Droplet Spreading Characteristics on Ultra-Slippery Solid Hydrophilic Surfaces with Ultra-Low Contact Angle Hysteresis
Abstract
:1. Introduction
2. Surface Fabrication and Methods
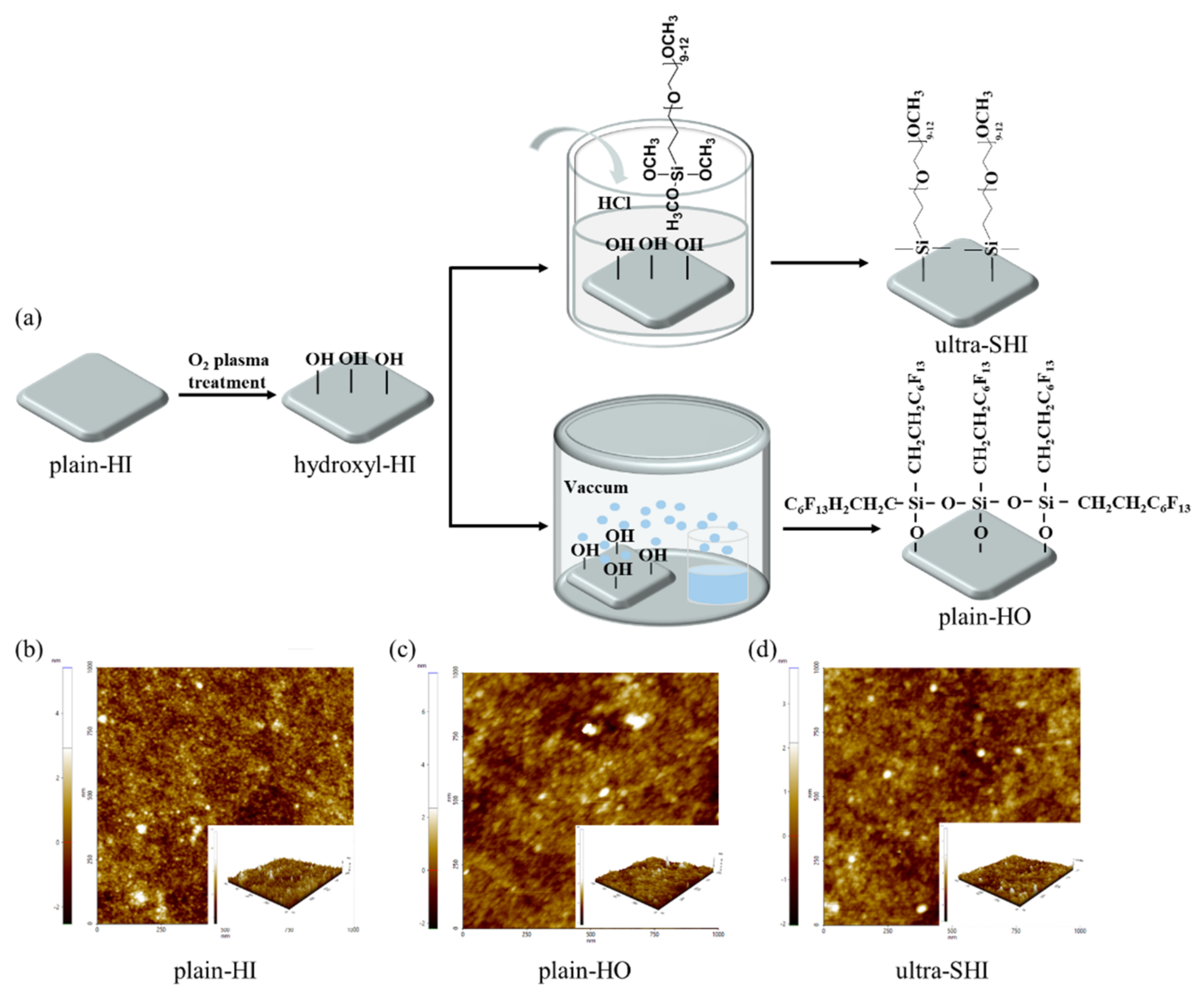
2.1. Materials and Surface Fabrication
2.2. Surface Wettability Characterization
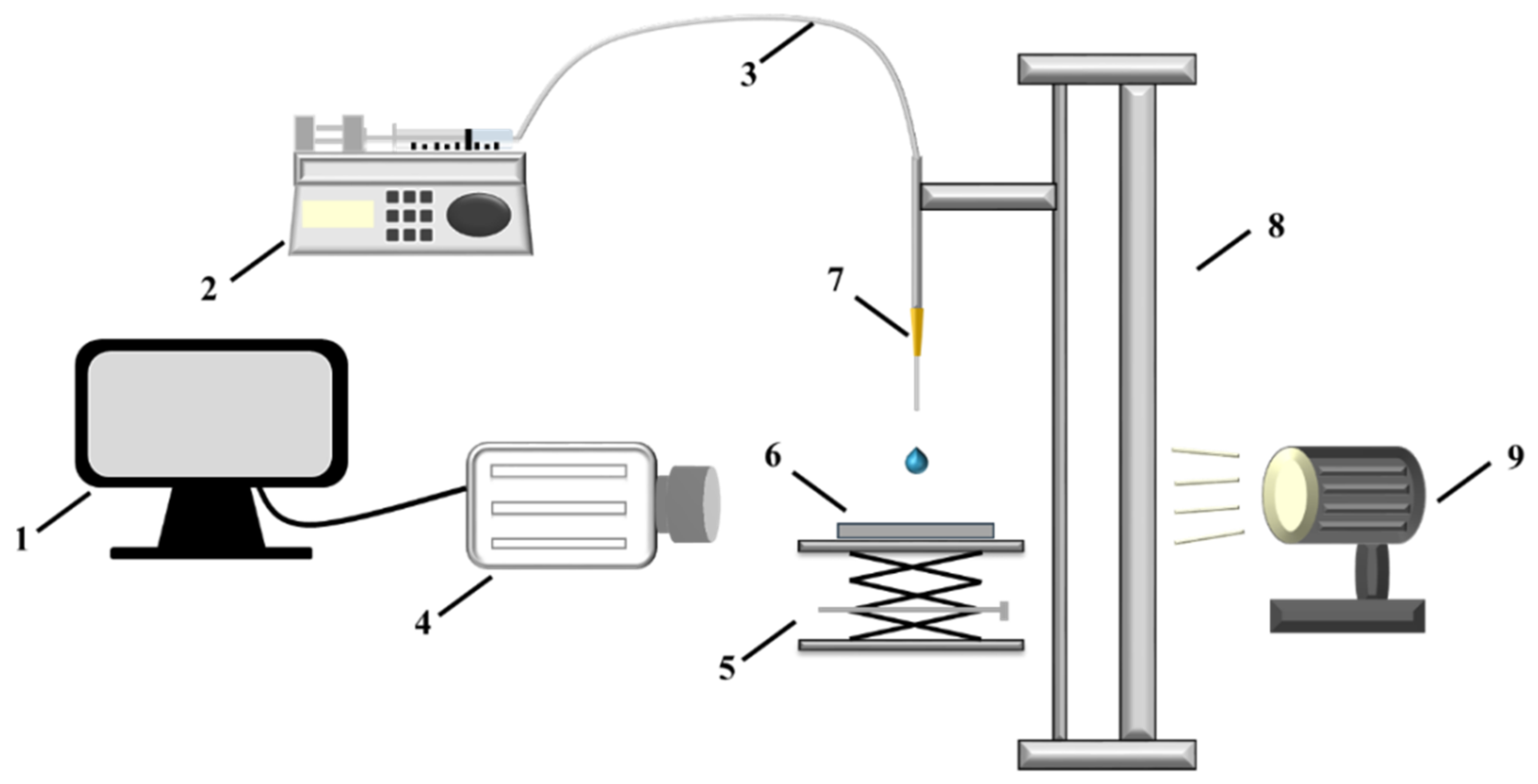
2.3. Experimental System and Method
3. Results and Discussion
3.1. Spreading Characteristics of the Droplet Impact on Horizontal Surfaces
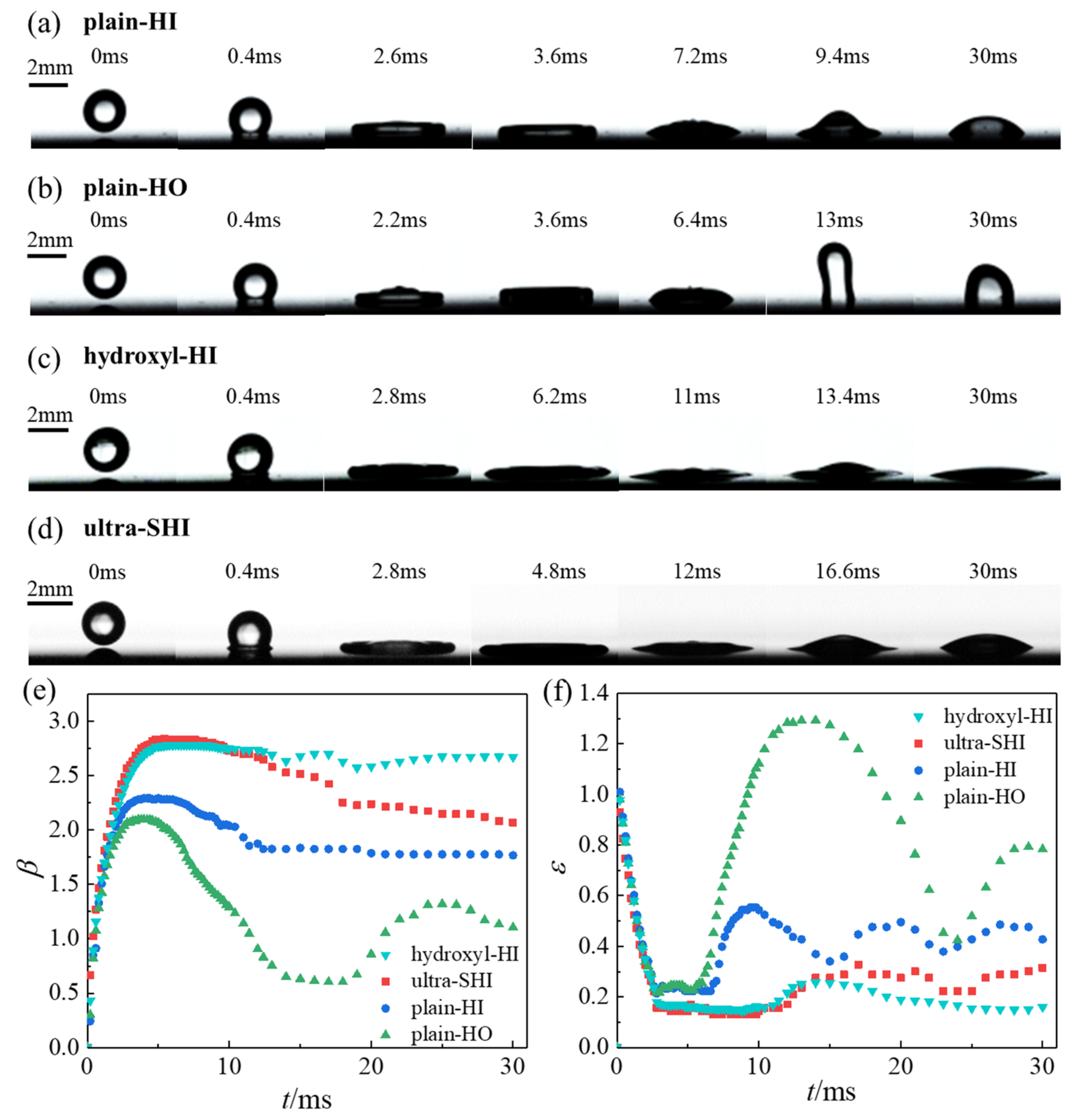
3.1.1. Effect of Surface Wettability on the Spreading Characteristics of the Droplet Impact
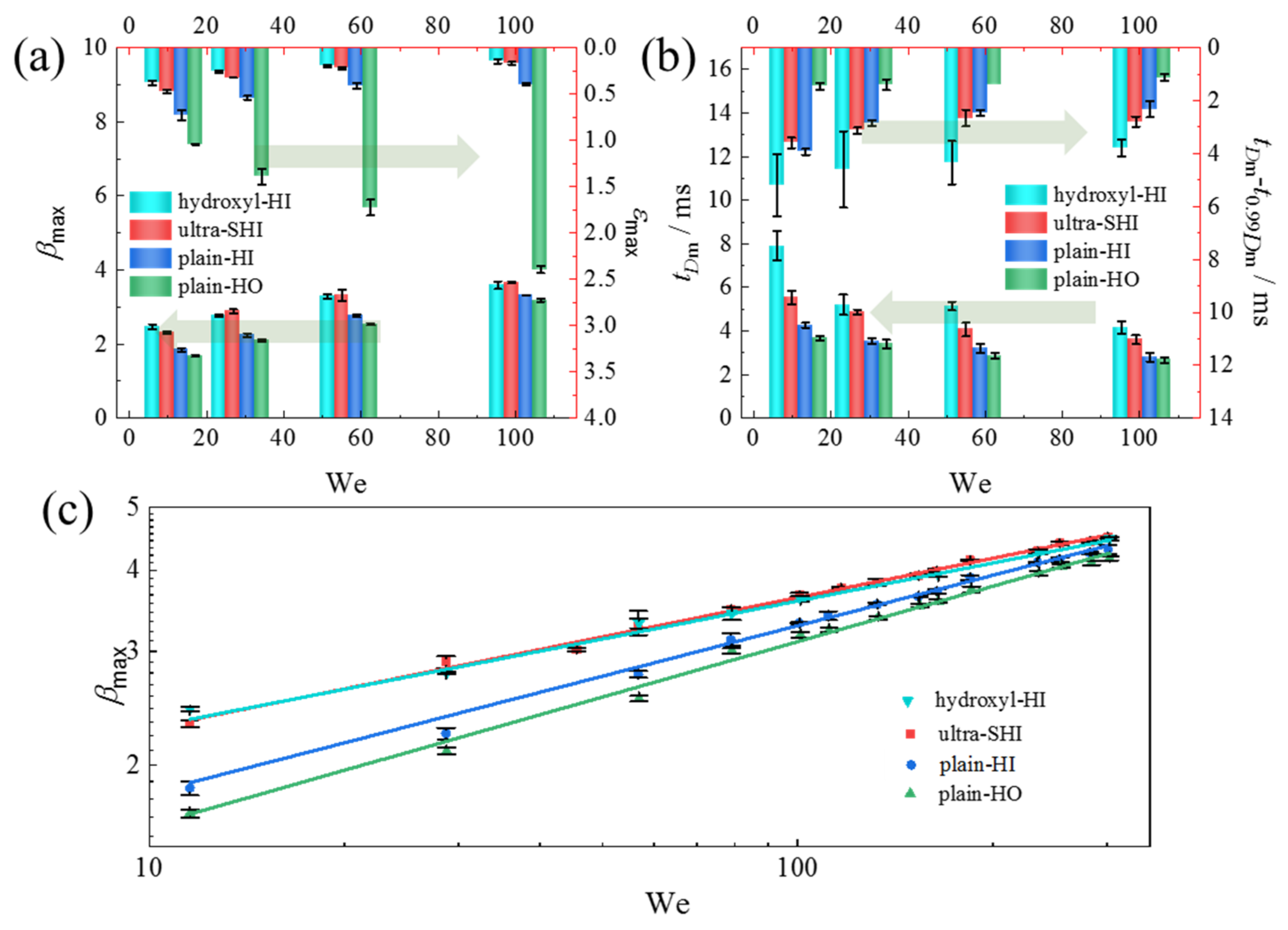
3.1.2. Effect of We Number on the Spreading Characteristics of the Droplet Impact
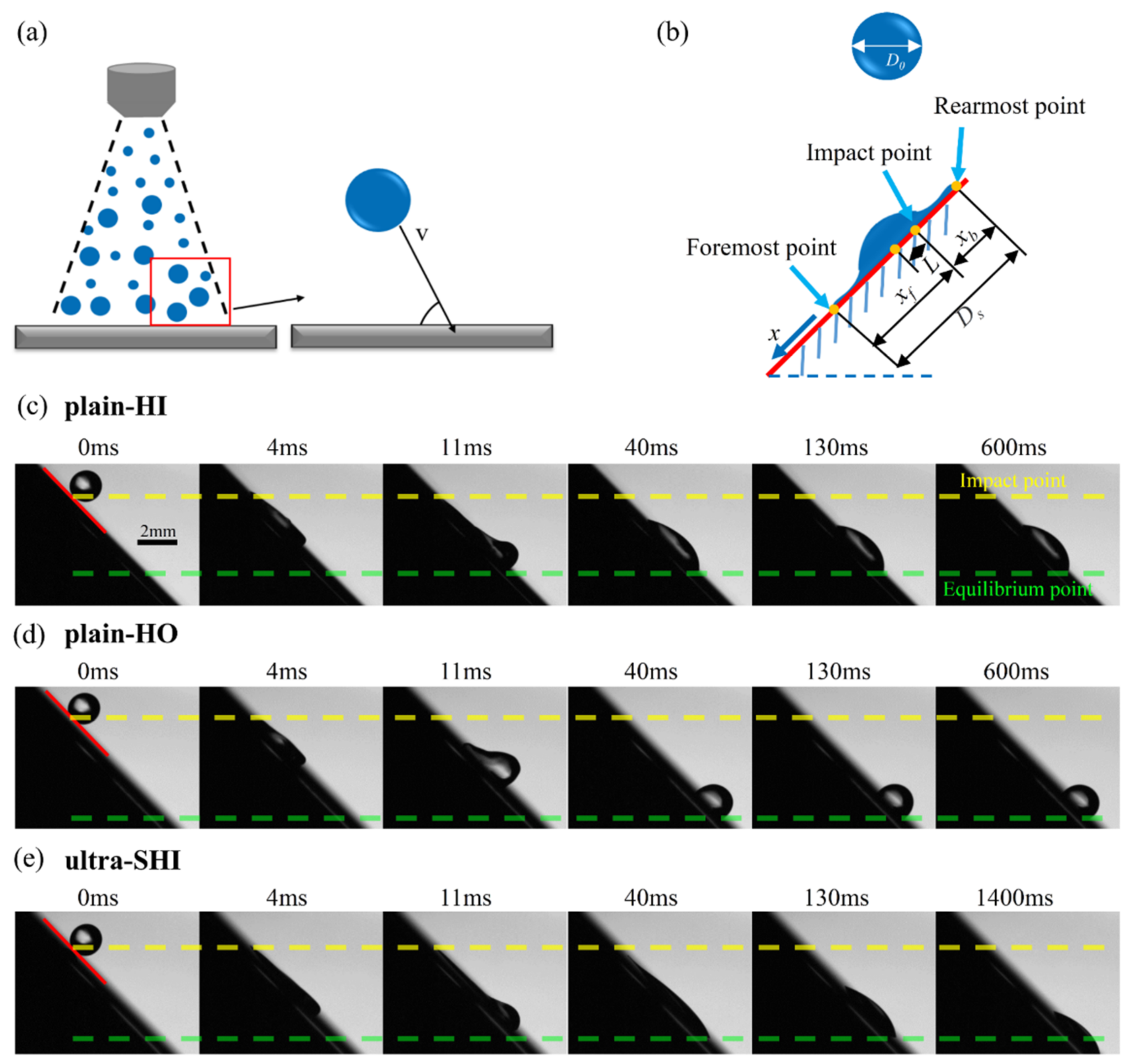
3.2. Spreading Characteristics of the Droplet Impact on Inclined Surfaces
3.2.1. Effect of Surface Wettability on the Spreading Characteristics of the Droplet Impact
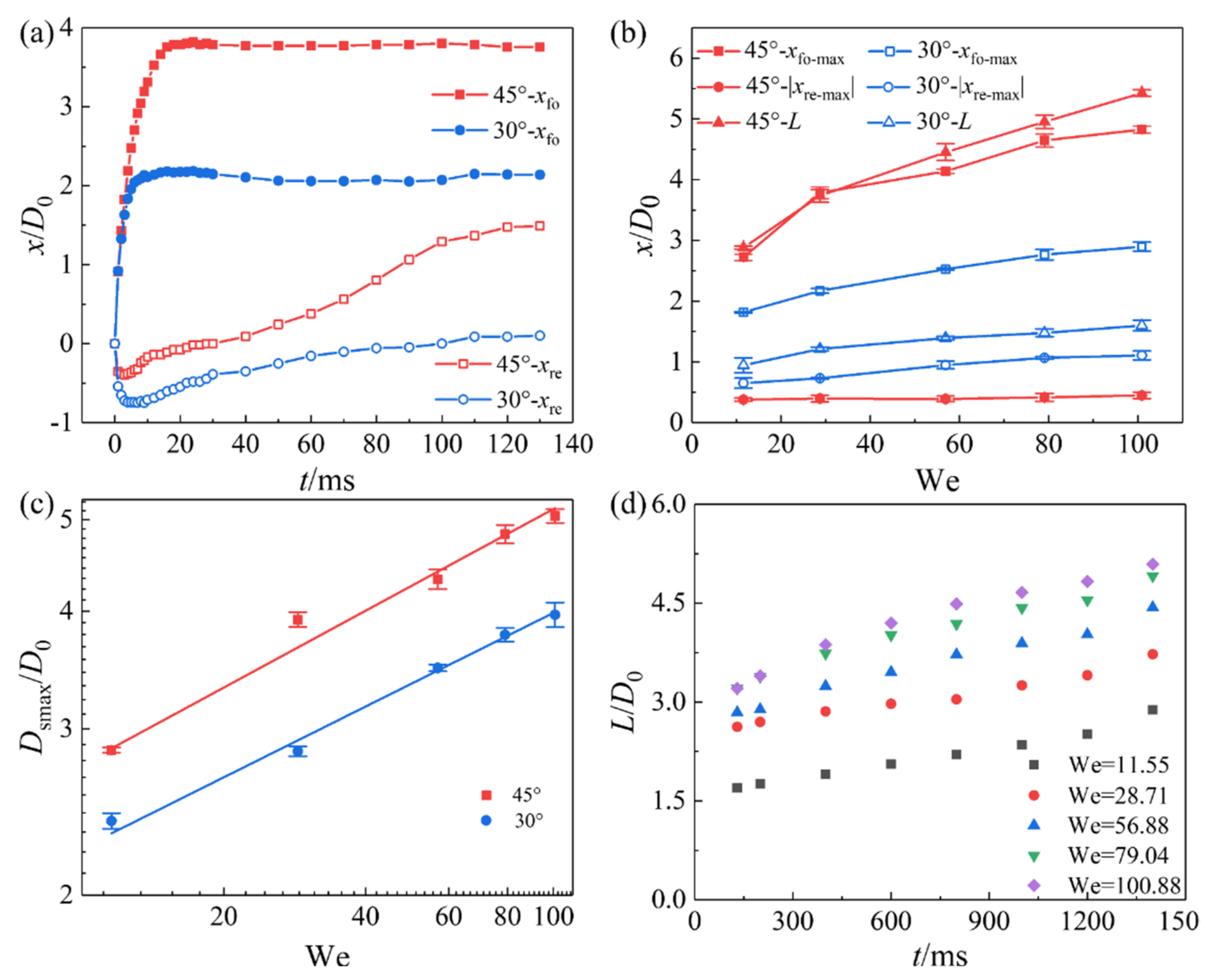
3.2.2. Effect of Inclination Angle and We Number on the Spreading Characteristics of the Droplet Impact
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.; Wang, S.; Jiang, L. Nature-inspired superwettability systems. Nat. Rev. Mater. 2017, 2, 17036. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discov. 2006, 5, 210–218. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef] [Green Version]
- Duprat, C.; Protiere, S.; Beebe, A.Y.; Stone, H.A. Wetting of flexible fibre arrays. Nature 2012, 482, 510–513. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Han, Z.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Silk, E.A.; Kim, J.; Kiger, K. Spray cooling of enhanced surfaces: Impact of structured surface geometry and spray axis inclination. Int. J. Heat Mass Transf. 2006, 49, 4910–4920. [Google Scholar] [CrossRef]
- Roisman, I.V. Fast forced liquid film spreading on a substrate: Flow, heat transfer and phase transition. J. Fluid Mech. 2010, 656, 189–204. [Google Scholar] [CrossRef]
- Ahn, H.S.; Jo, H.J.; Kang, S.H.; Kim, M.H. Effect of liquid spreading due to nano/microstructures on the critical heat flux during pool boiling. Appl. Phys. Lett. 2011, 98, 071908. [Google Scholar] [CrossRef] [Green Version]
- Worthington, A.M. On the forms assumed by drops of liquids falling vertically on a horizontal plate. Proc. R. Soc. Lond. 1876, 25, 261–272. [Google Scholar]
