therm. Anal. Calorim. 2012, 107, 1007–1013. [Google Scholar] [CrossRef]
- Rashidi, M.; Seyyedi Mansour, S.; Mostashari, P.; Ramezani, S.; Mohammadi, M.; Ghorbani, M. Electrospun nanofiber based on Ethyl cellulose/Soy protein isolated integrated with bitter orange peel extract for antimicrobial and antioxidant active food packaging. Int. J. Biol. Macromol. 2021, 193, 1313–1323. [Google Scholar] [CrossRef]
- Ahmad, B.; Stoyanov, S.; Pelan, E.; Stride, E.; Edirisinghe, M. Electrospinning of ethyl cellulose fibres with glass and steel needle configurations. Food Res. Int. 2013, 54, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-S.; Wu, D.-Y.; Wang, S.-S. Biodegradable Composite Nanofiber Containing Fish-Scale Extracts. ACS Appl. Bio Mater. 2021, 4, 462–469. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly(vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, M.; Dou, Y.; Ding, J.; Yue, H.; Yin, G.; Chen, X.; Cui, Y. Electrospun Silver Nanoparticles-Embedded Feather Keratin/Poly(vinyl alcohol)/Poly(ethylene oxide) Antibacterial Composite Nanofibers. Polymers 2020, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- de Souza, S.O.L.; Guerra, M.C.A.; Heneine, L.G.D.; de Oliveira, C.R.; Cunha Junior, A.d.S.; Fialho, S.L.; Oréfice, R.L. Biodegradable core-shell electrospun nanofibers containing bevacizumab to treat age-related macular degeneration. J. Mater. Sci. Mater. Med. 2018, 29, 173. [Google Scholar] [CrossRef]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Wijaya, W.; Altay, F.; Ceylan, Z. Fabrication and characterization of zein nanofibers integrated with gold nanospheres. LWT 2022, 155, 112976. [Google Scholar] [CrossRef]
- do Evangelho, J.A.; Crizel, R.L.; Chaves, F.C.; Prietto, L.; Pinto, V.Z.; Miranda, M.Z.d.; Dias, A.R.G.; Zavareze, E.d.R. Thermal and irradiation resistance of folic acid encapsulated in zein ultrafine fibers or nanocapsules produced by electrospinning and electrospraying. Food Res. Int. 2019, 124, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, M.; Song, W.-L.; Yu, D.-G.; Bligh, S.W.A. Electrospun Janus Beads-On-A-String Structures for Different Types of Controlled Release Profiles of Double Drugs. Biomolecules 2021, 11, 635. [Google Scholar] [CrossRef]
- Yan, T.; Tian, L.; Pan, Z. Structures and mechanical properties of plied and twisted polyacrylonitrile nanofiber yarns fabricated by a multi-needle electrospinning device. Fibers Polym. 2016, 17, 1627–1633. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Lim, L.-T.; Mendes, A.C.; Chronakis, I.S. Chapter Five-Electrospinning and electrospraying technologies for food applications. In Advances in Food and Nutrition Research; Lim, L.-T., Rogers, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 167–234. [Google Scholar]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Khamforoush, M.; Mahjob, M. Modification of the rotating jet method to generate highly aligned electrospun nanofibers. Mater. Lett. 2011, 65, 453–455. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Letters 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.-Z.; Li, H.-P.; Yang, J.-H.; Wan, J.; Yu, D.-G. Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers. Nanoscale Res. Lett. 2017, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Ghorani, B.; Tucker, N.; Yoshikawa, M. Approaches for the assembly of molecularly imprinted electrospun nanofibre membranes and consequent use in selected target recognition. Food Res. Int. 2015, 78, 448–464. [Google Scholar] [CrossRef]
- Marques, C.; Lise, C.C.; Bonadimann, F.S.; Mitterer-Daltoé, M.L. Flash Profile as an effective method for assessment of odor profile in three different fishes. J. Sci. Food Agric. 2019, 56, 4036–4044. [Google Scholar] [CrossRef]
- Lavanya, M.N.; Kathiravan, T.; Moses, J.A.; Anandharamakrishnan, C. Influence of spray-drying conditions on microencapsulation of fish oil and chia oil. Dry. Technol. 2020, 38, 279–292. [Google Scholar] [CrossRef]
- Kong, L.; Ziegler, G.R. Fabrication of pure starch fibers by electrospinning. Food Hydrocoll. 2014, 36, 20–25. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Radünz, M.; dos Santos Hackbart, H.C.; da Silva, F.T.; Camargo, T.M.; Bruni, G.P.; Monks, J.L.; da Rosa Zavareze, E.; Dias, A.R. Electrospun potato starch nanofibers for thyme essential oil encapsulation: Antioxidant activity and thermal resistance. J. Sci. Food Agric. 2020, 100, 4263–4271. [Google Scholar] [CrossRef] [PubMed]
- Nanda, P.K.; Das, A.K.; Dandapat, P.; Dhar, P.; Bandyopadhyay, S.; Dib, A.L.; Lorenzo, J.M.; Gagaoua, M. Nutritional aspects, flavour profile and health benefits of crab meat based novel food products and valorisation of processing waste to wealth: A review. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A. Chitosan-functionalized nanofibers: A comprehensive review on challenges and prospects for food applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef]
