Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties
Abstract
:1. Introduction
2. Basic Theories Related to MAM Research
2.1. Impedance Matching Characteristic
2.2. Attenuation Characteristic
2.3. Classification of MAM
3. Design Principles of Low-Frequency Broadband MAMs
3.1. Design of Low-Frequency Characteristics
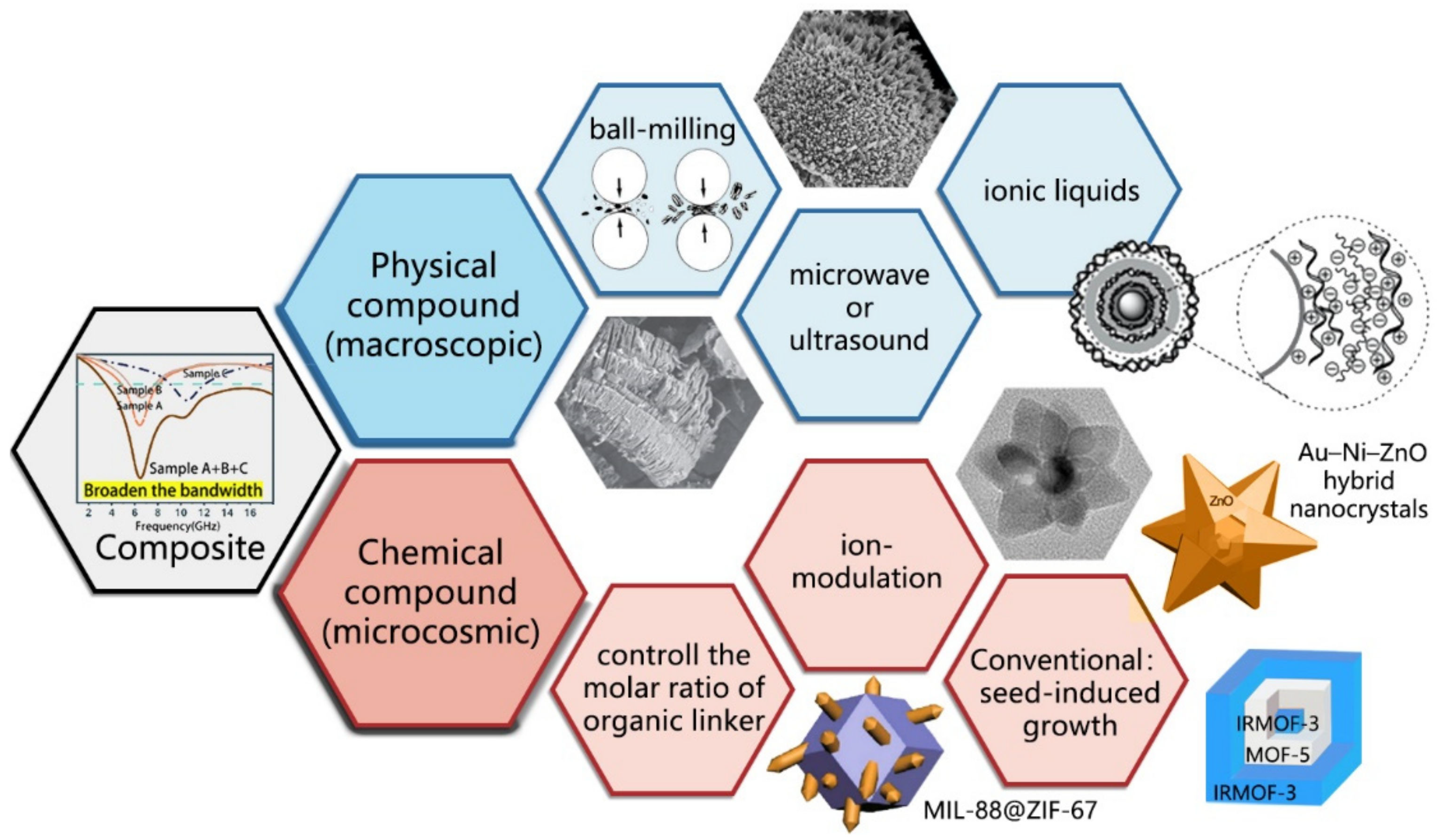
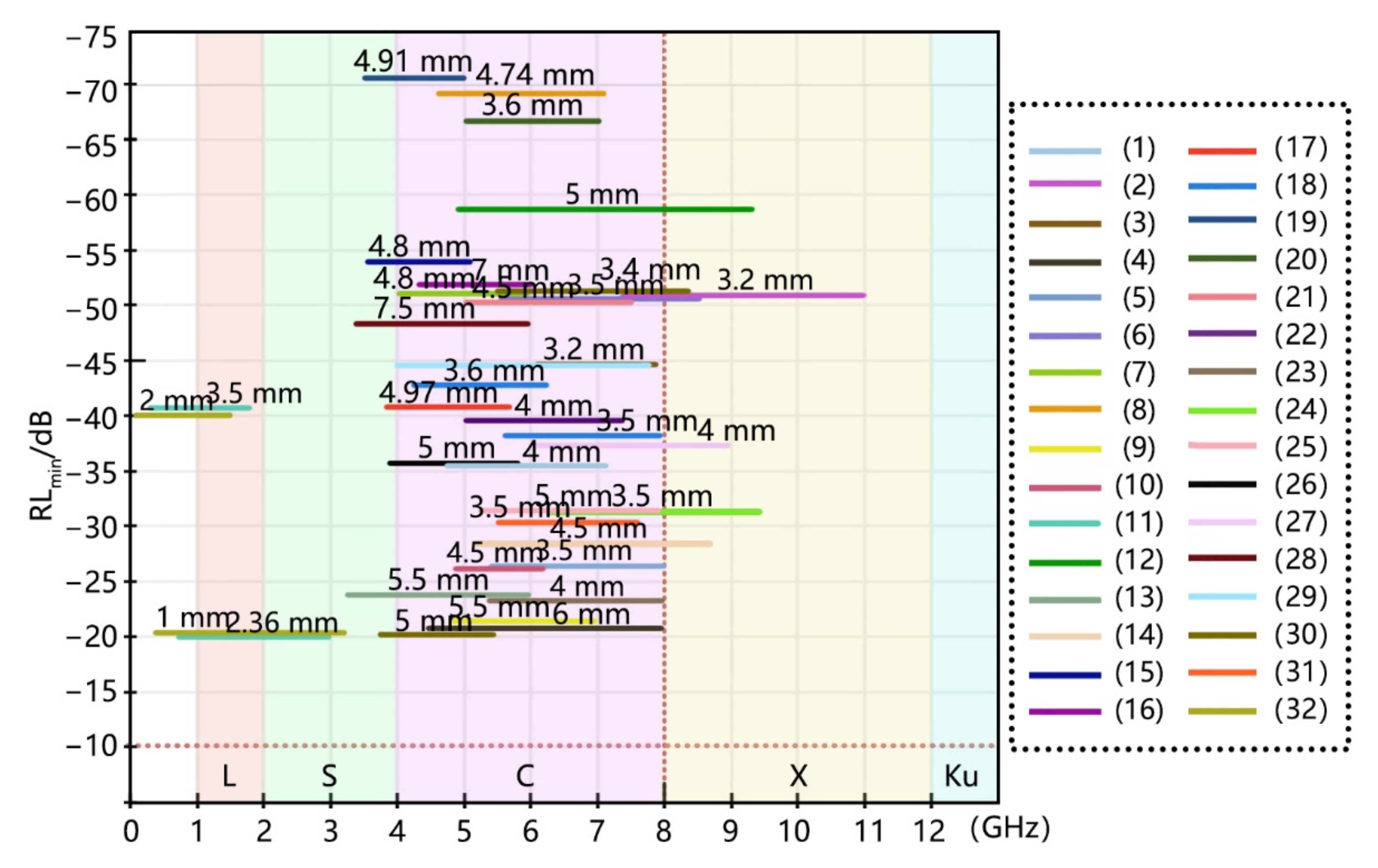
3.2. Realization of Broadband Characteristics
| Absorbing Agent | RLmin (dB) | Maximum Peak Position (GHz) | Frequency Range (GHz) (RL ≤ −10 dB) | Efficient Bandwidth (GHz) | Effective Absorption Frequency (<8 GHz) | EAB Range (>5 GHz) | Characteristic | Refs | |
|---|---|---|---|---|---|---|---|---|---|
| (a) | ZrO2/C-800 | −58.7 | 16.8 | 11.5–17.0 | 5.5 | √ | plentiful, dense, and equally distributed | [67] | |
| (b) | Fe3O4@NPC | −65.5 −50 | 9.8 6.9 | 7.7–12.2 5.4–8.2 | 4.5 2.8 | porous | [68] | ||
| (c) | Ni@C-ZIF | −86.8 | 13.2 | 6.1–8.1 | 2 | spherical-like hierarchical 3D nanostructures | [64] | ||
