A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
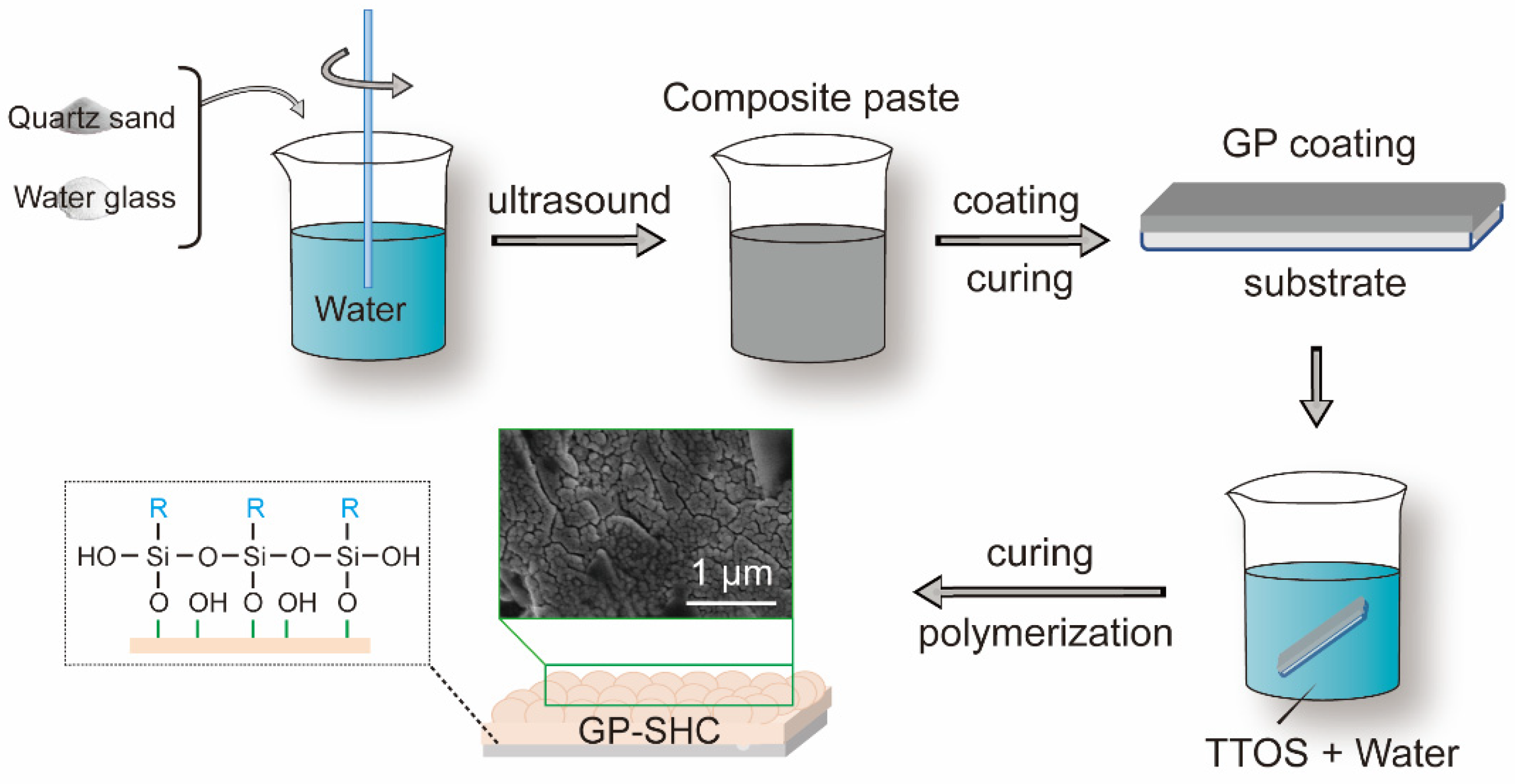
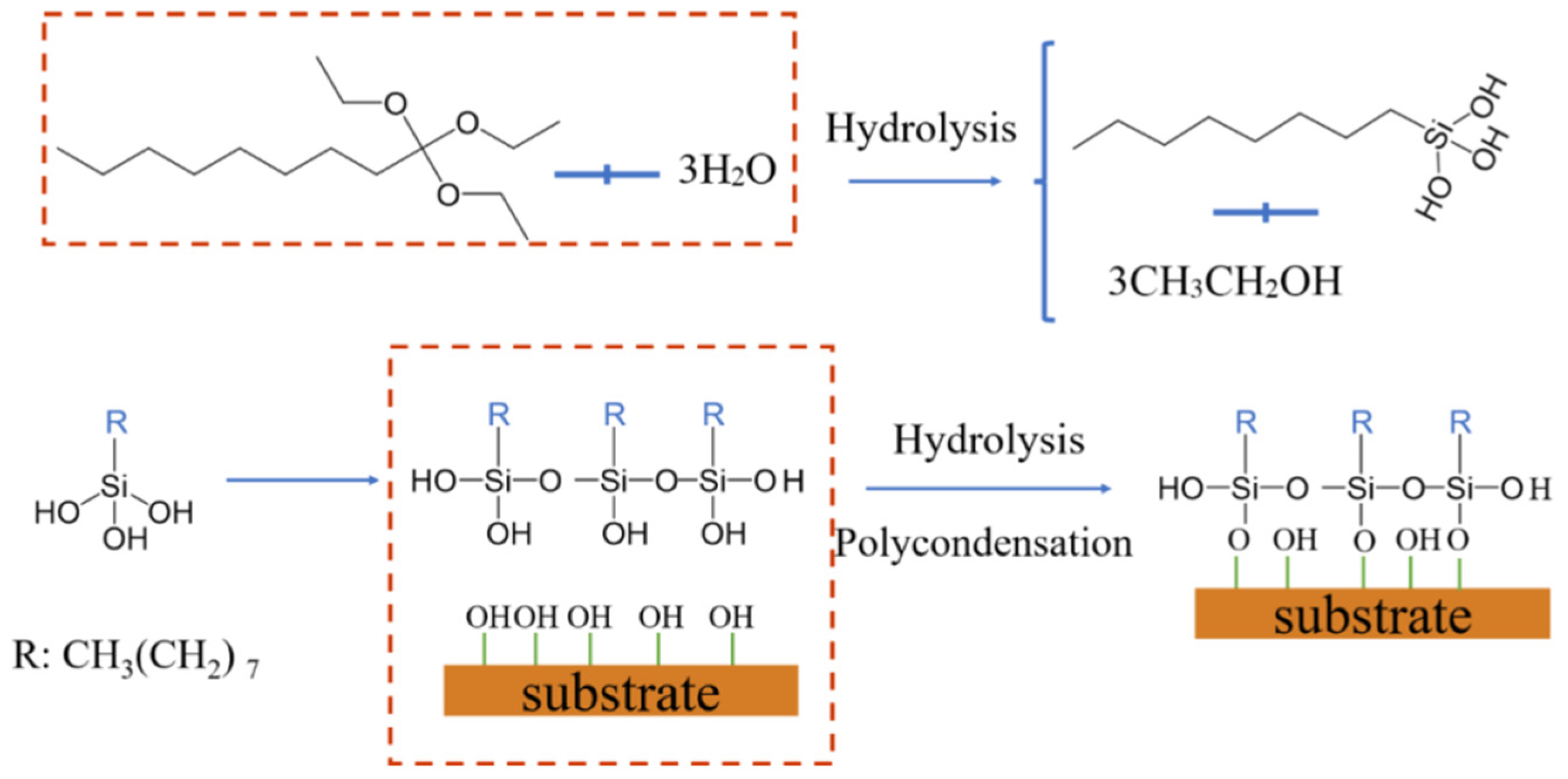
2.2. Fabrication of Superhydrophobic Alkali Activated Materials Coating
2.3. Characterizations
3. Results and Discussion