- Abid, S.; Hussain, T.; Nazir, A.; Zahir, A.; Ramakrishna, S.; Hameed, M.; Khenoussi, N. Enhanced antibacterial activity of PEO-chitosan nanofibers with potential application in burn infection management. Int. J. Biol. Macromol. 2019, 135, 1222–1236. [Google Scholar] [CrossRef]
- Hardiansyah, A.; Tanadi, H.; Yang, M.-C.; Liu, T.-Y. Electrospinning and antibacterial activity of chitosan-blended poly(lactic acid) nanofibers. J. Polym. Res. 2015, 22, 59. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Cheong, J.Y.; Kp, A.; Makvandi, P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Černík, M.; Kim, I.-D.; Varma, R.S. Electrospun fibers based on carbohydrate gum polymers and their multifaceted applications. Carbohydr. Polym. 2020, 247, 116705. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Faralli, A.; Ndoni, S.; Mendes, A.C.; Chronakis, I.S. Electrospinning of Xanthan Polysaccharide. Macromol. Mater. Eng. 2017, 302, 1700067. [Google Scholar] [CrossRef]
- Khan, M.J.; Kumari, S.; Selamat, J.; Shameli, K.; Sazili, A.Q. Reducing Meat Perishability through Pullulan Active Packaging. J. Food Qual. 2020, 2020, 8880977. [Google Scholar] [CrossRef]
- Poudel, D.; Swilley-Sanchez, S.; O’keefe, S.; Matson, J.; Long, T.; Fernández-Fraguas, C. Novel Electrospun Pullulan Fibers Incorporating Hydroxypropyl-β-Cyclodextrin: Morphology and Relation with Rheological Properties. Polymers 2020, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.; Introzzi, L.; Fuentes-Alventosa, J.M.; Santo, N.; Rocca, R.; Piergiovanni, L. Self-Assembled Pullulan–Silica Oxygen Barrier Hybrid Coatings for Food Packaging Applications. J. Agric. Food Chem. 2012, 60, 782–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moomand, K.; Lim, L.-T. Oxidative stability of encapsulated fish oil in electrospun zein fibres. Food Res. Int. 2014, 62, 523–532. [Google Scholar] [CrossRef]
- Dias Antunes, M.; da Silva Dannenberg, G.; Fiorentini, Â.M.; Pinto, V.Z.; Lim, L.-T.; da Rosa Zavareze, E.; Dias, A.R.G. Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int. J. Biol. Macromol. 2017, 104, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Nagasawa, N.; Nishida, H.; Furusawa, K.; Mori, Y.; Yoshii, F.; Dobashi, T. Reagent-free crosslinking of aqueous gelatin: Manufacture and characteristics of gelatin gels irradiated with gamma-ray and electron beam. J. Biomater. Sci. Polym. Ed. 2003, 14, 1197–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, F.; Ursini, O.; Lilla, E.; Angelini, G. Radiation-induced crosslinking of collagen gelatin into a stable hydrogel. J. Radioanal. Nucl. Chem. 2008, 275, 125–131. [Google Scholar] [CrossRef]
- Prasertsung, I.; Damrongsakkul, S.; Saito, N. Crosslinking of a Gelatin Solutions Induced by Pulsed Electrical Discharges in Solutions. Plasma Processes Polym. 2013, 10, 792–797. [Google Scholar] [CrossRef]
- Weiss, J.; Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. 13-Electrospun fibers: Fabrication, functionalities and potential food industry applications. In Nanotechnology in the Food, Beverage and Nutraceutical Industries; Huang, Q., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 362–397. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Cavidoglu, I.; Yagmur Karakas, C.; Tahsin Yilmaz, M. A new application on fatty acid stability of fish fillets: Coating with probiotic bacteria-loaded polymer-based characterized nanofibers. J. Food Saf. 2018, 38, e12547. [Google Scholar] [CrossRef]
- Ceylan, Z. A new cost-effective process for limitation of microbial growth in fish fleshes: Wrapping by aluminum foil coated with electrospun nanofibers. J. Food Saf. 2019, 39, e12697. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Lanno, G.-M.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef] [PubMed]
- Nitti, P.; Gallo, N.; Natta, L.; Scalera, F.; Palazzo, B.; Sannino, A.; Gervaso, F. Influence of Nanofiber Orientation on Morphological and Mechanical Properties of Electrospun Chitosan Mats. J. Healthc. Eng. 2018, 2018, 3651480. [Google Scholar] [CrossRef] [PubMed]
- Leidy, R.; Maria Ximena, Q.-C. Use of electrospinning technique to produce nanofibres for food industries: A perspective from regulations to characterisations. Trends Food Sci. Technol. 2019, 85, 92–106. [Google Scholar] [CrossRef]
