High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties
Abstract
1. Introduction
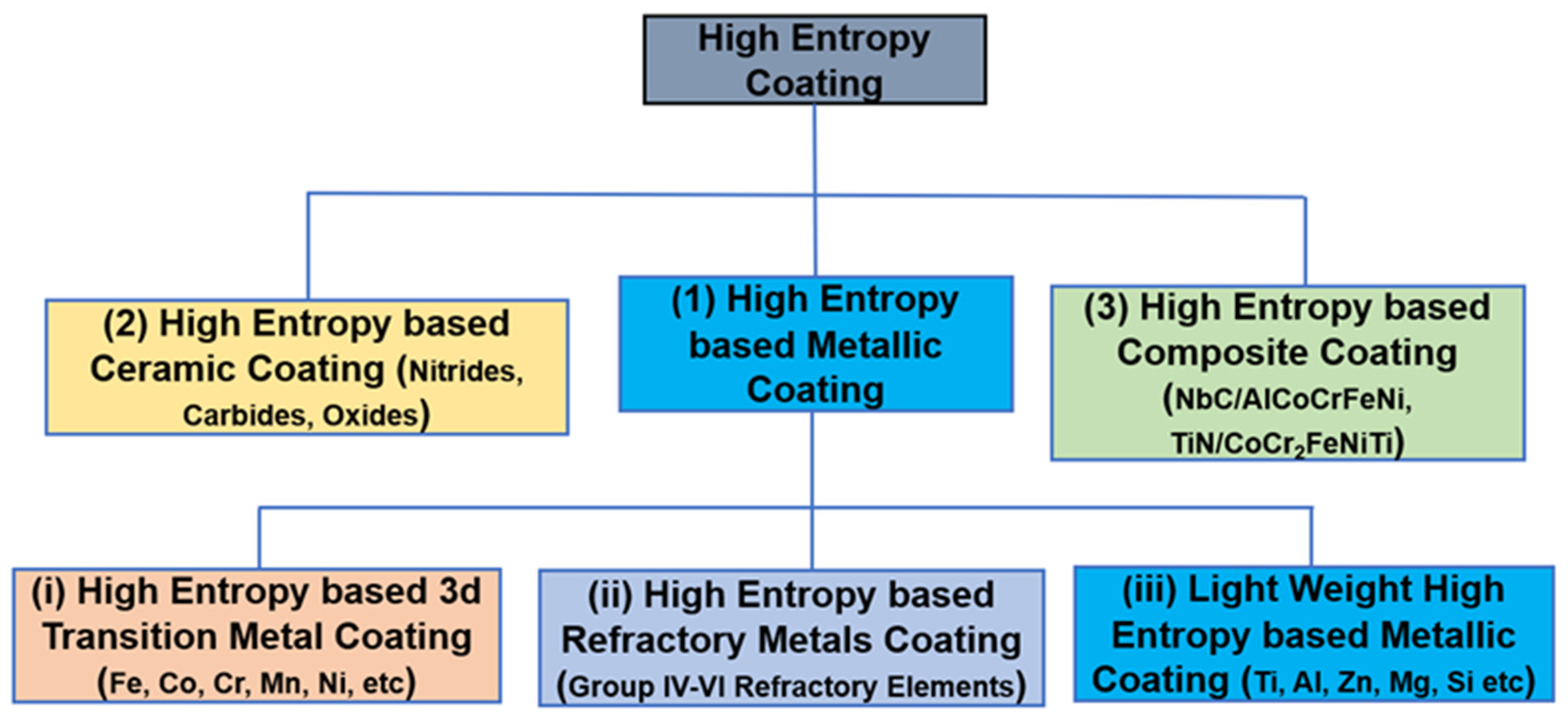
2. HEC Materials Classification
- HEM: high-entropy materials;
- HEA: high-entropy alloys;
- RHEA: refractory high-entropy alloys;
- HEC: high-entropy coatings;
- HEAC: high-entropy alloy coatings;
- RHEAC: refractory high-entropy alloy coatings;
- HECeC: high-entropy ceramic coatings;
- HECoC: high-entropy composite coatings;
- LWHEC: light weight high-entropy coatings.
2.1. HEA Metallic Coatings
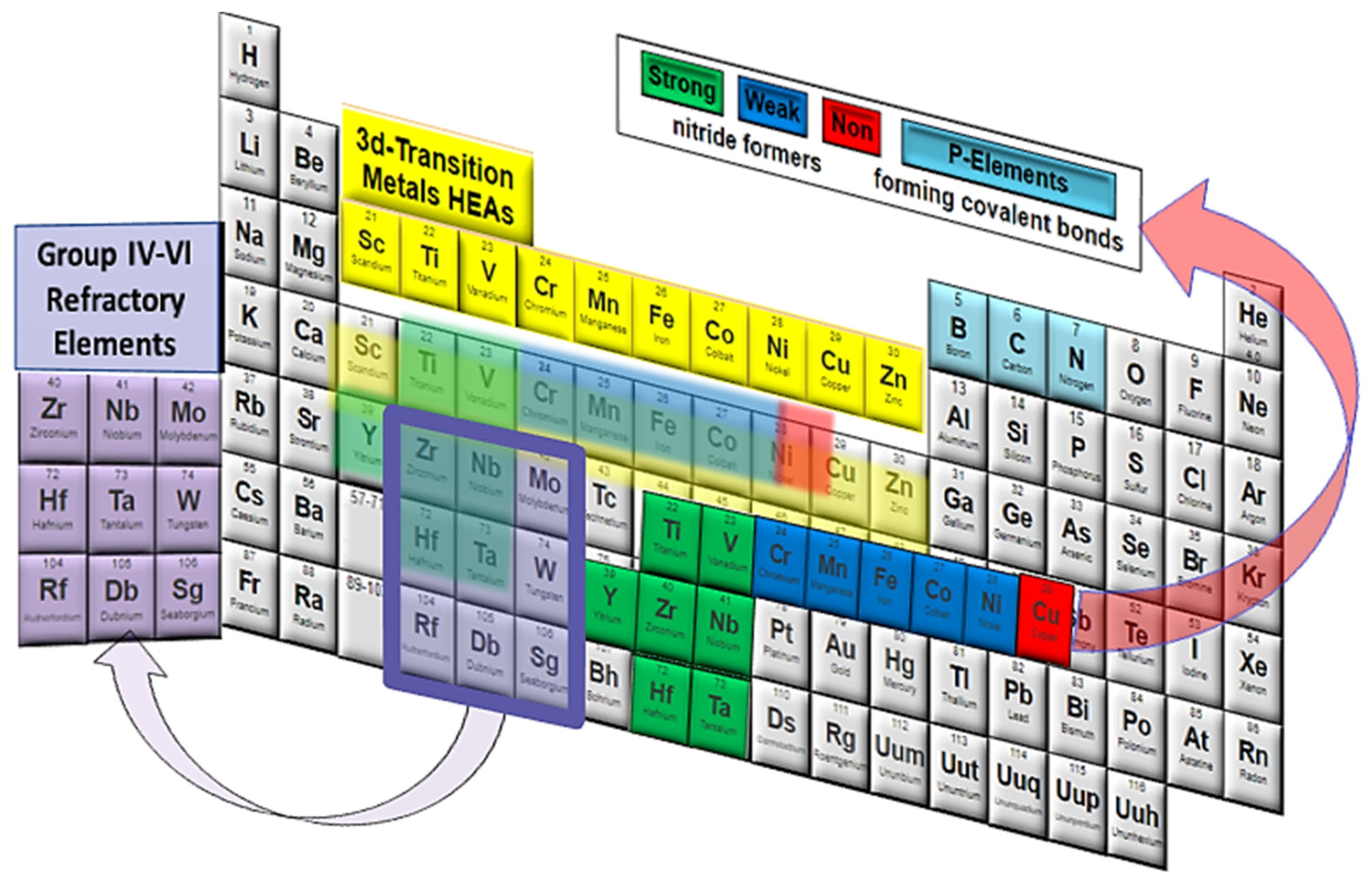
2.1.1. 3d-Transition Metal
2.1.2. Refractory High-Entropy Alloy (RHEAs)/Group IV–VI Metal Alloys
2.1.3. Light Weight High-Entropy Metals
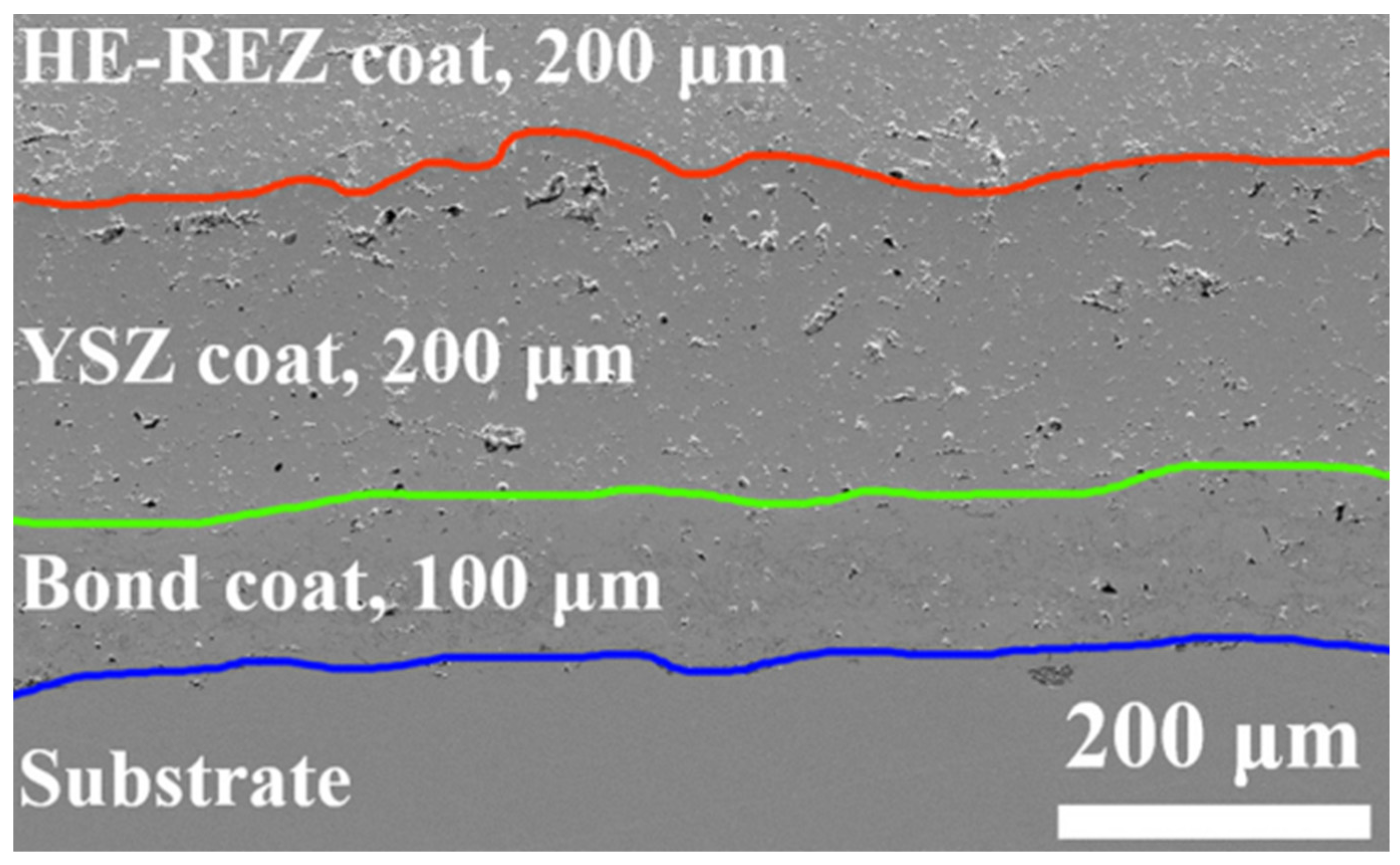
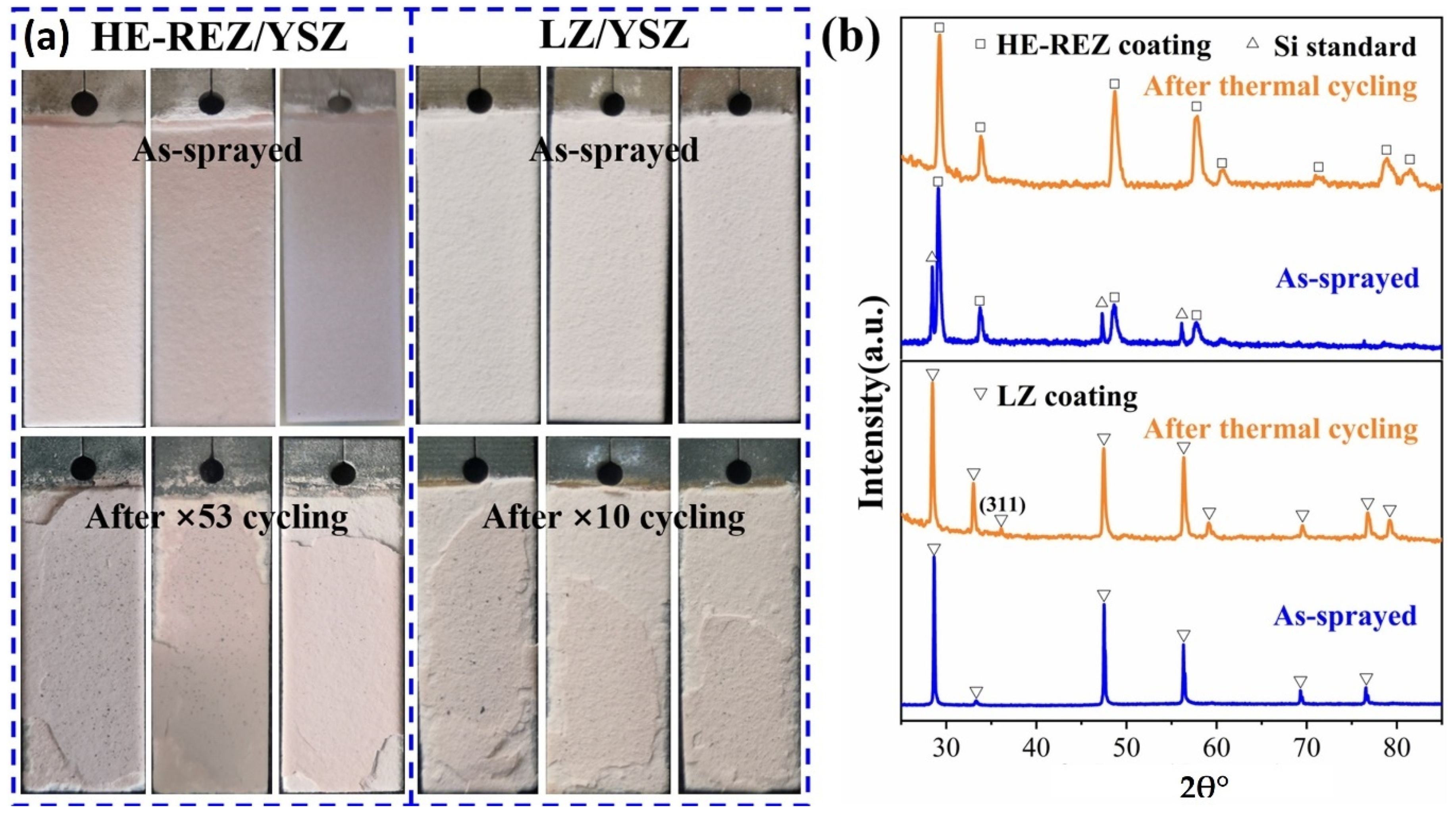
2.2. High-Entropy Ceramic Coatings
2.3. High-Entropy Composite Coating
3. HEC Fabrication Methods
3.1. Laser/Plasma Direct Deposition Methods
3.1.1. Laser Cladding
3.1.2. Laser Surface Alloying
3.1.3. Plasma Cladding
3.2. Thermal-Spraying Processes
3.2.1. Plasma Spraying
3.2.2. High-Velocity Oxygen-Fuel Spraying
3.2.3. Cold Spraying
3.2.4. HEAs Feedstock Synthesis
- I .
- Gas Atomisation
- II .
- Arc Melting Followed by Mechanical Milling (AM-MM)
- III .
- Mechanical Alloying (MA)
- IV .
- Blending
3.3. Vapour Deposition Methods
3.3.1. Magnetron Sputtering
3.3.2. Vacuum-Arc Deposition
4. Properties of High-Entropy Coatings
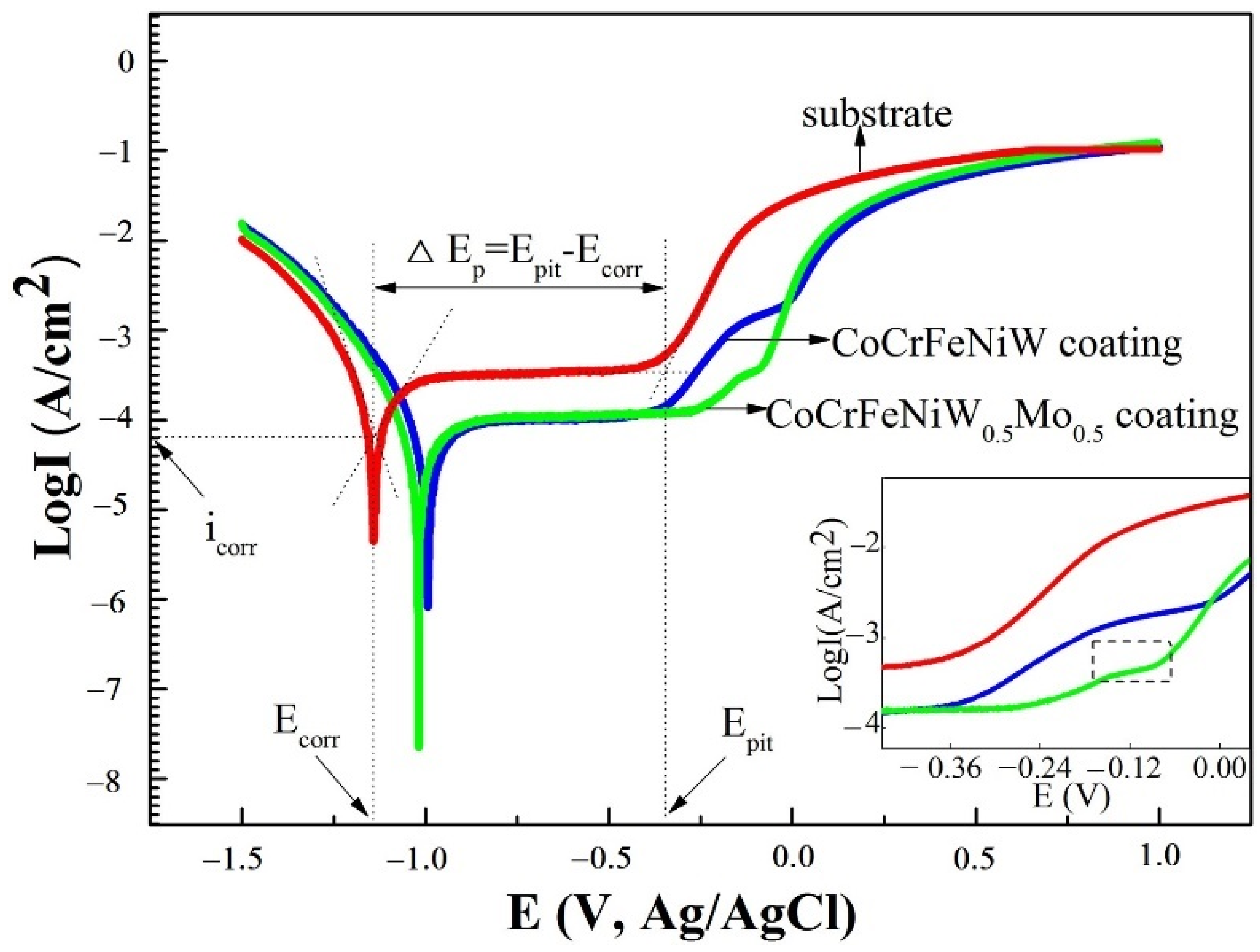
4.1. Corrosion Resistance
4.2. Oxidation Behaviour
4.3. Radiation Resistance
4.4. Diffusion Barriers
5. Suggested Future Work
- (1)
- According to the design principles, such as consisting of single solid solutions, the inclusion of the anti-corrosion elements, and strong bonding, homogeneous, and densified microstructures, in-depth research should be carried out on the design of high-entropy coatings (HECs) with superior corrosion resistance.
