Electrochemical and Optical Behavior of ZrN-Ag Coatings Deposited by Means of DC Reactive Magnetron Sputtering Technique
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Deposition Method
2.2. Coating Characterization
3. Results and Discussion
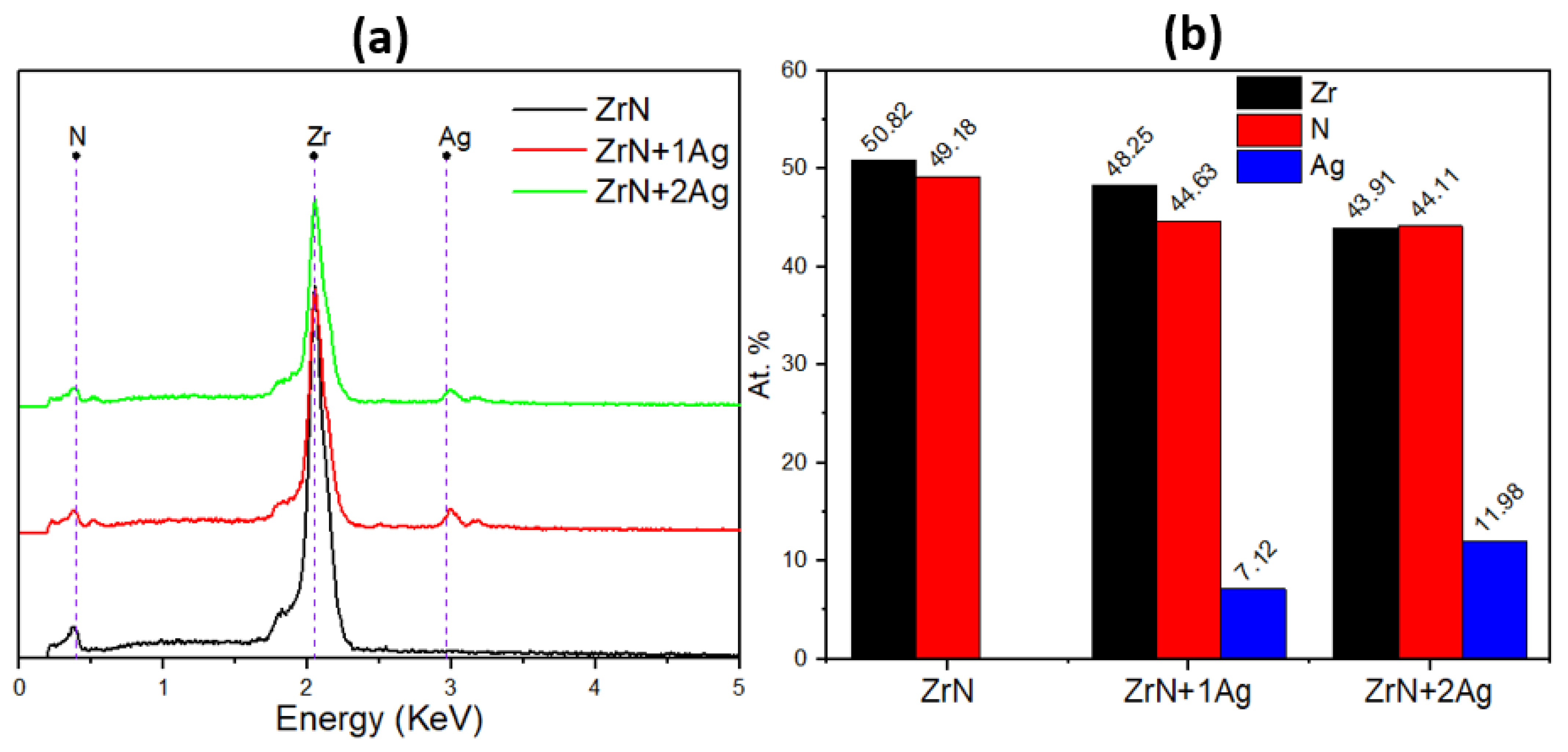
3.1. Chemical Composition of Coatings
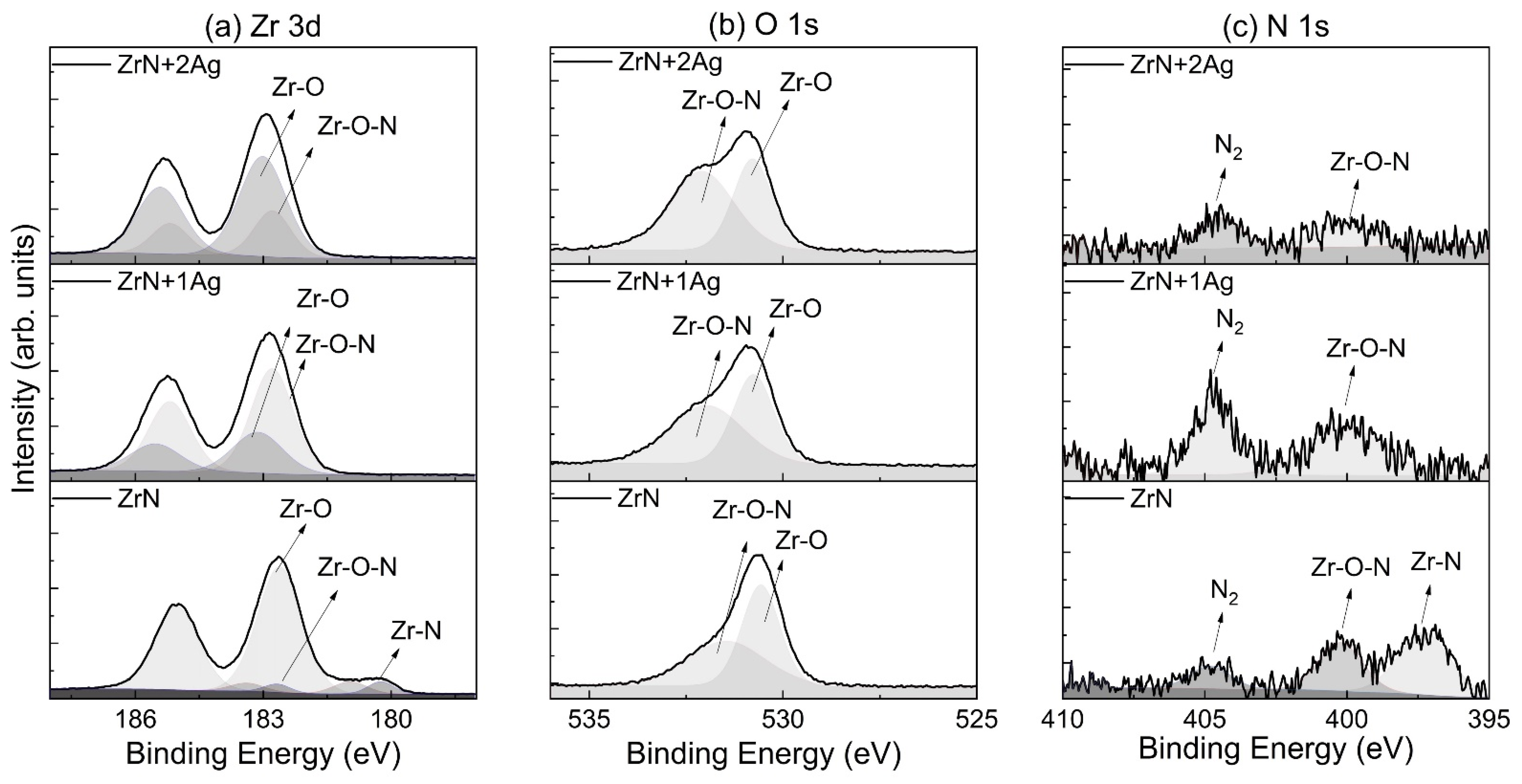
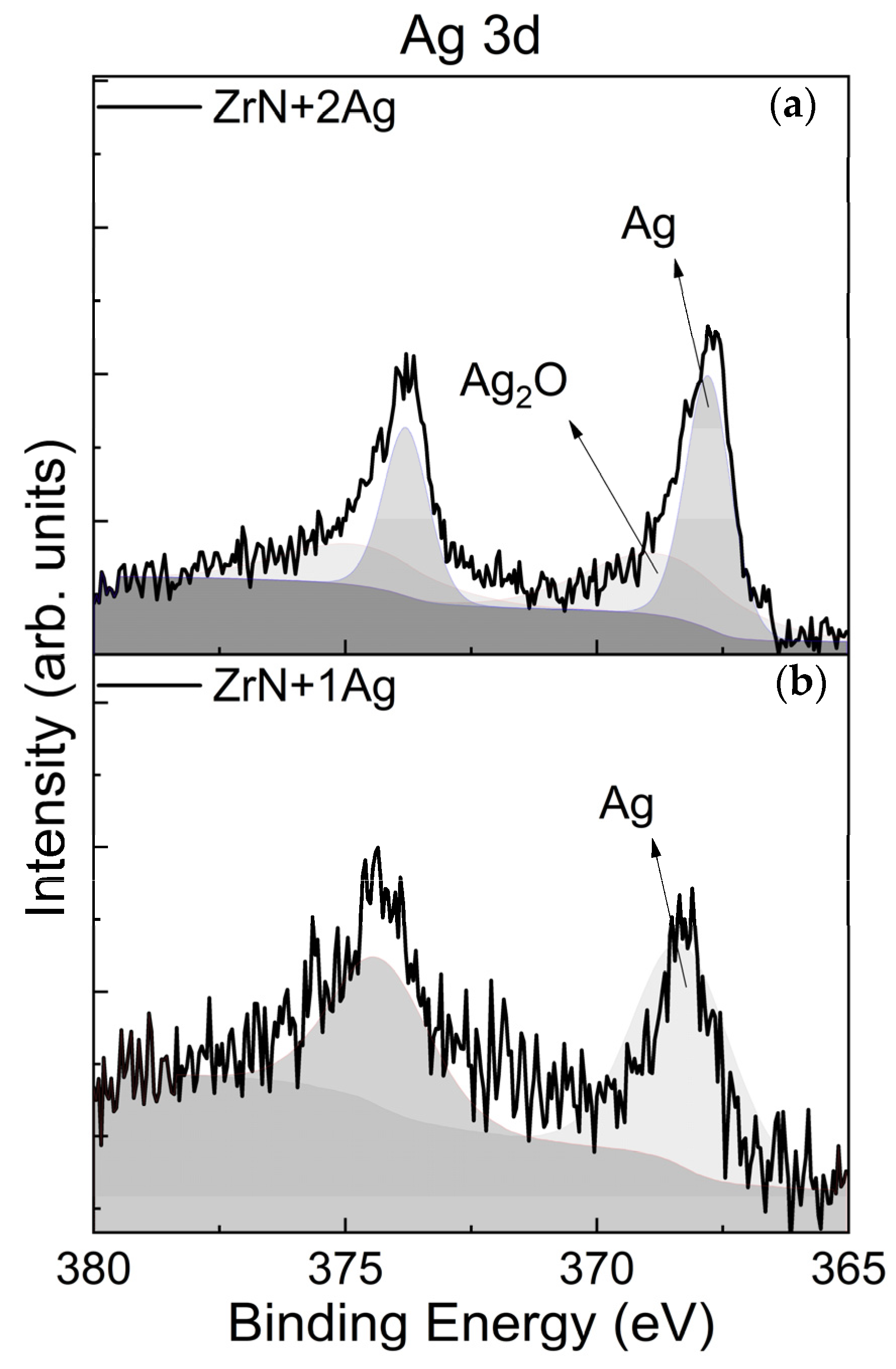
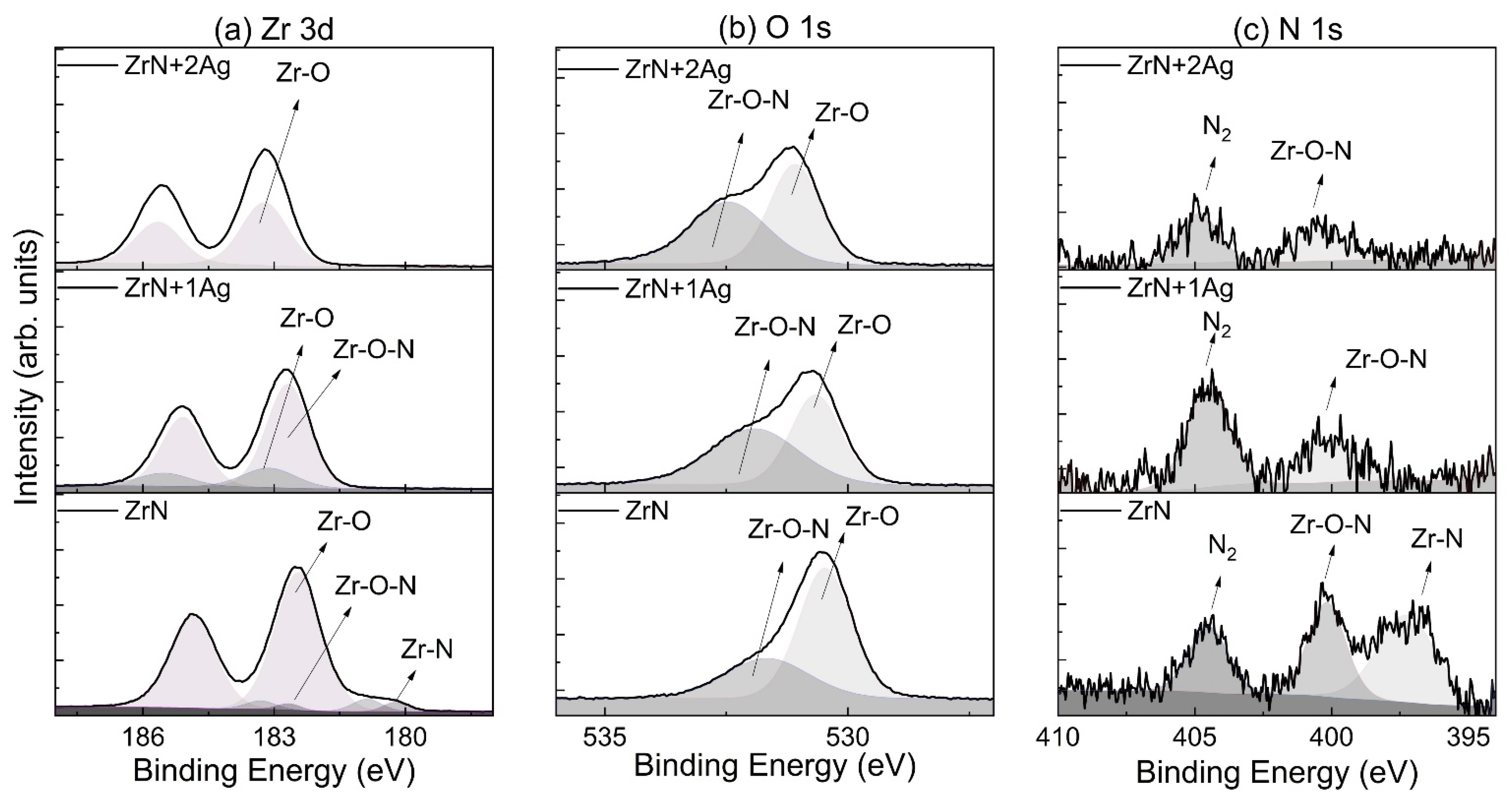
3.2. Chemical Bonding
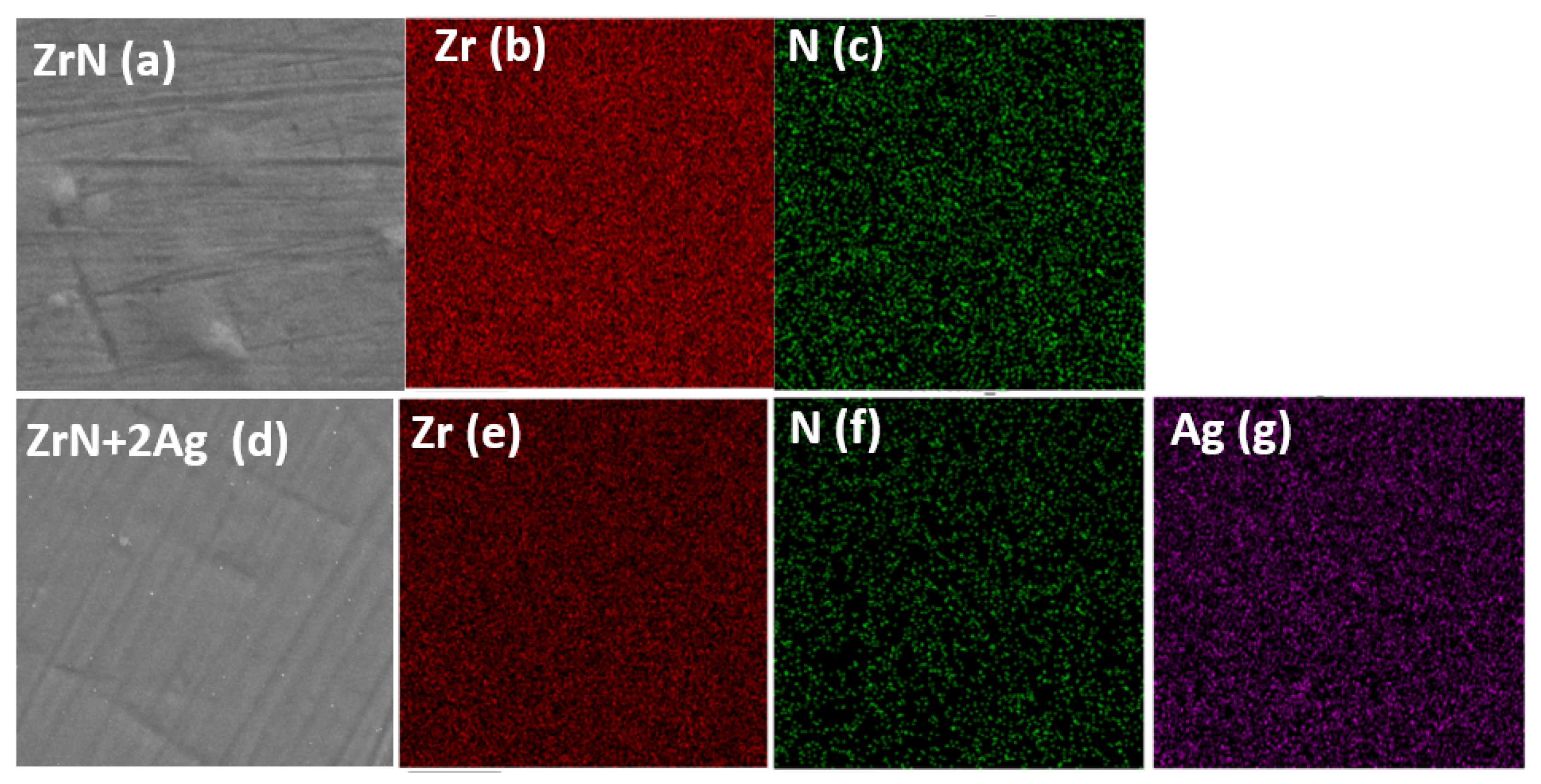
3.3. Surface Morphology of the Coatings
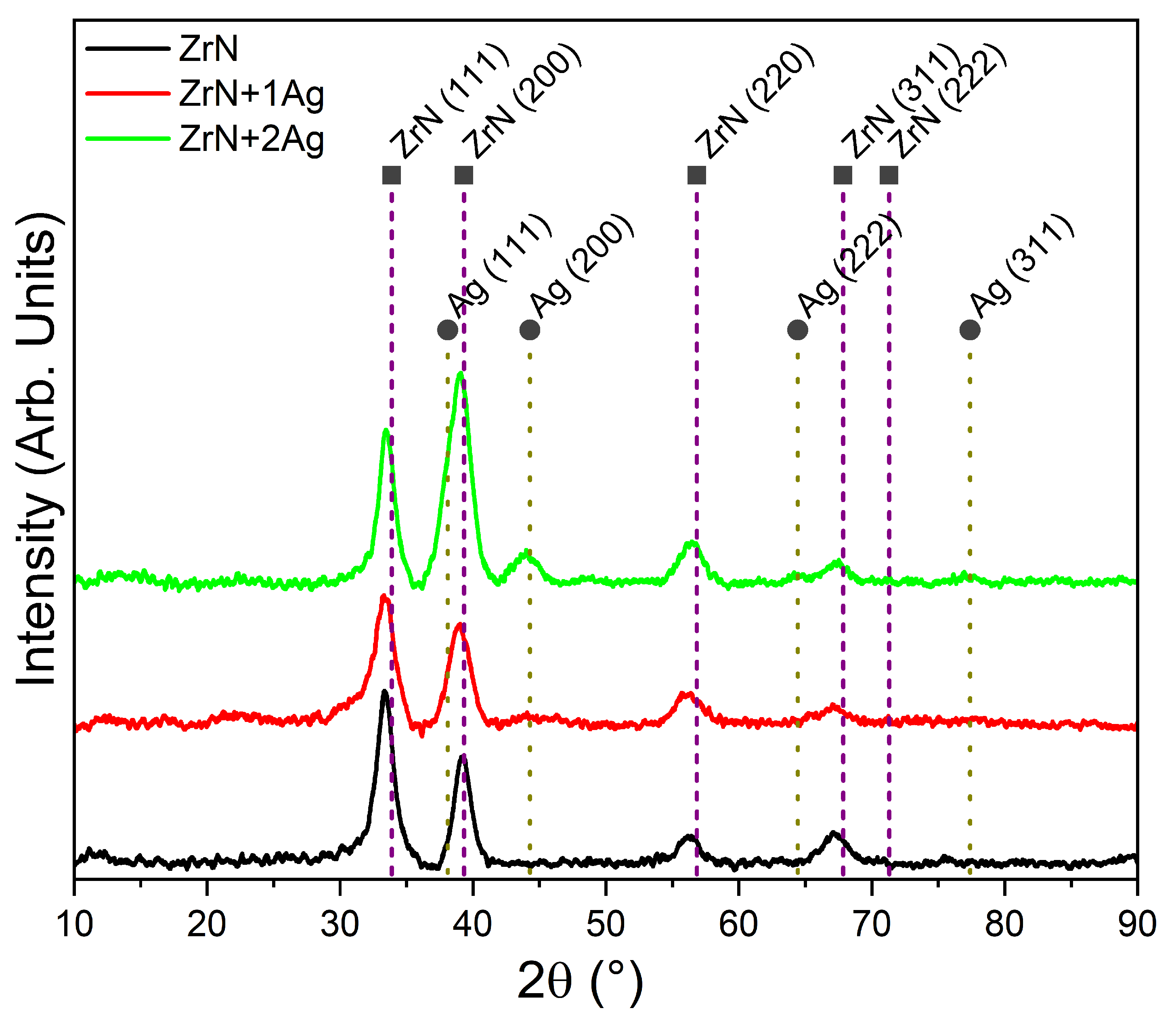
3.4. Microstructure of the Coatings
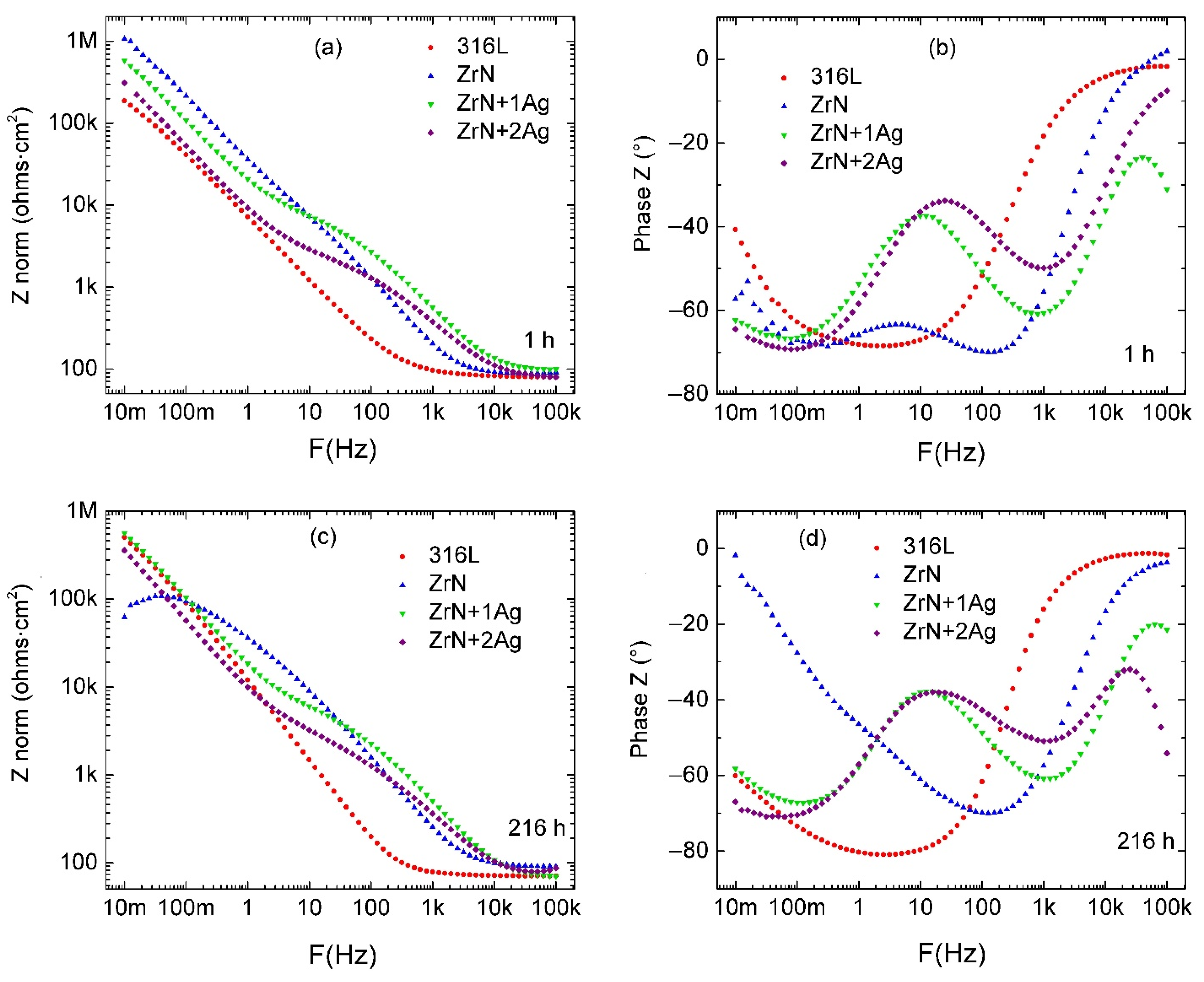
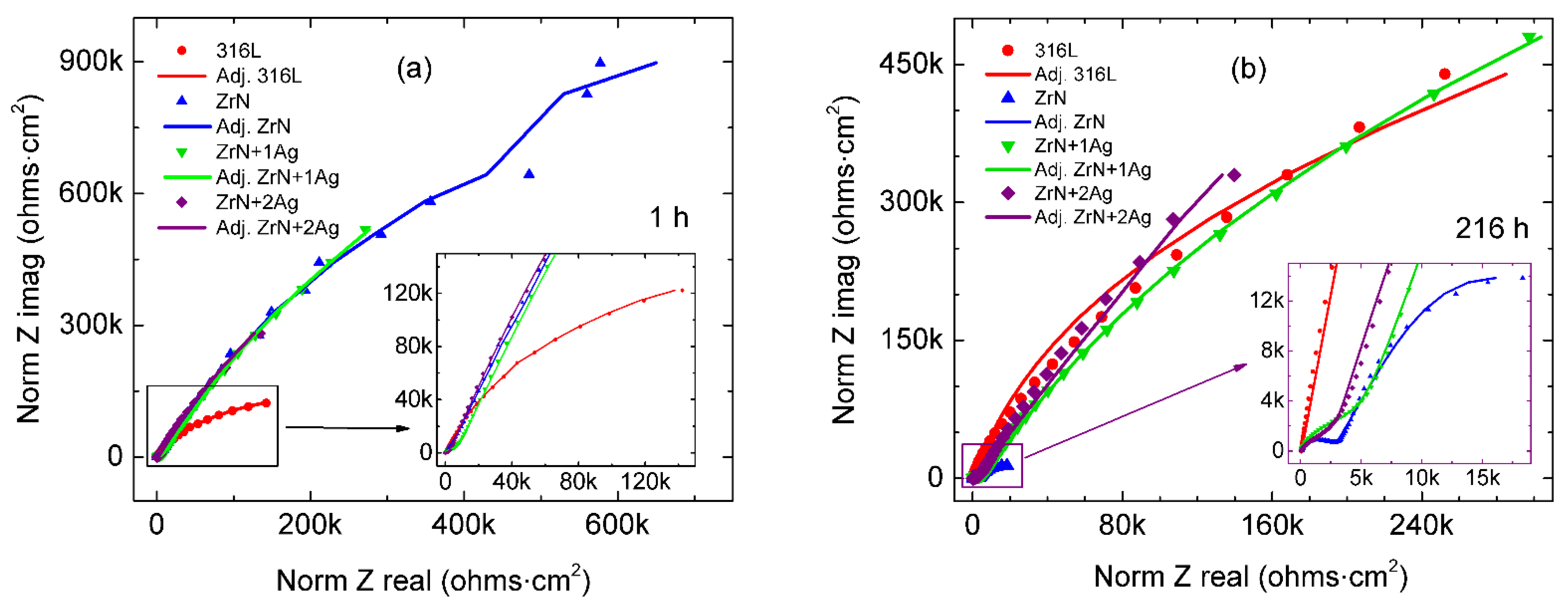
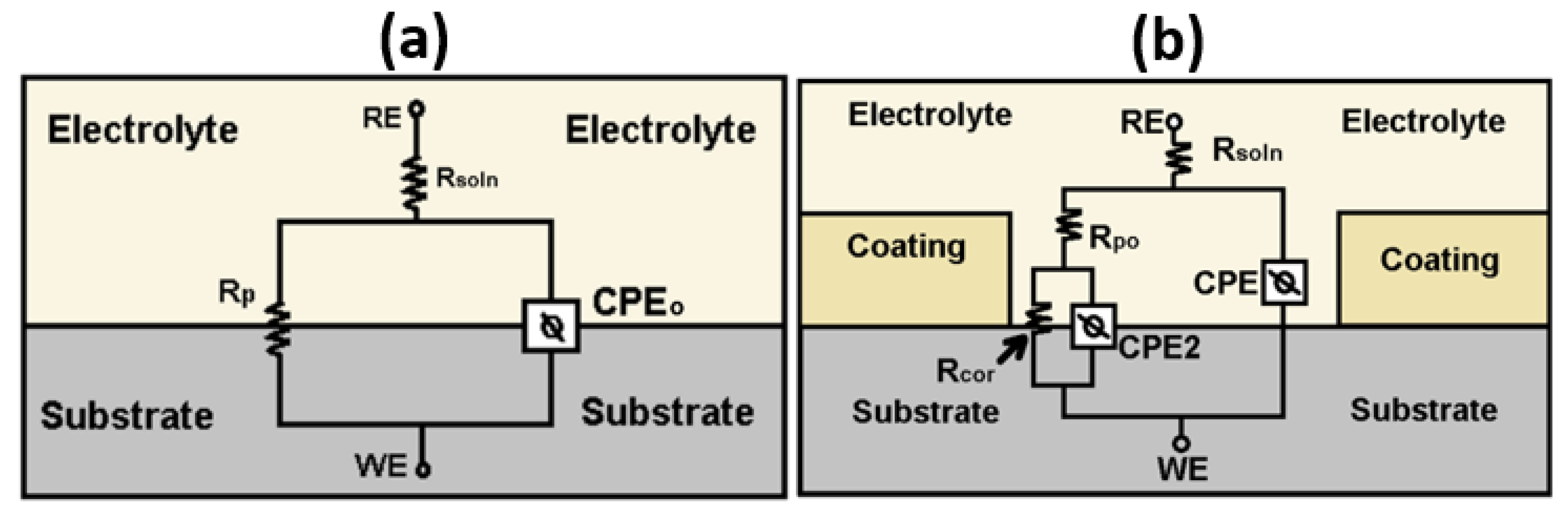
3.5. Corrosion Resistance of the Coatings
3.6. Optical Reflectance of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, Y.-C.; Katsuma, S.; Fujimoto, S.; Hiromoto, S. Electrochemical study of Type 304 and 316L stainless steels in simulated body fluids and cell cultures. Acta Biomater. 2006, 2, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Xi, T.; Shahzad, M.B.; Xu, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mater. Sci. Eng. C 2017, 71, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Ramadoss, A.; Kobayashi, A.; Muthirulandi, J. Nanocomposite Ti-Si-N Coatings Deposited by Reactive dc Magnetron Sputtering for Biomedical Applications. J. Am. Ceram. Soc. 2012, 95, 2746–2752. [Google Scholar] [CrossRef]
- Calderon, S.V.; Oliveira, J.; Evaristo, M.; Cavaleiro, A.; Carvalho, S. Prediction of optimized composition for enhanced mechanical and electrochemical response of Zr-C-N-Ag coatings for medical devices. Appl. Surf. Sci. 2014, 320, 570–580. [Google Scholar] [CrossRef]
- Zhou, R.; Ju, H.; Liu, S.; Zhao, Z.; Xu, J.; Yu, L.; Qian, H.; Jia, S.; Song, R.; Shen, J. The influences of Ag content on the friction and wear properties of TiCN–Ag films. Vacuum 2022, 196, 110719. [Google Scholar] [CrossRef]
