Self-Lubricating Property of TiB2-Ni Coating in the Hot Forging Die of Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Procedures
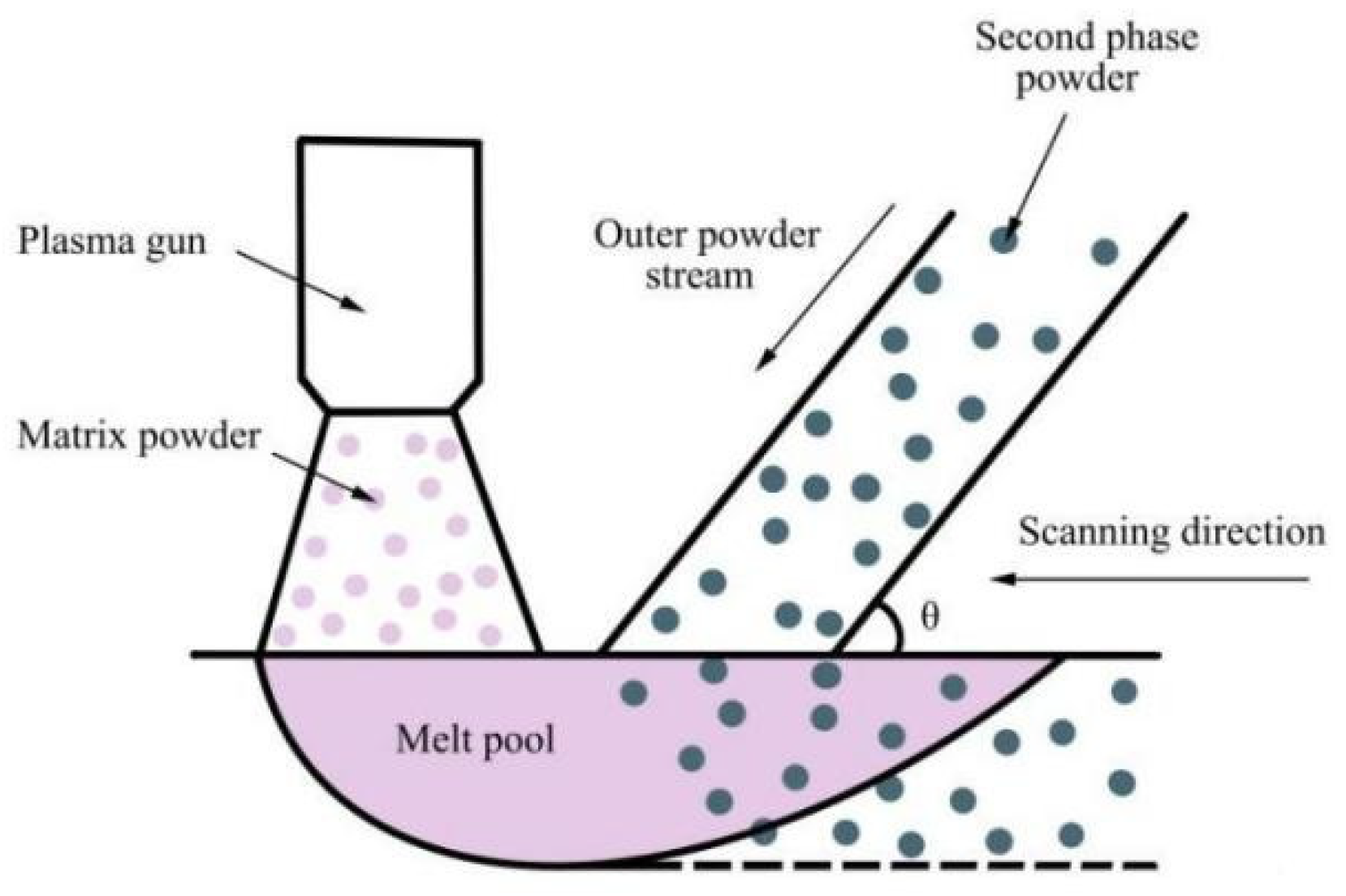
2.1. Materials and Methods
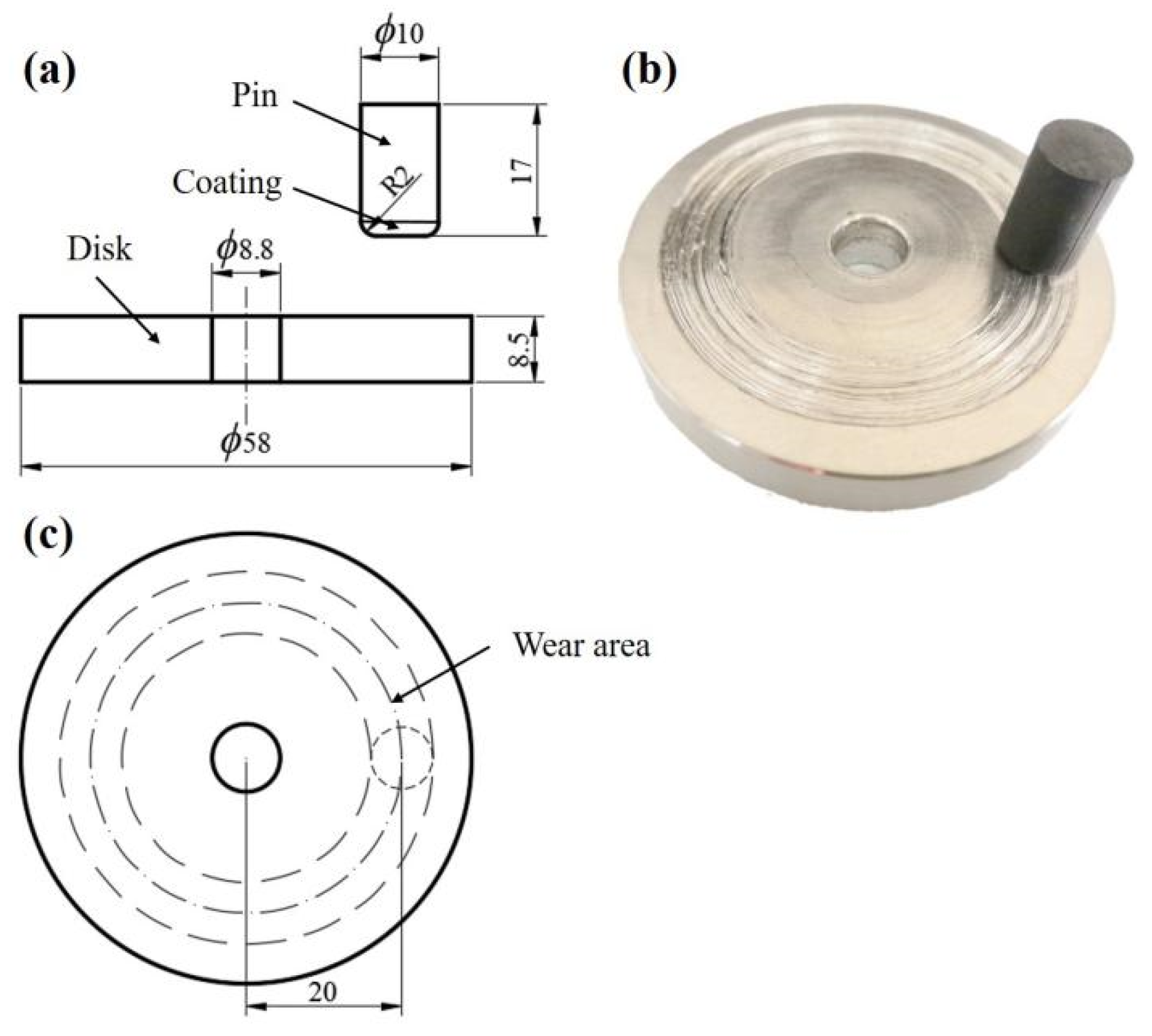