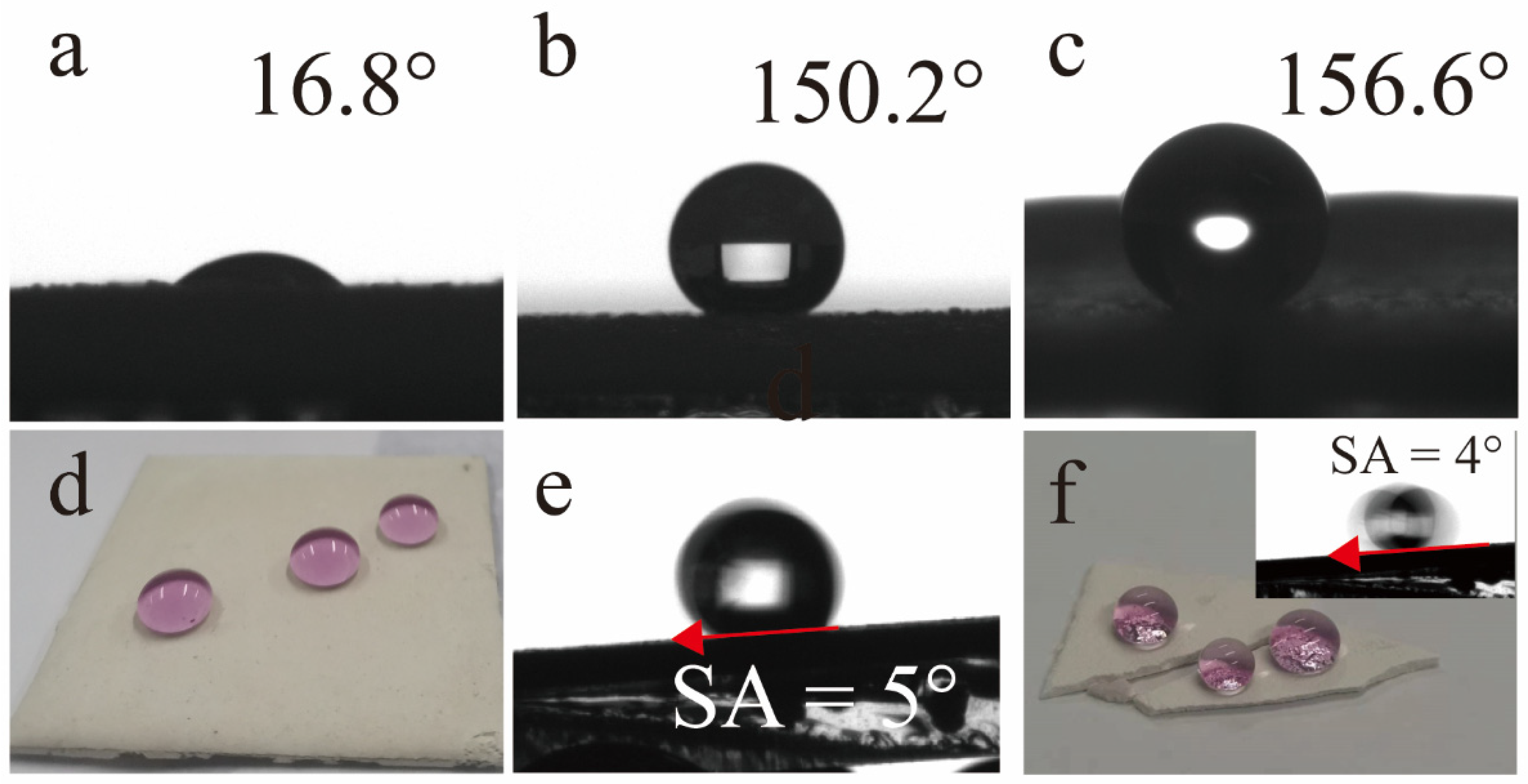
3.1. Wettability
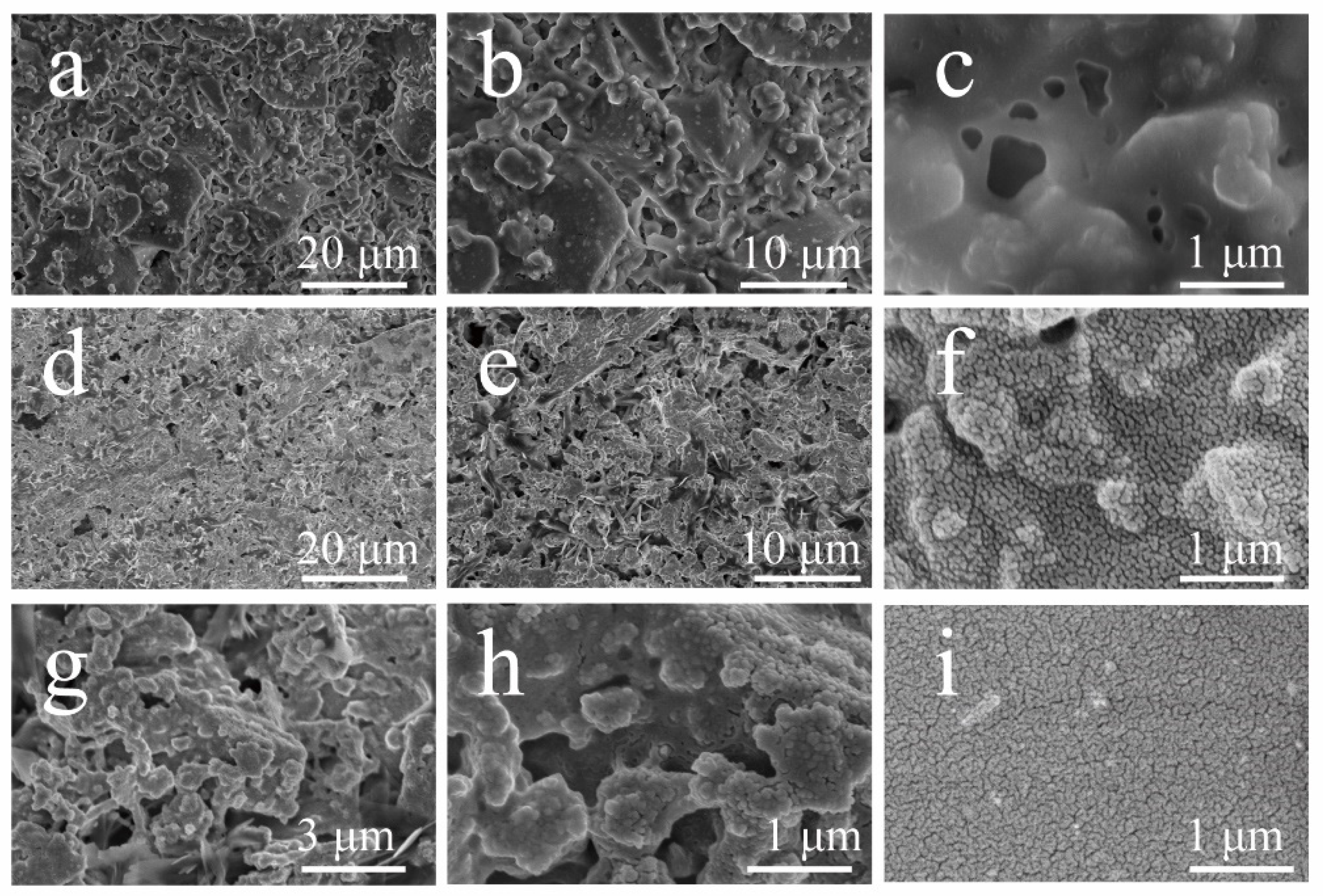
3.2. Surface Structure Characterization
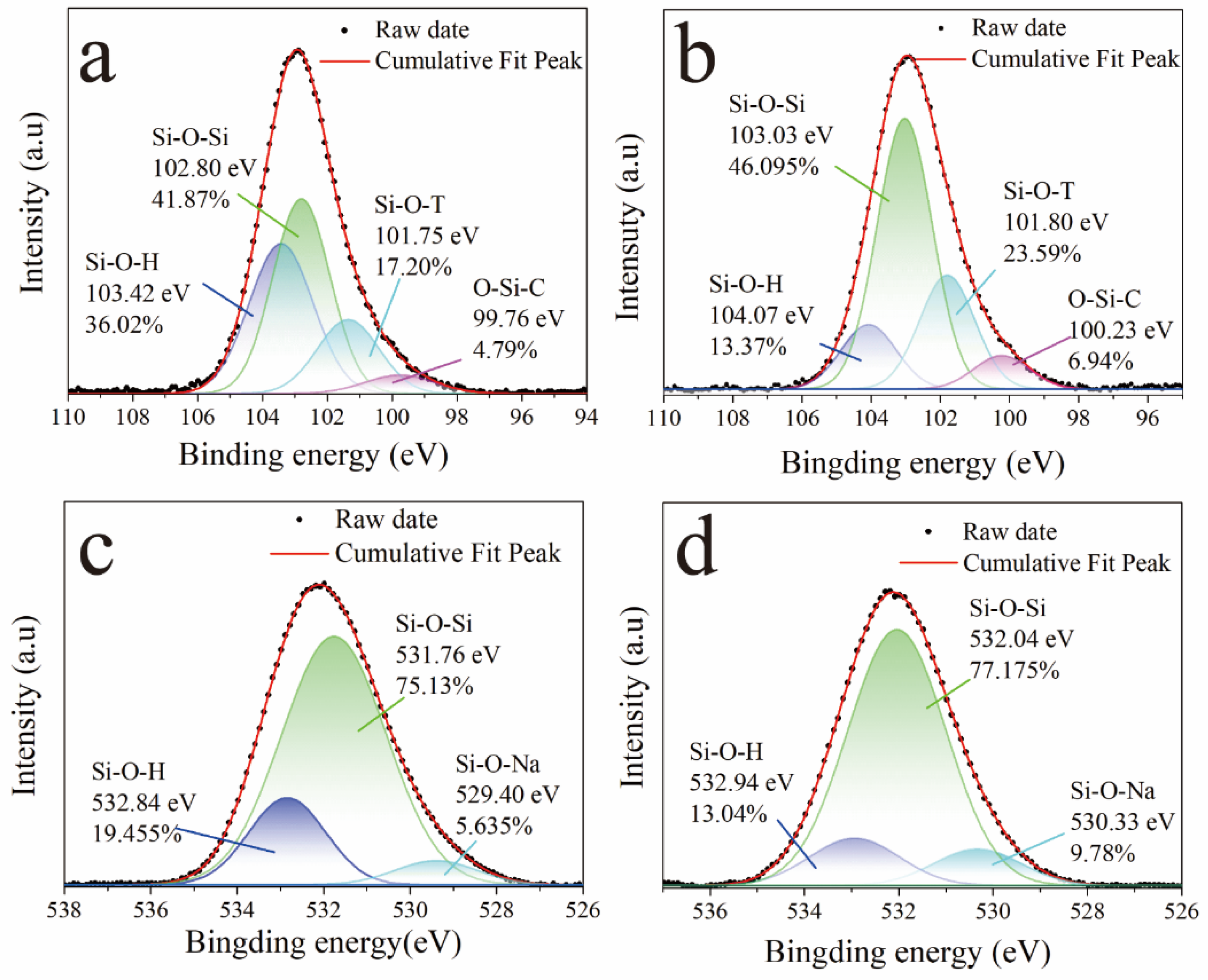
3.3. Crystalline Phases and Chemical Composition Analysis
3.4. Abrasion Resistance and Chemical Stability
4. Conclusions
- (1)
- TTOS were hydrolyzed and then continued to self-polymerize and copolymerize with the coating surface to grow nanoparticles, which provided excellent superhydrophobic property to coating surface with a contact angle of 150.2° and a roll angle of 5°.
- (2)
- The superhydrophobic coating expressed high stability against external force damage due to the overall hydrophobic modification. The results disclose that superhydrophobic alkali activated materials coatings still keep super-hydrophobicity after they were rubbed with 100-grit sandpaper until the substrate is exposed and were immersed in artificial seawater for a long time. And the coating can be regenerated by simple sandpaper rubbing after being damaged by chemical corrosion.