- Shirvani, F.; Shokri, A.; Ravan, B.A. An ab-initio study of structure and mechanical properties of rocksalt ZrN and its bilayers. Solid State Commun. 2021, 328, 114218. [Google Scholar] [CrossRef]
- Ju, H.; Yu, L.; Yu, D.; Asempah, I.; Xu, J. Microstructure, mechanical and tribological properties of TiN-Ag films deposited by reactive magnetron sputtering. Vacuum 2017, 141, 82–88. [Google Scholar] [CrossRef]
- Hong, C.; Huan, Y.; Zhang, P.; Zhang, K.; Dai, P. Effect of silver content on the microstructure, thermal stability and mechanical properties of CrNx/Ag nanocomposite films. Ceram. Int. 2021, 47, 25324–25336. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, K.; Du, S.; Meng, Q.; He, X.; Wang, S.; Wen, M.; Zheng, W. Tailoring the surface chemical bond states of the NbN films by doping Ag: Achieving hard hydrophobic surface. Appl. Surf. Sci. 2017, 407, 434–439. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Kratochvil, J.; Kaftan, D.; Ctvrtlik, R.; Stranak, V. Growth of hard nanostructured ZrN surface induced by copper nanoparticles. Appl. Surf. Sci. 2021, 562, 150230. [Google Scholar] [CrossRef]
- Chen, T.; Yu, L.H.; Ju, H.B.; Geng, Y.X.; Xu, J.H.; Koyama, S.J. Influence of Ag Content on the Microstructure, Mechanical, and Tribological Properties of ZrN-Ag Films. J. Nano Res. 2018, 54, 88–97. [Google Scholar] [CrossRef]
- Huang, J.-H.; Ho, C.-H.; Yu, G.-P. Effect of nitrogen flow rate on the structure and mechanical properties of ZrN thin films on Si(100) and stainless steel substrates. Mater. Chem. Phys. 2007, 102, 31–38. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, N.; Kuppusami, P.; Prasanthi, T.; Chandramohan, P.; Dash, S.; Srinivasan, M.; Mohandas, E.; Tyagi, A. Tribological properties of sputter deposited ZrN coatings on titanium modified austenitic stainless steel. Wear 2012, 280–281, 22–27. [Google Scholar] [CrossRef]
