Facile Fabrication of F-Doped SnO2 Nanomaterials for Improved Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental Part
2.1. Instruments and Reagents
2.2. Preparation of F-doped SnO2
2.3. Characterization
2.4. Photocatalytic Study
3. Results and Discussion
3.1. Crystal Structure
3.2. Functional Group Analysis
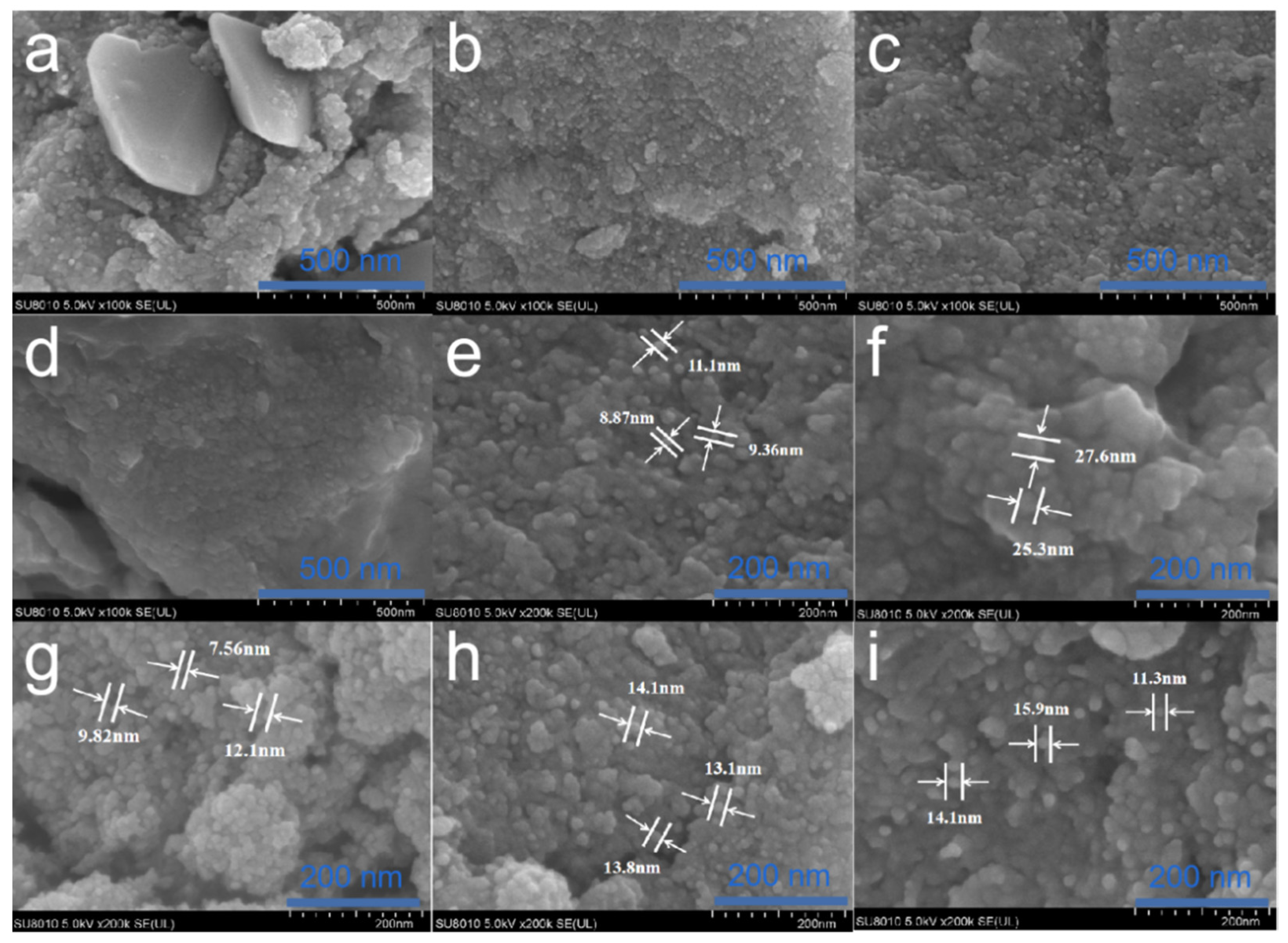
3.3. Morphology Analysis
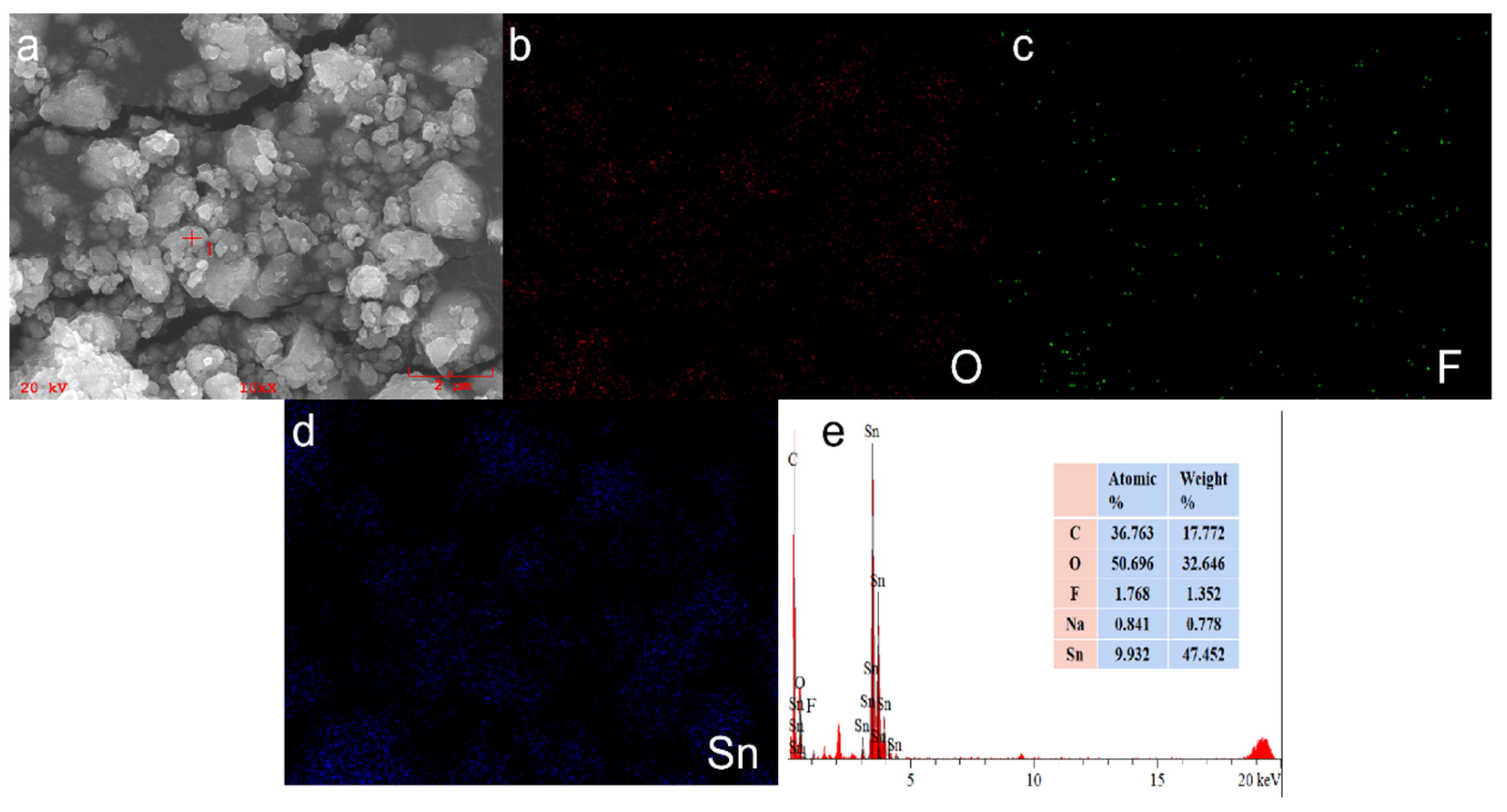
3.4. Elemental Analysis
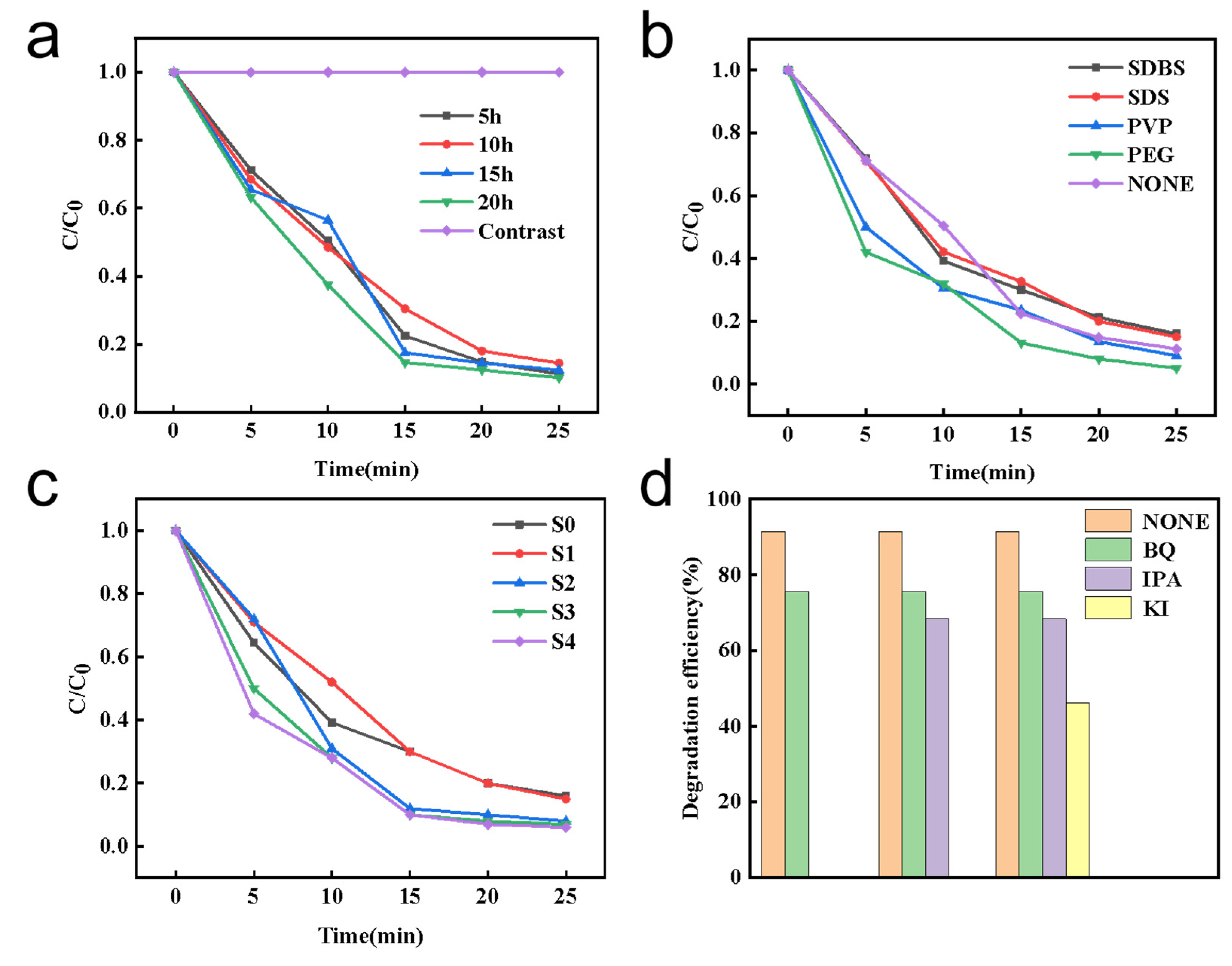
3.5. Photocatalytic Activity
3.6. Free Radical Capture Experiment
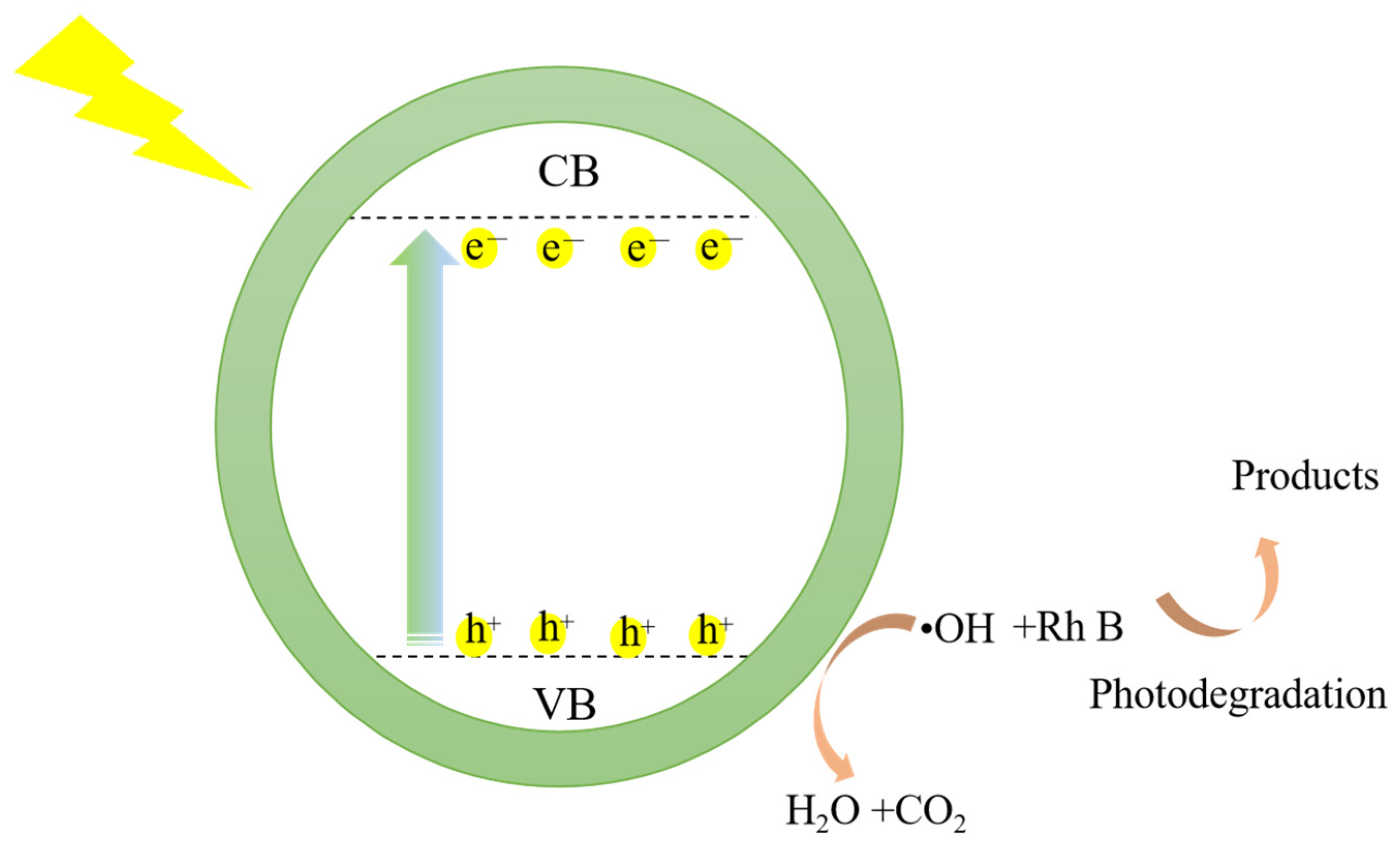
3.7. The Mechanism of the Rh B Degradation
3.8. Comparison of the Photocatalytic Degradation Efficiency of SnO2 Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Gao, J.; Chen, Z. A solid-state chemical reduction approach to synthesize graphitic carbon nitride with tunable nitrogen defects for efficient visible-light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2018, 535, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Min, Z.; Luo, Y.; Yin, H.; Liu, X. A Bi2WO6-based hybrid heterostructures photocatalyst with enhanced photodecomposition and photocatalytic hydrogen evolution through Z-scheme process–ScienceDirect. J. Ind. Eng. Chem. 2019, 69, 345–357. [Google Scholar] [CrossRef]