- Rydz, J.; Šišková, A.; Andicsová Eckstein, A. Scanning Electron Microscopy and Atomic Force Microscopy: Topographic and Dynamical Surface Studies of Blends, Composites, and Hybrid Functional Materials for Sustainable Future. Adv. Mater. Sci. Eng. 2019, 2019, 6871785. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Pathak, D.; Patil, D.S.; Dhiman, N.; Bhullar, V.; Mahajan, A. Electrospun PVP/TiO2 Nanofibers for Filtration and Possible Protection from Various Viruses like COVID-19. Technologies 2021, 9, 89. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef] [Green Version]
- Haru, Y.; Tomioka, A. Luminescent electrospun nanofibers doped with organic dye: Toward a disentangled deposition (Phys. Status Solidi B 6/2017). Phys. Status Solidi (B) 2017, 254, 1770230. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, R.M. Surface Characterization and Optical Study on Electrospun Nanofibers of PVDF/PAN Blends. Fiber Integr. Opt. 2017, 36, 78–90. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Pellerin, C. Raman spectroscopy of individual poly(ethylene oxide) electrospun fibers: Effect of the collector on molecular orientation. Vib. Spectrosc. 2017, 91, 92–98. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Ekiz, H.I.; López-Rubio, A. Phytochemical-loaded electrospun nanofibers as novel active edible films: Characterization and antibacterial efficiency in cheese slices. Food Control 2020, 112, 107133. [Google Scholar] [CrossRef]
- Nauman, S.; Lubineau, G.; Alharbi, H.F. Post Processing Strategies for the Enhancement of Mechanical Properties of ENMs (Electrospun Nanofibrous Membranes): A Review. Membranes 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Susanto, H.; Samsudin, A.M.; Faz, M.W.; Rani, M.P.H. Impact of post-treatment on the characteristics of electrospun poly (vinyl alcohol)/chitosan nanofibers. AIP Conf. Proc. 2016, 1725, 020087. [Google Scholar] [CrossRef] [Green Version]
- Orasugh, J.T.; Ghosh, S.K.; Chattopadhyay, D. Chapter 10—Nanofiber-reinforced biocomposites. In Fiber-Reinforced Nanocomposites: Fundamentals and Applications; Han, B., Sharma, S., Nguyen, T.A., Longbiao, L., Bhat, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–233. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aytac, Z.; Ipek, S.; Durgun, E.; Tekinay, T.; Uyar, T. Antibacterial electrospun zein nanofibrous web encapsulating thymol/cyclodextrin-inclusion complex for food packaging. Food Chem. 2017, 233, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Liu, X.; Baldursdottir, S.G.; Aho, J.; Qu, H.; Christensen, L.P.; Rantanen, J.; Yang, M. Electrospinnability of Poly Lactic-co-glycolic Acid (PLGA): The Role of Solvent Type and Solvent Composition. Pharm. Res. 2017, 34, 738–749. [Google Scholar] [CrossRef]
- Tarus, B.K.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Investigation of mechanical properties of electrospun poly (vinyl chloride) polymer nanoengineered composite. J. Eng. Fibers Fabr. 2020, 15, 1558925020982569. [Google Scholar] [CrossRef]
- Piacentini, E. Encapsulation Efficiency. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 706–707. [Google Scholar] [CrossRef]
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef]
- Reddy, V.S.; Tian, Y.; Zhang, C.; Ye, Z.; Roy, K.; Chinnappan, A.; Ramakrishna, S.; Liu, W.; Ghosh, R. A Review on Electrospun Nanofibers Based Advanced Applications: From Health Care to Energy Devices. Polymers 2021, 13, 3746. [Google Scholar] [CrossRef]
- Antaby, E.; Klinkhammer, K.; Sabantina, L. Electrospinning of Chitosan for Antibacterial Applications—Current Trends. Appl. Sci. 2021, 11, 11937. [Google Scholar] [CrossRef]
- Domingues, J.M.; Teixeira, M.O.; Teixeira, M.A.; Freitas, D.; Silva, S.F.d.; Tohidi, S.D.; Fernandes, R.D.V.; Padrão, J.; Zille, A.; Silva, C.; et al. Inhibition of Escherichia Virus MS2, Surrogate of SARS-CoV-2, via Essential Oils-Loaded Electrospun Fibrous Mats: Increasing the Multifunctionality of Antivirus Protection Masks. Pharmaceutics 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Kara, H.H.; Xiao, F.; Sarker, M.; Jin, T.Z.; Sousa, A.M.M.; Liu, C.-K.; Tomasula, P.M.; Liu, L. Antibacterial poly(lactic acid) (PLA) films grafted with electrospun PLA/allyl isothiocyanate fibers for food packaging. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Zaitoon, A.; Lim, L.-T.; Scott-Dupree, C. Activated release of ethyl formate vapor from its precursor encapsulated in ethyl Cellulose/Poly(Ethylene oxide) electrospun nonwovens intended for active packaging of fresh produce. Food Hydrocoll. 2021, 112, 106313. [Google Scholar] [CrossRef]