- Hernández-Navarro, C.; Rivera, L.; Flores-Martínez, M.; Camps, E.; Muhl, S.; García, E. Tribological study of a mono and multilayer coating of TaZrN/TaZr produced by magnetron sputtering on AISI-316L stainless steel. Tribol. Int. 2019, 131, 288–298. [Google Scholar] [CrossRef]
- Dinu, M.; Parau, A.C.; Vladescu, A.; Kiss, A.E.; Pana, I.; Mouele, E.S.M.; Braic, V. Corrosion improvement of 304l stainless steel by zrsin and zrsi(N,o) mono-and double-layers prepared by reactive cathodic arc evaporation. Coatings 2021, 11, 179–189. [Google Scholar] [CrossRef]
- Ghafoor, N.; Petrov, I.; Klenov, D.O.; Freitag, B.; Jensen, J.; Greene, J.E.; Hultman, L.; Odén, M. Self-organized anisotropic (Zr1-xSix)Ny nanocomposites grown by reactive sputter deposition. Acta Mater. 2015, 82, 179–189. [Google Scholar] [CrossRef]
- Kelly, P.J.; Li, H.; Benson, P.S.; Whitehead, K.A.; Verran, J.; Arnell, R.D.; Iordanova, I. Comparison of the tribological and antimicrobial properties of CrN/Ag, ZrN/Ag, TiN/Ag, and TiN/Cu nanocomposite coatings. Surf. Coat. Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Ju, H.; Yu, D.; Yu, L.; Ding, N.; Xu, J.; Zhang, X.; Zheng, Y.; Yang, L.; He, X. The influence of Ag contents on the microstructure, mechanical and tribological properties of ZrN-Ag films. Vacuum 2018, 148, 54–61. [Google Scholar] [CrossRef]
- Baghriche, O.; Kiwi, J.; Pulgarin, C.; Sanjinés, R. Antibacterial Ag–ZrN surfaces promoted by subnanometric ZrN-clusters deposited by reactive pulsed magnetron sputtering. J. Photochem. Photobiol. A Chem. 2012, 229, 39–45. [Google Scholar] [CrossRef]
- Domingues, R.P.; Rodrigues, M.S.; Lopes, C.; Pedrosa, P.; Alves, E.; Barradas, N.P.; Borges, J.; Vaz, F. Thin films composed of metal nanoparticles (Au, Ag, Cu) dispersed in AlN: The influence of composition and thermal annealing on the structure and plasmonic response. Thin Solid Film. 2019, 676, 12–25. [Google Scholar] [CrossRef]