- (2)
- To meet the demands at high temperatures, it is necessary to investigate the oxidation properties of HECs by altering alloying additions and tailoring their microstructure to comprehend and establish fundamental theories/mechanisms of HEM coatings involved in its oxidation behaviour.
- (3)
- To understand the radiation control mechanism at elevated temperature and high doses of irradiation, a set of different HECs needs to be investigated to correlate HEM intrinsic properties with defect dynamics of radiation-tolerant material.
- (4)
- There is limited literature on the modelling and simulations of HEM films and coatings, which help explain the complex relationships among the preparation methods, microstructures, and properties. Further studies associated with the predictive computational modelling of the HEM films and coatings are urgently required.
6. Conclusions
- (1)
- It is concluded that relative to HEM bulk preparation technologies, the required mechanical and functional properties can be easily achieved in HECs owing to their smaller thickness, hence the more rapid cooling rate.
- (2)
- Similar to HEM bulk materials, the HECs are also have the tendency to form the solid-solution phase or amorphous phase due to the high-entropy effect and the ‘fast quenching’ of coating processes. The formation of the single solid-solution phase is discussed regarding the four core effects.
- (3)
- The functional properties of HECs are that they exhibit excellent corrosion resistance, oxidation resistance, diffusion retardation, and high phase stability at elevated temperature.