| (d) | CoNi/C-650 | −74.7 | 15.6 | 10.9–13.2 | 3.3 | multi-metal | [69] | ||
| (e) | FeCoNi-MOF-600 FeCoNi-MOF-700 | −23.4 −69.3 | 6.0 5.52 | 5.1–7.9 4.7–7.2 | 2.8 2.5 | √ | multi-metal | [70] | |
| (f) | Porous CZC-800 | −21.60 | 5.5 | 4.9–7.0 | 2.1 | √ | porous | [71] | |
| (g) | CNC-1:1 | −44.8 | 10.7 | 6–7.8 | 1.8 | √ | porous and hollow | [65] | |
| (h) | Co3O4/Co/RGO | −52.8 | 13.12 | – | – | √ | two-dimensional growth | [72] | |
| (i) | Co/C-RGO | −52 −27 | 9.6 13.48 | 7.2–13 10.3–18 | 5.8 7.7 | √ | two-dimensional growth | [66] |
4. Low-Frequency Broadband Characteristics of MOFs
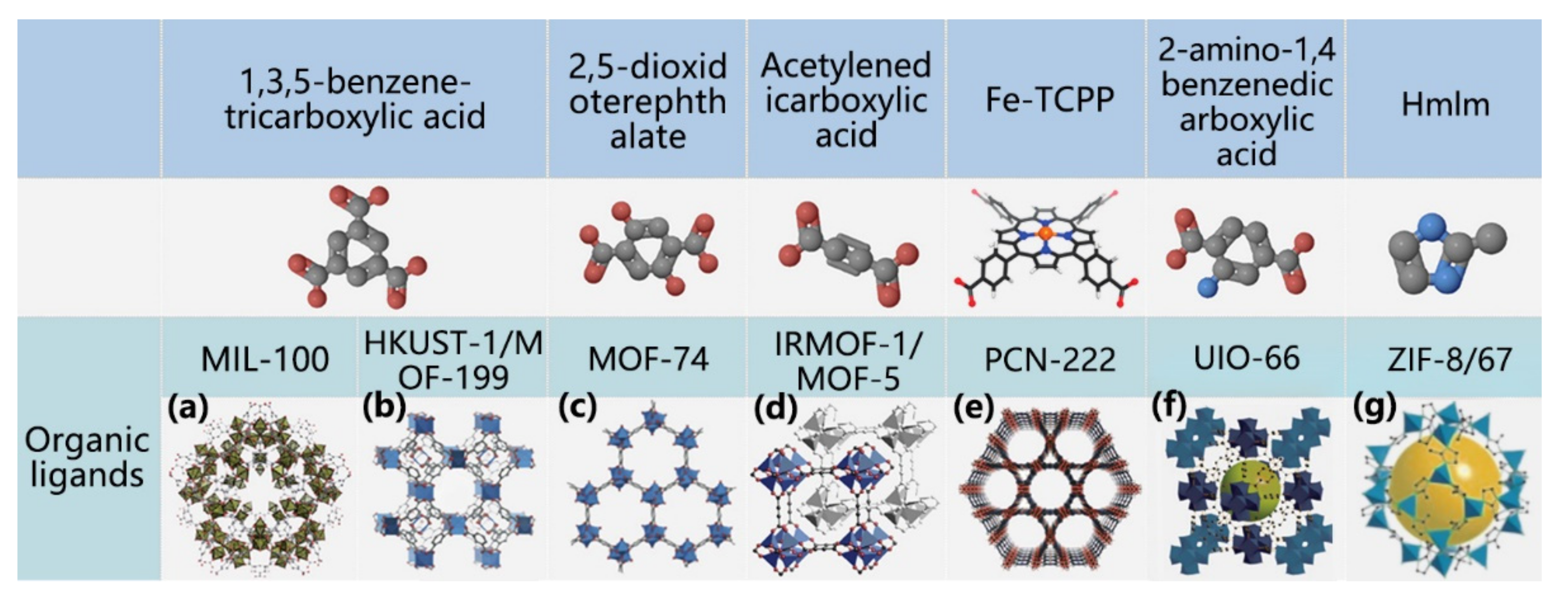
4.1. Classification of MOF

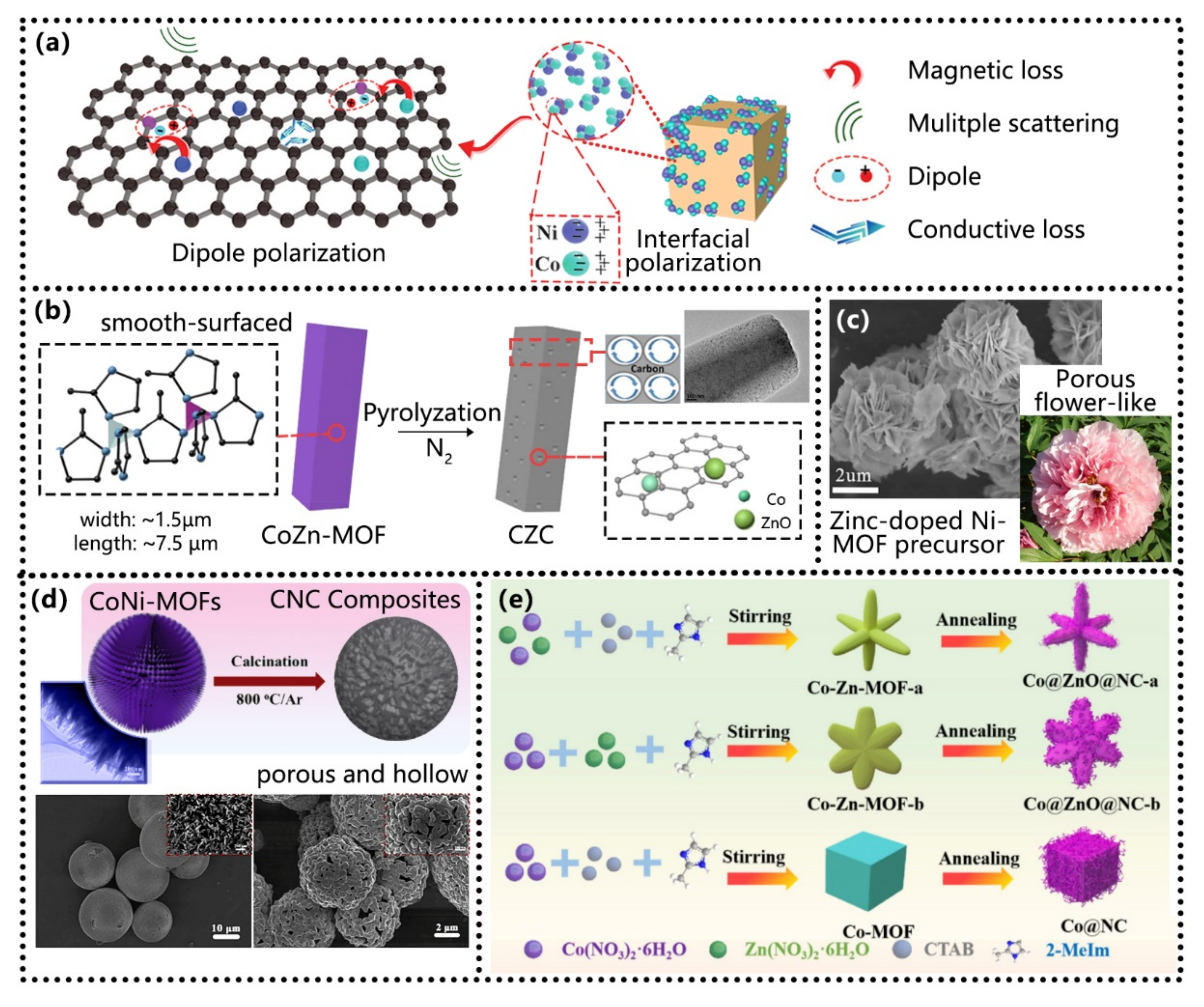
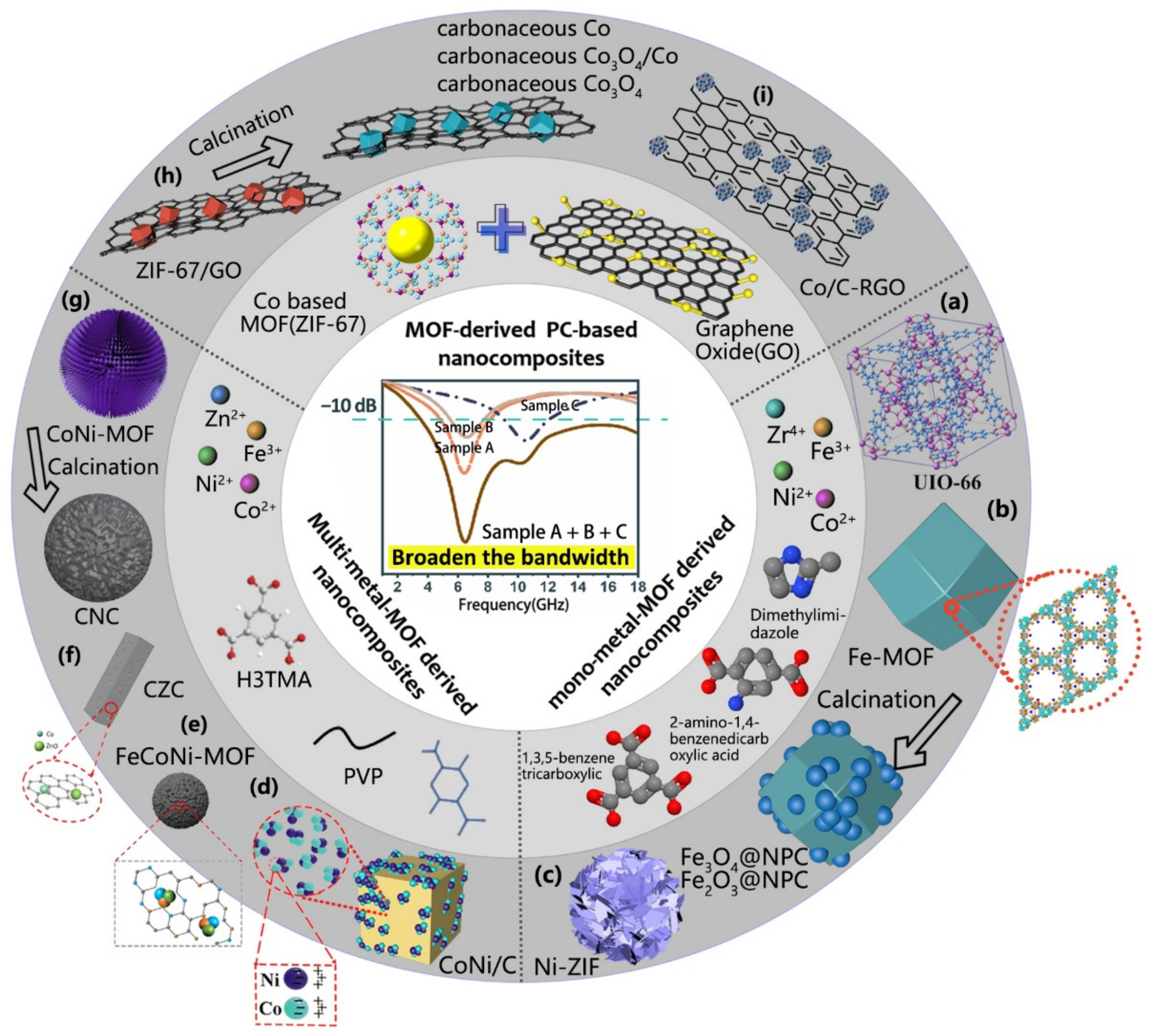
4.2. Compound Materials Based on MOFs
5. Process Controls in Preparation
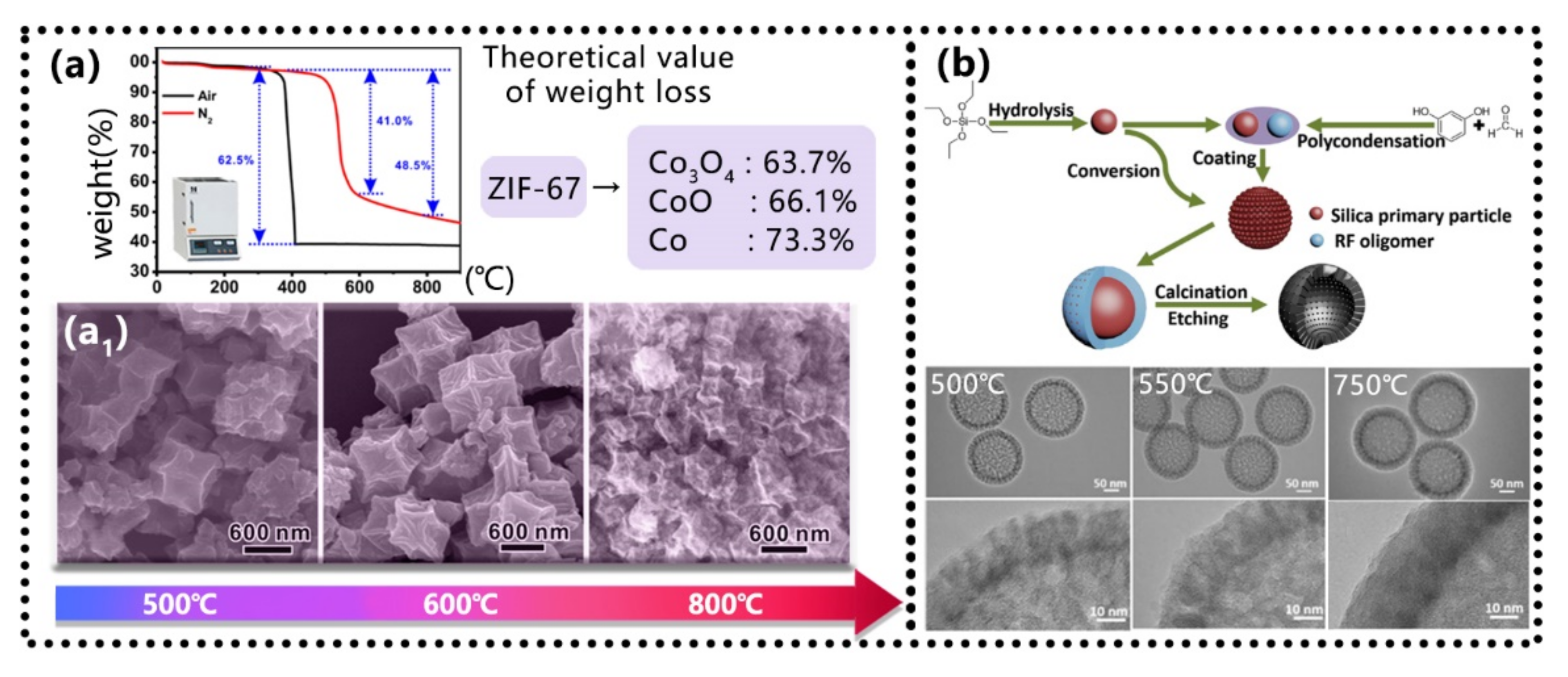
5.1. Sintering Temperatures Control
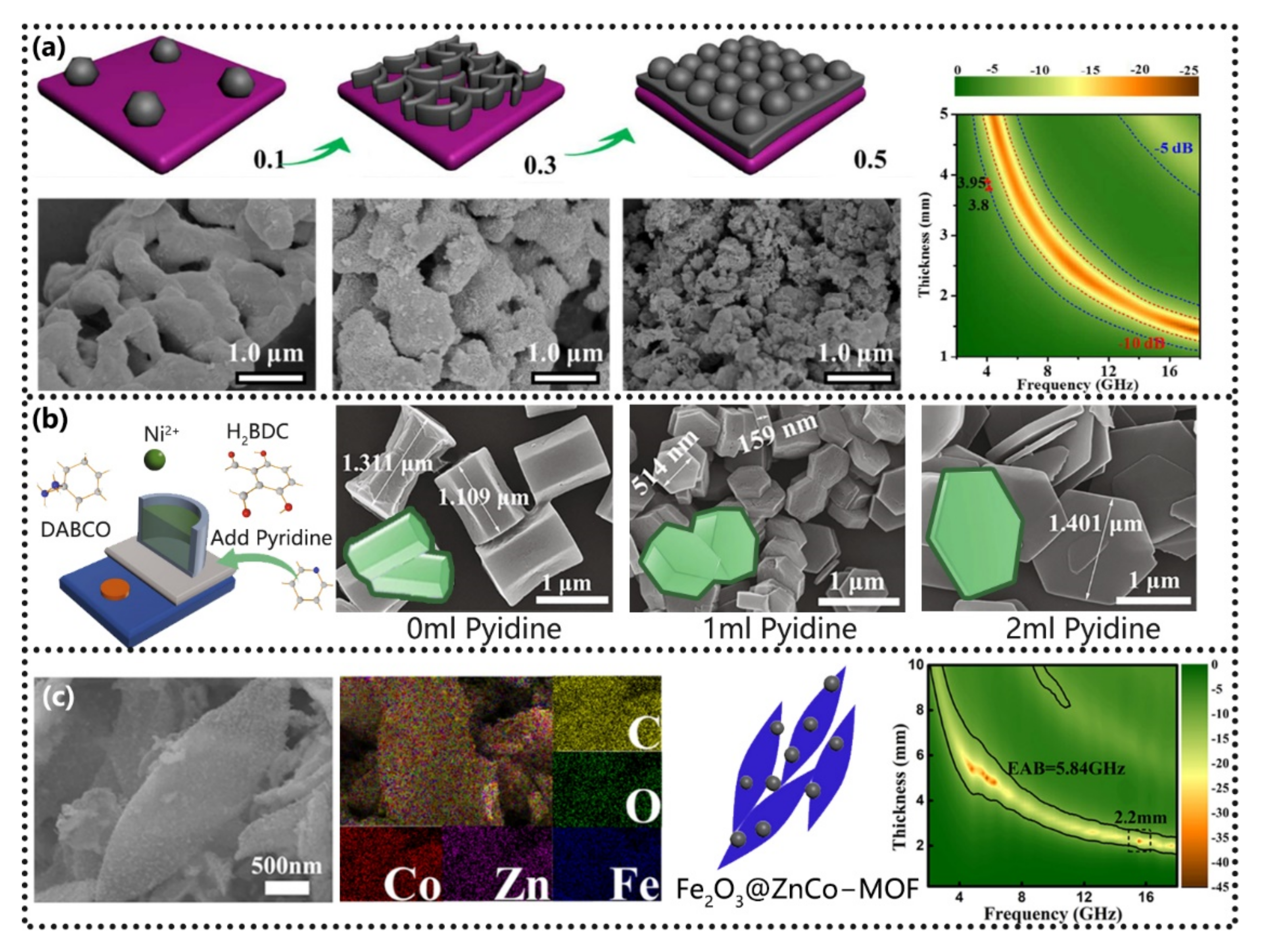