- Ren, P.; Zhang, K.; He, X.; Du, S.; Yang, X.; An, T.; Wen, M.; Zheng, W. Toughness enhancement and tribochemistry of the Nb-Ag-N films actuated by solute Ag. Acta Mater. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Enrichi, F.; Quandt, A.; Righini, G. Plasmonic enhanced solar cells: Summary of possible strategies and recent results. Renew. Sustain. Energy Rev. 2018, 82, 2433–2439. [Google Scholar] [CrossRef]
- Borges, J.; Rodrigues, M.S.; Lopes, C.; Costa, D.; Couto, F.; Kubart, T.; Martins, B.; Duarte, N.; Dias, J.; Cavaleiro, A.; et al. Thin films composed of Ag nanoclusters dispersed in TiO2: Influence of composition and thermal annealing on the microstructure and physical responses. Appl. Surf. Sci. 2015, 358, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S.; Abadias, G.; Bellas, D.V.; Lekka, C.; Lidorikis, E. Conductive nitrides: Growth principles, optical and electronic properties, and their perspectives in photonics and plasmonics. Mater. Sci. Eng. R Rep. 2018, 123, 1–55. [Google Scholar] [CrossRef]
- Guler, U.; Shalaev, V.M.; Boltasseva, A. Nanoparticle plasmonics: Going practical with transition metal nitrides. Mater. Today 2015, 18, 227–237. [Google Scholar] [CrossRef]
- Das, S.; Alford, T.L. Structural and optical properties of Ag-doped copper oxide thin films on polyethylene napthalate substrate prepared by low temperature microwave annealing. J. Appl. Phys. 2013, 113, 1–7. [Google Scholar] [CrossRef]
- Borges, J.; Costa, D.; Antunes, E.; Lopes, C.; Rodrigues, M.S.; Apreutesei, M.; Alves, E.; Barradas, N.P.; Pedrosa, P.; Moura, C.; et al. Biological behaviour of thin films consisting of Au nanoparticles dispersed in a TiO2 dielectric matrix. Vacuum 2015, 122, 360–368. [Google Scholar] [CrossRef]
- Placzek-Popko, E.; Gwozdz, K.; Gumienny, Z.; Zielony, E.; Pietruszka, R.; Witkowski, B.S.; Wachnicki, L.; Gieraltowska, S.; Godlewski, M.; Jacak, W.; et al. Si/ZnO nanorods/Ag/AZO structures as promising photovoltaic plasmonic cells. J. Appl. Phys. 2015, 117, 193101. [Google Scholar] [CrossRef]