2.2. Characterization
3. Results and Discussion
3.1. Composite Coating
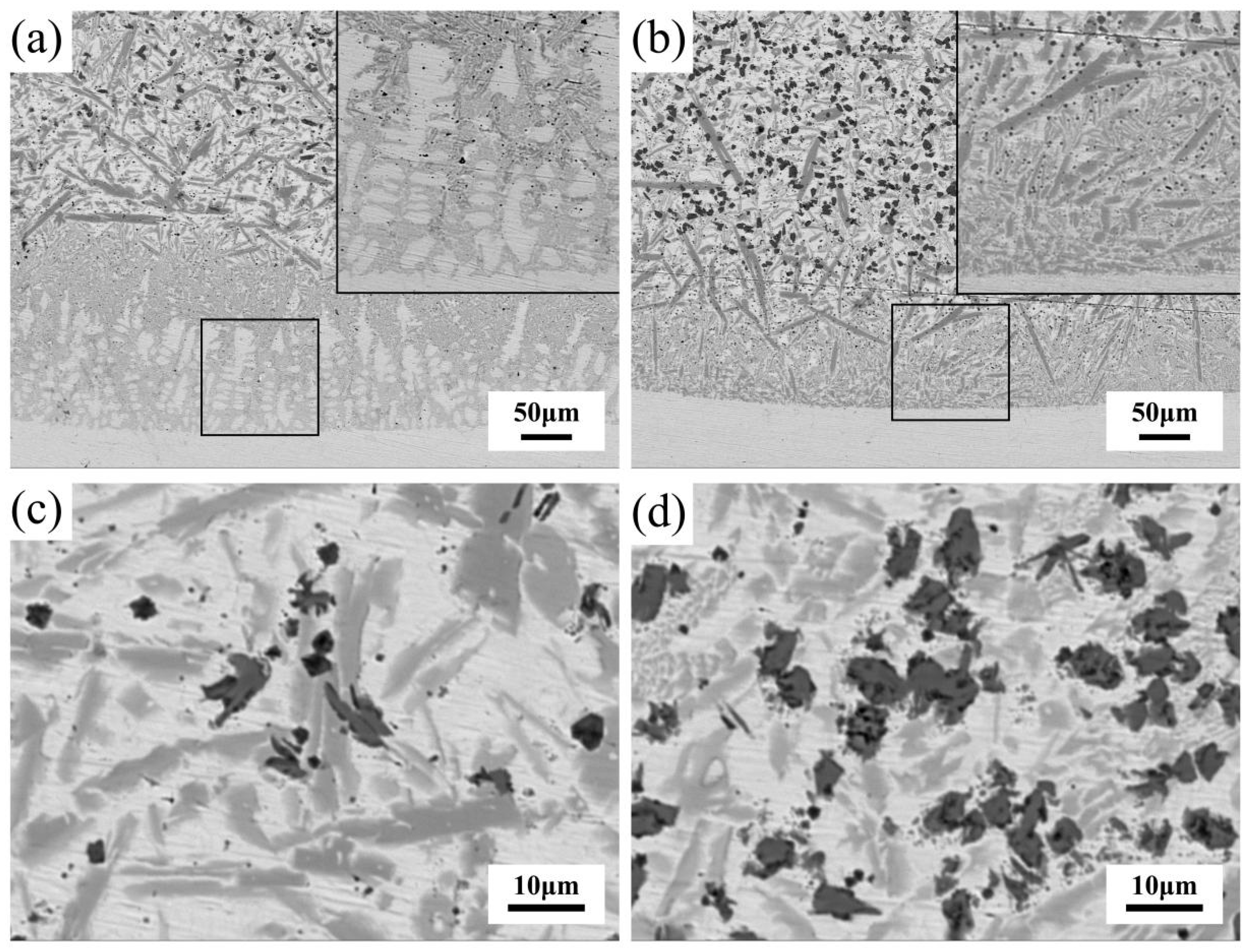
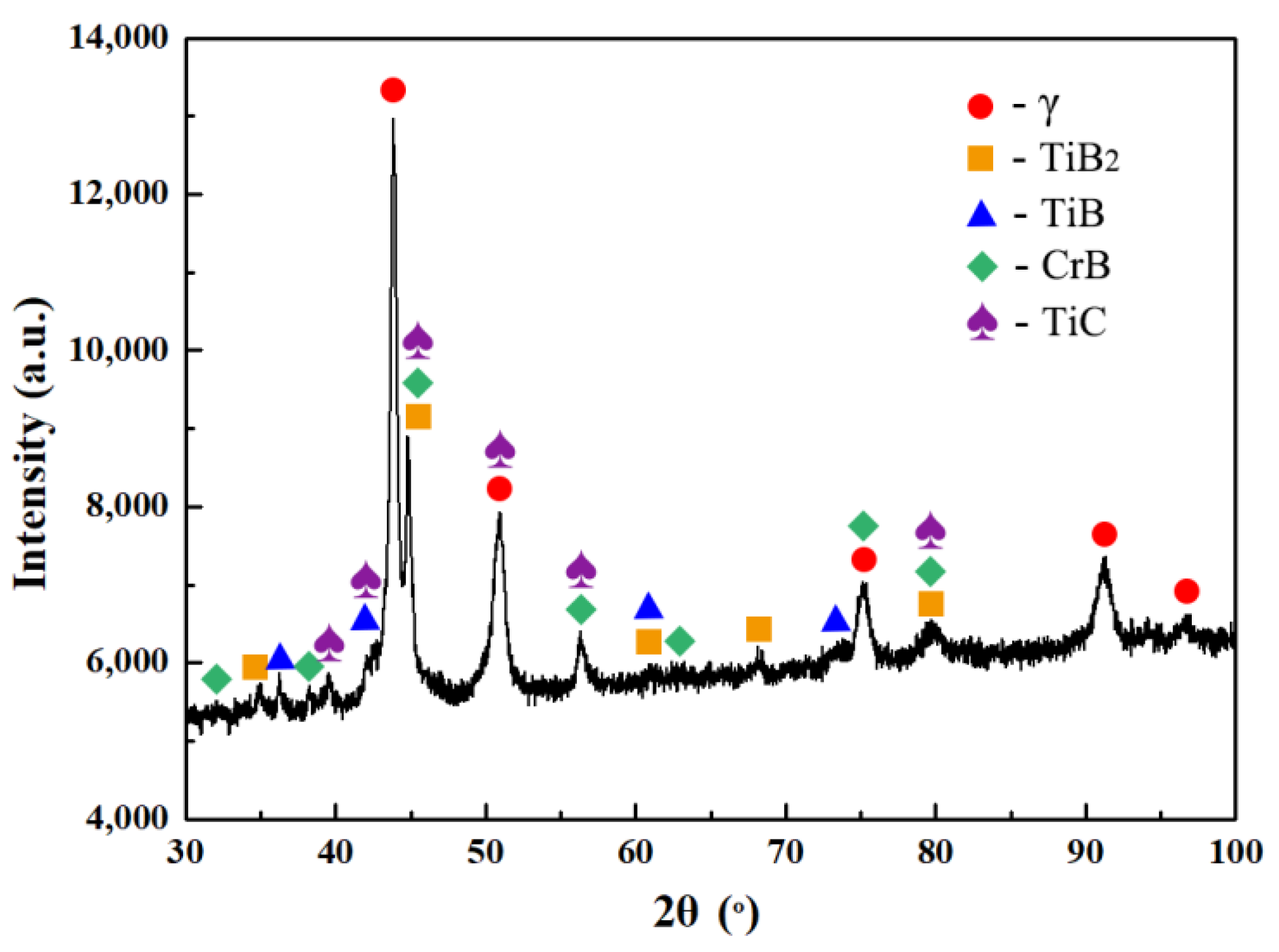
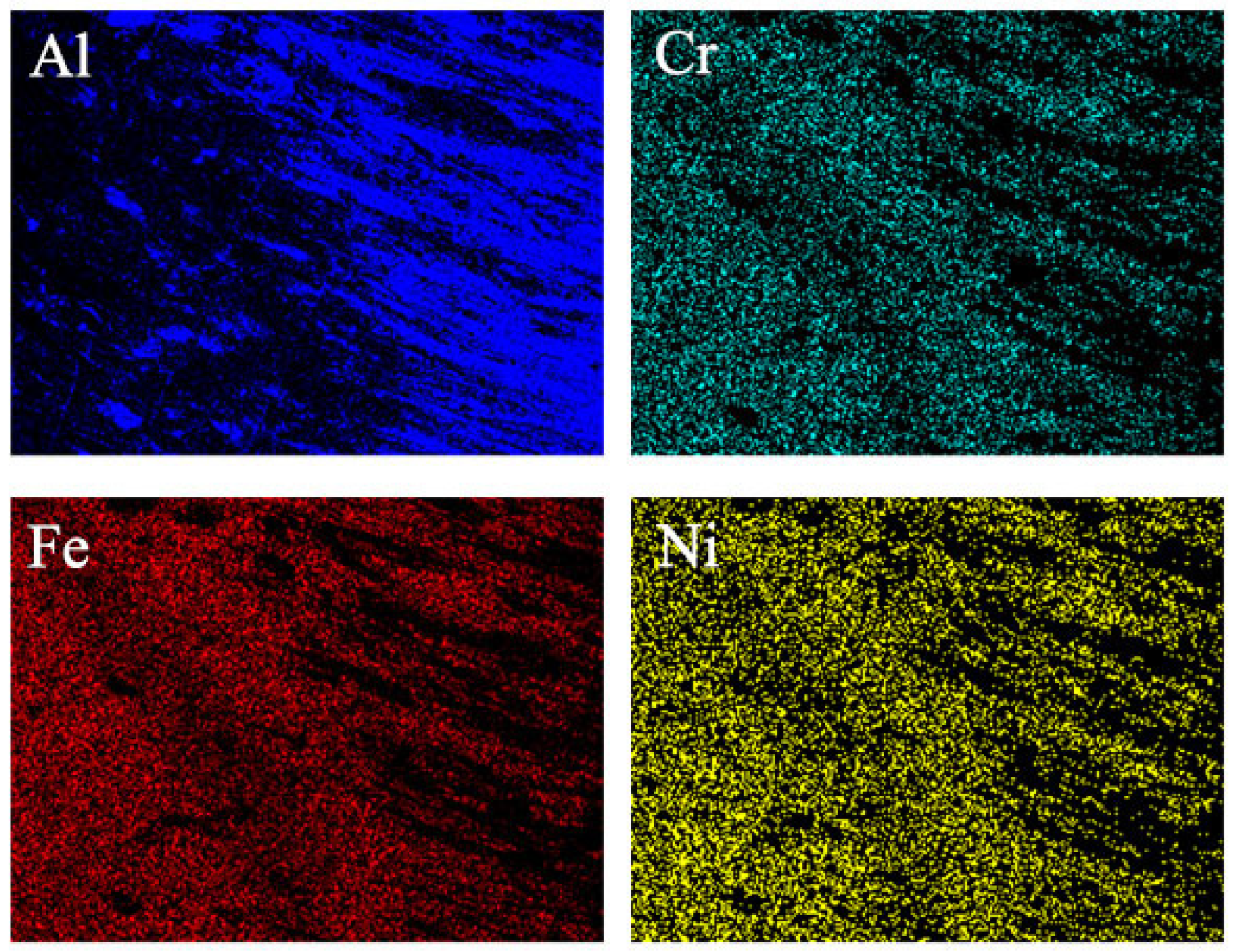