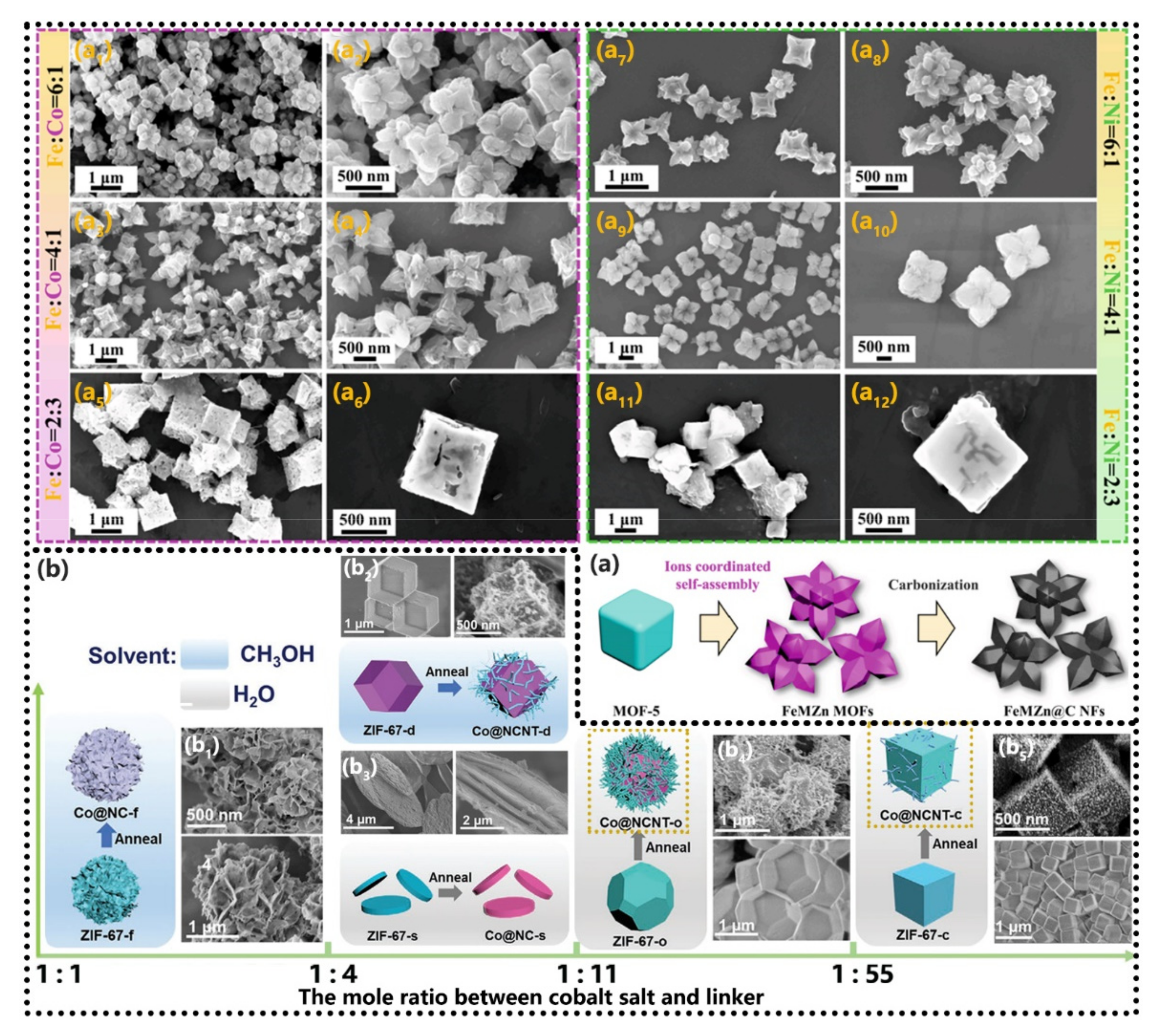
5.2. Materials Ratios Control
6. Challenges and Prospects
- Design and selection of composites. At present, MOF derivatives applied in the field of absorption can be divided into single-metal derivatives, multi-metal derivatives, oxide/sulfide derivatives, and other composite materials according to their compositions. The metal atoms mainly include Fe, Co, Ni, and Zn. Subsequent research on other metal elements should be increased, and combinations of different metals should be tried to achieve synergistic effects. Meanwhile, we should also pay attention to the ionic potential of metals combined with ligands and explore its corresponding relationship with low-frequency absorption ability [22]. In addition, MOFs products are also closely related to the ratio between metals and organic ligands, which should be recorded as their volume ratios for the ease of repeated experiments and large-scale productions.
