Intelligent Evaluation of Marine Corrosion of Q420 Steel Based on Image Recognition Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material and Solution
2.2. Corrosion Measurement
2.3. Image Recognition Method
2.3.1. Gray-Level Co-Occurrence Matrix
2.3.2. Binary Image Method
2.3.3. Fractal Model
3. Results and Discussion
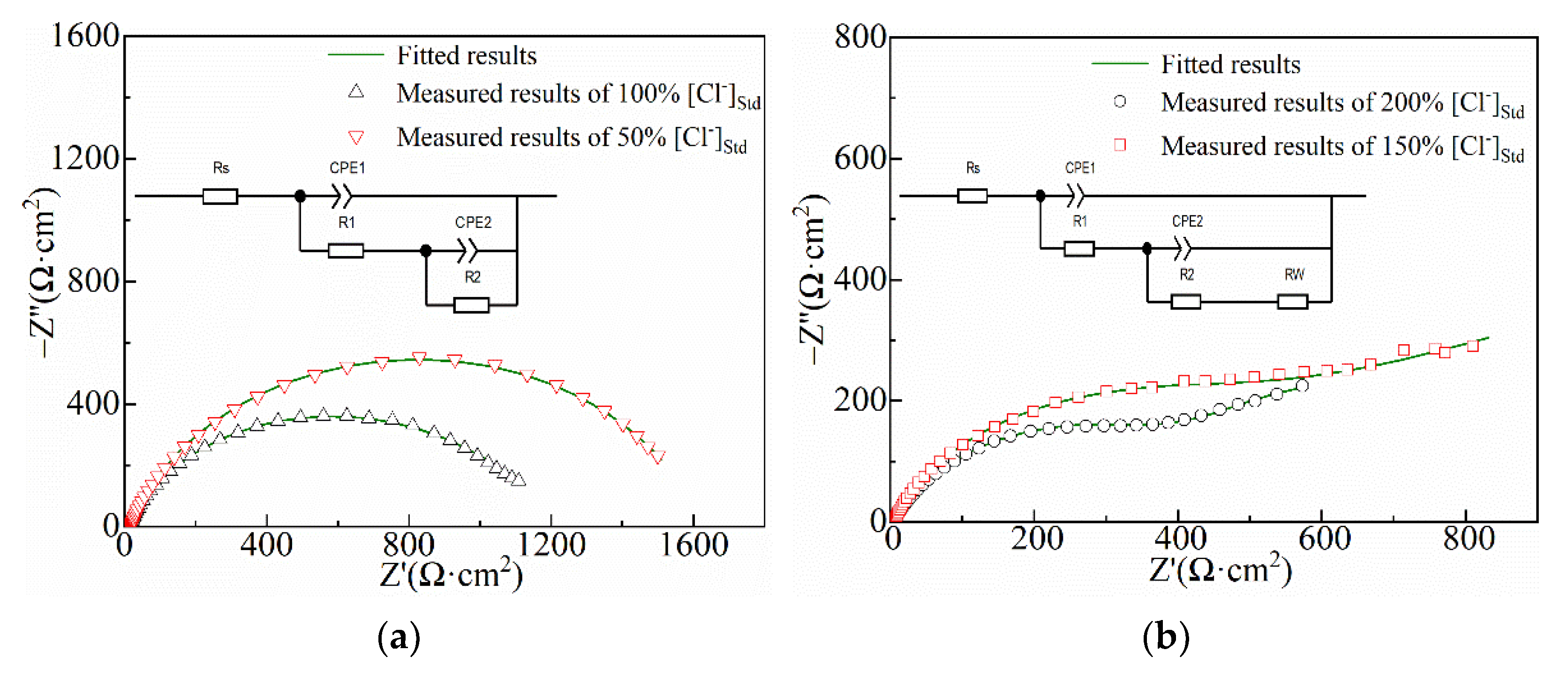
3.1. Electrochemical Analysis
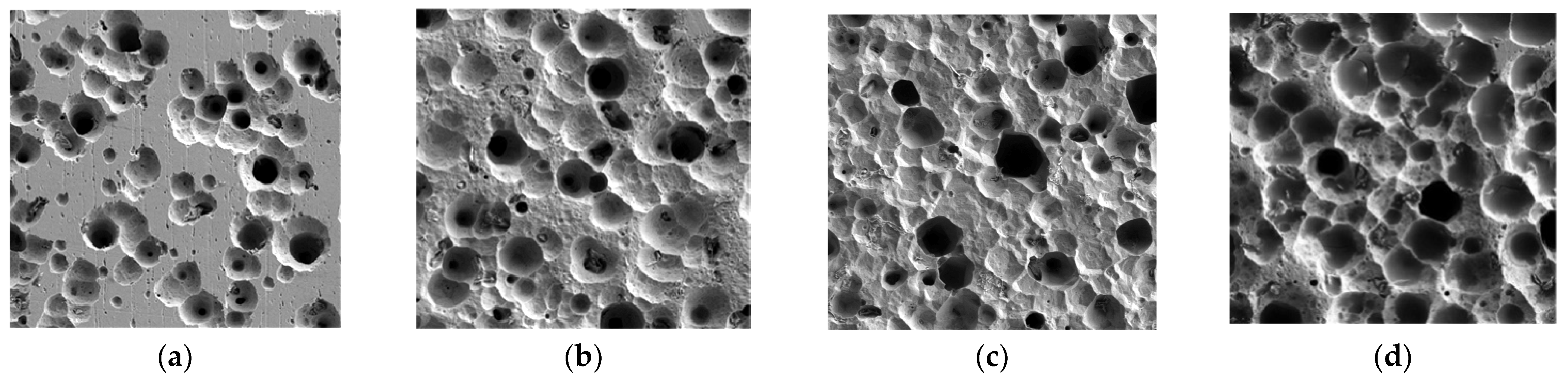
3.2. Image Recognition of Corrosion Morphology
3.2.1. Characteristic Parameter Analysis Based on GLCM
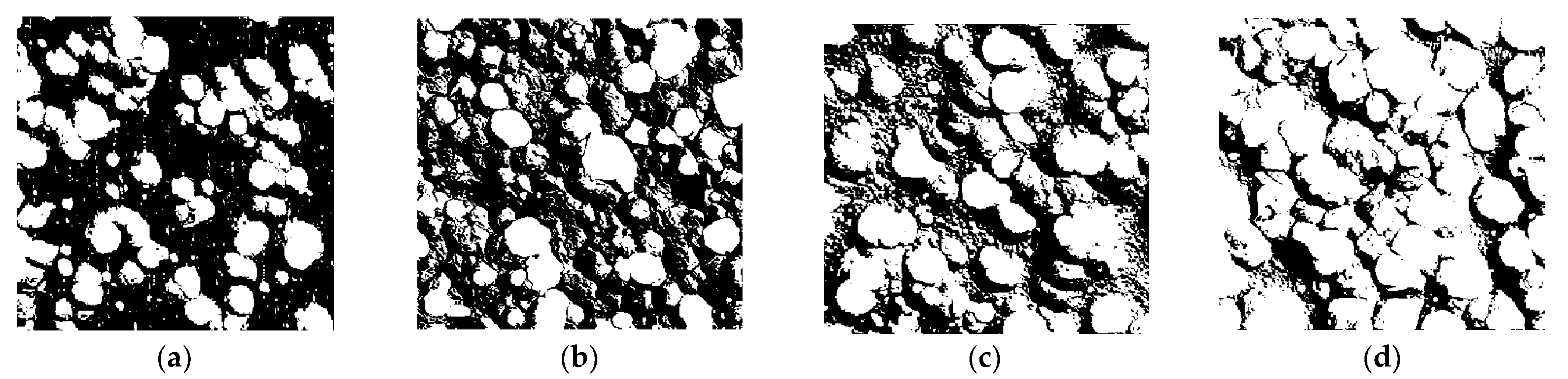
3.2.2. Characteristic Parameter Analysis Based on Binary Image
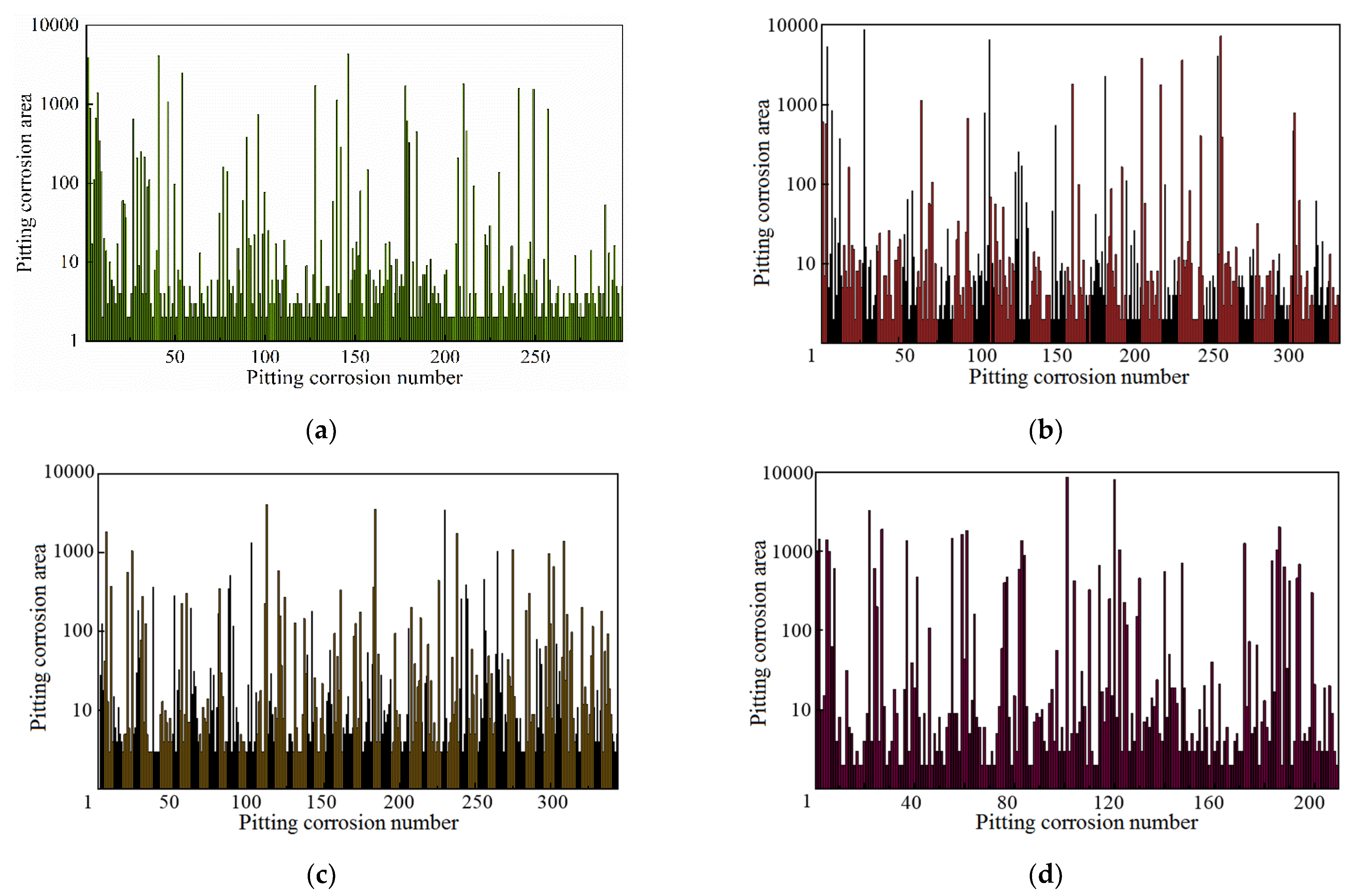
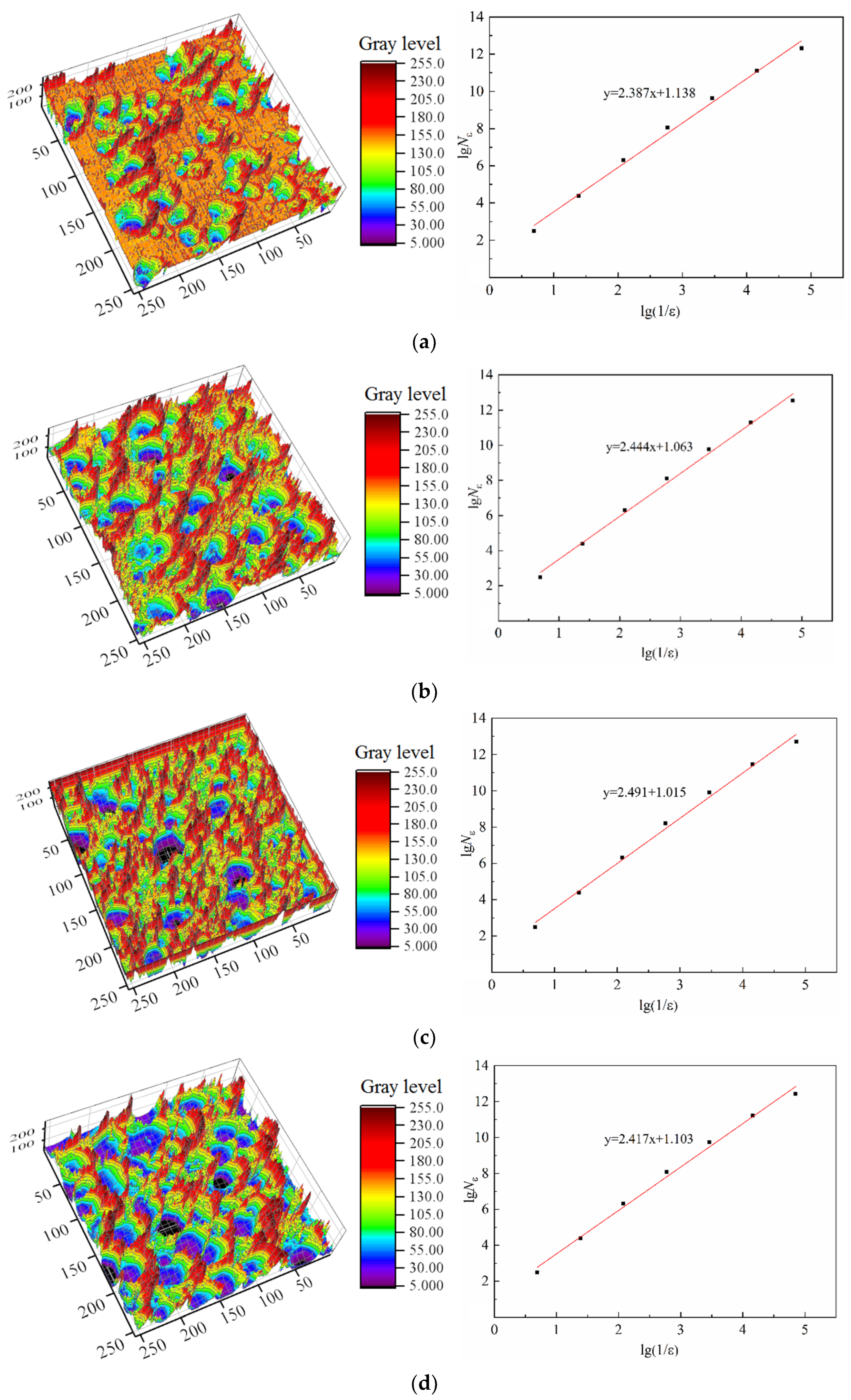