- Worthington, A.M. A second paper on the forms assumed by drops of liquids falling vertically on a horizontal plate. Proc. R. Soc. Lond. 1877, 25, 498–503. [Google Scholar]
- Yuan, Q.; Huang, X.J.; Zhao, Y. Dynamic spreading on pillar-arrayed surfaces: Viscous resistance versus molecular friction. Phys. Fluids 2014, 26, 092104. [Google Scholar] [CrossRef]
- McHale, G.; Shirtcliffe, N.J.; Aqil, S.; Perry, C.C.; Newton, M.I. Topography driven spreading. Phys. Rev. Lett. 2004, 93, 036102. [Google Scholar] [CrossRef]
- Tavakoli, F.; Davis, S.H.; Kavehpour, H.P. Spreading and arrest of a molten liquid on cold substrates. Langmuir 2014, 30, 10151–10155. [Google Scholar] [CrossRef]
- Vaikuntanathan, V.; Sivakumar, D. Transition from Cassie to impaled state during drop impact on groove-textured solid surfaces. Soft Matter 2014, 10, 2991–3002. [Google Scholar] [CrossRef]
- Hirvi, J.T.; Pakkanen, T.A. Nanodroplet impact and sliding on structured polymer surfaces. Surf. Sci. 2008, 602, 1810–1818. [Google Scholar] [CrossRef]
- Wang, Z.; Orejon, D.; Sefiane, K.; Takata, Y. Water vapor uptake into hygroscopic lithium bromide desiccant droplets: Mechanisms of droplet growth and spreading. Phys. Chem. Chem. Phys. 2018, 21, 1046–1058. [Google Scholar] [CrossRef] [Green Version]
- McHale, G.; Newton, M.I.; Shirtcliffe, N.J. Dynamic wetting and spreading and the role of topography. J. Phys. Condens. Matter 2009, 21, 464122. [Google Scholar] [CrossRef]
- Courbin, L.; Bird, J.C.; Reyssat, M.; Stone, H.A. Dynamics of wetting: From inertial spreading to viscous imbibition. J. Phys. Condens. Matter 2009, 21, 464127. [Google Scholar] [CrossRef]
- Edwards, A.M.J.; Ledesma-Aguilar, R.; Newton, M.I.; Brown, C.V.; McHale, G. Not spreading in reverse: The dewetting of a liquid film into a single drop. Sci. Adv. 2016, 2, e1600183. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.Y.; Zhang, L.; Zhang, Y.; Zhang, P.; Zhang, D.; Jiang, L. Uni-directional liquid spreading control on a bio-inspired surface from the peristome of Nepenthes alata. J. Mater. Chem. 2017, 5, 6914–6920. [Google Scholar] [CrossRef]
- Negeed, E.-S.R.; Albeirutty, M.; Takata, Y. Dynamic behavior of micrometric single water droplets impacting onto heated surfaces with TiO2 hydrophilic coating. Int. J. Therm. Sci. 2014, 79, 1–17. [Google Scholar] [CrossRef]