- Structural optimization. After different treatment processes, MOF precursors can be compounded with one-dimensional and two-dimensional materials. One-dimensional MOF derivatives have large specific surface areas due to their large length and diameter. Two-dimensional MOFs derivatives have a network structure, which allows multiple reflections of electromagnetic waves inside and can effectively improve the attenuation ability. In addition, the application of MOF is not limited to the field of microwave absorption, MOFs@MOFs, MOFs of core–shell structure and multilayer core–shell structure, CelloMOFs [116], Zr-MOF [117], MOF/COF-based hybrid nanomaterials [118] and other MOF materials are also widely used in water treatment, air purification, biology, energy storage, catalysts, membrane separation [116,117,118,119], supercapacitors [116,120] and other fields that have broad development prospects.
- Controlled preparation. With the aim of obtaining materials with the “low-frequency broadband”, comparative experiments should be conducted with variables of different materials (metal and organic ligand), material ratios, proportions of the dissolved agent, pyrolysis temperatures, and environment, and paraffin filling amounts.
7. Concluding Remarks
- In order to realize effective absorption of low-frequency waves, the impedance matching at low frequencies should be realized, so that the electromagnetic wave can enter the MAM to realize the energy conversion. Because the way of EM wave loss in a good conductor is mainly magnetic loss and the value of the permeability of the absorber is usually much smaller than the dielectric constant, the magnetic loss is more important than the dielectric loss. The low-frequency impedance matching can be improved by adding magnetic materials.
- In order to make full use of magnetic loss materials to effectively attenuate EM waves, nanostructured MOFs materials that meet the critical radius of single domains and produce resonance absorption size can be added to the polymer matrix, composed of low-dimensional or multi-dimensional materials, so as to produce excellent microwave absorption properties.
- In order to realize multiple reflections and interface polarization, the material size should be controlled at the nanometer level. Due to the large specific surface area of nanomaterials, attention should also be paid to the treatment of the interface, because agglomeration (cluster) will lead to the decrease in dielectric constants.
- When preparing MOF materials, a variety of metal ions can be compounded. In addition, a variety of materials with good microwave absorbing properties in different frequencies can be mixed. In this way, the microwave absorbing properties of overlapping parts can be enhanced and the broadband can be broadened.
- The sintering temperature and the matching thickness in the preparation process are also very important. At present, researchers are still not very clear about the reaction principle in the material preparation process, where there are many influencing factors; therefore, the comparative tests aiming at low frequencies and broadband are extremely critical.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Zhang, Z.; Qi, L.; Liao, Q.; Kang, Z.; Zhang, Y. Toward the application of high frequency electromagnetic wave absorption by carbon nanostructures. Adv. Sci. 2019, 6, 1801057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, H.; Zhang, Q.; Zhao, B.; Marken, F.; Gao, Q.; Lu, H.; Guan, L.; Wang, H.; Shao, G.; Xu, H.; et al. Novel hierarchical structure of MoS2/TiO2/Ti3C2Tx composites for dramatically enhanced electromagnetic absorbing properties. J. Adv. Ceram. 2021, 10, 1042–1051. [Google Scholar] [CrossRef]
- Xia, F.; Liu, J.; Gu, D.; Zhao, P.; Zhang, J.; Che, R. Microwave absorption enhancement and electron microscopy characterization of BaTiO3 nano-torus. Nanoscale 2011, 3, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, J.; Che, R.; Xu, J.; Liu, M.; Liu, Z. Double-shelled yolk–shell microspheres with Fe3O4 cores and SnO2 double shells as high-performance microwave absorbers. J. Phys. Chem. C 2013, 117, 489–495. [Google Scholar] [CrossRef]
- Ye, X.; Chen, Z.; Ai, S.; Hou, B.; Zhang, J.; Liang, X.; Zhou, Q.; Liu, H.; Cui, S. Porous SiC/melamine-derived carbon foam frameworks with excellent electromagnetic wave absorbing capacity. J. Adv. Ceram. 2019, 8, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xu, J.; Liu, X.; Zhang, S.; Yuan, H.; Zhu, C.; Chen, Y. Metal organic framework-derived three-dimensional graphene-supported nitrogen-doped carbon nanotube spheres for electromagnetic wave absorption with ultralow filler mass loading. Carbon 2019, 155, 233–242. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, X.; Li, B.; Han, Y.; Zheng, Y.; Yeung, K.W.K.; Li, C.; Cui, Z.; Liang, Y.; Li, Z.; et al. Treatment of MRSA-infected osteomyelitis using bacterial capturing, magnetically targeted composites with microwave-assisted bacterial killing. Nat. Commun. 2020, 11, 4446. [Google Scholar] [CrossRef]
- Kundi, M.; Hutter, H.P. Mobile phone base stations-Effects on wellbeing and health. Pathophysiology 2009, 16, 123–135. [Google Scholar] [CrossRef]
- Zhao, J. Biological effects of radiofrequency electromagnetic pollution on inner ear. J. Audiol. Speech Pathol. 2012, 20, 5. [Google Scholar]
- Ma, J.; Zhao, M.; Zhang, K.; Mu, S. Aircraft stealth technology and radar countermeasures. Natl. Def. Sci. Technol. 2009, 3, 8. [Google Scholar]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2018, 4, 9180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S. Metal-Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Chen, E.X.; Yang, H.; Zhang, J. Zeolitic imidazolate framework as formaldehyde gas sensor. Inorganic chemistry. Inorg. Chem. 2014, 53, 5411–5413. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zheng, L. MOF-derived porous Co/C nanocomposites with excellent electromagnetic wave absorption properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Hsu, F.K.; Lee, W.D. Magnetic cobalt-graphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate. J. Mater. Chem. A. 2015, 3, 9480–9490. [Google Scholar]