- (4)
- Several critical issues related to the reasons and design criteria for achieving the excellent functional and mechanical properties of the HECs are suggested, including the effects of stable oxide-forming elements on oxidation resistance as well as strong and non-nitride-forming elements on the hardness of HENs (high entropy nitrides).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Alvi, S.A. Refractory High-entropy Alloys and Films for High Temperature Applications. Ph.D. Thesis, Luleå University of Technology, Luleå, Sweden, 2021. [Google Scholar]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Zhao, M.; Jiang, Q. Effect of alloying elements on microstructure and properties of multiprincipal elements high-entropy alloys. J. Alloys Compd. 2009, 475, 752–757. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Tsai, R.-C.; Chang, T.; Huang, W.-F. Intermetallic Phases in High-Entropy Alloys: Statistical Analysis of their Prevalence and Structural Inheritance. Metals 2019, 9, 247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Al Rashid, A.; ur Rehman, E.; Atif, M. Laser deposition of high-entropy alloys: A comprehensive review. Opt. Laser Technol. 2021, 145, 107447. [Google Scholar] [CrossRef]
- Guruvidyathri, K.; Vaidya, M.; Murty, B. Challenges in design and development of high entropy alloys: A thermodynamic and kinetic perspective. Scr. Mater. 2020, 188, 37–43. [Google Scholar] [CrossRef]
- Reddy, S.; Bapari, S.; Bhattacharjee, P.P.; Chokshi, A. Superplastic-like flow in a fine-grained equiatomic CoCrFeMnNi high-entropy alloy. Mater. Res. Lett. 2017, 5, 408–414. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic Ti-Nb-Ta-Zr-Mo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Ren, Y.; Hirata, A.; Fujita, T.; Liaw, P.; Chen, M. Temperature-dependent compression behavior of an Al0.5CoCrCuFeNi high-entropy alloy. Materialia 2019, 5, 100243. [Google Scholar] [CrossRef]
- Pickering, E.; Jones, N. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A review on high entropy alloys coatings: Fabrication processes and property assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- Duchaniya, R.K.; Pandel, U.; Rao, P. Coatings based on high entropy alloys: An overview. Mater. Today Proc. 2021, 44, 4467–4473. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Cann, J.L.; De Luca, A.; Dunand, D.C.; Dye, D.; Miracle, D.B.; Oh, H.S.; Olivetti, E.A.; Pollock, T.M.; Poole, W.J.; Yang, R. Sustainability through alloy design: Challenges and opportunities. Prog. Mater. Sci. 2021, 117, 100722. [Google Scholar] [CrossRef]
- Ma, X.; Ruggiero, P.; Bhattacharya, R.; Senkov, O.N.; Rai, A.K. Evaluation of new high entropy alloy as thermal sprayed bondcoat in thermal barrier coatings. J. Therm. Spray Technol. 2022, 31, 1011–1020. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Yang, Y.-C.; Chen, C.-Y.; Yeh, J.-W. Thermal sprayed high-entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 coating with improved mechanical properties and oxidation resistance. Intermetallics 2017, 89, 105–110. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Yeh, J.-W.; Yeh, A.-C.; Shimoda, K. On the study of thermal-sprayed Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 HEA overlay coating. Surf. Coat. Technol. 2017, 316, 71–74. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Yeh, J.-W.; Yeh, A.-C.; Shimoda, K. A Heat-Resistant NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Overlay Coating for High-Temperature Applications. J. Electrochem. Soc. 2016, 163, C752–C758. [Google Scholar] [CrossRef]
- Hsu, W.-L.; Murakami, H.; Araki, H.; Watanabe, M.; Kuroda, S.; Yeh, A.-C.; Yeh, J.-W. A study of NiCo0.6Fe0.2CrxSiAlTiy high-entropy alloys for applications as a high-temperature protective coating and a bond coat in thermal barrier coating Systems. J. Electrochem. Soc. 2018, 165, C524–C531. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Liu, K.-C.; Wang, K.-Y.; Lou, L.-Y.; Zhang, Y.; Li, C.-J.; Li, C.-X. TGO and Al diffusion behavior of CuAlxNiCrFe high-entropy alloys fabricated by high-speed laser cladding for TBC bond coats. Corros. Sci. 2021, 192, 109781. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Jin, G.; Liu, Z.; Zheng, W.; Li, Y.; Wang, L. Microstructure and thermal stability of a Ni-Cr-Co-Ti-V-Al high-entropy alloy coating by laser surface alloying. Met. Mater. Int. 2017, 23, 1012–1018. [Google Scholar] [CrossRef]
- Ye, F.; Jiao, Z.; Yan, S.; Guo, L.; Feng, L.; Yu, J. Microbeam plasma arc remanufacturing: Effects of Al on microstructure, wear resistance, corrosion resistance and high temperature oxidation resistance of AlxCoCrFeMnNi high-entropy alloy cladding layer. Vacuum 2020, 174, 109178. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.; Yang, J.; Zhang, W.; He, L.; Zhang, R.; Yang, H.; Wang, J.; Long, J.; Chang, H. Mechanical and high-temperature corrosion properties of AlTiCrNiTa high entropy alloy coating prepared by magnetron sputtering for accident-tolerant fuel cladding. Surf. Coat. Technol. 2021, 417, 127228. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Chen, Y.; Liu, X.; Zhao, X.; Guo, F.; Xiao, P. Y-Hf co-doped AlCoCrFeNi high-entropy alloy coating with superior oxidation and spallation resistance at 1100 °C. Corros. Sci. 2021, 182, 109267. [Google Scholar] [CrossRef]
- Anupam, A.; Kumar, S.; Chavan, N.M.; Murty, B.S.; Kottada, R.S. First report on cold-sprayed AlCoCrFeNi high-entropy alloy and its isothermal oxidation. J. Mater. Res. 2019, 34, 796–806. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, Y.; Zhu, S.; Zhang, Z.; Tao, X.; Wang, Z.; Lu, B. Microstructure and wear properties of TiN–Al2O3–Cr2B multiphase ceramics in-situ reinforced CoCrFeMnNi high-entropy alloy coating. Mater. Chem. Phys. 2022, 276, 125352. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q. MoFeCrTiWAlNb refractory high-entropy alloy coating fabricated by rectangular-spot laser cladding. Intermetallics 2018, 102, 78–87. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Hao, X.; Zhang, X.; Liu, H. Lightweight refractory high entropy alloy coating by laser cladding on Ti–6Al–4V surface. Vacuum 2021, 183, 109823. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Yu, X.; Li, J. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
- Junjie, H.; Guo, H.; Jing, L.; Jingchao, T. New class of high-entropy defect fluorite oxides RE2(Ce0.2Zr0.2Hf0.2Sn0.2Ti0.2)2O7 (RE = Y, Ho, Er, or Yb) as promising thermal barrier coatings. J. Eur. Ceram. Soc. 2021, 41, 6080–6086. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Liu, J.-X.; Hu, Q.; Bao, W.; Wu, Y.; Cao, X.; Xu, F.; Zhang, G.-J. High-entropy thermal barrier coating of rare-earth zirconate: A case study on (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 prepared by atmospheric plasma spraying. J. Eur. Ceram. Soc. 2020, 40, 5731–5739. [Google Scholar] [CrossRef]
- Meng, G.; Yue, T.M.; Lin, X.; Yang, H.; Xie, H.; Ding, X. Laser surface forming of AlCoCrCuFeNi particle reinforced AZ91D matrix composites. Opt. Laser Technol. 2015, 70, 119–127. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, B.-H.; Shiao, M.-H.; Chang, S.-Y.; Shieu, F.-S. Structural morphology and characterization of (AlCrMoTaTi)N coating deposited via magnetron sputtering. Appl. Surf. Sci. 2013, 282, 789–797. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, X.; Liu, Q. Microstructure and properties of in-situ TiN reinforced laser cladding CoCr2FeNiTix high-entropy alloy composite coatings. Surf. Coat. Technol. 2018, 344, 353–358. [Google Scholar] [CrossRef]