- Antonini, C.; Amirfazli, A.; Marengo, M. Drop impact and wettability: From hydrophilic to super-hydrophobic surfaces. Phys. Fluids 2012, 24, 102014. [Google Scholar] [CrossRef]
- Samsonov, V.M. On computer simulation of droplet spreading. Curr. Opin. Colloid Interface Sci. 2011, 16, 303–309. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Longtin, J.P.; Attinger, D. Interfacial temperature measurements, high-speed visualization and finite-element simulations of droplet impact and evaporation on a solid surface. Int. J. Heat Mass Transf. 2010, 53, 3733–3744. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Wang, H.; Zhu, X.; Chen, R.; Ding, Y.; Liao, Q. Numerical simulation of droplet impact on surfaces with different wettabilities. CIESC J. 2013, 64, 2745–2751. [Google Scholar]
- Quan, S.; Li, S.; Li, W. A simulation of impact of droplets on solid surfaces by using the lattice Boltzmann method. Chin. J. Comput. Mech. 2009, 26, 627–632. [Google Scholar]
- Du, J.; Zhang, Y.; Min, Q. Numerical investigations of the spreading and retraction dynamics of viscous droplets impact on solid surfaces. Colloids Surf. Physicochem. Eng. Asp. 2021, 609, 125649. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Yi, X.; He, F.; Niu, F.; Hao, P. Effect of wettability on droplet impact: Spreading and splashing. Exp. Therm. Fluid Sci. 2021, 124, 110369. [Google Scholar] [CrossRef]
- Lin, S.; Zhao, B.; Zou, S.; Guo, J.; Wei, Z.; Chen, L. Impact of viscous droplets on different wettable surfaces: Impact phenomena, the maximum spreading factor, spreading time and post-impact oscillation. J. Colloid Interface Sci. 2018, 516, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fang, T. Retraction dynamics of water droplets after impacting upon solid surfaces from hydrophilic to superhydrophobic. Phys. Rev. Fluid 2020, 5, 033604. [Google Scholar] [CrossRef]
- Quere, D.; Meglio, J.-M.D.; Brochard-Wyart, F. Spreading of liquids on highly curved surfaces. Science 1990, 249, 1256–1260. [Google Scholar] [CrossRef]
- Stapelbroek, B.B.; Jansen, H.P.; Kooij, E.S.; Snoeijer, J.H.; Eddi, A. Universal spreading of water drops on complex surfaces. Soft Matter 2014, 10, 2641–2648. [Google Scholar] [CrossRef]
- Courbin, L.; Denieul, E.; Dressaire, E.; Roper, M.; Ajdari, A.; Stone, H.A. Imbibition by polygonal spreading on microdecorated surfaces. Nat. Mater. 2007, 6, 661–664. [Google Scholar] [CrossRef]