3.1.1. Microstructure and Composition
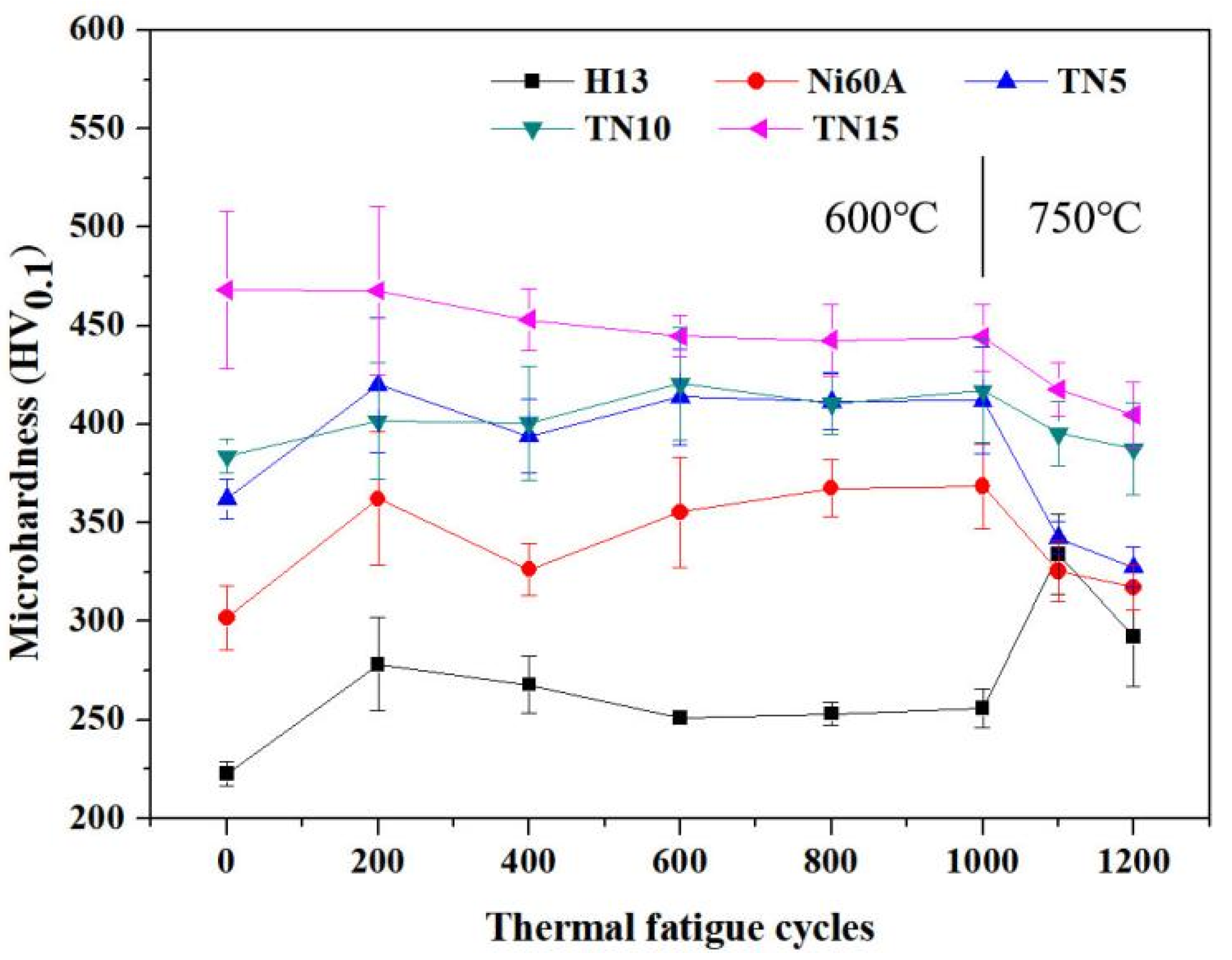
3.1.2. Microhardness
3.2. Thermal Fatigue Property
3.3. Wear Behavior and Adhesion
4. Conclusions
- (1)
- TiB2 was beneficial to the hardness of the Ni-based composite coating. The hardness of the nickel-based composite coatings increased by 15.61%, 27.29%, and 47.04% compared with the normal nickel-based coating when the content of TiB2 was 5, 10, and 15 wt.%, respectively.