- Tian, L.; Feng, Z.; Xiong, W. Microstructure, microhardness, and wear resistance of AlCoCrFeNiTi/Ni60 coating by plasma spraying. Coatings 2018, 8, 112. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC particles on microstructure and mechanical properties of AlCoCrFeNi high-entropy alloy coatings prepared by laser cladding. J. Alloys Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Lin, S.-J.; Chin, T.-S.; Gan, J.-Y.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chou, S.-Y. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements. Metall. Mater. Trans. A 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.-W.; Ranganathan, S.; Bhattacharjee, P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Jien-Wei, Y. Recent progress in high entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, M.-R.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Chang, S.-Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Gorsse, S.; Nguyen, M.; Senkov, O.N.; Miracle, D.B. Database on the mechanical properties of high entropy alloys and complex concentrated alloys. Data Brief 2018, 21, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.; Woodward, C.; Miracle, D. Microstructure and properties of aluminum-containing refractory high-entropy alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Moravcikova-Gouvea, L.; Kovacova, Z.; Kitzmantel, M.; Neubauer, E.; Jan, V.; Dlouhy, I. Influence of hot pressing sintering temperature on the properties of low-density Al0.5NbTa0.8Ti1.5V0.2Zr refractory high-entropy alloy. Mater. Sci. Forum 2021, 1016, 940–945. [Google Scholar] [CrossRef]
- Senkov, O.; Jensen, J.; Pilchak, A.; Miracle, D.; Fraser, H. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Senkov, O.N.; Zhang, F.; Miller, J.D. Phase Composition of a CrMo0.5NbTa0.5TiZr High Entropy Alloy: Comparison of Experimental and Simulated Data. Entropy 2013, 15, 3796–3809. [Google Scholar] [CrossRef]
- Gao, M.; Yeh, J.W.; Liaw, P.; Zhang, Y. High-Entropy Alloys (HEA): Fundamentals and Applications; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2018; Volume 8. [Google Scholar]
- Wang, L.; Chen, S.; Li, B.; Cao, T.; Wang, B.; Wang, L.; Ren, Y.; Liang, J.; Xue, Y. Lightweight Zr1.2V0.8NbTixAly high-entropy alloys with high tensile strength and ductility. Mater. Sci. Eng. A 2021, 814, 141234. [Google Scholar] [CrossRef]
- Maulik, O.; Kumar, D.; Kumar, S.; Dewangan, S.K.; Kumar, V. Structure and properties of lightweight high entropy alloys: A brief review. Mater. Res. Express 2018, 5, 052001. [Google Scholar] [CrossRef]
- Li, R.; Gao, J.C.; Fan, K. Study to microstructure and mechanical properties of mg containing high entropy alloys. Mater. Sci. Forum 2010, 650, 265–271. [Google Scholar] [CrossRef]
- Jia, Y.; Jia, Y.; Wu, S.; Ma, X.; Wang, G. Novel ultralight-weight complex concentrated alloys with high strength. Materials 2019, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, N.Y.; Stepanov, N.D.; Zherebtsov, S.V.; Tikhonovsky, M.A.; Salishchev, G.A. Structure and mechanical properties of B2 ordered refractory AlNbTiVZrx (x = 0–1.5) high-entropy alloys. Mater. Sci. Eng. A 2017, 704, 82–90. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Sokolovsky, V.S.; Tikhonovsky, M.A.; Salishchev, G.A. An AlNbTiVZr0.5 high-entropy alloy combining high specific strength and good ductility. Mater. Lett. 2015, 161, 136–139. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, M.; Li, Y.; Wu, C.; Yang, Y. A single-phase V0.5Nb0.5ZrTi refractory high-entropy alloy with outstanding tensile properties. Mater. Sci. Eng. A 2020, 792, 139774. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.-P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Lin, S.-Y.; Huang, Y.-C.; Wu, C.-L. Mechanical properties, deformation behaviors and interface adhesion of (AlCrTaTiZr)Nx multi-component coatings. Surf. Coat. Technol. 2010, 204, 3307–3314. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Lewin, E. Multi-component and high-entropy nitride coatings—A promising field in need of a novel approach. J. Appl. Phys. 2020, 127, 160901. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Li, F.; Zhou, L.; Liu, J.-X.; Liang, Y.; Zhang, G.-J. High-entropy pyrochlores with low thermal conductivity for thermal barrier coating materials. J. Adv. Ceram. 2019, 8, 576–582. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K.J.M.S.; Reports, E.R. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiang, H.; Dai, F.-Z.; Peng, Z.; Zhou, Y. (La0.2Ce0.2Nd0.2Sm0.2Eu0.2)2Zr2O7: A novel high-entropy ceramic with low thermal conductivity and sluggish grain growth rate. J. Mater. Sci. Technol. 2019, 35, 2647–2651. [Google Scholar] [CrossRef]
- Ren, X.; Tian, Z.; Zhang, J.; Wang, J. Equiatomic quaternary (Y1/4Ho1/4Er1/4Yb1/4)2SiO5 silicate: A perspective multifunctional thermal and environmental barrier coating material. Scr. Mater. 2019, 168, 47–50. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Ko, S.-T.; Chung, K.M.; Chen, R.; Luo, J.J.S.M. Size disorder as a descriptor for predicting reduced thermal conductivity in medium-and high-entropy pyrochlore oxides. Scr. Mater. 2020, 181, 76–81. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.-C.; Liu, J.-X.; Wei, X.-F.; Li, F.; Zhang, G.-J.; Jing, C.; Zhao, J.; Wu, H. High-entropy silicide ceramics developed from (TiZrNbMoW)Si2 formulation doped with aluminum. J. Eur. Ceram. Soc. 2020, 40, 2752–2759. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, J.-X.; Li, F.; Wei, X.; Wu, H.; Zhang, G.-J. A high entropy silicide by reactive spark plasma sintering. J. Adv. Ceram. 2019, 8, 148–152. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A high-entropy silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Wen, T.; Liu, H.; Ye, B.; Liu, D.; Chu, Y. High-entropy alumino-silicides: A novel class of high-entropy ceramics. Sci. China Mater. 2019, 63, 300–306. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Beresnev, V.; Smyrnova, K.; Kravchenko, Y.O.; Zukowski, P.; Bondarenko, G. The influence of nitrogen pressure on the fabrication of the two-phase superhard nanocomposite (TiZrNbAlYCr)N coatings. Mater. Lett. 2018, 211, 316–318. [Google Scholar] [CrossRef]
- Bagdasaryan, A.; Pshyk, A.; Coy, L.; Konarski, P.; Misnik, M.; Ivashchenko, V.; Kempiński, M.; Mediukh, N.; Pogrebnjak, A.; Beresnev, V. A new type of (TiZrNbTaHf)N/MoN nanocomposite coating: Microstructure and properties depending on energy of incident ions. Compos. Part B Eng. 2018, 146, 132–144. [Google Scholar] [CrossRef]
- Chang, C.-C.; Hsiao, Y.-T.; Chen, Y.-L.; Tsai, C.-Y.; Lee, Y.-J.; Ko, P.-H.; Chang, S.-Y.J. Lattice distortion or cocktail effect dominates the performance of Tantalum-based high-entropy nitride coatings. Appl. Surf. Sci. 2022, 577, 151894. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides: Properties, Characteristics, Processing and Applications; William Andrew: Norwich, NY, USA, 1996. [Google Scholar]