- Chu, K.H.; Xiao, R.; Wang, E.N. Uni-directional liquid spreading on asymmetric nanostructured surfaces. Nat. Mater. 2010, 9, 413–417. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Wang, R. Visualization study on capillary-spreading behavior of liquid droplet in vertically aligned carbon nanotube array. Int. J. Heat Mass Transf. 2018, 120, 1055–1064. [Google Scholar] [CrossRef]
- Wen, R.; Ma, X.; Lee, Y.-C.; Yang, R. Liquid-vapor phase-change heat transfer on functionalized nanowired surfaces and beyond. Joule 2018, 2, 2307–2347. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Xu, C.; Zhang, Y.; Li, Q.; Wen, R.; Ma, X. Fast capillary wicking on hierarchical copper nanowired surfaces with interconnected V-grooves: Implications for thermal management. ACS Appl. Nano Mater. 2021, 4, 5360–5371. [Google Scholar] [CrossRef]
- Semenov, S.; Trybala, A.; Rubio, R.G.; Kovalchuk, N.; Starov, V.; Velarde, M.G. Simultaneous spreading and evaporation: Recent developments. Adv. Colloid Interface Sci. 2014, 206, 382–398. [Google Scholar] [CrossRef] [Green Version]
- Nasto, A.; Brun, P.T.; Hosoi, A.E. Drop impact on hairy surfaces. Phys. Rev. Fluids 2019, 4, 064004. [Google Scholar] [CrossRef]
- Ding, B.; Wang, H.; Zhu, X.; Chen, R.; Liao, Q. How supercooled superhydrophobic surfaces affect dynamic behaviors of impacting water droplets? Int. J. Heat Mass Transf. 2018, 124, 1025–1032. [Google Scholar] [CrossRef]
- Gauthier, A.; Symon, S.; Clanet, C.; Quere, D. Water impacting on superhydrophobic macrotextures. Nat. Commun. 2015, 6, 8001. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.; Moon, M.W.; Lee, K.; Kim, H. Experimental study of drop spreading on textured superhydrophilic surfaces. Phys. Fluids 2013, 25, 092110. [Google Scholar] [CrossRef] [Green Version]
- Xiao, R.; Chu, K.; Wang, E.N. Multilayer liquid spreading on superhydrophilic nanostructured surfaces. Appl. Phys. Lett. 2009, 94, 193104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Bagal, A.; Dandley, E.C.; Oldham, C.J.; Fang, T.; Parsons, G.N.; Chang, C.-H. Wicking enhancement in three-dimensional hierarchical nanostructures. Langmuir 2016, 32, 8029–8033. [Google Scholar] [CrossRef]