- (3)
- The work provides new ideas for the superhydrophobic modification of alkali activated materials and novel vote for the practical application of superhydrophobic alkali activated materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibuichi, S.; Yamamoto, T.; Onda, T.; Tsujii, K. Super Water- and Oil-Repellent Surfaces Resulting from Fractal Structure. J. Colloid Interface Sci. 1998, 208, 287–294. [Google Scholar] [CrossRef] [PubMed]
- López-Ortega, A.; Areitioaurtena, O.; Alves, S.A.; Goitandia, A.M.; Elexpe, I.; Arana, J.L.; Bayón, R. Development of a superhydrophobic and bactericide organic topcoat to be applied on thermally sprayed aluminum coatings in offshore submerged components. Prog. Org. Coat. 2019, 137, 105376. [Google Scholar] [CrossRef]
- Liu, T.; Yin, B.; He, T.; Guo, N.; Dong, L.; Yin, Y. Complementary effects of nanosilver and superhydrophobic coatings on the prevention of marine bacterial adhesion. ACS Appl. Mater. Interfaces 2012, 4, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Nanostructured superhydrophobic polysiloxane coating for high barrier and anticorrosion applications in marine environment. J. Colloid Interface Sci. 2018, 512, 674–685. [Google Scholar] [CrossRef]
- He, X.; Cao, P.; Tian, F.; Bai, X.; Yuan, C. Autoclaving-induced in-situ grown hierarchical structures for construction of superhydrophobic surfaces: A new route to fabricate antifouling coatings. Surf. Coat. Technol. 2019, 357, 180–188. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Zhang, X.; Wang, C.; Lv, C.; Zhu, Y. Durable and self-healing superhydrophobic surface with bistratal gas layers prepared by electrospinning and hydrothermal processes. Chem. Eng. J. 2017, 326, 578–586. [Google Scholar] [CrossRef]
- Shen, L.; Fan, M.; Qiu, M.; Jiang, W.; Wang, Z. Superhydrophobic nickel coating fabricated by scanning electrodeposition. Appl. Surf. Sci. 2019, 483, 706–712. [Google Scholar] [CrossRef]
- Forooshani, H.M.; Aliofkhazraei, M.; Bagheri, H. Fabrication of hierarchical dual structured (HDS) nickel surfaces and their corrosion behavior. J. Alloy. Compd. 2019, 784, 556–573. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, C.; Song, L.; Ma, Y.; Ali, Z.; Gu, J.; Zhang, B.; Zhang, H.; Zhang, Q. A stable 3D sol-gel network with dangling fluoroalkyl chains and rapid self-healing ability as a long-lived superhydrophobic fabric coating. Chem. Eng. J. 2018, 334, 598–610. [Google Scholar] [CrossRef]
- Lin, H.; Qiu, Z.; Huang, P.; Zeng, L.; Liang, Y.; Zeng, C.; Lin, R.; Yuan, M.; Hong, R. Preparation of antireflective coatings with moisture resistance and a widely tunable refractive index by combining the sol-gel method with evaporation concentration. Constr. Build. Mater. 2022, 318, 125810. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Shen, Z.; Chen, H. Fabrication of Self-healing Superhydrophobic Surfaces from Water-Soluble Polymer Suspensions Free of Inorganic Particles through Polymer Thermal Reconstruction. Coatings 2018, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Mu, B.; Xu, J.; Wang, A. Reversible Thermochromic Superhydrophobic BiVO4 Hybrid Pigments Coatings with Self-Cleaning Performance and Environmental Stability Based on Kaolinite. ACS Appl. Mater. Interfaces 2021, 13, 3228–3236. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Zhu, Q.; Sun, Y.; Li, Y. Mechanically robust superhydrophobic porous anodized AA5083 for marine corrosion protection. Corros. Sci. 2019, 158, 108083. [Google Scholar] [CrossRef]
- Selim, M.S.; El-Safty, S.A.; Fatthallah, N.A.; Shenashen, M.A. Silicone/graphene oxide sheet-alumina nanorod ternary composite for superhydrophobic antifouling coating. Prog. Org. Coat. 2018, 121, 160–172. [Google Scholar] [CrossRef]