- Buinevich, V.; Nepapushev, A.; Moskovskikh, D.; Trusov, G.; Kuskov, K.; Vadchenko, S.; Rogachev, A.; Mukasyan, A. Fabrication of ultra-high-temperature nonstoichiometric hafnium carbonitride via combustion synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 16068–16073. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Chang, S.-Y.; Huang, Y.-C.; Shieu, F.-S.; Yeh, J.-W. Mechanical performance and nanoindenting deformation of (AlCrTaTiZr)NCy multi-component coatings co-sputtered with bias. Surf. Coat. Technol. 2012, 206, 5096–5102. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chang, Z.-C.; Kuo, L.-Y.; Lin, T.-J.; Lin, T.-N.; Shieu, F.-S. Solid solution coating of (TiVCrZrHf)N with unusual structural evolution. Surf. Coat. Technol. 2013, 217, 84–87. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Li, C.-E.; Chiang, S.-C.; Huang, Y.-C. 4-nm thick multilayer structure of multi-component (AlCrRuTaTiZr)Nx as robust diffusion barrier for Cu interconnects. J. Alloys Compd. 2012, 515, 4–7. [Google Scholar] [CrossRef]
- Dippo, O.F.; Mesgarzadeh, N.; Harrington, T.J.; Schrader, G.D.; Vecchio, K.S. Bulk high-entropy nitrides and carbonitrides. Sci. Rep. 2020, 10, 21288. [Google Scholar] [CrossRef] [PubMed]
- Braic, M.; Braic, V.; Balaceanu, M.; Zoita, C.; Vladescu, A.; Grigore, E. Characteristics of (TiAlCrNbY)C films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2010, 204, 2010–2014. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure and corrosion properties of MnCrFeCoNi high entropy alloy-graphene oxide composite coatings. Materialia 2019, 5, 100249. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, D.; Liang, X.; Chen, Y. Evolution of microstructure and mechanical properties of in situ synthesized TiC–TiB2/CoCrCuFeNi high entropy alloy coatings. Surf. Coat. Technol. 2015, 281, 109–116. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wan, L.; Meng, X.; Xie, Y. Strengthening and toughening mechanisms of CNTs/Mg-6Zn composites via friction stir processing. Mater. Sci. Eng. A 2018, 732, 205–211. [Google Scholar] [CrossRef]
- Meng, G.; Lin, X.; Xie, H.; Yue, T.M.; Ding, X.; Sun, L.; Qi, M. The effect of Cu rejection in laser forming of AlCoCrCuFeNi/Mg composite coating. Mater. Des. 2016, 108, 157–167. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Han, T.; Ranabhat, J.; Feng, X.; Shen, Y. Microstructures and oxidation behavior of NiCrAlCoY-Al composite coatings on Ti-6Al-4V alloy substrate via high-energy mechanical alloying method. J. Alloys Compd. 2017, 697, 268–281. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Q.; Wang, C.; Zhang, X. Corrosion and wear behavior of Ni60CuMoW coatings fabricated by combination of laser cladding and mechanical vibration processing. J. Alloys Compd. 2015, 621, 357–363. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, K.; Yao, C.; Nie, P.; Huang, J.; Li, Z. Microstructure and tribological properties of laser cladded self-lubricating nickel-base composite coatings containing nano-Cu and h-BN solid lubricants. Surf. Coat. Technol. 2019, 359, 485–494. [Google Scholar] [CrossRef]
- Fu, B.; Feng, K.; Li, Z. Study on the effect of Cu addition on the microstructure and properties of NiTi alloy fabricated by laser cladding. Mater. Lett. 2018, 220, 148–151. [Google Scholar] [CrossRef]
- Gopinath, M.; Thota, P.; Nath, A.K. Role of molten pool thermo cycle in laser surface alloying of AISI 1020 steel with in-situ synthesized TiN. Surf. Coat. Technol. 2019, 362, 150–166. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Zhang, Y.; Liu, S.-H.; Li, C.-J.; Li, C.-X. High-temperature oxidation behavior of CuAlNiCrFe high-entropy alloy bond coats deposited using high-speed laser cladding process. Surf. Coat. Technol. 2020, 398, 126093. [Google Scholar] [CrossRef]
- Wang, M.; Cui, H.; Wei, N.; Ding, L.; Zhang, X.; Zhao, Y.; Wang, C.; Song, Q. A new design of in situ Ti(C,N) reinforced composite coatings and their microstructures, interfaces, and wear resistances. ACS Appl. Mater. Interfaces 2018, 10, 4250–4265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, C.; Zhang, C.; Guan, M.; Tan, J. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Khorram, A.; Taheri, M.; Fasahat, M. Laser cladding of Inconel 713 LC with Stellite 31 powder: Statistical modeling and optimization. Laser Phys. 2021, 31, 096001. [Google Scholar] [CrossRef]
- Khorram, A.; Davoodi Jamaloei, A.; Jafari, A.; Paidar, M.; Cao, X. Microstructural evolution of laser-clad 75Cr3C2+25(80Ni20Cr) powder on Inconel 718 superalloy. J. Mater. Processing Technol. 2020, 284, 116735. [Google Scholar] [CrossRef]
- Moradi, M.; Ashoori, A.; Hasani, A. Additive manufacturing of stellite 6 superalloy by direct laser metal deposition—Part 1: Effects of laser power and focal plane position. Opt. Laser Technol. 2020, 131, 106328. [Google Scholar] [CrossRef]
- Moradi, M.; Hasani, A.; Malekshahi Beiranvand, Z.; Ashoori, A. Additive manufacturing of stellite 6 superalloy by direct laser metal deposition—Part 2: Effects of scanning pattern and laser power reduction in differrent layers. Opt. Laser Technol. 2020, 131, 106455. [Google Scholar] [CrossRef]
- Moradi, M.; Hasani, A.; Pourmand, Z.; Lawrence, J. Direct laser metal deposition additive manufacturing of Inconel 718 superalloy: Statistical modelling and optimization by design of experiments. Opt. Laser Technol. 2021, 144, 107380. [Google Scholar] [CrossRef]
- Ren, X.; Fu, H.; Xing, J.; Yi, Y. Research on high-temperature dry sliding friction wear behavior of CaTi modified high boron high speed steel. Tribol. Int. 2019, 132, 165–176. [Google Scholar] [CrossRef]
- Ha, T.K.; Jeong, H.T.; Jung, J.Y. High temperature deformation behavior of M2 high-speed tool steel. Solid State Phenom. 2007, 124–126, 1365–1368. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Liu, Q. Microstructure evolution and strengthening mechanism of laser-cladding MoFexCrTiWAlNby refractory high-entropy alloy coatings. J. Alloys Compd. 2020, 834, 155147. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Ley, N.; Joshi, S.S.; Zhang, B.; Ho, Y.-H.; Dahotre, N.B.; Young, M.L. Laser coating of a CrMoTaWZr complex concentrated alloy onto a H13 tool steel die head. Surf. Coat. Technol. 2018, 348, 150–158. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, S.; Zhang, C.; Zhang, H.; Dong, S. Phase evolution and properties in laser surface alloying of FeCoCrAlCuNix high-entropy alloy on copper substrate. Surf. Coat. Technol. 2017, 315, 368–376. [Google Scholar] [CrossRef]
- Cai, Z.; Cui, X.; Jin, G.; Lu, B.; Zhang, D.; Zhang, Z. In situ TEM tensile testing on high-entropy alloy coating by laser surface alloying. J. Alloys Compd. 2017, 708, 380–384. [Google Scholar] [CrossRef]
- Cai, Z.; Jin, G.; Cui, X.; Liu, Z.; Zheng, W.; Li, Y.; Wang, L. Synthesis and microstructure characterization of Ni-Cr-Co-Ti-V-Al high entropy alloy coating on Ti-6Al-4V substrate by laser surface alloying. Mater. Charact. 2016, 120, 229–233. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Rajamure, R.S.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.; Zhang, C. Phase evolution characteristics of FeCoCrAlCuVxNi high entropy alloy coatings by laser high-entropy alloying. Mater. Lett. 2015, 141, 7–9. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Cui, X.; Jin, G.; Li, Y.; Liu, Z.; Dong, M. Design and microstructure characterization of FeCoNiAlCu high-entropy alloy coating by plasma cladding: In comparison with thermodynamic calculation. Surf. Coat. Technol. 2017, 330, 163–169. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, Y.; Li, J.; Liu, Y.; Liu, Y. Microstructure and mechanical properties of FeCoCrNiNbX high-entropy alloy coatings. Phys. B Condens. Matter 2018, 550, 112–116. [Google Scholar] [CrossRef]