- Humayun, M.; Lei, X.; Ling, Z.; Zheng, Z.; Fu, Q.; Wei, L. Exceptional co-catalyst free photocatalytic activities of B and Fe co-doped SrTiO3 for CO2 conversion and H2 evolution. Nano Res. 2018, 11, 6391–6404. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Liu, J.; Wang, Z.; Chen, S.; Fu, X. Photocatalytic degradation of benzene over different morphology BiPO4: Revealing the significant contribution of high–energy facets and oxygen vacancies. Appl. Catalysis. B Environ. 2019, 243, 780–789. [Google Scholar] [CrossRef]
- Yang, L.; Huang, J.; Shi, L.; Cao, L.; Zhou, W.; Chang, K.; Ye, J. Efficient hydrogen evolution over Sb doped SnO2 photocatalyst sensitized by Eosin Y under visible light irradiation. Nano Energy 2017, 36, 331–340. [Google Scholar] [CrossRef]
- Lu, C.; Wang, J.; Xu, F.; Wang, A.; Meng, D. Zn-doped SnO2 hierarchical structures formed by a hydrothermal route with remarkably enhanced photocatalytic performance. Ceram. Int. 2018, 44, 15145–15152. [Google Scholar] [CrossRef]
- Cong, H.-P.; Xin, S.; Yu, S.-H. Flexible nitrogen-doped graphene/SnO2 foams promise kinetically stable lithium storage. Nano Energy 2015, 13, 482–490. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, Y.; Liu, X.; Chueh, C.-C.; Yang, S.; Jen, A.K.-Y. Enhanced efficiency and stability of inverted perovskite solar cells using highly crystalline SnO2 nanocrystals as the robust electron-transporting layer. Adv. Mater. 2016, 28, 6478–6484. [Google Scholar] [CrossRef]