- Xu, W.; Hu, Y.; Bao, W.; Xie, X.; Liu, Y.; Song, A.; Hao, J. Superhydrophobic copper surfaces fabricated by fatty acid soaps in aqueous solution for excellent corrosion resistance. Appl. Surf. Sci. 2017, 399, 491–498. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Lin, Z.X.; Tian, Z.K.; Cui, X.M. Inhibition of Efflorescence in Na-Based Geopolymer Inorganic Coating. ACS Omega 2020, 5, 14822–14830. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Qiu, J.; Cao, J.; Zou, Y.; Wang, S.; Jia, D.; Zhou, Y. Robust Inorganic Daytime Radiative Cooling Coating Based on a Phosphate Geopolymer. ACS Appl. Mater. Interfaces 2020, 12, 54963–54971. [Google Scholar] [CrossRef]
- Jamil, N.H.; Abdullah, M.M.A.B.; Pa, F.C.; Hasmaliza, M.; Ibrahim, W.M.A.W. Phase Transformation of Kaolin-Ground Granulated Blast Furnace Slag from Geopolymer ization to Sintering Process. Magnetochemistry 2021, 7, 32. [Google Scholar] [CrossRef]
- Lv, X.; Wang, K.; He, Y.; Cui, X. A green drying powder inorganic coating based on Geopolymer technology. Constr. Build. Mater. 2019, 214, 441–448. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, J.; Wang, Y. Durability properties of sustainable alkali-activated cementitious materials as marine engineering material: A review. Mater. Today Sustain. 2022, 17, 10009. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Xu, G.; Chen, Y.; You, G. Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious. Materials 2022, 15, 727. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, Y.-L.; Dai, J.-G.; Poon, C.-S.; Zhang, W.-D.; Zhang, P. Inhibiting efflorescence formation on fly ash–based Geopolymer via silane surface modification. Cem. Concr. Compos. 2018, 94, 43–52. [Google Scholar] [CrossRef]
- Lv, X.S.; Qin, Y.; Liang, H.; Han, Y.C.; Li, J.; He, Y.; Cui, X.M. Potassium methyl silicate (CH5SiO3Na) assisted activation and modification of alkali-activated-slag-based drying powder coating for protecting cement concrete. Constr. Build. Mater. 2022, 326, 126858. [Google Scholar] [CrossRef]
- Cirstea, N.F.; Badanoiu, A.; Boscornea, A.C. Intumescent Silicate Coatings with the Addition of Alkali-Activated Materials. Polymers 2022, 14, 1937. [Google Scholar] [CrossRef]
- Lashkari, S.; Yazdipanah, F.; Shahri, M.; Sarker, P. Mechanical and durability assessment of cement-based and alkali-activated coating mortars in an aggressive marine environment. SN Appl. Sci. 2021, 3, 618. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, S.; Sui, Y.; Lv, Z. Alkali-activated materials as coatings deposited on various substrates: A review. Int. J. Adhes. Adhes. 2021, 110, 102934. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, B.; Zhang, M.; Li, X.; Yang, J. A novel colorful sepiolite-based superhydrophobic coating with excellent mechanical and chemical stability and self-cleaning property. Mater. Lett. 2019, 254, 340–343. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Shu, X.; Yang, Y.; She, W.; Ran, Q. A review on recent advances in the fabrication and evaluation of superhydrophobic concrete. Chem. Eng. J. 2022, 237, 1098674. [Google Scholar] [CrossRef]
- Li, Q.; Yang, K.; Yang, C. An alternative admixture to reduce sorptivity of alkali-activated slag cement by optimising pore structure and introducing hydrophobic film. Cem. Concr. Compos. 2019, 95, 183–192. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Li, X.; Lei, L.; Qu, L.; Mao, J.; Zhang, H. Utilization of super-hydrophobic steel slag in mortar to improve water repellency and corrosion resistance. J. Clean. Prod. 2022, 341, 13078. [Google Scholar] [CrossRef]