- Mejía, V.C.P.; Chellali, M.R.; Garzón, C.M.; Olaya, J.J.; Hahn, H.; Velasco, L. Effect of discharge current on the corrosion resistance and microstructure of ZrTiSiN coatings deposited by magnetron co-sputtering. Mater Today Commun. 2021, 26, 102151. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Debessai, M.; Filip, P. Zirconium nitride/silver nanocomposite structures for biomedical applications. J. Vac. Sci. Technol. B Microelectron Nanom Struct. 2004, 22, 1134. [Google Scholar] [CrossRef]
- Dang, C.; Yao, Y.; Olugbade, T.; Li, J.; Wang, L. Effect of multi-interfacial structure on fracture resistance of composite TiSiN/Ag/TiSiN multilayer coating. Thin Solid Film. 2018, 653, 107–112. [Google Scholar] [CrossRef]
- Mejía, V.H.D.; Perea, D.; Bejarano, G.G.B. Development and characterization of TiAlN (Ag, Cu) nanocomposite coatings deposited by DC magnetron sputtering for tribological applications. Surf Coat. Technol. 2020, 381, 125095. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu oxidation mechanism on Cu-Zr(O)N coatings: Role on functional properties. Appl. Surf. Sci. 2021, 555, 149704. [Google Scholar] [CrossRef]
- Cubillos, G.; Mendoza, M.; Alfonso, J.; Blanco, G.; Bethencourt, M. Chemical composition and microstructure of zirconium oxynitride thin layers from the surface to the substrate-coating interface. Mater. Charact. 2017, 131, 450–458. [Google Scholar] [CrossRef]
- Yu, L.; Zhao, H.; Xu, J. Influence of silver content on structure, mechanical and tribological properties of WCN-Ag films. Mater. Charact. 2016, 114, 136–145. [Google Scholar] [CrossRef]
- Vanegas, P.H.S.; Calderon, V.S.; Alfonso, O.J.E.; Olaya, F.J.J.; Ferreira, P.J.; Carvalho, S. Influence of silicon on the microstructure and the chemical properties of nanostructured ZrN-Si coatings deposited by means of pulsed-DC reactive magnetron sputtering. Appl. Surf. Sci. 2019, 481, 1249–1259. [Google Scholar] [CrossRef]
- Cavaleiro, D.; Carvalho, S.; Cavaleiro, A.; Fernandes, F. TiSiN(Ag) films deposited by HiPIMS working in DOMS mode: Effect of Ag content on structure, mechanical properties and thermal stability. Appl. Surf. Sci. 2019, 478, 426–434. [Google Scholar] [CrossRef]