- Lu, J.; Wang, B.; Qiu, X.; Peng, Z.; Ma, M. Microstructure evolution and properties of CrCuFexNiTi high-entropy alloy coating by plasma cladding on Q235. Surf. Coat. Technol. 2017, 328, 313–318. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, X.; Xu, B. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, X.; Wang, Z.; Xu, B. Formation and mechanical properties of CoNiCuFeCr high-entropy alloys coatings prepared by plasma transferred arc cladding process. Plasma Chem. Plasma Process. 2013, 33, 979–992. [Google Scholar] [CrossRef]
- Jin, G.; Li, Y.; Cui, H.; Cui, X.; Cai, Z. Microstructure and tribological properties of in situ synthesized TiN reinforced Ni/Ti alloy clad layer prepared by plasma cladding technique. J. Mater. Eng. Perform. 2016, 25, 2412–2419. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Yu, Y.; Zhang, Z.; Zhu, S.; Lou, X.; Wang, Z. Study of high temperature friction and wear performance of (CoCrFeMnNi)85Ti15 high-entropy alloy coating prepared by plasma cladding. Surf. Coat. Technol. 2020, 384, 125337. [Google Scholar] [CrossRef]
- Fauchais, P. Current status and future directions of thermal spray coatings and techniques. In Future Development of Thermal Spray Coatings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–49. [Google Scholar]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal barrier coatings—A state of the art review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Ojha, S.; Thakare, J.; Pandey, C.; Giri, A.; Mahapatra, M.; Mulik, R. Experimental and numerical investigation of residual stress in coatings on steel. J. Test. Eval. 2019, 48, 4370–4386. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Song, J.; Deng, C.; Deng, C. Microstructure and corrosion behavior of Fe-based amorphous coating prepared by HVOF. J. Alloys Compd. 2017, 721, 506–511. [Google Scholar] [CrossRef]
- Wang, G.; Huang, Z.; Xiao, P.; Zhu, X. Spraying of Fe-based amorphous coating with high corrosion resistance by HVAF. J. Manuf. Processes 2016, 22, 34–38. [Google Scholar] [CrossRef]
- Jahani, B.; Azarmi, F.; Brooks, A. Development of antibacterial surfaces via thermal spray coating techniques. Biomed. Sci. Instrum. 2018, 54, 116–122. [Google Scholar] [CrossRef]
- Wang, R.; Duan, J.; Ye, F. Effect of spraying parameters on the crystallinity and microstructure of solution precursor plasma sprayed coatings. J. Alloys Compd. 2018, 766, 886–893. [Google Scholar] [CrossRef]
- Hu, Z.-C.; Liu, B.; Wang, L.; Cui, Y.-H.; Wang, Y.-W.; Ma, Y.-D.; Sun, W.-W.; Yang, Y. Research progress of failure mechanism of thermal barrier coatings at high temperature via finite element method. Coatings 2020, 10, 732. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mulik, R.S.; Mahapatra, M.M. Mechanical property evaluation of carbon nanotubes reinforced plasma sprayed YSZ-alumina composite coating. Ceram. Int. 2018, 44, 6980–6989. [Google Scholar] [CrossRef]
- Wang, W.; Qi, W.; Xie, L.; Yang, X.; Li, J.; Zhang, Y. Microstructure and corrosion behavior of (CoCrFeNi)95Nb5 high-entropy alloy coating fabricated by plasma spraying. Materials 2019, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Oete, M.; Königstein, T.; Tan, Z.; He, D. Wear behavior of HVOF-sprayed Al0.6TiCrFeCoNi high entropy alloy coatings at different temperatures. Surf. Coat. Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Pala, Z.; Bai, M.; Lukac, F.; Hussain, T. Laser clad and HVOF-sprayed stellite 6 coating in chlorine-rich environment with KCl at 700 °C. Oxid. Met. 2017, 88, 749–771. [Google Scholar] [CrossRef]
- Kay, C.M.; Karthikeyan, J. Introduction to cold spray. In High Pressure Cold Spray: Principles and Applications; ASM International: Almere, The Netherlands, 2016. [Google Scholar]
- Boruah, D. Structural Integrity Assessment of Cold Spray Additive Manufactured Titanium Alloy Ti6Al4V. Ph.D. Thesis, Theoretical and Experimental Coventry University, Coventry, UK, 2020. [Google Scholar]
- Luzin, V.; Spencer, K.; Zhang, M.; Matthews, N.; Davis, J.; Saleh, M. Residual Stresses in Cold Spray Coatings. In Cold-Spray Coatings; Springer: Cham, Switzerland, 2018; pp. 451–480. [Google Scholar]
- Rokni, M.; Nutt, S.; Widener, C.; Champagne, V.; Hrabe, R. Review of relationship between particle deformation, coating microstructure, and properties in high-pressure cold spray. J. Therm. Spray Technol. 2017, 26, 1308–1355. [Google Scholar] [CrossRef]
- Boruah, D.; Ahmad, B.; Lee, T.L.; Kabra, S.; Syed, A.; McNutt, P.; Doré, M.; Zhang, X. Evaluation of residual stresses induced by cold spraying of Ti-6Al-4V on Ti-6Al-4V substrates. Surf. Coat. Technol. 2019, 374, 591–602. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn high entropy alloy (HEA) coating via cold spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Ashgriz, N. Handbook of Atomization and Sprays: Theory and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1,5Co1,5CrFeTi0,5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Haas, S.; Mosbacher, M.; Senkov, O.N.; Feuerbacher, M.; Freudenberger, J.; Gezgin, S.; Völkl, R.; Glatzel, U. Entropy determination of single-phase high entropy alloys with different crystal structures over a wide temperature range. Entropy 2018, 20, 654. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Fu, M.; Xiong, W. Microstructural evolution of AlCoCrFeNiSi high-entropy alloy powder during mechanical alloying and its coating performance. Materials 2018, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-entropy alloys by mechanical alloying: A review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Bai, M.; Namus, R.; Xu, Y.; Guan, D.; Rainforth, M.W.; Inkson, B.J. In-situ Ti-6Al-4V/TiC composites synthesized by reactive spark plasma sintering: Processing, microstructure, and dry sliding wear behaviour. Wear 2019, 432, 202944. [Google Scholar] [CrossRef]
- He, B.; Zhang, N.; Lin, D.; Zhang, Y.; Dong, F.; Li, D. The phase evolution and property of FeCoCrNiAlTix high-entropy alloying coatings on Q253 via laser cladding. Coatings 2017, 7, 157. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and mechanical properties of multi-element (AlCrMnMoNiZr)Nx coatings by reactive magnetron sputtering. J. Alloys Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Deng, M.-J.; Chang, Z.-C.; Kuo, B.-H.; Chen, E.-C.; Chang, S.-Y.; Shieu, F.-S. Oxidation resistance and characterization of (AlCrMoTaTi)-Six-N coating deposited via magnetron sputtering. J. Alloys Compd. 2015, 647, 179–188. [Google Scholar] [CrossRef]
- Pogrebnjak, A.; Yakushchenko, I.; Bagdasaryan, A.; Bondar, O.; Krause-Rehberg, R.; Abadias, G.; Chartier, P.; Oyoshi, K.; Takeda, Y.; Beresnev, V. Microstructure, physical and chemical properties of nanostructured (Ti–Hf–Zr–V–Nb) N coatings under different deposition conditions. Mater. Chem. Phys. 2014, 147, 1079–1091. [Google Scholar] [CrossRef]