- Singkammo, S.; Wisitsoraat, A.; Sriprachuabwong, C.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. Electrolytically exfoliated graphene-loaded flame-made Ni-doped SnO2 composite film for acetone sensing. ACS Appl. Mater. Interfaces 2015, 7, 3077–3092. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Wang, Q.; Han, X.; Li, X.; Liu, J.; Lin, H.; Qu, F. The synthesis of ZnO/SnO2 porous nanofibers for dye adsorption and degradation. Dalton Trans. 2014, 44, 3034–3042. [Google Scholar] [CrossRef] [Green Version]
- Babu, B.; Kadam, A.; Ravikumar, R.; Byon, C. Enhanced visible light photocatalytic activity of Cu-doped SnO2 quantum dots by solution combustion synthesis. J. Alloy. Compd. 2017, 703, 330–336. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, Z.; Yang, L.; Wang, S.; Liu, H.; Feng, Y.; Zhao, Y.; Feng, F. A simple method for synthesis of highly efficient flower-like SnO2 photocatalyst nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 30, 50–55. [Google Scholar] [CrossRef]
- Hou, L.; Lian, L.; Zhou, L.; Zhang, L.; Yuan, C. Interfacial hydrothermal synthesis of SnO2 nanorods towards photocatalytic degradation of methyl orange. Mater. Res. Bull. 2014, 60, 1–4. [Google Scholar] [CrossRef]