- Zheng, D.; Choi, C.-H.; Sun, G.; Zhao, X. Superwicking on Nanoporous Micropillared Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 30925–30931. [Google Scholar] [CrossRef]
- Narhe, R.D.; Beysens, D.; Nikolayev, V.S. Contact line dynamics in drop coalescence and spreading. Langmuir 2004, 20, 1213–1221. [Google Scholar] [CrossRef]
- Chlarke, A.; Blake, T.D.; Carruthers, B.K.; Woodward, A. Spreading and imbibition of liquid droplets on porous surfaces. Langmuir 2002, 18, 2980–2984. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, F.; Zhao, C.; Wang, S.; Liu, M.; Jiang, L. Superspreading on immersed gel surfaces for the confined synthesis of thin polymer films. Angew. Chem. Int. Ed. 2016, 55, 3615–3619. [Google Scholar] [CrossRef]
- Park, K.C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Sun, N.; Nielsen, S.O.; Stogin, B.B.; Wang, J.; Yang, S.; Wong, T.S. Hydrophilic directional slippery rough surfaces for water harvesting. Sci. Adv. 2018, 4, eaaq0919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Zhang, L.; Monga, D.; Stone, H.A.; Dai, X. Hydrophilic slippery surface enabled coarsening effect for rapid water harvesting. Cell Rep. Phys. Sci. 2021, 2, 100387. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Z.; Sarma, J.; Zhao, W.; Dai, X. Gradient quasi-liquid surface enabled self-propulsion of highly wetting liquids. Adv. Funct. Mater. 2021, 31, 2008614. [Google Scholar] [CrossRef]
- Ho, T.A.; Papavassiliou, D.V.; Lee, L.L.; Striolo, A. Liquid water can slip on a hydrophilic surface. Proc. Natl. Acad. Sci. USA 2011, 108, 16170–16175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, K.; Soto, D.; Farnham, T.; Paxson, A.; Katmis, A.U.; Gleason, K.; Varanasi, K.K. Grafted Nanofilms Promote Dropwise Condensation of Low-Surface-Tension Fluids for High-Performance Heat Exchangers. Joule 2019, 3, 1377–1388. [Google Scholar] [CrossRef]
- Cha, H.; Vahabi, H.; Wu, A.; Chavan, S.; Kim, M.-K.; Sett, S.; Bosch, S.A.; Wang, W.; Kota, A.K.; Miljkovic, N. Dropwise condensation on solid hydrophilic surfaces. Sci. Adv. 2020, 6, eaax0746. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, S.; Urata, C.; Sato, T.; Hones, R.; Hozumi, A. Smooth and transparent films showing paradoxical surface properties: The lower the static contact angle, the better the water sliding performance. Langmuir 2019, 35, 6822–6829. [Google Scholar] [CrossRef]