- Ahari, H.; Anvar, A.A.; Ataee, M.; Naeimabadi, M. Employing Nanosilver, Nanocopper, and Nanoclays in Food Packaging Production: A Systematic Review. Coatings 2021, 11, 509. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of Electrospinning to Develop Antimicrobial Biodegradable Multilayer Systems: Encapsulation of Cinnamaldehyde and Their Physicochemical Characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Pateiro, M.; Barba, F.J.; Dominguéz, R.; Gagaoua, M.; Lorenzo, J.M. Chapter Three-Development of new food and pharmaceutical products: Nutraceuticals and food additives. In Advances in Food and Nutrition Research; Lorenzo, J.M., Barba, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 92, pp. 53–96. [Google Scholar]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, N.; Romero, J.; Torres, A.; López de Dicastillo, C.; Rojas, A.; Galotto, M.J.; Guarda, A. Supercritical impregnation of thymol in poly(lactic acid) filled with electrospun poly(vinyl alcohol)-cellulose nanocrystals nanofibers: Development an active food packaging material. J. Food Eng. 2018, 217, 1–10. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken. Foods 2021, 10, 1277. [Google Scholar] [CrossRef]
- Vargas Romero, E.; Lim, L.-T.; Suárez Mahecha, H.; Bohrer, B.M. The Effect of Electrospun Polycaprolactone Nonwovens Containing Chitosan and Propolis Extracts on Fresh Pork Packaged in Linear Low-Density Polyethylene Films. Foods 2021, 10, 1110. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Y.; Cui, H. Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT 2018, 97, 711–718. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, A.; Fazlara, A.; Maktabi, S.; Pourmahdi, M.; Bavarsad, N. The effects of chitosan containing nano-capsulated Cuminum cyminum essential oil on the shelf-life of veal in modified atmosphere packaging. J. Food Meas. Charact. 2022, 16, 920–933. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Yin, C.; Khan, M.R.; Zhao, H.; Xu, Y.; Huang, L.; Zheng, D.; Qi, M. Preparation and characterization of β-cyclodextrin–oregano essential oil microcapsule and its effect on storage behavior of purple yam. J. Sci. Food Agric. 2020, 100, 4849–4857. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Sohany, M.; Tawakkal, I.S.M.A.; Ariffin, S.H.; Shah, N.N.A.K.; Yusof, Y.A. Characterization of Anthocyanin Associated Purple Sweet Potato Starch and Peel-Based pH Indicator Films. Foods 2021, 10, 2005. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.; Wang, Q.; Chen, M.; Li, L.; Li, X.; Jiang, S. Intelligent double-layer fiber mats with high colorimetric response sensitivity for food freshness monitoring and preservation. Food Hydrocoll. 2020, 101, 105468. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Jia, L.; Saldaña, M.D.A.; Dong, T.; Jin, Y.; Sun, W. A smart nanofibre sensor based on anthocyanin/poly-l-lactic acid for mutton freshness monitoring. Int. J. Food Sci. Technol. 2021, 56, 342–351. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Wu, H.; Teng, C.; Liu, B.; Tian, H.; Wang, J. Characterization and long term antimicrobial activity of the nisin anchored cellulose films. J. Biol. Macromol. 2018, 113, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Du, L.; Wang, L. Tara gum/polyvinyl alcohol-based colorimetric NH3 indicator films incorporating curcumin for intelligent packaging. Sens. Actuators B Chem. 2017, 244, 759–766. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Larasati, T.S.; Abdullah, A.; Heng, L.Y. Real-Time Monitoring of Shrimp Spoilage Using On-Package Sticker Sensor Based on Natural Dye of Curcumin. Food Anal. Methods 2012, 5, 881–889. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT 2019, 113, 108292. [Google Scholar] [CrossRef]
- Ceylan, Z. Use of characterized chitosan nanoparticles integrated in poly(vinyl alcohol) nanofibers as an alternative nanoscale material for fish balls. J. Food Saf. 2018, 38, e12551. [Google Scholar] [CrossRef]
- Ionescu, O.M.; Iacob, A.-T.; Mignon, A.; Van Vlierberghe, S.; Baican, M.; Danu, M.; Ibănescu, C.; Simionescu, N.; Profire, L. Design, preparation and in vitro characterization of biomimetic and bioactive chitosan/polyethylene oxide based nanofibers as wound dressings. Int. J. Biol. Macromol. 2021, 193, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of Action of Electrospun Chitosan-Based Nanofibers against Meat Spoilage and Pathogenic Bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Aktürk, A.; Erol Taygun, M.; Karbancıoğlu Güler, F.; Goller, G.; Küçükbayrak, S. Fabrication of antibacterial polyvinylalcohol nanocomposite mats with soluble starch coated silver nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 255–262. [Google Scholar] [CrossRef]