- Nouri, A.; Fakhri, A. Synthesis, characterization and photocatalytic applications of N-, S-, and C-doped SnO2 nanoparticles under ultraviolet (UV) light illumination. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 138, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Ansari, S.G.; Foaud, H.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]
- Tuan, P.V.; Trung, H.L.; Chu, H.; Hoang, T.B.; Khiem, T.N. Effects of annealing temperature on the structure, morphology, and photocatalytic properties of SnO2/rGO nanocomposites. Nanotechnology 2020, 32, 15201. [Google Scholar] [CrossRef]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; De Stefano, L.; Vaiano, V. F-doped ZnO nano- and meso-crystals with enhanced photocatalytic activity in diclofenac degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Chen, B.; Ye, F.; Duan, H.; Cui, H. Facile fabrication of N-doped carbon quantum dots modified SnO2 composites for improved visible light photocatalytic activity. Vacuum 2021, 191, 110371. [Google Scholar] [CrossRef]
- Ramakrishna, D.; Rajkumar, B.; Reddy, G.B.; Veerabhadram, G. Construction of in situ self-assembled FeWO4/g-C3N4 nanosheet heterostructured Z-scheme photocatalysts for enhanced photocatalytic degradation of rhodamine B and tetracycline. Nanoscale Adv. 2019, 1, 10. [Google Scholar]
- Janani, R.; Menon, S.S.; Bhalerao, G.; Gupta, B.; Singh, S. Zn1-xGaxO1-yNy—Graphene oxide nanocomposite for enhanced visible—Light photocatalytic activity. Dye. Pigment. 2019, 165, 249–255. [Google Scholar] [CrossRef]