- Li, R.; Li, M.; Jiang, C.; Qiao, B.; Zhang, W.; Xu, J. Thermal stability of AlCrTaTiZrMo-nitride high entropy film as a diffusion barrier for Cu metallization. J. Alloys Compd. 2019, 773, 482–489. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.; Braic, M. Nanostructured multi-element (TiZrNbHfTa)N and (TiZrNbHfTa)C hard coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Liu, C.; Xu, J.; Liu, Z.; Ning, X.; Jiang, S.; Miao, D. Fabrication of highly electrically conductive Ti/Ag/Ti tri-layer and Ti–Ag alloy thin films on PET fabrics by multi-target magnetron sputtering. J. Mater. Sci. Mater. Electron. 2018, 29, 19578–19587. [Google Scholar] [CrossRef]
- Chen, T.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188, 193–200. [Google Scholar] [CrossRef]
- Lin, M.-I.; Tsai, M.-H.; Shen, W.-J.; Yeh, J.-W. Evolution of structure and properties of multi-component (AlCrTaTiZr)Ox films. Thin Solid Film. 2010, 518, 2732–2737. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti6Al4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Lin, C.; Duh, J. Corrosion behavior of (Ti–Al–Cr–Si–V)xNy coatings on mild steels derived from RF magnetron sputtering. Surf. Coat. Technol. 2008, 203, 558–561. [Google Scholar] [CrossRef]
- Xin, H.; Yang, J.; Zhang, W.; Yang, J.; Mao, J.; Teng, C.; Kong, X.; Si, J.; Xu, X.; Zhang, W.; et al. Effect of Au ion irradiation on the surface morphology, microstructure and mechanical properties of AlNbTiZr medium-entropy alloy coatings with various Al content for ATF. Surf. Coat. Technol. 2022, 434, 128157. [Google Scholar] [CrossRef]
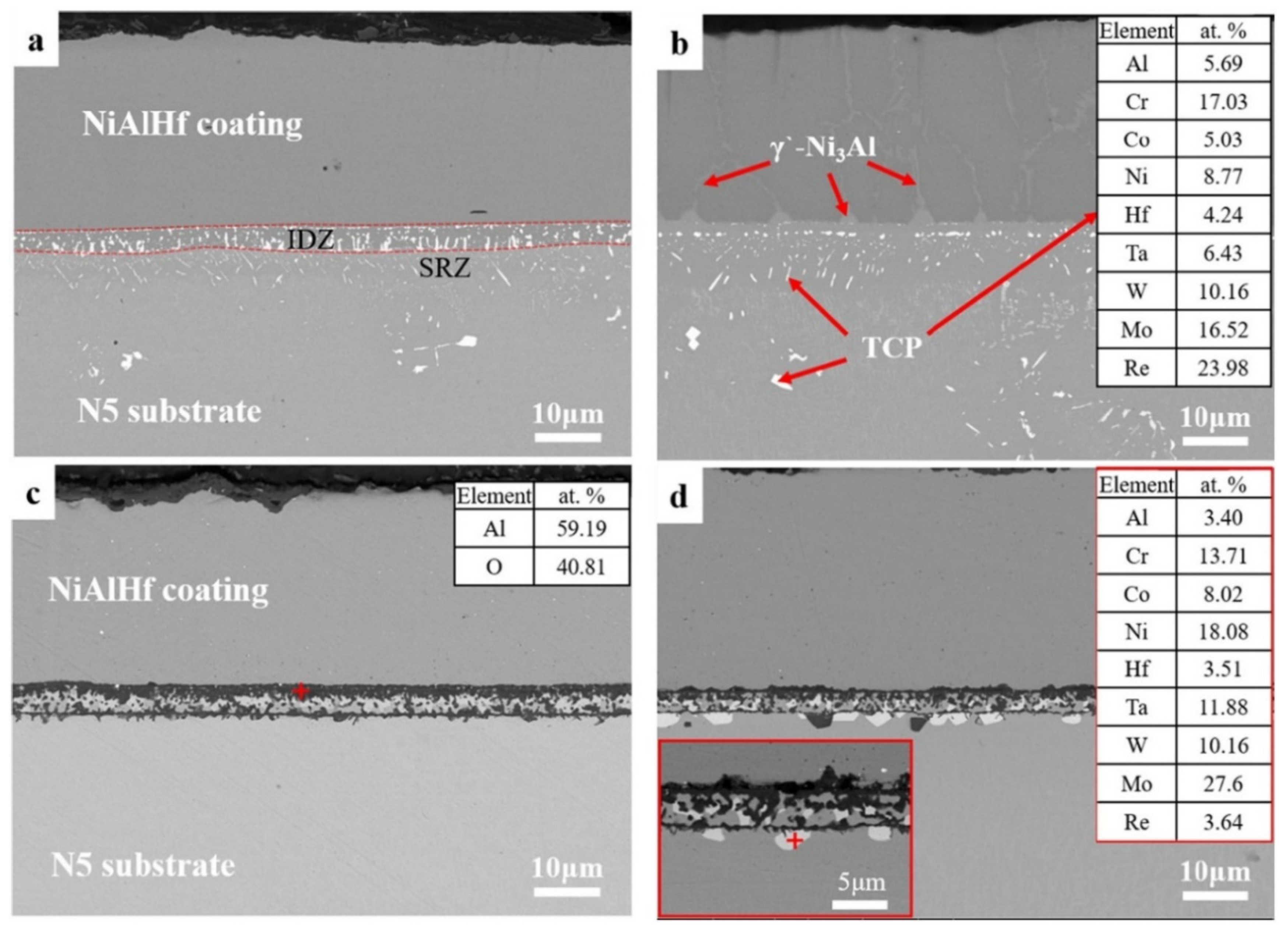
- Xu, Z.; Zhang, P.; Wang, W.; Shi, Q.; Yang, H.; Wang, D.; Hong, Y.; Wang, L.; Guo, C.; Lin, S.; et al. AlCoCrNiMo high-entropy alloy as diffusion barrier between NiAlHf coating and Ni-based single crystal superalloy. Surf. Coat. Technol. 2021, 414, 127101. [Google Scholar] [CrossRef]
- Yan, X.H.; Li, J.S.; Zhang, W.R.; Zhang, Y. A brief review of high-entropy films. Mater. Chem. Phys. 2018, 210, 12–19. [Google Scholar] [CrossRef]
- Kong, D.; Guo, J.; Liu, R.; Zhang, X.; Song, Y.; Li, Z.; Guo, F.; Xing, X.; Xu, Y.; Wang, W. Effect of remelting and annealing on the wear resistance of AlCoCrFeNiTi0.5 high entropy alloys. Intermetallics 2019, 114, 106560. [Google Scholar] [CrossRef]
- Lu, P.; Saal, J.E.; Olson, G.B.; Li, T.; Swanson, O.J.; Frankel, G.; Gerard, A.Y.; Quiambao, K.F.; Scully, J.R. Computational materials design of a corrosion resistant high entropy alloy for harsh environments. Scr. Mater. 2018, 153, 19–22. [Google Scholar] [CrossRef]
- Quiambao, K.F.; McDonnell, S.J.; Schreiber, D.K.; Gerard, A.Y.; Freedy, K.M.; Lu, P.; Saal, J.E.; Frankel, G.S.; Scully, J.R. Passivation of a corrosion resistant high entropy alloy in non-oxidizing sulfate solutions. Acta Mater. 2019, 164, 362–376. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Sun, J.; Li, X.; Li, P.; Du, J.; Qiao, P.; Wang, Y. CoCrFeNi(W1−xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering. Mater. Des. 2017, 117, 193–202. [Google Scholar] [CrossRef]
- Fu, Y.; Dai, C.; Luo, H.; Li, D.; Du, C.; Li, X. The corrosion behavior and film properties of Al-containing high-entropy alloys in acidic solutions. Appl. Surf. Sci. 2021, 560, 149854. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, H.; Li, W.; Wu, J.; Zhong, X.; Chen, G.; Guo, S. Novel high-entropy and medium-entropy stainless steels with enhanced mechanical and anti-corrosion properties. Mater. Sci. Technol. 2018, 34, 572–579. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Shabani, M.; Jablonski, P.D.; Pataky, G.J.; Poursaee, A. The influence of incorporation of Mn on the pitting corrosion performance of CrFeCoNi High Entropy Alloy at different temperatures. Mater. Des. 2019, 184, 108170. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Liu, C. Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloys Compd. 2014, 585, 282–286. [Google Scholar] [CrossRef]
- Kashkarov, E.; Afornu, B.; Sidelev, D.; Krinitcyn, M.; Gouws, V.; Lider, A. Recent advances in protective coatings for accident tolerant Zr-based fuel claddings. Coatings 2021, 11, 557. [Google Scholar] [CrossRef]
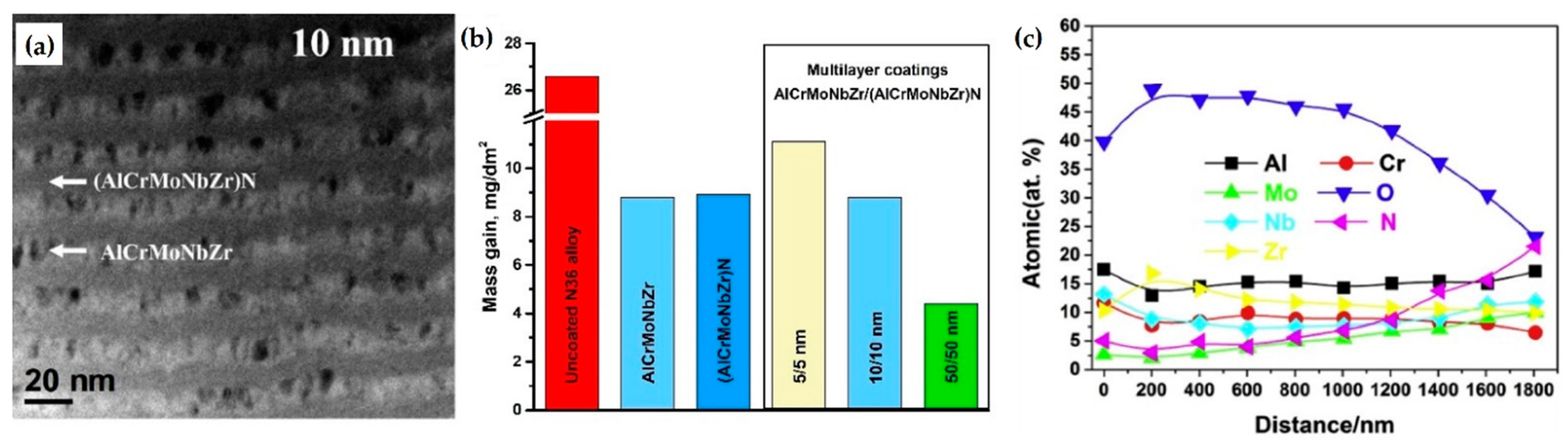
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: A case study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Xiao, P. Effect of microstructure on early oxidation of MCrAlY coatings. Acta Mater. 2018, 159, 150–162. [Google Scholar] [CrossRef]
- Cai, J.; Guan, Q.; Hou, X.; Wang, Z.; Su, J.; Han, Z. Isothermal oxidation behaviour of thermal barrier coatings with CoCrAlY bond coat irradiated by high-current pulsed electron beam. Appl. Surf. Sci. 2014, 317, 360–369. [Google Scholar] [CrossRef]
- Lance, M.J.; Unocic, K.A.; Haynes, J.A.; Pint, B.A. APS TBC performance on directionally-solidified superalloy substrates with HVOF NiCoCrAlYHfSi bond coatings. Surf. Coat. Technol. 2015, 284, 9–13. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Zhao, X.; Guo, F.; Xiao, P. Effect of microstructure of a NiCoCrAlY coating fabricated by high-velocity air fuel on the isothermal oxidation. Corros. Sci. 2019, 159, 108126. [Google Scholar] [CrossRef]
- Shen, Z.; He, L.; Xu, Z.; Mu, R.; Huang, G. Morphological evolution and failure of LZC/YSZ DCL TBCs by electron beam-physical vapor deposition. Materialia 2018, 4, 340–347. [Google Scholar] [CrossRef]
- Golightly, F.A.; Stott, F.H.; Wood, G.C. The relationship between oxide grain morphology and growth mechanisms for Fe-Cr-Al and Fe-Cr-Al-Y alloys. J. Electrochem. Soc. 1979, 126, 1035–1042. [Google Scholar] [CrossRef]
- Golightly, F.A.; Stott, F.H.; Wood, G.C. The influence of yttrium additions on the oxide-scale adhesion to an iron-chromium-aluminum alloy. Oxid. Met. 1976, 10, 163–187. [Google Scholar] [CrossRef]
- Chen, H.; Hou, X.; He, J.; Guo, H. Bioxidant corrosion behaviour of CoNiCrAlY coated IN738 at 1100 °C. Corros. Sci. 2019, 151, 154–162. [Google Scholar] [CrossRef]
- Meng, G.-H.; Liu, H.; Liu, M.-J.; Xu, T.; Yang, G.-J.; Li, C.-X.; Li, C.-J. Large-grain α-Al2O3 enabling ultra-high oxidation-resistant MCrAlY bond coats by surface pre-agglomeration treatment. Corros. Sci. 2020, 163, 108275. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Audigié, P.; Rouaix-Vande Put, A.; Malié, A.; Thouron, C.; Monceau, D. High-temperature cyclic oxidation of Pt-rich γ-γ’ bond-coatings. Part II: Effect of Pt and Al on TBC system lifetime. Corros. Sci. 2019, 150, 1–8. [Google Scholar] [CrossRef]
- Meng, G.-H.; Liu, H.; Xu, P.-Y.; Li, G.-R.; Xu, T.; Yang, G.-J.; Li, C.-J. Superior oxidation resistant MCrAlY bond coats prepared by controlled atmosphere heat treatment. Corros. Sci. 2020, 170, 108653. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Beake, B.D.; Harris, A.J. Nanomechanics to 1000 °C for high temperature mechanical properties of bulk materials and hard coatings. Vacuum 2019, 159, 17–28. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Horodek, P.; Tarelnyk, V.; Buranich, V.V.; Amekura, H.; Okubo, N.; Ishikawa, N.; Beresnev, V.M. Positron annihilation studies of defect structure of (TiZrHfNbV)N nitride coatings under Xe14+ 200 MeV ion irradiation. Mater. Lett. 2021, 303, 130548. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Liu, X.; Chen, Y.; Yeh, J.-W.; Tseng, K.-K.; Natesan, K. Irradiation effects in high entropy alloys and 316H stainless steel at 300 °C. J. Nucl. Mater. 2018, 510, 421–430. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.; Zhang, J.; Xiong, T.; Jin, Q.; Zhou, Y.; Shao, X.; Zhang, B.; Zhu, Z.; Zheng, S.; et al. High He-ion irradiation resistance of CrMnFeCoNi high-entropy alloy revealed by comparison study with Ni and 304SS. J. Mater. Sci. Technol. 2019, 35, 300–305. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gao, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C.; et al. A promising new class of irradiation tolerant materials: Ti2ZrHfV0.5Mo0.2 high-entropy alloy. J. Mater. Sci. Technol. 2019, 35, 369–373. [Google Scholar] [CrossRef]