- Tra Thanh, N.; Ho Hieu, M.; Tran Minh Phuong, N.; Do Bui Thuan, T.; Nguyen Thi Thu, H.; Thai, V.P.; Do Minh, T.; Nguyen Dai, H.; Vo, V.T.; Nguyen Thi, H. Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. C 2018, 91, 318–329. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, J.; Bai, J.; Lai, J. Meat packaging, preservation, and marketing implications: Consumer preferences in an emerging economy. Meat Sci. 2018, 145, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Rantsiou, K.; Kathariou, S.; Winkler, A.; Skandamis, P.; Saint-Cyr, M.J.; Rouzeau-Szynalski, K.; Amézquita, A. Next generation microbiological risk assessment: Opportunities of whole genome sequencing (WGS) for foodborne pathogen surveillance, source tracking and risk assessment. Int. J. Food Microbiol. 2018, 287, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, D.; Ren, L.; Mei, J.; Xu, Y.; Li, J. Preparation of pH-sensitive polylactic acid-naringin coaxial electrospun fiber membranes for maintaining and monitoring salmon freshness. Int. J. Biol. Macromol. 2021, 188, 708–718. [Google Scholar] [CrossRef]
- Ding, T.; Li, T.; Li, J. Preparation of Coaxial Polylactic Acid–Propyl Gallate Electrospun Fibers and the Effect of Their Coating on Salmon Slices during Chilled Storage. ACS Appl. Mater. Interfaces 2019, 11, 6463–6474. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Dong, Q.; Li, L. Fabrication of antibacterial fibrous films by electrospinning and their application for Japanese sea bass (Lateolabrax japonicus) preservation. LWT 2021, 149, 111870. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Alp Erbay, E.; Dağtekin, B.B.; Türe, M.; Yeşilsu, A.F.; Torres-Giner, S. Quality improvement of rainbow trout fillets by whey protein isolate coatings containing electrospun poly(ε-caprolactone) nanofibers with Urtica dioica L. extract during storage. LWT 2017, 78, 340–351. [Google Scholar] [CrossRef]
- Nazari, M.; Majdi, H.; Milani, M.; Abbaspour-Ravasjani, S.; Hamishehkar, H.; Lim, L.-T. Cinnamon nanophytosomes embedded electrospun nanofiber: Its effects on microbial quality and shelf-life of shrimp as a novel packaging. Food Packag. Shelf Life 2019, 21, 100349. [Google Scholar] [CrossRef]
- Ceylan, Z.; Yaman, M.; Sağdıç, O.; Karabulut, E.; Yilmaz, M.T. Effect of electrospun thymol-loaded nanofiber coating on vitamin B profile of gilthead sea bream fillets (Sparus aurata). LWT 2018, 98, 162–169. [Google Scholar] [CrossRef]
- Gudjónsdóttir, M.; Gacutan, M.D.; Mendes, A.C.; Chronakis, I.S.; Jespersen, L.; Karlsson, A.H. Effects of electrospun chitosan wrapping for dry-ageing of beef, as studied by microbiological, physicochemical and low-field nuclear magnetic resonance analysis. Food Chem. 2015, 184, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Dai, Y.; Cui, H. Antibacterial poly(ethylene oxide) electrospun nanofibers containing cinnamon essential oil/beta-cyclodextrin proteoliposomes. Carbohydr. Polym 2017, 178, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Gu, Y.; Cui, H. Novel electrospun gelatin-glycerin-ε-Poly-lysine nanofibers for controlling Listeria monocytogenes on beef. Food Packag. Shelf Life 2018, 18, 21–30. [Google Scholar] [CrossRef]
- Taktak, W.; Nasri, R.; López-Rubio, A.; Chentir, I.; Gómez-Mascaraque, L.G.; Boughriba, S.; Nasri, M.; Karra-Chaâbouni, M. Design and characterization of novel ecofriendly European fish eel gelatin-based electrospun microfibers applied for fish oil encapsulation. Process Biochem. 2021, 106, 10–19. [Google Scholar] [CrossRef]
- Li, T.; Shen, Y.; Chen, H.; Xu, Y.; Wang, D.; Cui, F.; Han, Y.; Li, J. Antibacterial Properties of Coaxial Spinning Membrane of Methyl ferulate/zein and Its Preservation Effect on Sea Bass. Foods 2021, 10, 2385. [Google Scholar] [CrossRef]
- Çetinkaya, T.; Ceylan, Z.; Meral, R.; Kılıçer, A.; Altay, F. A novel strategy for Au in food science: Nanoformulation in dielectric, sensory properties, and microbiological quality of fish meat. Food Biosci. 2021, 41, 101024. [Google Scholar] [CrossRef]
- Weng, R.; Sun, L.; Jiang, L.; Li, N.; Ruan, G.; Li, J.; Du, F. Electrospun Graphene Oxide–Doped Nanofiber-Based Solid Phase Extraction followed by High-Performance Liquid Chromatography for the Determination of Tetracycline Antibiotic Residues in Food Samples. Food Anal. Methods 2019, 12, 1594–1603. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Y.; Qu, B.; Li, Y.; Lu, Y.; Tian, L.; Shen, W.; Ramakrishna, S. Rapid determination of sulfonamide residues in pork by surface-modified hydrophilic electrospun nanofibrous membrane solid-phase extraction combined with ultra-performance liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 5499–5511. [Google Scholar] [CrossRef]
- Chen, S.Y.; Harrison, M.; Ng, E.K.; Sauvageau, D.; Elias, A. Immobilized Reporter Phage on Electrospun Polymer Fibers for Improved Capture and Detection of Escherichia coli O157:H7. ACS Food Sci. Technol. 2021, 1, 1085–1094. [Google Scholar] [CrossRef]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Monitoring freshness of chicken breast by using natural halochromic curcumin loaded chitosan/PEO nanofibers as an intelligent package. Int. J. Biol. Macromol. 2021, 170, 437–446. [Google Scholar] [CrossRef]