- Sudhaparimala, S. Green synthesis of tin based nano medicine: Assessment of microstructure and surface property. Am. J. Nanosci. Nanotechnol. 2014, 2, 75. [Google Scholar] [CrossRef] [Green Version]
- Haritha, E.; Roopan, S.M.; Madhavi, G.; Elango, G.; Al-Dhabi, N.A.; Arasu, M.V. Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol. B Biol. 2016, 162, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Kumaran, S.M.; Kumar, S.S.; Muthuraja, S.; Roopan, S.M. Green synthesis of SnO2 nanoparticles and its photocatalytic activity of phenolsulfonphthalein dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, M.; Golmohammadi, M.; Naeimi, A. Biosynthesis of tin oxide (SnO2) nanoparticles using jujube fruit for photocatalytic degradation of organic dyes. Adv. Powder Technol. 2019, 30, 1551–1557. [Google Scholar] [CrossRef]
- Chen, X.; Chu, D.; Wang, L.; Hu, W.; Yang, H.; Sun, J.; Zhu, S.; Wang, G.; Tao, J.; Zhang, S. One-step synthesis of novel hierarchical flower-like SnO2 nanostructures with enhanced photocatalytic activity. J. Alloy. Compd. 2017, 729, 710–715. [Google Scholar] [CrossRef]
- Gui-Bing, H.; Chun-Jie, J. Biosynthesis of SnO nanoparticles based on response surface methodology and the study of their dye removal. J. Nanosci. Nanotechnol. 2018, 18, 5020. [Google Scholar]