- Mahmood, A.; Guo, W.; Tabassum, H.; Zou, R. Metal-organic framework-based nanomaterials for electrocatalysis. Adv. Energy Mater. 2016, 6, 1600423. [Google Scholar] [CrossRef]
- Tong, X.; Cherif, M.; Zhang, G.; Zhan, X.; Ma, J.; Almesrati, A.; Vidal, F.; Song, Y.; Claverie, J.P.; Sun, S. N, P-codoped graphene dots supported on N-Doped 3D graphene as metal-free catalysts for oxygen reduction. ACS Appl. Mater. Inter. 2021, 13, 30512–30523. [Google Scholar] [CrossRef]
- Ma, J.; Wang, L.; Deng, Y.; Zhang, W.; Wu, T.; Song, Y. Mass production of high-performance single atomic FeNC electrocatalysts via sequenced ultrasonic atomization and pyrolysis process. Sci. China Mater. 2021, 64, 631–641. [Google Scholar] [CrossRef]
- Ma, M.; Bi, Y.; Tong, Z.; Liu, Y.; Lyu, P.; Wang, R.; Chen, Y. Recent progress of MOF-derived porous carbon materials for microwave absorption. RSC. Adv. 2021, 11, 16572–16591. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y. Thin Films and/or Coating for Electromagnetic Interference and Stealth. Inorganic and Organic Thin Films: Fundamentals. Fabr. Appl. 2021, 2, 587–614. [Google Scholar]
- Duan, W.; Yin, X.; Li, Q.; Schlier, L.; Greil, P.; Travitzky, N. A review of absorption properties in silicon-based polymer derived ceramics. J. Eur. Ceram. Soc. 2016, 36, 3681–3689. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Peng, K. Research progress on electromagnetic microwave absorption properties of MOFs derived composites. J. Anhui Univ. Nat. Sci. 2020, 44, 15. [Google Scholar]
- Naito, Y.; Suetake, K. Application of ferrite to electromagnetic wave absorber and its characteristics. IEEE Trans. Microw. Theory Tech. 1971, 19, 65–72. [Google Scholar] [CrossRef]
- Cao, M.S.; Song, W.L.; Hou, Z.L.; Wen, B.; Yuan, J. The effects of temperature and frequency on the dielectric properties, electromagnetic interference shielding and microwave-absorption of short carbon fiber/silica composites. Carbon 2010, 48, 788–796. [Google Scholar] [CrossRef]
- Wang, L.; Guan, Y.; Qiu, X.; Zhu, H.; Pan, S.; Yu, M.; Zhang, Q. Efficient ferrite/Co/porous carbon microwave absorbing material based on ferrite@metal–organic framework. Chem. Eng. J. 2017, 326, 945–955. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.W.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Che, R. Dimensional Design and Core–Shell Engineering of Nanomaterials for Electromagnetic Wave Absorption. Adv. Mater. 2021, 34, 2107538. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Zhang, X.; Gao, Q.; Li, Y.; Du, Y. A novel Co/TiO2 nanocomposite derived from a metal–organic framework: Synthesis and efficient microwave absorption. J. Mater. Chem. C 2016, 4, 1860–1870. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Chen, C.; Liu, X.; Liu, H. The 3D CoNi alloy particles embedded in N-doped porous carbon foams for high-performance microwave absorbers. Carbon 2019, 152, 545–555. [Google Scholar] [CrossRef]
- Zhang, X.F.; Dong, X.L.; Huang, H.; Zhang, X.F.; Zhu, X.G.; Li, J.P.; Sun, J.P. Microwave absorption properties of the carbon-coated nickel nanocapsules. Appl. Phys. Lett. 2006, 89, 053115. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and tunable high-performance microwave absorption of an ultralight and highly compressible graphene foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Chen, X. Recent progress of nanomaterials for microwave absorption. J. Mater. 2019, 5, 503–541. [Google Scholar] [CrossRef]
- Chambers, B. Optimum design of a Salisbury screen radar absorber. Electron. Lett. 1994, 30, 1353–1354. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z.; Han, Y.; Zhang, T. Electromagnetic wave absorbing properties of multi-walled carbon nanotube/cement composites. Constr. Build. Mater. 2013, 46, 98–103. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Liu, S.; Chen, L.; Guo, J. Microwave absorption properties of one thin sheet employing carbonyl–iron powder and chlorinated polyethylene. J. Magn. Magn. Mater. 2010, 322, 1736–1740. [Google Scholar] [CrossRef]
- Fan, M.; He, Z.; Pang, H. Microwave absorption enhancement of CIP/PANI composites. Synthetic. Met. 2013, 166, 1–6. [Google Scholar] [CrossRef]
- Xu, J.S.; Zhou, W.C.; Luo, F.; Zhu, D.; Su, J.; Jiang, S. Research progress on radar stealth technique and radar absorbing materials. Mater. Rev. 2014, 28, 46–49. [Google Scholar]
- Zhang, X.; He, Y.; Liu, S.; Yan, Q.; Huang, Y. Research progress of MOF derived materials in electromagnetic absorption field. Dev. Appl. Mater. 2020, 35, 11. [Google Scholar]
- Wei, H.; Zhang, Z.; Hussain, G.; Zhou, L.; Li, Q.; Ostrikov, K.K. Techniques to enhance magnetic permeability in microwave absorbing materials. Appl. Mater. Today 2020, 19, 100596. [Google Scholar] [CrossRef]
- Liu, W.; Wei, G.; Ji, G. Construction and electromagnetic properties of lightweight and efficient Radar absorbing materials derived from Fe-MOF. Aeronaut. Sci. Technol. 2021, 32, 8. [Google Scholar]
- Hossain, A.A.; Biswas, T.S.; Mahmud, S.Y.; Yanagida, T.; Tanaka, H.; Kawai, T. Influence of Mg and Cr substitution on structural and magnetic properties of polycrystalline Ni0.50Zn0.50-x-yMgxCryFe2O4. Mater. Chem. Phys. 2009, 113, 172–178. [Google Scholar] [CrossRef]
- Pinho, M.S.; Gregori, M.L.; Nunes, R.C.R.; Soares, B.G. Performance of radar absorbing materials by waveguide measurements for X-and Ku-band frequencies. Eur. Polym. J. 2002, 38, 2321–2327. [Google Scholar] [CrossRef]
- Joseph, N.; Sebastian, M.T. Electromagnetic interference shielding nature of PVDF-carbonyl iron composites. Mater. Lett. 2013, 90, 64–67. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Han, X.; Gao, X.; Liu, P. Metal organic framework (ZIF-67)-derived hollow CoS2/N-doped carbon nanotube composites for extraordinary electromagnetic wave absorption. Compos. Part B Eng. 2019, 163, 67–76. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, F.; Li, Z.; Zheng, C.; Zeng, Y.; Zheng, X.; Luo, M.; Shi, H. Research progress of core-shell electromagnetic wave absorbing materials. Mater. Rep. 2020, 34, 7061–7070. [Google Scholar]