- (2)
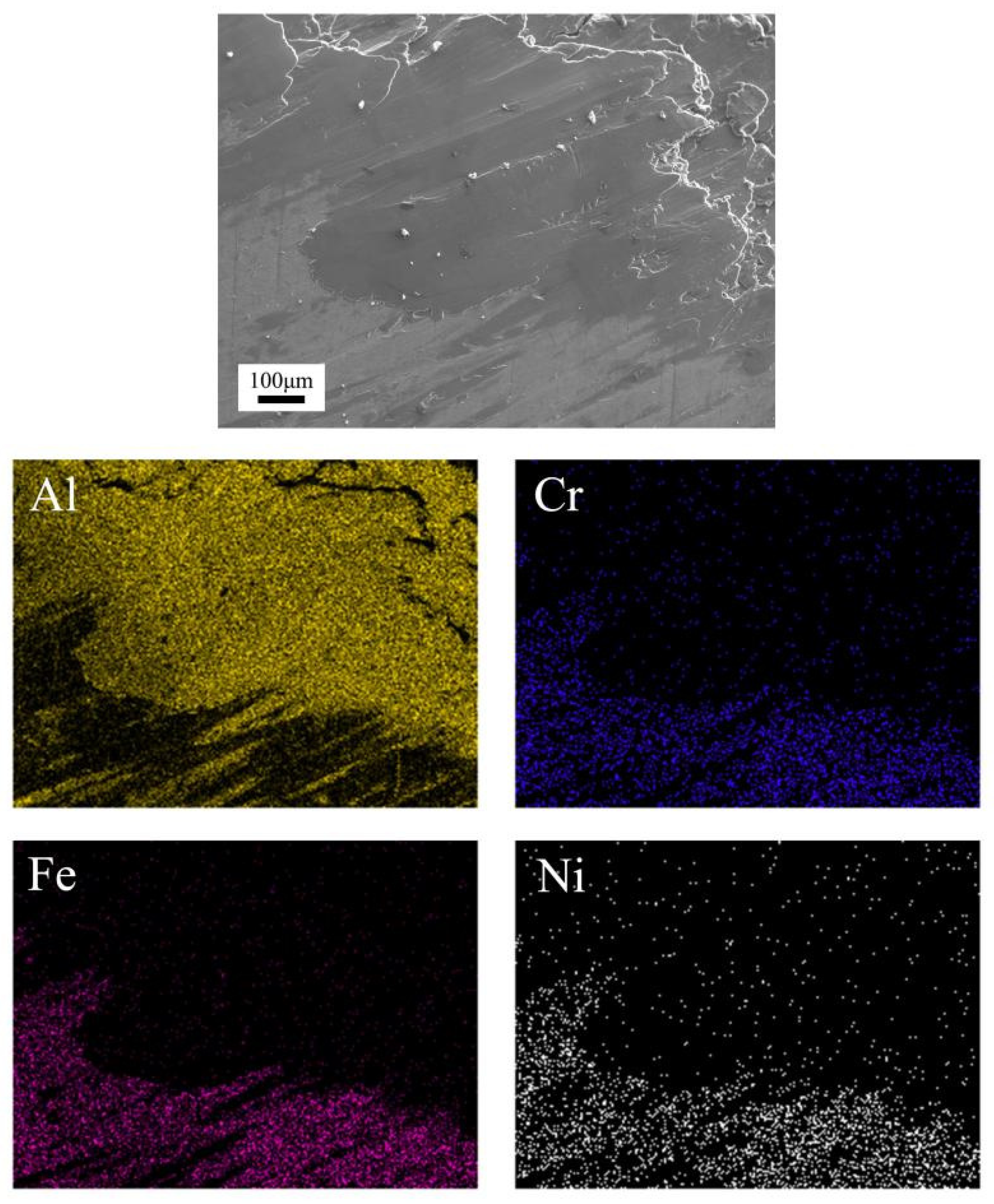
- Both the normal nickel-based coating and the nickel-based coatings reinforced with 5, 10, and 15 wt.% TiB2 had no crack in the first 1000 thermal fatigue cycles at 600 °C. In the subsequent 200 cycles at 750 °C, thermal fatigue cracks appeared in all coatings and were more severe in the TiB2 reinforced nickel-based composite coatings.