- Zhang, W.-N.; Zuo, H.-J.; Wang, N.-P.; Gao, Y. Preparation and photocatalytic properties of SnO2 nanomicrospheres. Main Group Chem. 2016, 15, 295–299. [Google Scholar] [CrossRef]
- Luque, P.; Garrafa-Gálvez, H.; Nava, O.; Olivas, A.; Martínez-Rosas, M.; Vilchis-Nestor, A.; Villegas-Fuentes, A.; Chinchillas-Chinchillas, M. Efficient sunlight and UV photocatalytic degradation of Methyl Orange, Methylene Blue and Rhodamine B, using Citrus×paradisi synthesized SnO2 semiconductor nanoparticles. Ceram. Int. 2021, 47, 23861–23874. [Google Scholar] [CrossRef]

| Conditions | Reagent | Time | Surfactant |
|---|---|---|---|
| Different solvent–thermal times | 48 mL oleic acid; 32 mL lauryl amine; 1.4 g SnCl4·5H2O | 5 h | None |
| 10 h | |||
| 15 h | |||
| 20 h | |||
| Different surfactants | 48 mL oleic acid; 32 mL lauryl amine; 1.4 g SnCl4·5H2O | 5 h | 1 mmol PEG (6000) |
| 1 mmol PVP | |||
| 1 mmol SDBS | |||
| 1 mmol SDS |
| Samples | SnCl4·5H2O (g) | NaF (g) | n(F)/n(Sn) | F (wt.%) |
|---|---|---|---|---|
| S0 | 1.4 | 0.0000 | 0:1 | 0.00 |
| S1 | 1.4 | 0.1680 | 1:1 | 12.70 |
| S2 | 1.4 | 0.0336 | 1:5 | 2.50 |
| S3 | 1.4 | 0.0168 | 1:10 | 1.70 |
| S4 | 1.4 | 0.0112 | 1:15 | 0.84 |
| Year | Sample | Pollutant | Degradation/Time | Reference |
|---|---|---|---|---|
| 2015 | SnO2 | Phenolsulfonphthalein | 100% (120 min) | [23] |
| 2019 | SnO2 | Rh B | 95% (90 min) | [12] |