- Yılmaz, M.; Altan, A. Optimization of functionalized electrospun fibers for the development of colorimetric oxygen indicator as an intelligent food packaging system. Food Packag. Shelf Life 2021, 28, 100651. [Google Scholar] [CrossRef]
- Aghaei, Z.; Ghorani, B.; Emadzadeh, B.; Kadkhodaee, R.; Tucker, N. Protein-based halochromic electrospun nanosensor for monitoring trout fish freshness. Food Control 2020, 111, 107065. [Google Scholar] [CrossRef]
- Aghaei, Z.; Emadzadeh, B.; Ghorani, B.; Kadkhodaee, R. Cellulose Acetate Nanofibres Containing Alizarin as a Halochromic Sensor for the Qualitative Assessment of Rainbow Trout Fish Spoilage. Food Bioprocess Technol. 2018, 11, 1087–1095. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Zaitoon, A.; Luo, X.; Lim, L.-T. Triggered and controlled release of active gaseous/volatile compounds for active packaging applications of agri-food products: A review. Compre. Rev. Food Sci. Food Saf. 2022, 21, 541–579. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Li, C.; Chen, X.; Cui, H. Fabrication of a dual-response intelligent antibacterial nanofiber and its application in beef preservation. LWT 2022, 154, 112606. [Google Scholar] [CrossRef]
- Luo, X.; Zaitoon, A.; Lim, L.-T. A review on colorimetric indicators for monitoring product freshness in intelligent food packaging: Indicator dyes, preparation methods, and applications. Compre. Rev. Food Sci. Food Saf. 2022. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kerry, J.P.; Hopkins, D.L. Meat packaging solutions to current industry challenges: A review. Meat Sci. 2018, 144, 159–168. [Google Scholar] [CrossRef]
- Kerry, J.; O’grady, M.; Hogan, S. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; Costa, J.A.V.; Brizio, A.P.D.R.; Morais, M.G.d. Development of a colorimetric pH indicator using nanofibers containing Spirulina sp. LEB 18. Food Chem. 2020, 328, 126768. [Google Scholar] [CrossRef]
- Oberdörster, G.; Castranova, V.; Asgharian, B.; Sayre, P. Inhalation Exposure to Carbon Nanotubes (CNT) and Carbon Nanofibers (CNF): Methodology and Dosimetry. J. Toxicol. Environ. Health Part B 2015, 18, 121–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaiser, B.K.; Hirn, S.; Kermanizadeh, A.; Kanase, N.; Fytianos, K.; Wenk, A.; Haberl, N.; Brunelli, A.; Kreyling, W.G.; Stone, V. Effects of Silver Nanoparticles on the Liver and Hepatocytes In Vitro. Toxicol. Sci. 2012, 131, 537–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.T.; Cho, Y.B.; Park, J.-S.; Yang, Y.; Kim, Y.S. Sensitive naked-eye detection of gaseous ammonia based on dye-impregnated nanoporous polyacrylonitrile mats. Sens. Actuators B Chem. 2016, 230, 250–259. [Google Scholar] [CrossRef]
- Dainelli, D.; Gontard, N.; Spyropoulos, D.; Zondervan-van den Beuken, E.; Tobback, P. Active and intelligent food packaging: Legal aspects and safety concerns. Trends Food Sci. Technol. 2008, 19, S103–S112. [Google Scholar] [CrossRef]
| Polymers | Solvent | Active Agent | Feeding Rate (mL h−1) | Distance (cm) | Electrical Potential (kV) | Fibres Diameter (nm/µm) | References |
|---|---|---|---|---|---|---|---|
| Polysaccharides | |||||||
| Starch | Formic acid 75% | Carvacrol | 0.6 | 20 | 25 | 73–95 nm | [62] |
| Cellulose acetate | 2:1 v/v acetone/ DMF and 2:1 v/v acetone/DMAc | Lemon myrtle essential oil | ~0.5 | 15 | 17.5–25 | 440–515 nm | [63] |
| Chitosan | HFIP/acetic acid mixture (80:20, v/v) | N/A | 0.3 | 17 | 12 | 202.10 ± 9.52 | [64] |
| Proteins | |||||||
| Zein | Ethanol 70% Isopropanol 70% | Fish oil preservation | 1 | 20 | 20 | 200–500 nm | [65] |
| Zein | Ethanol 70% | Ilex paraguariensis polyphenols | 1 | 20 | 23 | 106–158 nm | [66] |
| Zein | Ethanol 70% | Red cabbage anthocyanins | 1 | 16 | 16 | 444–510 nm | [67] |
| Zein | Lecithin loaded Cinnamaldehyde | 0.3 | 12 | 13–15 | 166–198 nm | [68] | |
| Zein + glycerol | Ethanol 80% | Resveratrol | 0.5 | 8 | 14 | 378–510 nm | [69] |
| Zein/gelatin blend | Bittergoard phenol | 0.25–0.75 | 17 | 15–20 | 160 ± 25 nm | [70] | |
| Gelatin from cold water-fish | Water, acetic acid/water (50:50, v/v), and 2,2,2-trifluoroethanol (TFE) | N/A | 0.1–0.5 | 15 | 15 | 91–200 nm | [71] |
| 240 bloom type Fish Gelatin | Citric acid | 0.1 | 15 | 23 | 2.19 ± 0.07 µm | [72] | |
| Leather trimmings | N/A | 1 | 20 | 20 | 229 ± 49 nm | [73] | |
| Hypophthalmichths molitrix sarcoplasmic protein | chitosan | 0.8 | 14 | 20 | 342.8–458.7 nm | [74] | |
| Biomass-Derivates and Synthetic Biopolymers | |||||||