- (3)
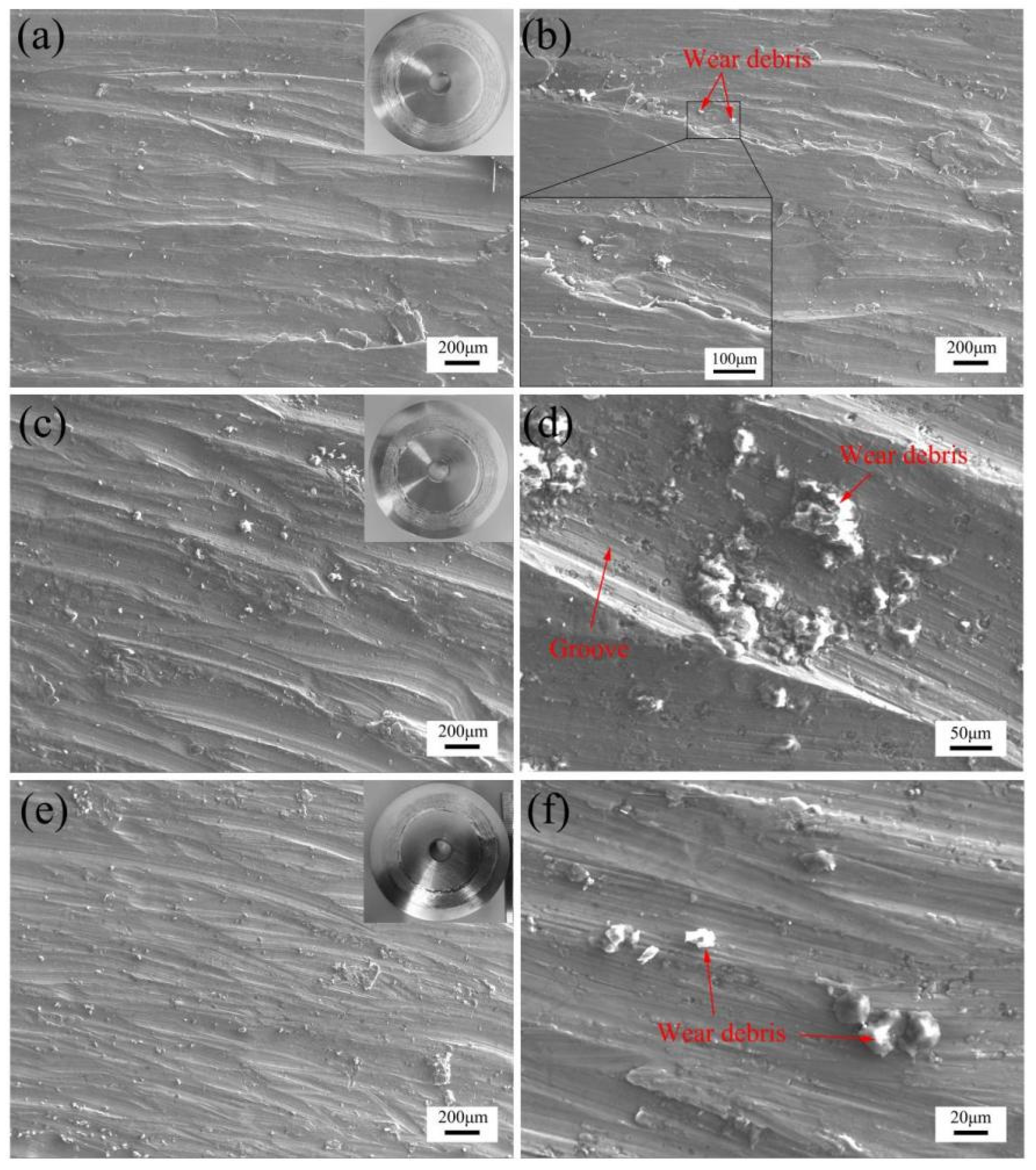
- The TiB2 reinforced phase promotes the generation of boron-rich products on the surface of nickel-based composite coatings during the dry sliding against the aluminum alloy under loads of 12 N at 430 °C. The boron-rich products formed self-lubricating film and debris on TiB2-Ni coatings, which was beneficial to reduce friction coefficient, mitigate the “mold sticking” defect of aluminum alloy, and transform the morphology of transferred aluminum alloy from rough plough to smooth layer.
- (4)
- With the increase of TiB2, a larger bulk hard phase tends to accumulate in the prepared nickel-based composite coatings, which led to the formation of hard debris on the surface. The increased hard debris may intensify the abrasive wear of the counterface if the self-lubricating film was incomplete or weak.