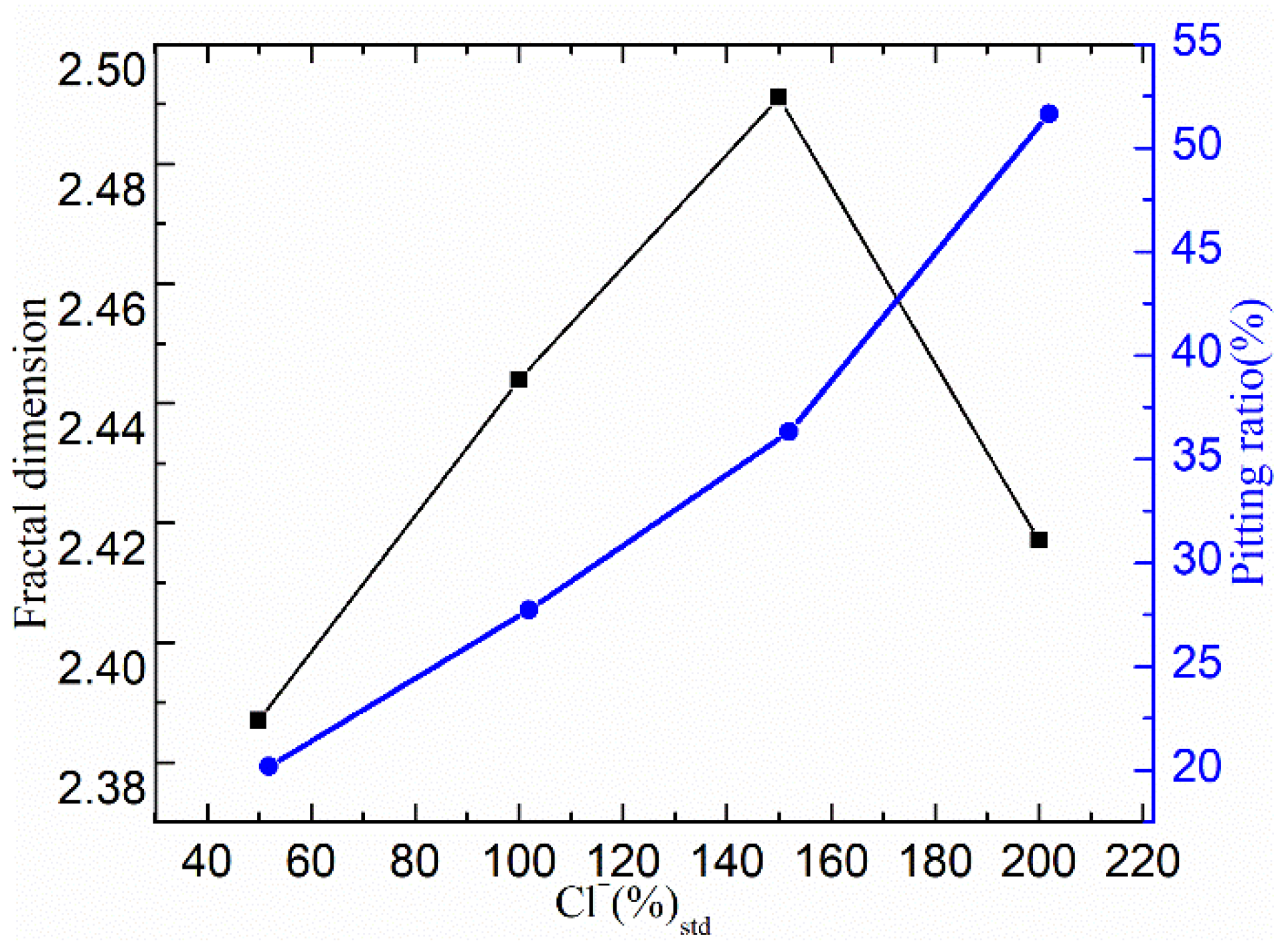
3.2.3. Characteristic Parameter Analysis Based on Fractal Theory
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Li, D.; Chang, W.; Wang, Z.; Hu, L.; Zhang, Y.; Wang, M.; Yang, Z.; Song, J.; Chen, S.; et al. In situ marine exposure study on corrosion behaviors of five alloys in coastal waters of western Pacific Ocean. J. Mater. Res. Technol. 2020, 9, 8104–8116. [Google Scholar] [CrossRef]
- Zhang, X.M.; Chen, Z.Y.; Luo, H.F.; Zhou, T.; Zhao, Y.L.; Ling, Z.C. Corrosion resistances of metallic materials in environments containing chloride ions: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 377–410. [Google Scholar] [CrossRef]