| PHB | Chloroform/DMF (3/7, v/v) | N/A | 5–20 | - | 1–1.75 kV cm−1 | 1310–2010 nm | [75] |
| PLA | Tea phenol | 20 | 15 | 20 | 493 ± 46 nm | [76] | |
| PLA | α tocopherol/ γ-CD inclusion complex | 1 | 10 | 15 | 430 ± 170 nm | [77] | |
| PVA | Aqueous acetic acid (1%, w/w) | Eugenol microemulsions | 1.2 | 10 | 20 | 57–126 nm | [78] |
| PVA | Water at 80 °C | CD/Cinnamon essential oil (80% trans-cinnamaldehyde) | 0.6–0.9 | 15 | 12–15 | 522.1–751.1 nm | [79] |
| PEO | Ethanol 80% | Aloe vera skin extract | 0.5 | 15 | 16 | 185–250 nm | [80] |
| PCL | NMP, acetone | N/A | 3 | 15 | 20 | 100–500 nm | [81] |
| Polymer Blends | |||||||
| Ethyl cellulose/Soy protein isolated | water/ethanol/acetic acid with volume ratio of 2/2/6 (v/v/v) | Bitter orange peel extract | 1 | 10 | 14–16 | 177.6–204 nm | [82,83] |
| Polyhydroxyalkanoate PHA or PHB/fish scale blend | N/A | 0.8 | 24 | 25 | 100–500 nm | [84] | |
| Gelatin/chitosan/PLA blends | acetic acid 80% | N/A | 0.5 | 10 | 20 | 50–70 nm | [85] |
| Chicken feather Keratin/PVA blend | Citric acid | 0.3 | 15 | 20 | 353 nm | [86] | |
| Pullulan-carboxymethylcellulose sodium | Water | Tea polyphenols | 0.36–0.6 | 15 | 19–21 | 100–300 nm | [87] |
| Keratin, PVA & PEO blend | AgNPs | 0.6 | 15 | 20 | 249.76 ± 38.02 nm | [88] | |
| PCL & gelatin blend | bevacizumab | 4.2 | 15.5 | 25 | 175–248 nm | [89] | |
| Polymers | Active Agent | Fibres Diameter (nm) | Muscle Food Types | Role of Nanofibres | References |
|---|---|---|---|---|---|
| Synthetic | |||||
| PLA | Naringin | 206–243 | Salmon | pH-monitoring and antibacterial activity against P. fluorescens | [183] |
| PLA | Acid-propyl gallate | <100 | Salmon slices | Antibacterial activity against P. fluorescens P07 | [184] |
| PVA | Chitosan | 200–2500 | Fish Balls | Psychrophilic bacterial count was inhibited thereby retaining freshness in fish balls | [174] |
| PVA | Nisin & curcumin | 288 ± 63 | Fish (Oncorhynchus mykiss) flesh | Retarded total viable bacteria, psychrophilic bacteria, yeast, and mould growth in the flesh | [126] |
| PVA | Essential oil from Laurus nobilis & Rosmarinus officinalis | - | Chicken breast fillets | Antibacterial activity Listeria monocytogenes | [14] |
| PVA | Poly(hexamethylene biguanide) hydrochloride (PHMB) | <1000 | Japanese sea bass Lateolabrax japonicus | Delayed total volatile basic nitrogen production, inhibited total viable count, Pseudomonas spp. and hydrogen sulphide producing bacteria | [185] |
| PVA | Curcumin, Nisin from Lactococcus lactis | 172 | Rainbow Trout fish fillet | Antibacterial activity against total mesophilic aerobic bacteria and lactic acid bacteria | [173] |
| PEO | Chitosan | 40.6–75.5 | Fresh meat | Antibacterial activity against E. coli, S. typhyimurium, S. aureus, L. inocua | [176] |
| PEO | Chitosan | - | Fresh red meat | In vitro antibacterial activity against E. coli, S. typhyimurium, S. aureus, L. inocua; in situ bioactivity against E. coli | [186] |
| PEO | Chrysanthemum essential oil | 50–250 | Beef | Antibacterial activity against L. monocytogenes; no effect on red colour and texture of meat | [161] |
| PCL | (Urtica dioica L.) extract | - | Rainbow trout fillets | Antibacterial activity against mesophilic, psychrophilic, and lactic acid bacteria as well as Enterobacteriaceae, low TVB-N, and TBA concentrations | [187] |
| PCL | Colombian Propolis extract & Chitosan | <5000 | Boneless pork loin chops | Antioxidant activity; delayed drip/purge loss; better colour stability; antibacterial activity against aerobic mesophilic and psychrophilic bacteria | [159] |
| Cross linked PVA | Cinnamon essential oil nanophytosomes | 66.48 | Shrimp | Antibacterial activity against S. aureus, P. aeruginosa, and E. coli | [188] |
| Natural | |||||
| Chitosan | Thymol | 135.94 ± 35.43 | Gilthead sea bream fillets (Sparus aurata) | Proximate analysis of Vitamin B complex (Riboflavin, thiamin Nictonimadie, pyridoxal, pyridoxine, and pyridoxamine) | [189] |
| Chitosan | Sodium carbonate | 446 ± 13 | Beef M. longissimus dorsi muscle | Antibacterial activity against total aerobic bacteria, lactic acid bacteria, yeast, and mould | [190] |
| PEO | Cinnamon essential oil/βCD loaded proteoliposomes | 317–364 | Beef | Antimicrobial activity against B. cereus, better colour stability, no impact on texture during storage | [191] |
| Zein + glycerol | Carvacrol | <5000 | Pork | Antibacterial activities against E. coli and S. aureus and antioxidant activities | [167] |
| Gelatin | Thyme essential oil Β-cyclodextrin | 150–195 | Chicken | Antibacterial activity against C. jejuni | [160] |
| Gelatin | Glycerine-Poly lysine | 154 | Beef | Antibacterial activity against L. monocytogens | [192] |