- (5)
- In view of the significantly reduced transferred aluminum alloy on coatings as well as the higher hardness than normal nickel-based coatings and the thermal fatigue performance under 600 °C, the TiB2 reinforced Ni-based coatings were considered as a potential self-lubricating coating in hot die forging of an aluminum alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoycheva, S.; Marchese, D.; Paul, C.; Padoan, S.; Juhmani, A.S.; Linkov, I. Multi-criteria decision analysis framework for sustainable manufacturing in automotive industry. Clean Prod. 2018, 187, 257–272. [Google Scholar] [CrossRef]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferrous Met. Soc. China 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Riahi, A.R.; Alpas, A.T. Adhesion of AA5182 aluminum sheet to DLC and TiN coatings at 25 °C and 420 °C. Surf. Coat. Technol. 2007, 202, 1055–1061. [Google Scholar] [CrossRef]
- Challen, J.M.; Oxley, P.L.B. An explanation of the different regimes of friction and wear using asperity deformation models. Wear 1979, 53, 229–243. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Donahue, R.J. The Metallurgy of Aluminum Alloys for Structural High-Pressure Die Castings. Int. J. Metalcast. 2020, 15, 1–16. [Google Scholar] [CrossRef]
- Rokni, M.R.; Zarei-Hanzaki, A.; Roostaei, A.A.; Abedi, H.R. An investigation into the hot deformation characteristics of 7075 aluminum alloy. Mater. Des. 2011, 32, 2339–2344. [Google Scholar] [CrossRef]
- Gali, O.A.; Shafiei, M.; Hunter, J.A.; Riahi, A.R. The tribological behavior of PVD coated work roll surfaces during rolling of aluminum. Surf. Coat. Technol. 2014, 260, 230–238. [Google Scholar] [CrossRef]
- Hampson, M.R.; Roberts, E.W.; Cropper, M.D.; Watters, R.B.; Forster, D.J. Towards the effective solid lubrication of ball bearings operating at high temperature. Proc. Inst. Mech. Eng. Part J.-J. Eng. Tribol. 2008, 222, 1041–1049. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.J.; Li, M.L.; Yan, S.S.; Yao, Z.H.; Liu, Q.Y. Preparation and Properties of High-Temperature Self-Lubricating Materials Based on H13 Steel. J. Mater. Eng. Perform. 2020, 29, 7830–7842. [Google Scholar] [CrossRef]
- Adam, P.; Mateusz, K.; Natalia, M.; Michał, K. Wear behavior of self-lubricating boride layers produced on Inconel 600-alloy by laser alloying. Wear 2019, 426–427, 919–933. [Google Scholar]
- Baran, O.; Bidev, F.; Cicek, H.; Kara, L.; Efeoglu, I.; Kucukomeroglu, T. Investigation of the friction and wear properties of Ti/TiB2/MOS2 graded-composite coatings deposited by CFUBMS under air and vacuum conditions. Surf. Coat. Technol. 2014, 260, 310–315. [Google Scholar] [CrossRef]
- Su, Y.F.; Zhang, Y.S.; Song, J.J.; Hu, L.T. Tribological Behavior and lubrication mechanism of self-lubricating ceramic/metal composites: The effect of matrix type on the friction and wear properties. Wear 2017, 372, 130–138. [Google Scholar] [CrossRef]
- Munro, R.G. Material properties of titanium diboride. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A. Tribological properties of boric acid and boric acid forming surfaces: Part 1, Crystal chemistry and self-lubricating mechanism of boric acid. In Society of Tribologists Lubrication Engineers Annual Conference; Argonne National Labs: Denver, CO, USA, 1990. [Google Scholar]
- Morón, R.C.V.; Rodriguez, G.; Jiménez-Tinoco, L.F.; Amador, A.M.; Méndez-Méndez, J.V.; Escobar-Hernández, J.; Calderon, C.D.R.; Nava-Sánchez, J.L. Multipass Scratch Behavior of Borided and Nitrided H13 Steel. J. Mater. Eng. Perform. 2018, 27, 3886–3899. [Google Scholar] [CrossRef]
- Karakas, M.S.; Gunen, A.; Kanca, E.; Yilmaz, E. Boride Layer Growth Kinetics of Aisi H13 Steel Borided with Nano-Sized Powders. Arch. Metall. Mater. 2018, 63, 159–165. [Google Scholar]
- Erdemir, A.; Bindal, C.; Zuiker, C.; Savrun, E. Tribology of naturally occuring boric acid films on boron carbide. Surf. Coat. Technol. 1996, 86–87, 507–510. [Google Scholar] [CrossRef]
- Erdemir, A.; Halter, M.; Fenske, G.R. Preparation of ultralow-friction surface films on vanadium diboride. Wear 1997, 205, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gu, W.; Pan, L.M.; Song, K.; Chen, X.; Qiu, T. Friction and wear properties of in situ (TiB2 + TiC)/Ti3SiC2 composites. Wear 2011, 271, 2940–2946. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.J.; Wang, H.P.; Li, M.P. Microstructure and mechanical properties of Ni-based composite coatings reinforced by in situ synthesized TiB2 + TiC by laser cladding. Int. J. Min. Met. Mater. 2013, 20, 57–63. [Google Scholar] [CrossRef]
- Rahul, K.; Maksim, A.; Le, L.; Irina, H. Sliding wear performance of in-situ spark plasma sintered Ti-TiBw composite at temperature up to 900 °C. Wear 2021, 476, 203663. [Google Scholar]
- Li, X.Q.; Gao, Y.M.; Yang, Q.X.; Pan, W.; Li, Y.F.; Zhong, Z.C.; Song, L.C. Evaluation of tribological behavior of B4C–hBN ceramic composites under water-lubricated condition. Ceram. Int. 2015, 41, 7387–7393. [Google Scholar] [CrossRef]