- Rajput, S.S.; Gangopadhyay, S.; Cavaleiro, A.; AL-Rjoub, A.; Kumar, C.S.; Fernandes, F. Influence of Ag additions on the structure, mechanical properties and oxidation behaviour of CrAlNAg coatings deposited by sputtering. Surf Coat. Technol. 2021, 426, 127767. [Google Scholar] [CrossRef]
- Al-Rjoub, A.; Cavaleiro, A.; Fernandes, F. Influence of Ag alloying on the morphology, structure, mechanical properties, thermal stability and oxidation resistance of multilayered TiSiN/Ti(Ag)N films. Mater. Des. 2020, 192, 108703. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, M.; Li, J.; Wang, L. The improved corrosion and tribocorrosion properties of TiSiN/Ag by thermal treatment. Surf. Coatings Technol. 2020, 385, 125437. [Google Scholar] [CrossRef]
- Yu, D.; Yu, L.; Asempah, I.; Ju, H.; Xu, J.; Koyama, S.; Gao, Y. Microstructure, mechanical and tribological properties of VCN-Ag composite films by reactive magnetron sputtering. Surf. Coatings Technol. 2020, 399, 126167. [Google Scholar] [CrossRef]
- Calderon, V.S.; Cavaleiro, A.; Carvalho, S. Chemical and structural characterization of Zr-C-N-Ag coatings: XPS, XRD and Raman spectroscopy. Appl. Surf. Sci. 2015, 346, 240–247. [Google Scholar] [CrossRef]
- Vanegas, H.S.; Alfonso, J.E.; Olaya, J.J. Effect of Si content on functional behavior of nanostructured coatings of Zr–Si–N. Mater. Res. Express 2019, 6, 115076. [Google Scholar] [CrossRef]
- Rios, J.F.; Calderón, J.A.; Nogueira, R.P. Electrochemical behavior of metals used in drinking water distribution systems: A rotating cylinder electrode’s study. Corrosion 2013, 69, 875–885. [Google Scholar] [CrossRef]
- Alves, C.A.; Calderon, S.; Dias, D.; Carvalho, S. Influence of Oxygen content on the electrochemical behavior of Ta1-xOx coatings. Electrochim. Acta 2016, 211, 385–394. [Google Scholar] [CrossRef] [Green Version]