| 2019 | SnO2 | MB | 90% (5 h) | [24] |
| 2017 | SnO2 | Rh B | 93.6% (120 min) | [25] |
| 2018 | SnO2 | Malachite green | 90% (60 min) | [26] |
| 2016 | SnO2 | MB | 97.1% (200 min) | [27] |
| 2021 | SnO2 | Rh B | 100% (60 min) | [28] |
| 2021 | SnO2/NCQDs | Rh B | 99% (120 min) | [18] |
| 2022 | F doped SnO2 | Rh B | 92.9% (25 min) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Liao, R.; Yang, S.; Qiu, Y.; Wang, M.; Zhang, Z.; Du, J.; Xie, Z. Facile Fabrication of F-Doped SnO2 Nanomaterials for Improved Photocatalytic Activity. Coatings 2022, 12, 795. https://doi.org/10.3390/coatings12060795
Xiao L, Liao R, Yang S, Qiu Y, Wang M, Zhang Z, Du J, Xie Z. Facile Fabrication of F-Doped SnO2 Nanomaterials for Improved Photocatalytic Activity. Coatings. 2022; 12(6):795. https://doi.org/10.3390/coatings12060795
Chicago/Turabian StyleXiao, Linfeng, Runhua Liao, Shu Yang, Yang Qiu, Meng Wang, Zheng Zhang, Jie Du, and Zhixiang Xie. 2022. "Facile Fabrication of F-Doped SnO2 Nanomaterials for Improved Photocatalytic Activity" Coatings 12, no. 6: 795. https://doi.org/10.3390/coatings12060795
APA StyleXiao, L., Liao, R., Yang, S., Qiu, Y., Wang, M., Zhang, Z., Du, J., & Xie, Z. (2022). Facile Fabrication of F-Doped SnO2 Nanomaterials for Improved Photocatalytic Activity. Coatings, 12(6), 795. https://doi.org/10.3390/coatings12060795