- Yang, Y.B.; Xie, Z.P.; Zhang, Z.M.; Li, X.B.; Wang, Q.; Zhang, Y.H. Processing maps for hot deformation of the extruded 7075 aluminum alloy bar: Anisotropy of hot workability. Mater. Sci. Eng. A 2014, 615, 183–190. [Google Scholar] [CrossRef]
- Guo, L.G.; Yang, S.; Yang, H.; Zhang, J. Processing map of as-cast 7075 aluminum alloy for hot working. Chin. J. Aeronaut. 2015, 28, 1774–1783. [Google Scholar] [CrossRef] [Green Version]
- Amado, J.M.; Tobar, M.J.; Alvarez, J.C.; Lamas, J.; Yañez, A. Laser cladding of tungsten carbides (Spherotene (R)) hardfacing alloys for the mining and mineral industry. Appl. Surf. Sci. 2009, 255, 5553–5556. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Zhao, S.S.; Wang, Y.B.; Liu, F.L.; Gao, W.Y.; Lin, X.C. Mitigation of pores generation at overlapping zone during laser cladding. J. Mater. Process. Technol. 2015, 216, 369–374. [Google Scholar] [CrossRef]
- Cao, L.L.; Xia, Y.Z.; Cui, H.Z.; Li, Q.P.; Zhu, B.W.; Wang, Q.B. Microstructural characteristics of TiB2-TiC-NiAl composite coatings via Plasma Cladding Process. Surf. Eng. 2019, 35, 997–1002. [Google Scholar] [CrossRef]
- Pan, C.G.; Liu, D.D.; Zhao, C.X.; Chang, Q.M.; He, P. Corrosion and Thermal Fatigue Behaviors of TiC/Ni Composite Coating by Self-Propagating High-Temperature Synthesis in Molten Aluminum Alloy. Coatings 2017, 7, 203. [Google Scholar] [CrossRef] [Green Version]
- Klobčar, D.; Tušek, J.; Taljat, B. Thermal Fatigue of Materials for Die Casting Tooling. Mater. Sci. Eng. A 2008, 472, 198–207. [Google Scholar] [CrossRef]
- Klobčar, D.; Kosec, L.; Kosec, B.; Tušek, J. Thermo fatigue cracking of die casting dies. Eng. Fail. Anal. 2012, 20, 43–53. [Google Scholar] [CrossRef]
- Persson, A.; Hogmark, S.; Bergstrom, J. Simulation and evaluation of thermal fatigue cracking of hot work tool steels. Int. J. Fatigue 2004, 26, 1095–1107. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Nuraliza, N.; Syahrullail, S.; Razak, D.M.; Azwadi, C.S.N. Influence of micro-pits on sliding motion under low speeds for block-on-disk tribotester. Particul. Sci. Technol. 2016, 34, 754–763. [Google Scholar] [CrossRef]
- Hu, Z.B.; Li, H.J.; Fu, Q.G.; Xue, H.; Sun, G.L. Fabrication and tribological properties of B2O3 as friction reducing coatings for carbon-carbon composites. New Carbon Mater. 2007, 22, 131–134. [Google Scholar] [CrossRef]
- Wei, J.J.; Erdemir, A.; Fenske, G.R. Dry Lubricant Films for Aluminum Forming. Tribol. Trans. 2000, 43, 535–541. [Google Scholar] [CrossRef]








| Parameters | Welding Current (A) | Carrier Gas (L/min) | Welding Speed (mm/min) | Vibration Extent (mm) | Vibration Frequency (times/min) | Spray Distance (mm) |
|---|---|---|---|---|---|---|
| TiB2-Ni60A | 120 | 3 | 20 | 7 | 50 | 10 |
| Period | Heating Temperature (℃) | Holding Time of Heating (s) | Cooling Temperature (℃) | Holding Time of Cooling (s) | Cycles |
|---|---|---|---|---|---|
| 1 | 600 | 120 | 20 | 10 | 1000 |
| 2 | 750 | 600 | 20 | 10 | 200 |
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Other | Al |
|---|---|---|---|---|---|---|---|---|---|
| 0.4 | 0.5 | 1.2–2.0 | 0.3 | 2.1–2.9 | 0.18–0.28 | 5.1–6.1 | 0.2 | 0.15 | Bal. |
| Sample | Ni60A | TN5 | TN10 | TN15 |
|---|---|---|---|---|
| Crack depth (μm) | 223.2 | 920.0 | 897.5 | 1302.6 |
| Debris | B | C | O | Al | Ti | Fe | Mg | Si | Cu | Mn | Ni | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disc (TN5) | 0 | 19.10 | 24.20 | 11.41 | 0 | 16.19 | 1.84 | 27.27 | 0 | 0 | 0 | 0 |
| 0 | 0 | 9.55 | 1.58 | 0 | 88.39 | 0 | 0 | 0 | 0.48 | 0 | 0 | |
| Disc (TN10) | 0 | 0 | 1.82 | 93.75 | 0 | 0.43 | 1.59 | 0 | 1.80 | 0.33 | 0.29 | 0 |
| 0 | 0 | 1.84 | 93.56 | 0 | 0.61 | 1.74 | 0 | 1.57 | 0.22 | 0.27 | 0.19 | |
| Disc (TN15) | 60.17 | 21.60 | 1.89 | 14.09 | 0 | 0.42 | 0.34 | 0.40 | 1.08 | 0 | 0 | 0 |
| 46.17 | 9.25 | 1.03 | 39.94 | 0 | 0.35 | 0.45 | 0 | 2.35 | 0.46 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Fu, T.; Xie, B.; Wang, H.; Ye, P.; Pan, X. Self-Lubricating Property of TiB2-Ni Coating in the Hot Forging Die of Aluminum Alloy. Coatings 2022, 12, 829. https://doi.org/10.3390/coatings12060829
Wang Z, Fu T, Xie B, Wang H, Ye P, Pan X. Self-Lubricating Property of TiB2-Ni Coating in the Hot Forging Die of Aluminum Alloy. Coatings. 2022; 12(6):829. https://doi.org/10.3390/coatings12060829
Chicago/Turabian StyleWang, Zhehan, Tao Fu, Bing Xie, Huajun Wang, Pingyuan Ye, and Xudong Pan. 2022. "Self-Lubricating Property of TiB2-Ni Coating in the Hot Forging Die of Aluminum Alloy" Coatings 12, no. 6: 829. https://doi.org/10.3390/coatings12060829
APA StyleWang, Z., Fu, T., Xie, B., Wang, H., Ye, P., & Pan, X. (2022). Self-Lubricating Property of TiB2-Ni Coating in the Hot Forging Die of Aluminum Alloy. Coatings, 12(6), 829. https://doi.org/10.3390/coatings12060829