- von Fieandt, K.; Pilloud, D.; Fritze, S.; Osinger, B.; Pierson, J.F.; Lewin, E. Optical and electrical properties of hard (Hf,Nb,Ti,V,Zr)Nx thin films. Vacuum 2021, 193, 110517. [Google Scholar] [CrossRef]
- Popović, M.; Novaković, M.; Rakočević, Z.; Bibić, N. Tailoring the structural and optical properties of TiN thin films by Ag ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 389–390, 33–39. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Maeruf, T.; Debessai, M.; Filip, P. Zirconium nitride/nickel nanocomposite structures. Vacuum 2005, 79, 186–193. [Google Scholar] [CrossRef]
- Hu, C.; Guo, K.; Li, Y.; Gu, Z.; Quan, J.; Zhang, S.; Zheng, W. Optical coatings of durability based on transition metal nitrides. Thin Solid Film. 2019, 688, 137339. [Google Scholar] [CrossRef]
- Guler, U.; Boltasseva, A.; Shalaev, V.M. Refractory plasmonics. Science 2014, 344, 263–264. [Google Scholar] [CrossRef]
- Tseng, C.C.; Hsieh, J.H.; Liu, S.J.; Wu, W. Effects of Ag contents and deposition temperatures on the electrical and optical behaviors of Ag-doped Cu2O thin films. Thin Solid Film. 2009, 518, 1407–1410. [Google Scholar] [CrossRef]
- Jalili, S.; Hajakbari, F.; Hojabri, A. Effect of silver thickness on structural, optical and morphological properties of nanocrystalline Ag/NiO thin films. J. Theor. Appl. Phys. 2018, 12, 15–22. [Google Scholar] [CrossRef] [Green Version]


| Cr (at.%) | Fe (at.%) | Ni (at.%) | Mn (at.%) | Mo (at.%) |
|---|---|---|---|---|
| 16.4 | 69.9 | 10.1 | 1.5 | 2.1 |
| Samples | Silver Pellets | Power (W) | Substrate Temperature (°C) | Thickness (nm) | Deposition Rate (nm/s) |
|---|---|---|---|---|---|
| ZrN | 0 | 140 | 200 | 544.9 | 0.15 |
| ZrN+1Ag | 1 | 140 | 200 | 633.7 | 0.18 |
| ZrN+2Ag | 2 | 140 | 200 | 845.1 | 0.23 |
| NaCl (g/L) | C3H5O3Na (g/L) | CaCl (g/L) | KCl (g/L) |
|---|---|---|---|
| 6.0 | 3.1 | 0.2 | 1.5 |
| Coating | Ag Content | Plane | 2θ (°) | FWHM | Lattice Parameter (nm) |
|---|---|---|---|---|---|
| ZrN | 0 at.% | ZrN (111) | 33.39 | 1.58 | 0.464 |
| ZrN+1Ag | 7 at.% | ZrN (111) | 33.41 | 1.54 | 0.464 |