- Cheng, Y.; Li, Z.; Li, Y.; Dai, S.; Ji, G.; Zhao, H.; Cao, J.; Du, Y. Rationally regulating complex dielectric parameters of mesoporous carbon hollow spheres to carry out efficient microwave absorption. Carbon 2018, 127, 643–652. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Kumar, D.; Sinha, G.R.; Moon, A.P. Carbonyl iron powders as absorption material for microwave interference shielding: A review. J. Alloys Compd. 2021, 853, 157251. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, S.M.; Seo, J.M.; Chang, D.W.; Dai, L.; Baek, J.B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Song, C.; Yin, X.; Han, M.; Li, X.; Hou, Z.; Zhang, L.; Chengv, L. Three-dimensional reduced graphene oxide foam modified with ZnO nanowires for enhanced microwave absorption properties. Carbon 2017, 116, 50–58. [Google Scholar] [CrossRef]
- Li, N.; Huang, G.; Xiao, H.; Feng, Q.; Fu, S. Investigations on structure-dependent microwave absorption performance of nano-Fe3O4 coated carbon-based absorbers. Carbon 2019, 144, 216–227. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Zhu, M.; Li, M.; Zhang, H.; Wei, H.; Zhang, L.; Cheng, L. Constructing hollow graphene nano-spheres confined in porous amorphous carbon particles for achieving full X band microwave absorption. Carbon 2019, 142, 346–353. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Ma, L.; Liu, S.; Yu, Z. Microwave absorption properties of a wave-absorbing coating employing carbonyl-iron powder and carbon black. Appl. Surf. Sci. 2010, 257, 842–846. [Google Scholar] [CrossRef]
- Leslie-Pelecky, D.L.; Rieke, R.D. Magnetic properties of nanostructured materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Lu, M.; Xia, G.; Cao, W. Iron removal from kaolin using thiourea dioxide: Effect of ball grinding and mechanism analysis. Appl. Clay. Sci. 2017, 143, 354–361. [Google Scholar] [CrossRef]
- Kholkina, E.; Kumar, N.; Eränen, K.; Peurla, M.; Palonen, H.; Salonen, J.; Murzin, D.Y. Ultrasound irradiation as an effective tool in synthesis of the slag-based catalysts for carboxymethylation. Ultrason. Sonochem. 2021, 73, 105503. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; Napolitano-Tabares, P.I.; Šulc, J.; Pacheco-Fernández, I.; Pino, V. Role of ionic liquids in composites in analytical sample preparation. Separations 2020, 7, 37. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Wu, H.B.; Lou, X.W.D. Metal–organic framework-assisted synthesis of compact Fe2O3 nanotubes in Co3O4 host with enhanced lithium storage properties. Nano-Micro Lett. 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zeng, D.; Cortie, M.B.; Dowd, A.; Guo, H.; Wang, J.; Peng, D.L. Seed-Induced Growth of Flower-Like Au–Ni–ZnO Metal–Semiconductor Hybrid Nanocrystals for Photocatalytic Applications. Small 2015, 11, 1460–1469. [Google Scholar] [CrossRef]
- Mudinepalli, V.R.; Feng, L.; Lin, W.C.; Murty, B.S. Effect of grain size on dielectric and ferroelectric properties of nanostructured Ba0.8Sr0.2TiO3 ceramics. J. Adv. Ceram. 2015, 4, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Rivas, D.F.; Cintas, P.; Gardeniers, H.J. Merging microfluidics and sonochemistry: Towards greener and more efficient micro-sono-reactors. Chem. Commun. 2012, 48, 10935–10947. [Google Scholar] [CrossRef]
- Wu, S.; Zhuang, G.; Wei, J.; Zhuang, Z.; Yu, Y. Shape control of core–shell MOF@MOF and derived MOF nanocages via ion modulation in a one-pot strategy. J. Mater. Chem. A 2018, 6, 18234–18241. [Google Scholar] [CrossRef]
- Zhuang, J.; Chou, L.Y.; Sneed, B.T.; Cao, Y.; Hu, P.; Feng, L.; Tsung, C.K. Surfactant-Mediated Conformal Overgrowth of Core–Shell Metal–Organic Framework Materials with Mismatched Topologies. Small 2015, 11, 5551–5555. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. MOF@MOF: Microporous core–shell architectures. Chem. Commun. 2009, 41, 6162–6164. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, Y.; Yan, Y.; Ding, L.; Liu, P. High-performance electromagnetic wave absorbers based on two kinds of nickel-based MOF-derived Ni@C microspheres. ACS. Appl. Mater. Inter. 2019, 11, 40781–40792. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Xie, W.; Qiu, F.; Zhang, Y.; Song, S.; Dong, L. Enhanced microwave absorption performance of porous and hollow CoNi@C microspheres with controlled component and morphology. J. Alloy. Compd. 2019, 809, 151837. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, A.; Sun, M.; Jiang, W.; Wu, F.; Dong, W. Electromagnetic dissipation on the surface of metal organic framework (MOF)/reduced graphene oxide (RGO) hybrids. Mater. Chem. Phys. 2017, 199, 340–347. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, J.; Liu, C.; Wang, F.; Jiang, Y.; Cui, P.; Liu, J. A MOF-derived ZrO2/C nanocomposite for efficient electromagnetic wave absorption. Inorg. Chem. Front. 2020, 7, 385–393. [Google Scholar] [CrossRef]
- Xiang, Z.; Song, Y.; Xiong, J.; Pan, Z.; Wang, X.; Liu, L.; Lu, W. Enhanced electromagnetic wave absorption of nanoporous Fe3O4@carbon composites derived from metal-organic frameworks. Carbon 2019, 142, 20–31. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, J.; Zhang, X.; Xu, D.; Wu, N.; Lv, L.; Liu, J. Bimetallic MOF-derived porous CoNi/C nanocomposites with ultra-wide band microwave absorption properties. New J. Chem. 2019, 43, 16546–16554. [Google Scholar] [CrossRef]
- Ouyang, J.; He, Z.; Zhang, Y.; Yang, H.; Zhao, Q. Trimetallic FeCoNi@ C nanocomposite hollow spheres derived from metal–organic frameworks with superior electromagnetic wave absorption ability. ACS Appl. Mater. Interfaces 2019, 11, 39304–39314. [Google Scholar] [CrossRef]
- Liao, Q.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Hu, S.; Tong, Y. Highly cuboid-shaped heterobimetallic metal–organic frameworks derived from porous Co/ZnO/C microrods with improved electromagnetic wave absorption capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Q.; Li, S.; Lu, Y.; Jin, S.; Li, K.; Chen, H.; Zhang, H. Metal organic framework (MOF)-derived carbonaceous Co3O4/Co microframes anchored on RGO with enhanced electromagnetic wave absorption performances. Synth Met. 2017, 228, 32–40. [Google Scholar] [CrossRef]