- Nakamura, S.; Archer, R.J.; Dunderdale, G.J.; Hozumi, A. Perfluorinated compounds are not necessary: Pegylated organosilanes can endow good water sliding/removal properties. J. Hazard. Mater. 2020, 398, 122625. [Google Scholar] [CrossRef]
- Papra, A.; Gadegaard, N.; Larsen, N.B. Characterization of ultrathin poly (ethylene glycol) monolayers on silicon substrates. Langmuir 2001, 17, 1457–1460. [Google Scholar] [CrossRef]
- Jo, S.; Park, K. Surface modification using silanated poly (ethylene glycol) s. Biomaterials 2000, 21, 605–616. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; McCarthy, T.J. Trialkylsilane monolayers covalently attached to silicon surfaces: Wettability studies indicating that molecular topography contributes to contact angle hysteresis. Langmuir 1999, 15, 3759–3766. [Google Scholar] [CrossRef]
- Sharma, S.; Johnson, R.W.; Desai, T.A. Ultrathin poly (ethylene glycol) films for silicon-based microdevices. Appl. Surf. Sci. 2003, 206, 218–229. [Google Scholar] [CrossRef]
- Sharma, S.; Johnson, R.W.; Desai, T.A. XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors. Biosens. Bioelectron. 2004, 20, 227–239. [Google Scholar] [CrossRef]
- Zhang, F.; Kang, E.; Neoh, K.; Wang, P.; Tan, K. Modification of Si (100) surface by the grafting of poly (ethylene glycol) for reduction in protein adsorption and platelet adhesion. J. Biomed. Mater. Res. 2001, 56, 324–332. [Google Scholar] [CrossRef]
- Yan, H.; Yuanhao, W.; Hongxing, Y. TEOS/silane coupling agent composed double layers structure: A novel super-hydrophilic coating with controllable water contact angle value. Appl. Energy 2017, 185, 2209–2216. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Zhou, X.; Li, X.; Kappl, M.; Steffen, W.; Butt, H.J. One-Step Synthesis of a Durable and Liquid-Repellent Poly (dimethylsiloxane) Coating. Adv. Mater. 2021, 33, 2100237. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol–coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef]
- Krumpfer, J.W.; McCarthy, T.J. Rediscovering silicones: “Unreactive” silicones react with inorganic surfaces. Langmuir 2011, 27, 11514–11519. [Google Scholar] [CrossRef]
- Xu, Y.; Vincent, S.; He, Q.C.; Le-Quang, H. Spread and recoil of liquid droplets impacting on solid surfaces with various wetting properties. Surf. Coat. Technol. 2019, 357, 140–152. [Google Scholar] [CrossRef]