| ZrN+2Ag | 12 at.% | ZrN (111) | 33.48 | 1.70 | 0.463 |
| Ag (200) | 43.66 | 1.54 | 0.414 |
| Parameter | Unit | 316L 1 h | 316L 216 h |
|---|---|---|---|
| Rsoln | ohm·cm2 | 79.9 | 70.0 |
| Rp | ohm·cm2 | 3.4 × 105 | 1.4 × 106 |
| CPE0 | S·sa/cm2 | 3.3 × 10−5 | 1.82 × 10−5 |
| M | 7.81 × 10−1 | 8.71 × 10−1 |
| Parameter | Unit | ZrN | ZrN+1Ag | ZrN+2Ag |
|---|---|---|---|---|
| Rsoln | ohm·cm2 | 86.9 | 77.9 | 71.8 |
| Rcor | ohm·cm2 | 3.91 × 106 | 4.87 × 106 | 3.94 × 106 |
| Rpo | ohm·cm2 | 1.06 × 104 | 8.60 × 103 | 2.66 × 103 |
| Ccor | S·sa/cm2 | 4.47 × 10−6 | 1.13 × 10−5 | 2.27 × 10−5 |
| n | 7.27 × 10−1 | 7.74 × 10−1 | 7.96 × 10−1 | |
| Cc | S·sa/cm2 | 2.37 × 10−6 | 2.30 × 10−6 | 4.81 × 10−6 |
| ñ | 8.95 × 10−1 | 7.65 × 10−1 | 7.32 × 10−1 |
| Parameter | Unit | ZrN | ZrN+1Ag | ZrN+2Ag |
|---|---|---|---|---|
| Rsoln | ohm·cm2 | 81.4 | 58.0 | 42.0 |
| Rcor | ohm·cm2 | 5.44 × 104 | 2.44 × 106 | 4.58 × 107 |
| Rpo | ohm·cm2 | 2.83 × 103 | 6.30 × 103 | 4.94 × 103 |
| Ccor | S·sa/cm2 | 3.22 × 10−4 | 1.17 × 105 | 1.46 × 10−5 |
| n | 7.54 × 10−1 | 7.93 × 10−1 | 8.89 × 10−1 | |
| Cc | S·sa/cm2 | 4.13 × 10−6 | 2.36 × 10−6 | 1.06 × 10−5 |
| ñ | 7.76 × 10−1 | 7.77 × 10−1 | 6.39 × 10−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejía, C.P.; Vanegas, H.S.; Olaya, J.J. Electrochemical and Optical Behavior of ZrN-Ag Coatings Deposited by Means of DC Reactive Magnetron Sputtering Technique. Coatings 2022, 12, 754. https://doi.org/10.3390/coatings12060754
Mejía CP, Vanegas HS, Olaya JJ. Electrochemical and Optical Behavior of ZrN-Ag Coatings Deposited by Means of DC Reactive Magnetron Sputtering Technique. Coatings. 2022; 12(6):754. https://doi.org/10.3390/coatings12060754
Chicago/Turabian StyleMejía, Claudia P., Henry S. Vanegas, and Jhon J. Olaya. 2022. "Electrochemical and Optical Behavior of ZrN-Ag Coatings Deposited by Means of DC Reactive Magnetron Sputtering Technique" Coatings 12, no. 6: 754. https://doi.org/10.3390/coatings12060754