- Chen, X.; Huang, S.; Sun, F.; Lai, N. Modifications of metal and ligand to modulate the oxygen reduction reaction activity of two-dimensional MOF catalysts. J. Phys. Chem. C 2019, 124, 1413–1420. [Google Scholar] [CrossRef]
- Xie, L.S.; Skorupskii, G.; Dinca, M. Electrically conductive metal–organic frameworks. Chem. Rev. 2020, 120, 8536–8580. [Google Scholar] [CrossRef] [Green Version]
- Hmadeh, M.; Lu, Z.; Liu, Z.; Gándara, F.; Furukawa, H.; Wan, S.; Augustyn, V.; Chang, R.; Liao, L.; Zhou, F.; et al. New porous crystals of extended metal-catecholates. Chem. Mater. 2012, 24, 3511–3513. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Bahlakeh, G. Development of metal-organic framework (MOF) decorated graphene oxide nanoplatforms for anti-corrosion epoxy coatings. Carbon 2020, 161, 231–251. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef]
- Glover, T.G.; Peterson, G.W.; Schindler, B.J.; Britt, D.; Yaghi, O. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.; Zhou, H.C. Zirconium-metalloporphyrin PCN-222: Mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yao, Z.; Zhou, J.; Jin, L.; Wei, B.; He, X. Facile synthesis of MOF-derived concave cube nanocomposite by self-templated toward lightweight and wideband microwave absorption. Carbon 2022, 186, 574–588. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, L.; Tang, Y.; Liu, J. Earthworm-like (Co/CoO)@C composite derived from MOF for solving the problem of low-frequency microwave radiation. J. Alloy. Compd. 2021, 881, 160556. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Li, M.; Yang, L.; Liu, J.; Hou, Y.; Yang, Y. Surface architecture of Ni-based metal organic framework hollow spheres for adjustable microwave absorption. ACS. Appl. Nano. Mater. 2019, 2, 7888–7897. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, X.; Liu, C.; Lyu, L.; Yang, Y.; Wang, Z.; Liu, J. Non-magnetic bimetallic MOF-derived porous carbon-wrapped TiO2/ZrTiO4 composites for efficient electromagnetic wave absorption. Nano-Micro Lett. 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M.; Bessarabov, D. Modulated synthesis of zirconium-metal organic framework (Zr-MOF) for hydrogen storage applications. Int. J. Hydrogen. Energ. 2014, 39, 890–895. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, Y.; Chen, X.; Wu, Z.; He, Y.; Zhang, L.; Zou, Y. Porous flower-like Ni/C composites derived from MOFs toward high-performance electromagnetic wave absorption. J. Magn. Magn. Mater. 2019, 487, 165334. [Google Scholar] [CrossRef]
- Yang, K.; Cui, Y.; Wan, L.; Zhang, Q.; Zhang, B. MOF-derived magnetic-dielectric balanced Co@ZnO@ N-doped carbon composite materials for strong microwave absorption. Carbon 2022, 190, 366–375. [Google Scholar] [CrossRef]
- Hen, J.; Zheng, J.; Wang, F.; Huang, Q.; Ji, G. Carbon fibers embedded with FeIII-MOF-5-derived composites for enhanced microwave absorption. Carbon 2021, 174, 509–517. [Google Scholar]
- Ding, J.; Wang, L.; Zhao, Y.; Xing, L.; Yu, X.; Chen, G.; Che, R. Boosted interfacial polarization from multishell TiO2@Fe3O4@PPy heterojunction for enhanced microwave absorption. Small 2019, 15, 1902885. [Google Scholar] [CrossRef]
- Guo, H.; Wang, L.; You, W.; Yang, L.; Li, X.; Chen, G.; Che, R. Engineering phase transformation of MoS2/RGO by N-doping as an excellent microwave absorber. ACS. Appl. Mater. Inter. 2020, 12, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Xu, L.; Zuraiqi, K.; Daeneke, T.; Yao, Z.; Zavabeti, A. Liquid metal derived MOF functionalized nanoarrays with ultra-wideband electromagnetic absorption. J. Colloid. Inter. Sci. 2022, 606, 1852–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, W.; Wang, J.; Zhai, J.; Lei, X.; Zhao, W.; Yan, J. Constructing multiple heterogeneous interfaces in the composite of bimetallic MOF-derivatives and rGO for excellent microwave absorption performance. Carbon 2021, 173, 1059–1072. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guan, B.Y.; Lou, X.W. Co-Fe alloy/N-doped carbon hollow spheres derived from dual metal–organic frameworks for enhanced electrocatalytic oxygen reduction. Small 2019, 15, 1805324. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Z.; Zhang, F.; Xia, Z.; Zou, J.; Gu, Z.; Wu, G. MOF-derived NiFe2S4/porous carbon composites as electromagnetic wave absorber. J. Colloid. Inter. Sci. 2022, 610, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, A.; Chen, L.; Jia, Q.; Zhou, C.; Liu, J.; Wang, X. A novel multi-cavity structured MOF derivative/porous graphene hybrid for high performance microwave absorption. Carbon 2021, 176, 279–289. [Google Scholar] [CrossRef]
- Tao, J.; Jiao, Z.; Xu, L.; Yi, P.; Yao, Z.; Yang, F.; Li, Z. Construction of MOF-Derived Co/C shell on carbon fiber surface to enhance multi-polarization effect towards efficient broadband electromagnetic wave absorption. Carbon 2021, 184, 571–582. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Liu, Z.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of binary MOF-derived hybrid nanoflowers via selective assembly and their microwave absorbing properties. Carbon 2021, 182, 484–496. [Google Scholar] [CrossRef]
- Wu, F.; Wan, L.; Li, Q.; Zhang, Q.; Zhang, B. Ternary assembled MOF-derived composite: Anisotropic epitaxial growth and microwave absorption. Compos. Part B-Eng. 2022, 236, 109839. [Google Scholar] [CrossRef]
- Zhu, H.; Jiao, Q.; Fu, R.; Su, P.; Yang, C.; Feng, C.; Zhao, Y. Cu/NC@Co/NC composites derived from core-shell Cu-MOF@Co-MOF and their electromagnetic wave absorption properties. J. Colloid. Inter. Sci. 2022, 613, 182–193. [Google Scholar] [CrossRef]