- Li, J.; Chen, P.; Cai, H.; Xu, Y.; Rasulov, M. Investigation of Silane Impregnation for Protection of Alkali-Activated Slag Mortar. Adv. Mater. Sci. Eng. 2021, 144, 997573. [Google Scholar] [CrossRef]
- Liu, Z.H.; Pang, X.Q.; Wang, K.T.; Lv, X.S.; Cui, X.M. Superhydrophobic Coatings Prepared by the in Situ Growth of Silicone Nanofilaments on Alkali-Activated Geopolymers Surface. ACS Appl. Mater. Interfaces 2019, 11, 22809–22816. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.S.; Sun, K.C.; Ali, M.; Riaz, R.; Jeong, S.H. Fabrication of coral-reef structured nano silica for self-cleaning and super-hydrophobic textile applications. Chem. Eng. J. 2020, 401, 125859. [Google Scholar] [CrossRef]
- Fu, H.; Mo, R.; Wang, P.; Wang, Y.; Cao, Y.; Guang, W.; Ding, Y. Influence of Elevated Temperatures and Cooling Method on the Microstructure Development and Phase Evolution of Alkali-Activated Slag. Materials 2022, 15, 2022. [Google Scholar] [CrossRef]
- Li, F.; Chen, D.; Yang, Z.; Lu, Y.; Zhang, H.; Li, S. Effect of mixed fibers on fly ash-based geopolymer resistance against carbonation. Constr. Build. Mater. 2022, 322, 126394. [Google Scholar] [CrossRef]
- Khater, H.M. Hybrid slag geopolymer composites with durable characteristics activated by cement kiln dust. Constr. Build. Mater. 2019, 228, 116708. [Google Scholar] [CrossRef]
- Kanuchova, M.; Kozakova, L.; Drabova, M.; Sisol, M.; Estokova, A.; Kanuch, J.; Skvarla, J. Monitoring and characterization of creation of Geopolymer prepared from fly ash and metakaolin by X-ray photoelectron spectroscopy method. Environ. Prog. Sustain. Energy 2015, 34, 841–849. [Google Scholar] [CrossRef]
- Yap, S.W.; Johari, N.; Mazlan, S.A.; Hassan, N.A. Mechanochemical durability and self-cleaning performance of zinc oxide-epoxy superhydrophobic coating prepared via a facile one-step approach. Ceram. Int. 2021, 47, 15825–15833. [Google Scholar] [CrossRef]
- Deng, S.; Jia, S.; Deng, X.; Qing, Y.; Luo, S.; Wu, Y. New insight into island-like structure driven from hydroxyl groups for high-performance superhydrophobic surfaces. Chem. Eng. J. 2021, 416, 129078. [Google Scholar] [CrossRef]
- Yin, B.; Xu, T.; Hou, D.; Zhao, E.; Hua, X.; Han, K.; Zhang, Y.; Zhang, J. Superhydrophobic anticorrosive coating for concrete through in-situ bionic induction and gradient mineralization. Constr. Build. Mater. 2020, 257, 119510. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Li, Y.; Wang, C.; Zhu, Y.; Wang, H.; Wang, J. Robust superhydrophobic epoxy composite coating prepared by dual interfacial enhancement. Chem. Eng. J. 2019, 371, 276–285. [Google Scholar] [CrossRef]
- Jia, S.; Chen, H.; Luo, S.; Qing, Y.; Deng, S.; Yan, N.; Wu, Y. One-step approach to prepare superhydrophobic wood with enhanced mechanical and chemical durability: Driving of alkali. Appl. Surf. Sci. 2018, 455, 115–122. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Fang, Z.; Chai, X.; Cui, X. A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation. Coatings 2022, 12, 864. https://doi.org/10.3390/coatings12060864
Qin Y, Fang Z, Chai X, Cui X. A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation. Coatings. 2022; 12(6):864. https://doi.org/10.3390/coatings12060864
Chicago/Turabian StyleQin, Yao, Zhou Fang, Xinrui Chai, and Xuemin Cui. 2022. "A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation" Coatings 12, no. 6: 864. https://doi.org/10.3390/coatings12060864
APA StyleQin, Y., Fang, Z., Chai, X., & Cui, X. (2022). A Superhydrophobic Alkali Activated Materials Coating by Facile Preparation. Coatings, 12(6), 864. https://doi.org/10.3390/coatings12060864