- Tong, Y.; Velisa, G.; Zhao, S.; Guo, W.; Yang, T.; Jin, K.; Lu, C.; Bei, H.; Ko, J.Y.P.; Pagan, D.C.; et al. Evolution of local lattice distortion under irradiation in medium- and high-entropy alloys. Materialia 2018, 2, 73–81. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Cabot, A.; Trofimov, E.A. Does the pathway for development of next generation nuclear materials straightly go through high-entropy materials? Int. J. Refract. Met. Hard Mater. 2021, 97, 105504. [Google Scholar] [CrossRef]
- Komarov, F.F.; Pogrebnyak, A.D.; Konstantinov, S.V. Radiation Resistance of high-entropy nanostructured (Ti, Hf, Zr, V, Nb)N coatings. Tech. Phys. 2015, 60, 1519–1524. [Google Scholar] [CrossRef][Green Version]
- Narita, T.; Thosin, K.A.Z.; Fengqun, L.; Hayashi, S.; Murakami, H.; Gleeson, B.; Young, D. Development of Re based diffusion barrier coatings on nickel based superalloys. Mater. Corros.-Werkst. Und Korros. 2005, 56, 923–929. [Google Scholar] [CrossRef]
- Narita, T. Rhenium coating as a diffusion barrier on a nickel-based superalloy in high temperature oxidation. Mater. High Temp. 2001, 18, 245–251. [Google Scholar]
- Matsumura, Y.; Fukumoto, M.; Hayashi, S.; Kasama, A.; Iwanaga, I.; Tanaka, R.; Narita, T. Oxidation Behavior of a Re-Base Diffusion-Barrier/β-NiAl Coating on Nb–5Mo–15W at High Temperatures. Oxid. Met. 2004, 61, 105–124. [Google Scholar] [CrossRef]
- Fukumoto, M.; Matsumura, Y.; Hayashi, S.; Sakamoto, K.; Kasama, A.; Tanaka, R.; Narita, T. Formation of a Rhenium-Base Coating on a Nb-Base Alloy. Oxid. Met. 2003, 60, 335–346. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y. Functional properties and promising applications of high entropy alloys. Scr. Mater. 2020, 187, 188–193. [Google Scholar] [CrossRef]
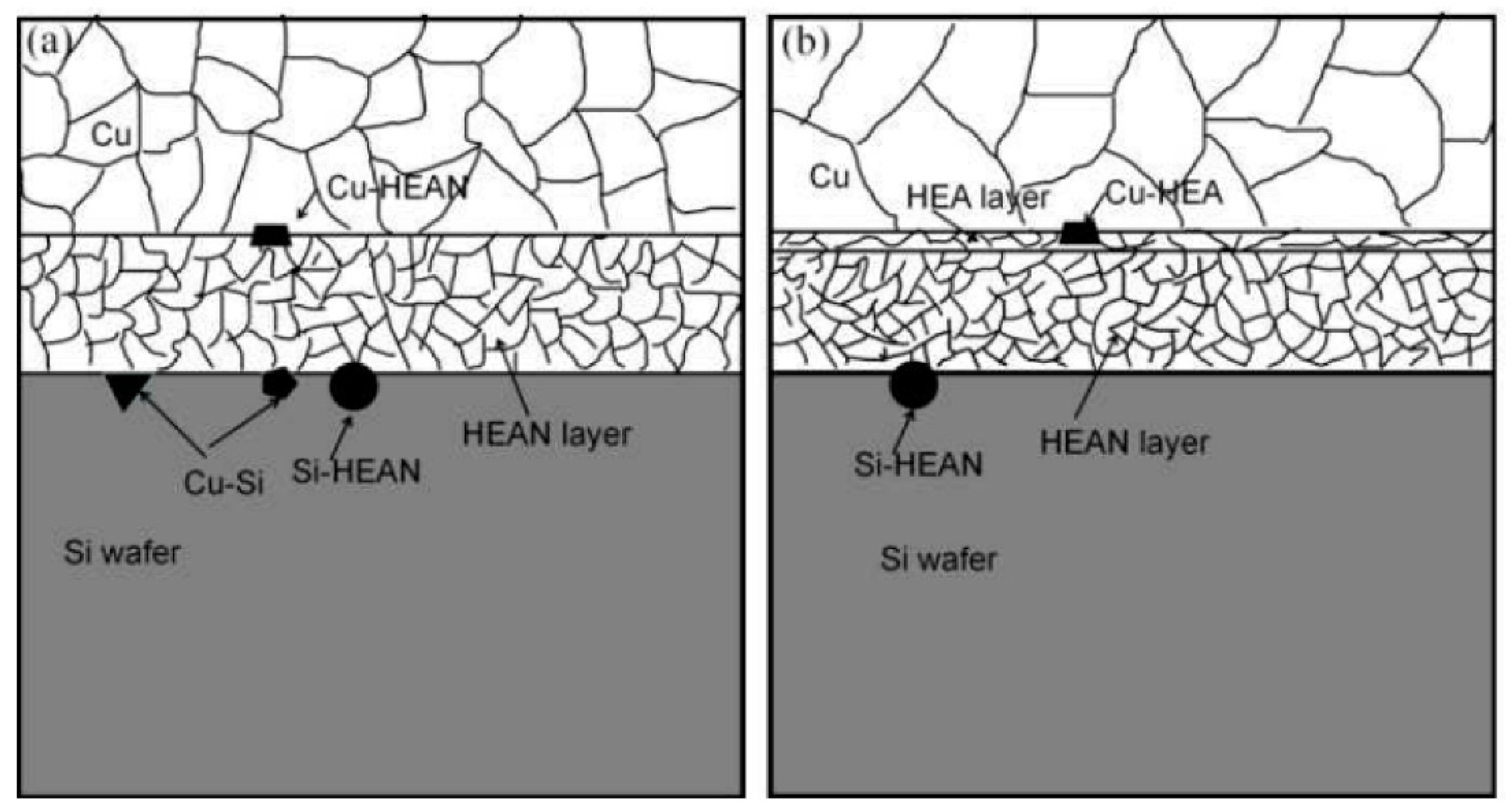
- Tsai, M.-H.; Yeh, J.-W.; Gan, J.-Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Film. 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Teng, C.; Cao, H. Recent Advances in Barrier Layer of Cu Interconnects. Materials 2020, 13, 5049. [Google Scholar] [CrossRef]
- Guo, C.; Wang, W.; Cheng, Y.; Zhu, S.; Wang, F. Yttria partially stabilised zirconia as diffusion barrier between NiCrAlY and Ni-base single crystal René N5 superalloy. Corros. Sci. 2015, 94, 122–128. [Google Scholar] [CrossRef]
- Jiang, C.; Li, R.; Wang, X.; Shang, H.; Zhang, Y.; Liaw, P.K. Diffusion barrier performance of AlCrTaTiZr/AlCrTaTiZr-N high-entropy alloy films for cu/si connect system. Entropy 2020, 22, 234. [Google Scholar] [CrossRef] [PubMed]




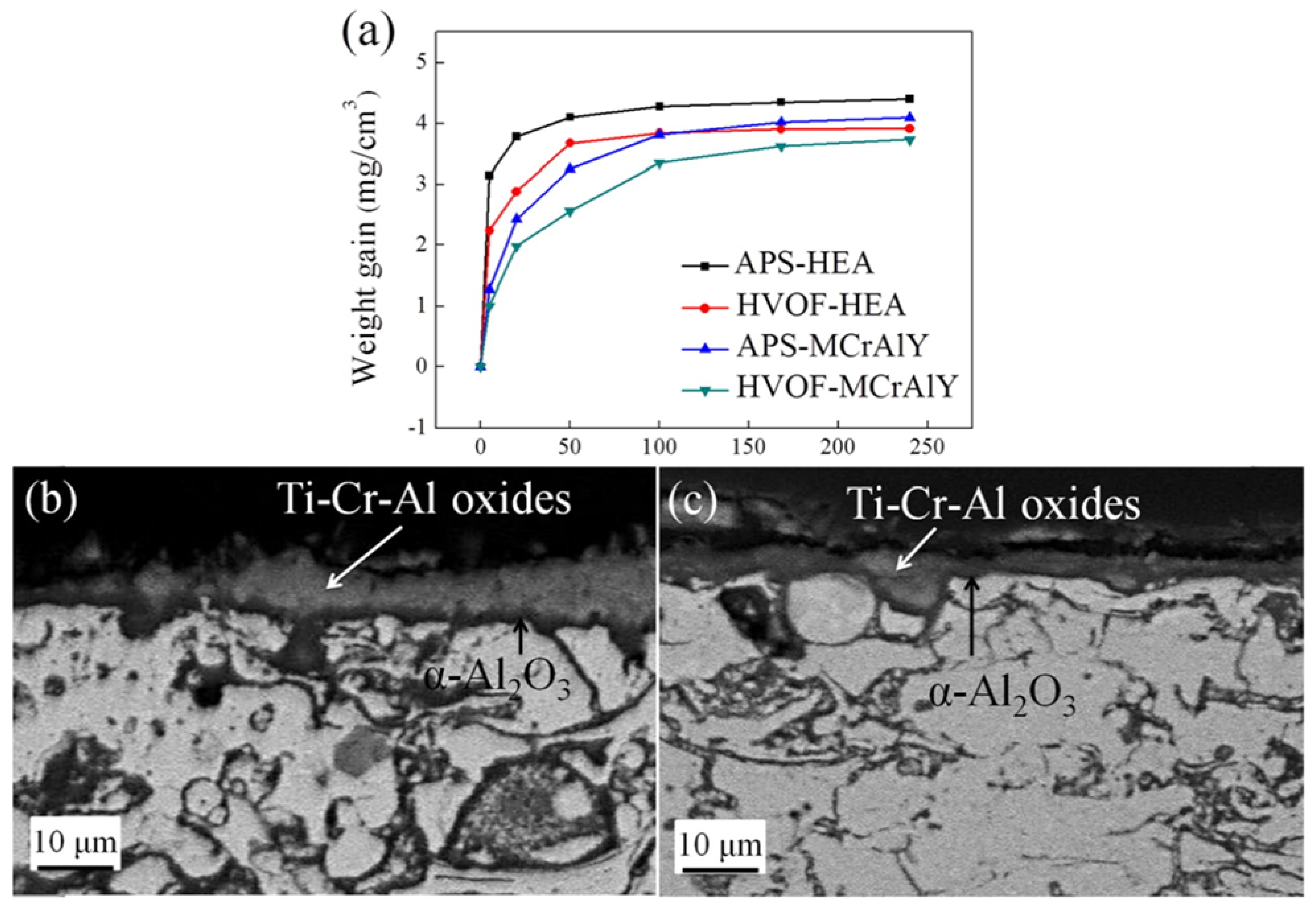

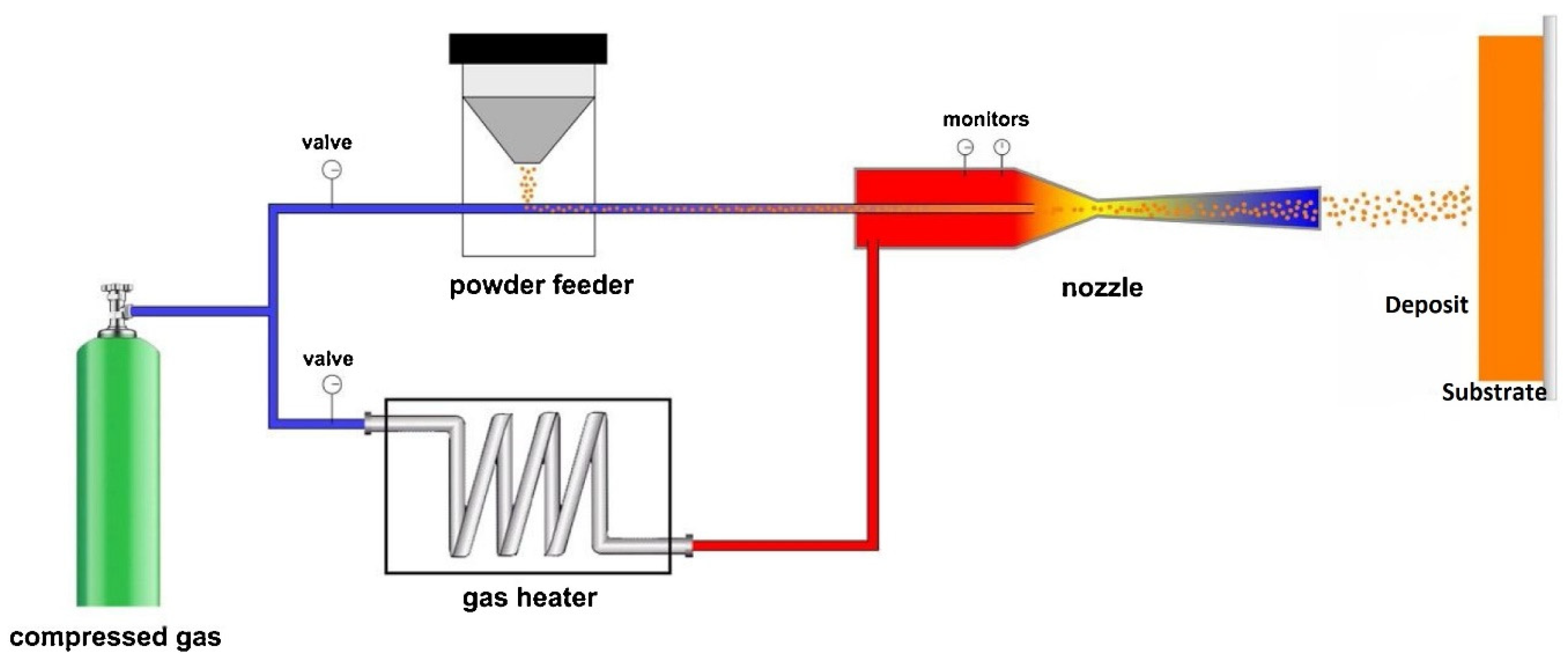
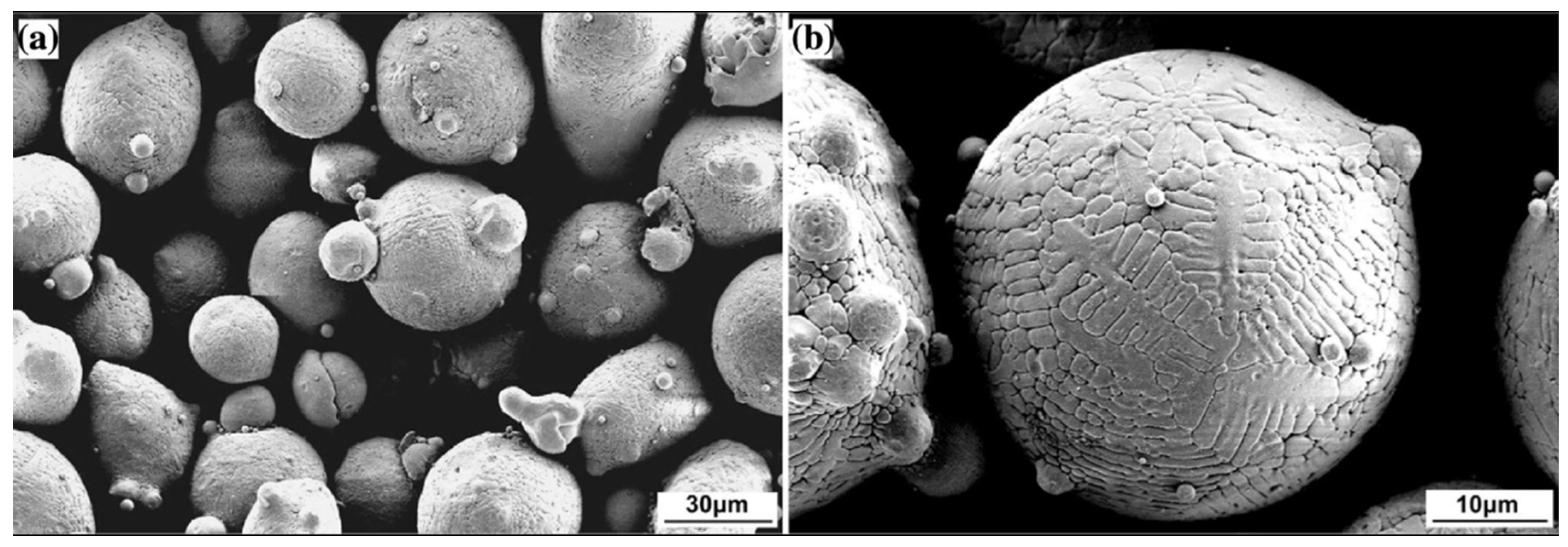


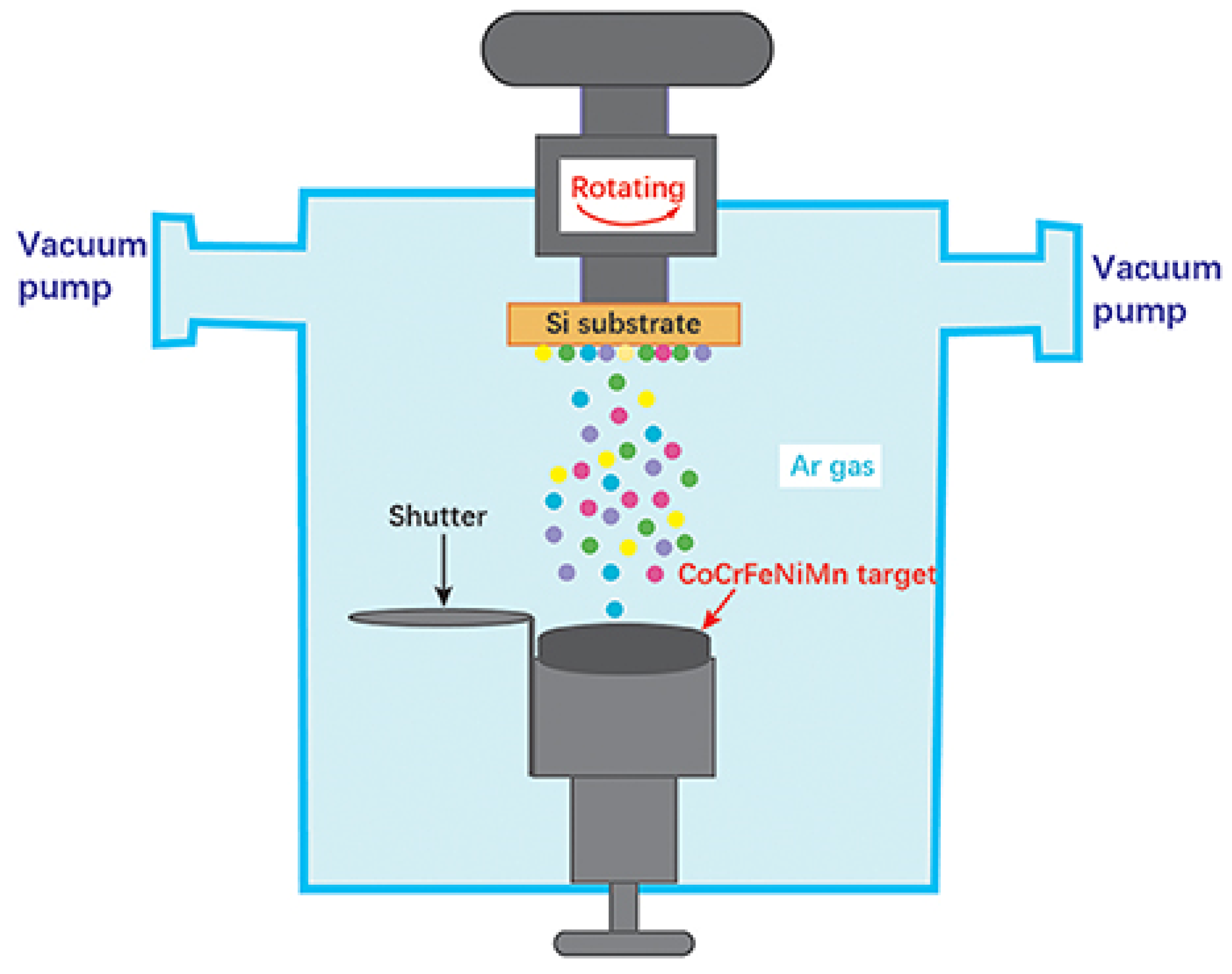


| HEC Type | HEC Composition | Substrate | Deposition Process | Phase/Structure | Temp (°C) | Outstanding Characteristics/Properties Exhibited | Ref | |
|---|---|---|---|---|---|---|---|---|
| High Entropy Metallic coatings | 3d Transition Metal Coatings | NiCoCrAlTi | Haynes 230 | HVOF | FCC, BCC | 1150 | Excellent oxidation resistance, | [19] |
| NiCo0.6Fe0.2Cr1.5SiAlTi0.2 | 304-SS/Inconel 718 | Plasma Spraying/ HVOF/SPS | BCC, FCC, Cr3Si-phase | 1100 | High oxidation resistance, High wear resistance, High hardness, Lower thermal conductivity, | [20,21,22,23] | ||
| CuAlxNiCrFe | Inconel 718 | Laser Cladding | FCC, BCC | 1100 | High thermal stability, High oxidation resistance, High diffusion resistance | [24] | ||
| NiCrCoTiVAl | Ti-6Al-4V | Laser Alloying | BCC, Intermetallic | ≤1005 | High oxidation resistance, High thermal stability | [25] | ||
| AlxCoCrFeMnNi | Q235 Steel | Plasma Cladding | FCC, BCC | 600 | High corrosion resistance, High oxidation resistance, High wear resistance | [26] | ||
| AlTiCrNiTa | Zr-4 | Magnetron sputtering | FCC, amorphous | 330 | High hardness, High corrosion resistance | [27] | ||
| Y-Hf doped AlCoCrFeNi | Ni-super alloy | Sintering process | FCC, BCC | 1100 | Low oxidation rate, High oxidation resistance | [28] | ||
| AlCoCrFeNi | Ni-super alloy | Cold Spray | FCC, BCC | 1100 | High thermal stability, High oxidation resistance, | [29] | ||
| (CoCrFeMnNi)0.85Ti0.15 | Q235 Steel | Plasma Cladding | FCC, BCC, Intermetallic (sigma) | 400 | High hardness, High wear resistance | [30] | ||
| Refractory High Entropy Metal Coatings | MoFeCrTiWAlNb | M2-Steel | Laser Cladding | BCC, HCP | 600 | High Hardness, High wear resistance | [31] | |
| AlTiVMoNb | Ti–6Al–4V | Laser Cladding | BCC | 800 | High Hardness Oxidation Resistance > Substrate Light weight | [32] | ||
| TiZrNbWMo | 45-Steel | Laser cladding | BCC, TiW ppt | 800 | High hardness, High thermal stability | [33] | ||
| High Entropy Ceramic Coatings | RE2(Ce0.2Zr0.2Hf0.2Sn0.2Ti0.2)2O7 (RE = Y, Ho, Er, Yb | Ni-super alloy | APS | Fluorite | 1200 | High thermal expansion coefficient, Low thermal conductivity, High hardness | [34] | |
| (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7 | Ni-super alloy | APS | Fluorite | 1100 | High thermal stability, High coefficient of thermal expansion. | [35] | ||
| AlCoCrCuFeNi/Mg-alloy | AZ91D | Laser cladding | a-Mg, intermetallic, BCC | - | High wear resistance | [36] | ||
| (AlCrMoTaTi)N | p-Si (100) | Magnetron sputtering | FCC, BCC, amorphous | 800 | High electrical resistivity | [37] | ||
| High Entropy Composite Coatings | CoCr2FeNiTix/TiN | 904L-steel | Laser cladding | FCC, TiN, Laves phase | - | High wear resistance, Low corrosion resistance | [38] | |
| AlCoCrFeNiTi/Ni60 | 316-SS | Plasma Spraying | FCC, BCC | 500 | High wear resistance | [39] | ||
| AlCoCrFeNi/NbC | Q235-Steel | Laser cladding | FCC, BCC | - | High hardness, High wear resistance | [40] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, M.; Amer, M.; Hayat, Q.; Janik, V.; Zhang, X.; Moradi, M.; Bai, M. High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings 2022, 12, 691. https://doi.org/10.3390/coatings12050691
Arshad M, Amer M, Hayat Q, Janik V, Zhang X, Moradi M, Bai M. High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings. 2022; 12(5):691. https://doi.org/10.3390/coatings12050691
Chicago/Turabian StyleArshad, Muhammad, Mohamed Amer, Qamar Hayat, Vit Janik, Xiang Zhang, Mahmoud Moradi, and Mingwen Bai. 2022. "High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties" Coatings 12, no. 5: 691. https://doi.org/10.3390/coatings12050691
APA StyleArshad, M., Amer, M., Hayat, Q., Janik, V., Zhang, X., Moradi, M., & Bai, M. (2022). High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings, 12(5), 691. https://doi.org/10.3390/coatings12050691