- Chen, J.-N.; Xu, R.-N.; Zhang, Z.; Chen, X.; Ouyang, X.-L.; Wang, G.-Y.; Jiang, P.-X. Phenomenon and mechanism of spray cooling on nanowire arrayed and hybrid micro/nanostructured surfaces. J. Heat Transf. 2018, 140, 112401. [Google Scholar] [CrossRef]
- Clanet, C.; Beguin, C.; Richard, D.; Quere, D. Maximal deformation of an impacting drop. J. Fluid Mech. 2004, 517, 199–208. [Google Scholar] [CrossRef]
- Lee, J.; Laan, N.; de Bruin, K.G.; Skantzaris, G.; Shahidzadeh, N.; Derome, D.; Carmeliet, J.; Bonn, D. Universal rescaling of drop impact on smooth and rough surfaces. J. Fluid Mech. 2016, 786, R4. [Google Scholar] [CrossRef]
- Bobinski, T.; Sobieraj, G.; Psarski, M.; Celichowski, G.; Rokicki, J. Droplet bouncing on the surface with micro-structure. Arch. Mech. 2017, 69, 177–193. [Google Scholar]
- Bartolo, D.; Josserand, C.; Bonn, D. Retraction dynamics of aqueous drops upon impact on non-wetting surfaces. J. Fluid Mech. 2005, 545, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.; Han, S.; Chung, J.; Chung, J.T.; Ko, S.; Grigoropoulos, C.P. Experimental study on spreading and evaporation of inkjet printed pico-liter droplet on a heated substrate. Int. J. Heat Mass Transf. 2009, 52, 431–441. [Google Scholar] [CrossRef]
- Roux, D.C.D.; Cooper-White, J.J. Dynamics of water spreading on a glass surface. J. Colloid Interface Sci. 2004, 277, 424–436. [Google Scholar] [CrossRef]
- Fujimoto, H.; Shiotani, Y.; Tong, A.Y.; Hama, T.; Takuda, H. Three-dimensional numerical analysis of the deformation behavior of droplets impinging onto a solid substrate. Int. J. Multiph. Flow 2007, 33, 317–332. [Google Scholar] [CrossRef]



| Substrate | Pretreatment | Reaction Conditions | θa/θr (°) Water | Ref. |
|---|---|---|---|---|
| Silicon wafers | Concentrated sulfuric acid containing sodium dichromate and hydrogen peroxide | Grafting: (CH3)3SiCl, toluene, room temperature, 72 h | 105/96 | [62] |
| Grafting: (CH3)3SiN(CH3)2, toluene, room temperature, 72 h | 106/98 | |||
| Grafting: (CH3)3SiOSO2CF3, toluene, room temperature, 72 h | 105/95 | |||
| Silicon wafers | Oxidation: hydrogen peroxide, sulfuric acid, 10 min, 120 °C | Grafting: 2-[Methoxypoly(ethyleneoxy)propyl]trimethoxysilane, toluene, room temperature, 18 h | 38 ± 2/34 ± 2 | [60] |