| Gelatin | Thiamine (vitamin B1) | 41.51 ±18.64 | Red meat and salmon | Provide thiamine stability and improve the bioaccessibility of raw and cooked meats | [14,38] |
| Gelatin/zein | Perillaldehyde, thymol, ɛ-polylysine | 52.32–78.54 | Chilled chicken | Antibacterial activity against S. aureus and S. enteritidis | [158] |
| Fish Eel skin gelatin | - | 1125 | European eel oil | Ecofriendly encapsulation material for synthetic polymer replacement | [193] |
| Zein | - | 190 | Fish oil | Oxidative stability of fish oil was enhanced after zein nanofibres encapsulation | [119] |
| Zein | Methyl ferulate | 185–342 | Sea bass | Antibacterial activity against S. putrefaciens and antioxidant activity | [194] |
| Zein | Gold Nanoparticles | 161–530 | Fish Dicentrarchus labrax | Retained dielectric properties, delayed both microbial infestation and sensory deterioration | [195] |
| Zein | Cinnamic aldehyde | 334.2–383 | Meat sausages | Antibactericidal against E. coli O157:H7 and S. aureus PTCC 1337, no effect on colour, texture profile, or sensory properties of sausages | [37,96] |
| Alginate /PEO | Phlorotannin | 282 | Chicken | Antibacterial activity against S. enteritidis | [16] |
| Sodium alginate/PVA | Lactobacillus rhamnosas | 60.09–522.1 | Fresh rainbow trout fillets | Stability of polyunsaturated fatty acids such as eicosapentaenoic acid docosa hexaenoic acid retained. Predominant change of Monounsaturated acid such as oleic acid is inhibited | [125] |
| Supporting Material | Fibres Diameter (nm) | Intelligent Mechanism | Muscle Foods | Role of Nanofibres | References |
|---|---|---|---|---|---|
| PANCMA fibres * | 200–1000 | SPE adsorbent membrane graphene oxide-doped | Chicken muscle | Recovery tetracycline antibiotics and volatile nitrogen to analytical determination by LC | [196] |
| PSSA fibres * | 407–469 | SPE adsorbent membrane plasma treated surface | Pork meat | Recovering 13 sulphonamide residues to analytical determination by LC | [197] |
| PHB porous fibres | 2780 | Portable biosensor of immobilized bacteriophage | - | Detecting the bioluminescent protein Nanoluc expression upon infection of E. coli O157:H7 | [198] |
| Chitosan/PEO fibres | 283–383 | Halochromic curcumin colour changes | Chicken breast | Quality indicator by monitoring surface pH changes based on total volatile basic nitrogen formation by microbial spoilage | [199] |
| PLLA fibres | 250–500 | Blueberry anthocyanin colour changes | Mutton meat | Freshness indication by TVB-N content changing colours distinguished by naked eyes from pink to a pale pink and finally colourless | [168] |
| Pullulan | Purple sweet potato extract anthocyanin-rich | Pork | Naked eyes visible pH-sensing reversible changes from purple and blue to green | [167] | |
| PVOH nanofibres and PS microfibres layers | 713–1259 | Methylene blue oxidation (MBox, blue)-reduction (MBrd, colorless) reaction activated by UV | Meatballs | Intelligent response of oxygen leakage by colour changing of colourless (no oxygen) to blue (oxygen in package headspace) | [200] |
| Zein fibres | 79–619.37 | Alizarin halochromic dye colour changes | Rainbow trout fillets | TVB-N detect by pH varying and consequent visual colour change from yellow to magenta monitoring freshness of fillets | [201] |
| Cellulose acetate nanofibres | 210–304 | Alizarin halochromic dye colour changes | Rainbow trout fillets | TVB-N detect by pH varying and consequent visual colour change from yellow to magenta monitoring freshness of fillets spoilage | [202] |
| Pullulan/chitin | 176.81–379.07 | Curcumin and anthocyanins dyes colour changes | Fish (Plectorhynchus cinctus) | Colour changes from pink (pH of 6–7) to powder blue (pale blue) | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagaoua, M.; Pinto, V.Z.; Göksen, G.; Alessandroni, L.; Lamri, M.; Dib, A.L.; Boukid, F. Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products. Coatings 2022, 12, 644. https://doi.org/10.3390/coatings12050644
Gagaoua M, Pinto VZ, Göksen G, Alessandroni L, Lamri M, Dib AL, Boukid F. Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products. Coatings. 2022; 12(5):644. https://doi.org/10.3390/coatings12050644
Chicago/Turabian StyleGagaoua, Mohammed, Vânia Zanella Pinto, Gülden Göksen, Laura Alessandroni, Melisa Lamri, Amira Leila Dib, and Fatma Boukid. 2022. "Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products" Coatings 12, no. 5: 644. https://doi.org/10.3390/coatings12050644
APA StyleGagaoua, M., Pinto, V. Z., Göksen, G., Alessandroni, L., Lamri, M., Dib, A. L., & Boukid, F. (2022). Electrospinning as a Promising Process to Preserve the Quality and Safety of Meat and Meat Products. Coatings, 12(5), 644. https://doi.org/10.3390/coatings12050644