- Peng, H.; Xiong, Z.; Gan, Z.; Liu, C.; Xie, Y. Microcapsule MOFs@MOFs derived porous “nut-bread” composites with broadband microwave absorption. Compos. Part B-Eng. 2021, 224, 109170. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, Z.; Wang, Z.; Peng, Y.; Xia, L.; Ma, S.; Lin, Z.; Huang, Y. A review on metal–organic framework-derived porous carbon-based novel microwave absorption materials. Nano-Micro Lett. 2021, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, S.; Ma, B.; Xiong, Y.; Luo, H.; Cheng, Y.; Gong, R. Bimetallic CoFe-MOF@ Ti3C2Tx MXene derived composites for broadband microwave absorption. Chem. Eng. J. 2022, 431, 134007. [Google Scholar] [CrossRef]
- Li, S.; Lin, L.; Yao, L.; Zheng, H.; Luo, Q.; Xu, W.; Zhang, C.; Xie, Q.; Wang, L.S.; Peng, D.L. MOFs-derived Co-C@ C hollow composites with high-performance electromagnetic wave absorption. J. Alloy. Compd. 2021, 856, 158183. [Google Scholar] [CrossRef]
- Rösler, C.; Aijaz, A.; Turner, S.; Filippousi, M.; Shahabi, A.; Xia, W.; Fischer, R.A. Hollow Zn/Co zeolitic imidazolate framework (ZIF) and yolk–shell metal@Zn/Co ZIF nanostructures. Chem.-Eur. J. 2016, 22, 3304–3311. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Z.; Li, X.; Ren, J.; Wang, Y.; Zhang, Q.; Zhang, B. MOF-derived yolk-shell Co@ZnO/Ni@NC nanocage: Structure control and electromagnetic wave absorption performance. J. Colloid Interf. Sci. 2021, 600, 99–110. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Li, X.; Zhang, J.; Wang, M.; Che, R. MOF-derived yolk-shell Ni@C@ZnO Schottky contact structure for enhanced microwave absorption. Chem. Eng. J. 2020, 383, 123099. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef]
- Jacobs, B.W.; Houk, R.J.; Anstey, M.R.; House, S.D.; Robertson, I.M.; Talin, A.A.; Allendorf, M.D. Ordered metal nanostructure self-assembly using metal–organic frameworks as templates. Chem. Sci. 2011, 2, 411–416. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Feng, A.; Zhang, N.; Jia, Z.; Huang, Z.; Wu, G. Mesoporous carbon hollow microspheres with tunable pore size and shell thickness as efficient electromagnetic wave absorbers. Compos. Part B Eng. 2019, 167, 690–699. [Google Scholar] [CrossRef]
- Liu, W.; Tan, S.; Yang, Z.; Ji, G. Enhanced low-frequency electromagnetic properties of MOF-derived cobalt through interface design. ACS Appl. Mater. Interfaces 2018, 10, 31610–31622. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yi, P.; Wan, L.; Hou, J.; Chen, P.; Zu, J.; Zhou, J. Thickness-controllable synthesis of MOF-derived Ni@N-doped carbon hexagonal nanoflakes with dielectric-magnetic synergy toward wideband electromagnetic wave absorption. Chem. Eng. J. 2022, 427, 130940. [Google Scholar] [CrossRef]
- Kong, M.; Liu, X.; Jia, Z.; Wang, B.; Wu, X.; Wu, G. Porous magnetic carbon CoFe alloys@ZnO@C composites based on Zn/Co-based bimetallic MOF with efficient electromagnetic wave absorption. J. Colloid Interf. Sci. 2021, 604, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ling, M.; Wan, L.; Liu, P.; Wang, Y.; Zhang, Q.; Zhang, B. Three-dimensional FeMZn (M=Co or Ni) MOFs: Ions coordinated self-assembling processes and boosting microwave absorption. Chem. Eng. J. 2022, 435, 134905. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Pei, K.; You, W.; Yu, X.; Wu, Z.; Che, R. Multidimension-Controllable Synthesis of MOF-Derived Co@N-Doped Carbon Composite with Magnetic-Dielectric Synergy toward Strong Microwave Absorption. Small 2020, 16, 2000158. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted Applications: A review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Du, Q.; Rao, R.; Bi, F.; Yang, Y.; Zhang, W.; Yang, Y.; Zhang, W.; Yang, Y.; Liu, N.; Zhang, X. Preparation of modified zirconium-based metal-organic frameworks (Zr-MOFs) supported metals and recent application in environment: A review and perspectives. Surf. Interfaces 2022, 28, 101647. [Google Scholar] [CrossRef]
- Guo, C.; Duan, F.; Zhang, S.; He, L.; Wang, M.; Chen, J.; Zhang, J.; Jia, Q.; Zhang, Z.; Du, M. Heterostructured hybrids of metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs). J. Mater. Chem. A 2022, 10, 475–507. [Google Scholar] [CrossRef]
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of various dimensional metal organic frameworks (MOFs) and their hybrid composites for emerging applications—A review. Chemosphere 2022, 298, 134184. [Google Scholar] [CrossRef]
- Hou, W.; Liao, Q.; Xie, S.; Song, Y.; Qin, L. Prospects and Challenges of Flexible Stretchable Electrodes for Electronics. Coatings 2022, 12, 558. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, P.; Wang, J.; Feng, X.; Dai, J. Low-frequency microwave absorption of MOF-derived Co/CoO/SrCO3@C composites. Mater. Chem. Phys. 2021, 264, 124457. [Google Scholar] [CrossRef]
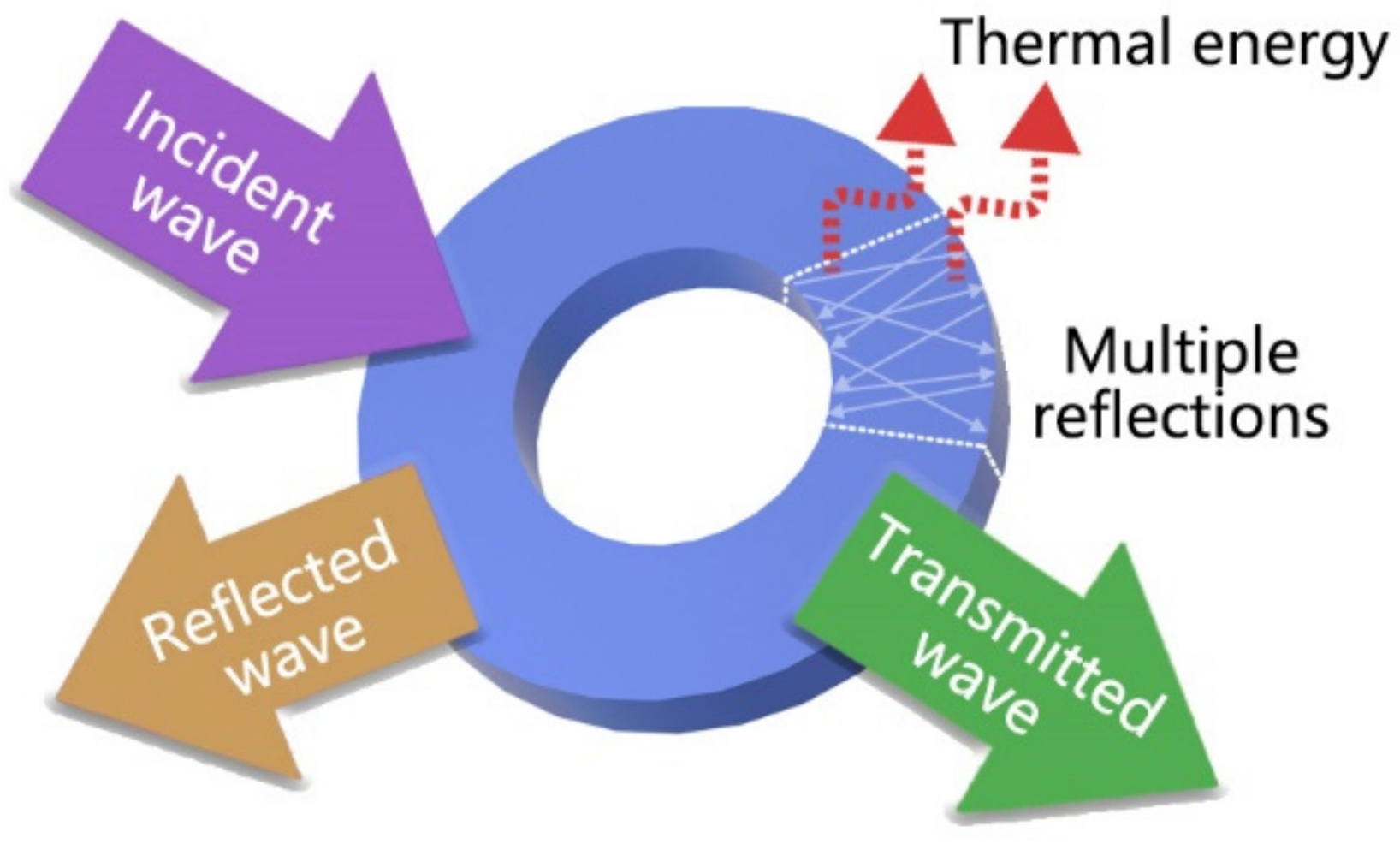
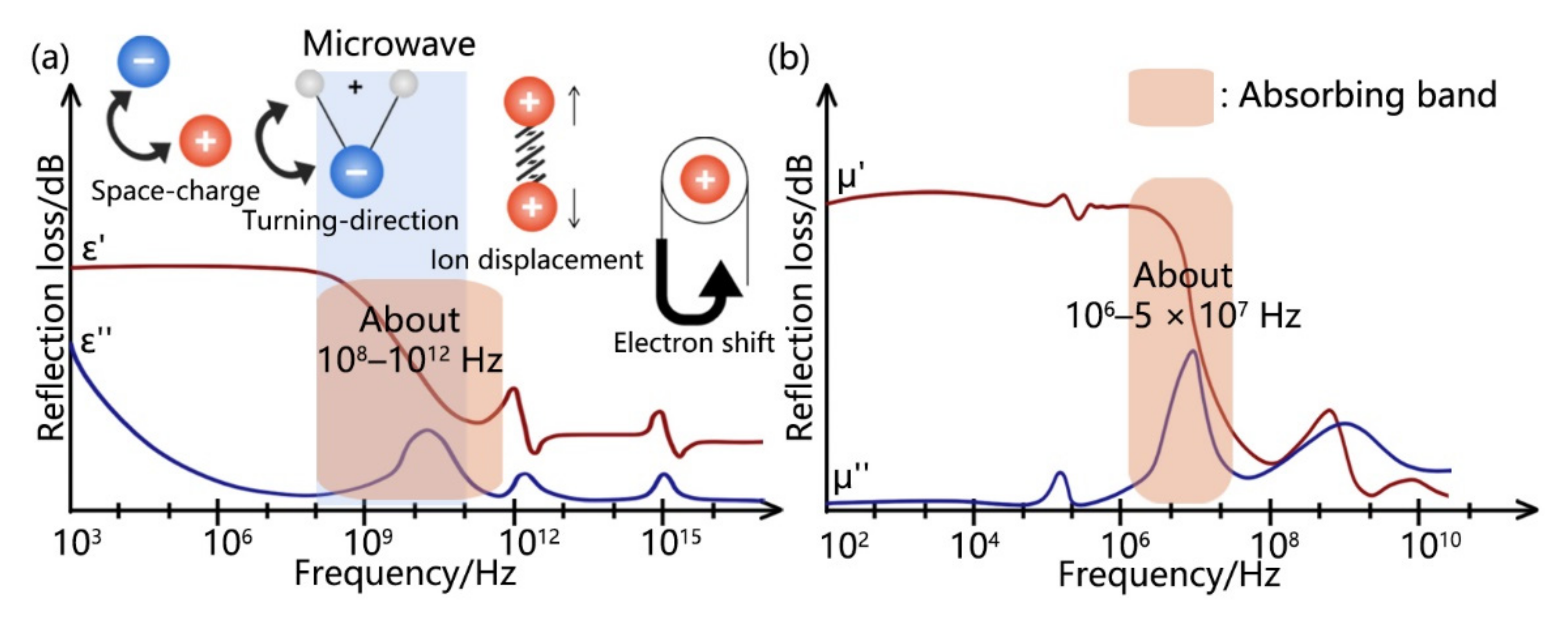
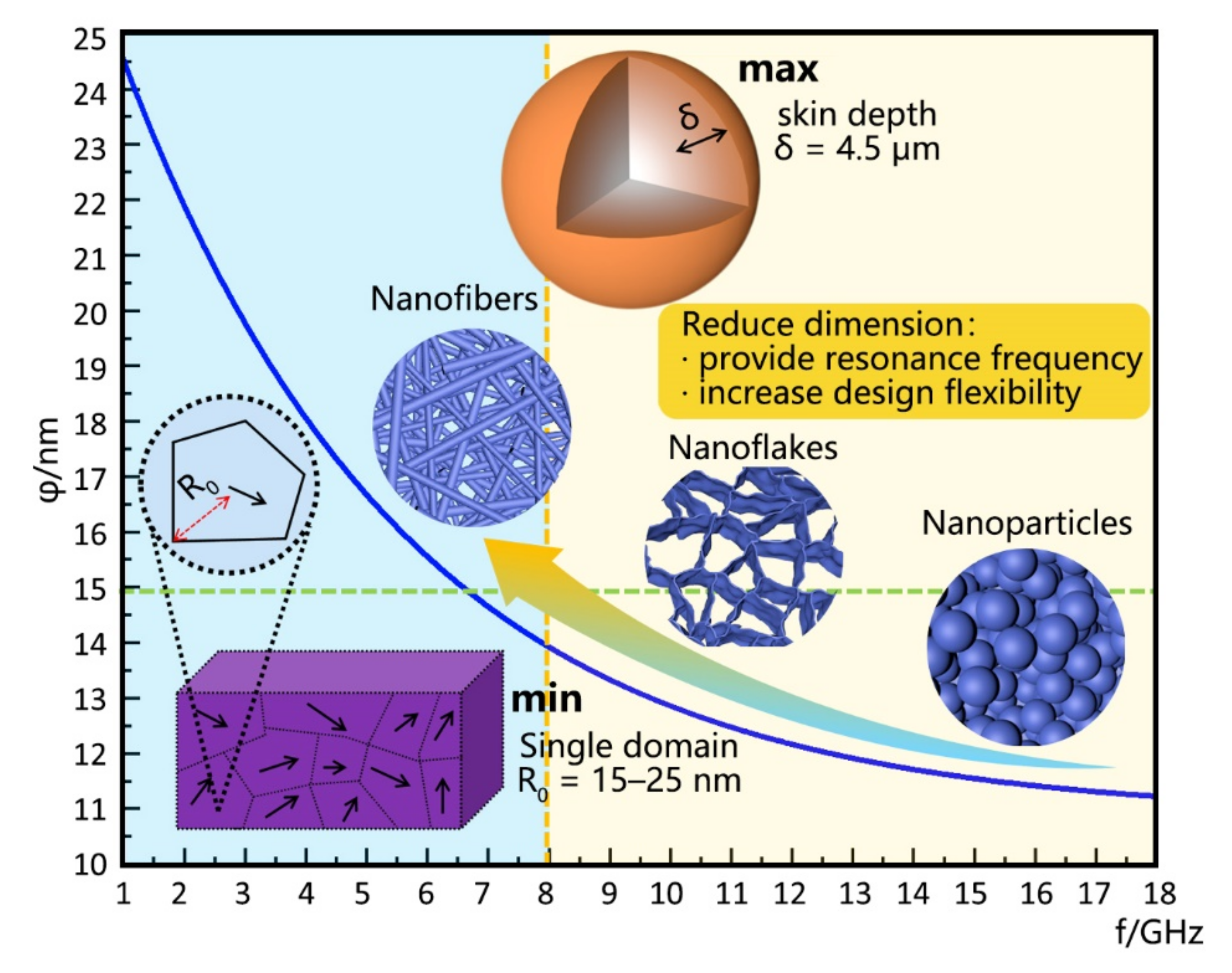


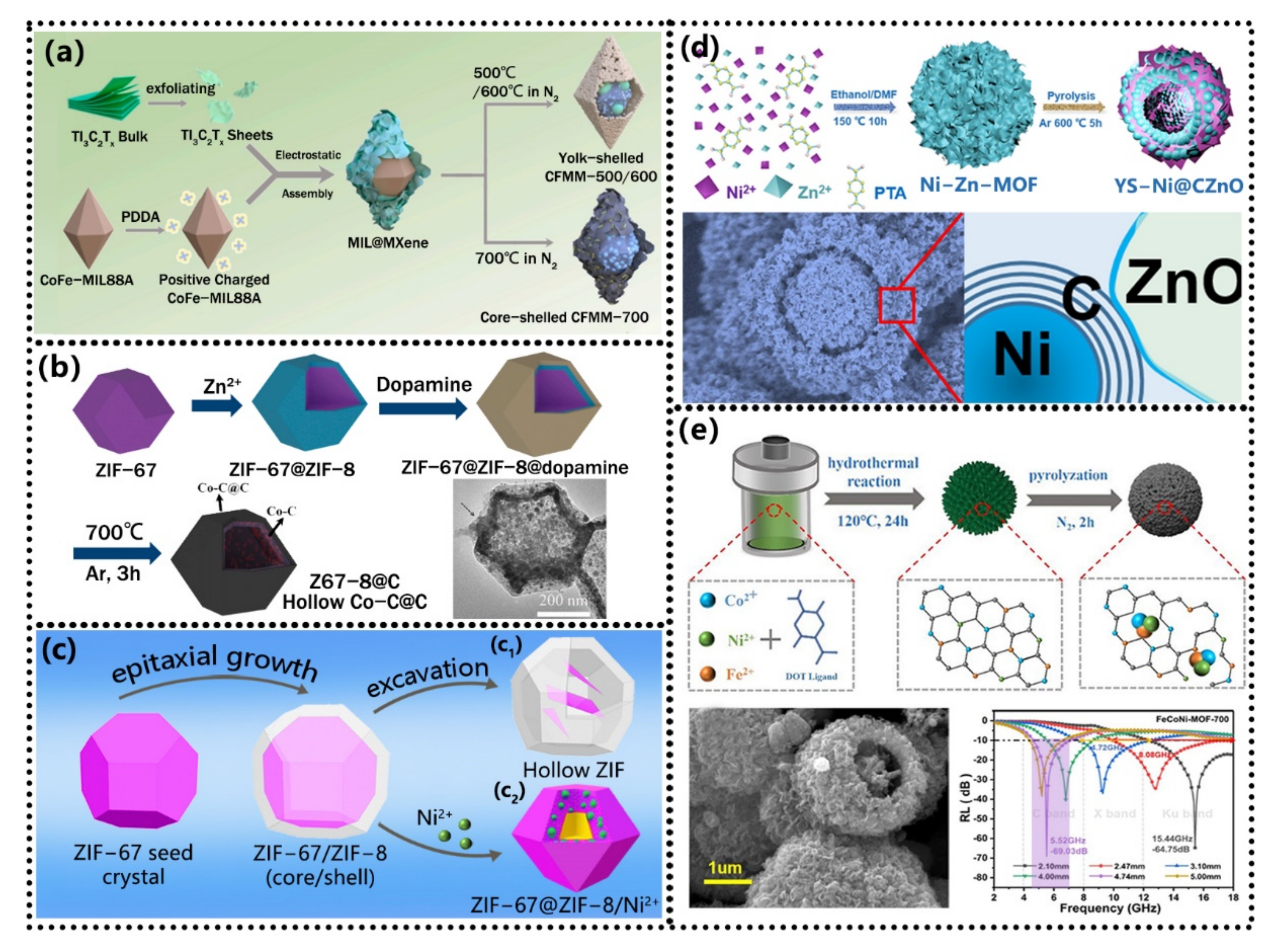
| Type | Mechanism (Main) | Materials | Disadvantage |
|---|---|---|---|
| dielectric loss | polarization process of electrons, ions, and interfaces. | barium carbonate electric ceramics, silicon nitride, manganese dioxide, etc. | large matching thickness [35,36]. |
| magnetic loss | domain wall resonance, natural resonance, and eddy current loss. | ferrite, carbonyl iron powder (CIP) magnetic metal powder, etc. [37] | prone to oxidize, high density, low saturation magnetization. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, W.; Liao, Q.; Hou, W.; Chen, L.; Li, X.; Zhang, Z.; Sun, M.; Song, Y.; Qin, L. Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties. Coatings 2022, 12, 766. https://doi.org/10.3390/coatings12060766
Si W, Liao Q, Hou W, Chen L, Li X, Zhang Z, Sun M, Song Y, Qin L. Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties. Coatings. 2022; 12(6):766. https://doi.org/10.3390/coatings12060766
Chicago/Turabian StyleSi, Wei, Qingwei Liao, Wei Hou, Liyin Chen, Xiaolu Li, Zhiwei Zhang, Minna Sun, Yujun Song, and Lei Qin. 2022. "Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties" Coatings 12, no. 6: 766. https://doi.org/10.3390/coatings12060766
APA StyleSi, W., Liao, Q., Hou, W., Chen, L., Li, X., Zhang, Z., Sun, M., Song, Y., & Qin, L. (2022). Low-Frequency Broadband Absorbing Coatings Based on MOFs: Design, Fabrication, Microstructure and Properties. Coatings, 12(6), 766. https://doi.org/10.3390/coatings12060766