| Glass | Soaking in chromic acid overnight | Grafting: silanated PEG I/silanated PEG II, anhydrous toluene, 70 °C, overnight | I: 32.3 ± 2.9/18.5 ± 2.0; II: 49.3 ± 0.6/24.4 ± 2.5 | [61] |
| Silicon wafers | Hydrophilization: concentrated hydrogen chloride, hydrogen peroxide, deionized water, 80 °C, 15 min | PEG–silane coupling to silicon: hydrolysis of PEG–OSiCl3, 120 min, room temperature | 42.33 ± 2.61 (CA) | [63] |
| - | [64] | |||
| Si(100) surface | Cleaning: concentrated sulfuric acid, hydrogen peroxide; argon plasma | Grafting: PEGMA macromonomer, riboflavin, ethanol/water, UV illumination, 0.5–3.0 h | - | [65] |
| Glass sheets | Cleaning: detergent and water | Grafting: 2-[acetoxy (polyethyleneoxy) propyl]triethoxysilane (pH 5.5), TEOS, 10 min, 75 °C | 10.7 (CA) | [66] |
| Silicon wafers | Oxygen plasma | Grafting: DCDMS, toluene, room temperature, 1800 s | 104 ± 1/100 ± 1 | [67] |
| Silicon wafers | Reacting SiH4 and O2 gases in a PECVD reactor | PEG 400 (vapor), water plasma, 100 °C, vacuum | 25 ± 2 (CA) | [68] |
| Silicon wafers | Cleaning: oxygen plasma, 250 mTorr, 20 min | PDMS2000, 100 °C, 24 h | 104/102 | [69] |
| Silicon wafers | Cleaning: UV/ozone | PEG9–12-Si, TEOS, ethanol, aqueous HCl; spin-coated, dried at 80 °C for 3 h | 42 ± 2/35 ± 1 | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, Q.; Ying, Y.; You, Z.; Wang, S.; Chun, J.; Ma, X.; Wen, R. Droplet Spreading Characteristics on Ultra-Slippery Solid Hydrophilic Surfaces with Ultra-Low Contact Angle Hysteresis. Coatings 2022, 12, 755. https://doi.org/10.3390/coatings12060755
Song Y, Wang Q, Ying Y, You Z, Wang S, Chun J, Ma X, Wen R. Droplet Spreading Characteristics on Ultra-Slippery Solid Hydrophilic Surfaces with Ultra-Low Contact Angle Hysteresis. Coatings. 2022; 12(6):755. https://doi.org/10.3390/coatings12060755
Chicago/Turabian StyleSong, Yajie, Qi Wang, Yushan Ying, Zhuo You, Songbai Wang, Jiang Chun, Xuehu Ma, and Rongfu Wen. 2022. "Droplet Spreading Characteristics on Ultra-Slippery Solid Hydrophilic Surfaces with Ultra-Low Contact Angle Hysteresis" Coatings 12, no. 6: 755. https://doi.org/10.3390/coatings12060755
APA StyleSong, Y., Wang, Q., Ying, Y., You, Z., Wang, S., Chun, J., Ma, X., & Wen, R. (2022). Droplet Spreading Characteristics on Ultra-Slippery Solid Hydrophilic Surfaces with Ultra-Low Contact Angle Hysteresis. Coatings, 12(6), 755. https://doi.org/10.3390/coatings12060755