- Cui, M.M.; Wang, P.Y.; Wang, Z.K.; Wang, B. Mangrove inspired anti-corrosion coatings. Coatings 2019, 9, 725. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Li, C.P.; Li, Y.H.; Lu, J.L.; Wang, Y.S.; Luo, X.Q. Multi-physics analysis of the galvanic corrosion of Mg-steel couple under the influence of time-dependent anisotropic deposition film. J. Magnes. Alloy. 2021, 9, 866–882. [Google Scholar] [CrossRef]
- Xing, W.; Deng, N.; Xin, B.; Chen, Y.; Zhang, Z. Investigation of a novel automatic micro image-based method for the recognition of animal fibers based on Wavelet and Markov Random Field. Micron 2019, 119, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Z.; Krys, D. Improving laser image resolution for pitting corrosion measurement using Markov random field method. Automat. Constr. 2012, 21, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Palm, B.G.; Bayer, F.M.; Cintra, R.J. 2-D Rayleigh autoregressive moving average model for SAR image modeling. Comput. Stat. Data. Anal. 2022, 171, 107453. [Google Scholar] [CrossRef]
- Sun, D.; Li, F.; Gao, Q.; Lu, Y. A dynamical analysis on non-local autoregressive model and its application on image reconstruction. Chaos Solitons Fractals 2020, 130, 109427. [Google Scholar] [CrossRef]
- Qi, Z.; Hongqi, Y.; Huan, Z.; Kaixuan, L.; Yi, H. Ultimate strength assessment of hull structural plates with general corrosion based on fractal theory. Ocean Eng. 2022, 245, 110417. [Google Scholar] [CrossRef]
- Peng, L.; Pan, B.; Liu, Z.; Liu, Z.; Tan, S. Investigation on abrasion-corrosion properties of WC-based composite with fractal theory. Int. J. Refract. Met. Hard Mater. 2020, 87, 105142. [Google Scholar] [CrossRef]
- Songbo, R.; Chao, K.; Ying, G.; Song, G.; Shenghui, Z.; Gang, L.; Tao, Y.; Yajie, Z. Measurement pitting morphology characteristic of corroded steel surface and fractal reconstruction model. Measurement 2022, 190, 110678. [Google Scholar] [CrossRef]
- Fajardo, J.I.; Paltán, C.A.; López, L.M.; Carrasquero, E.J. Textural analysis by means of a gray level co-occurrence matrix method. Case: Corrosion in steam piping systems. Mater. Today Proc. 2022, 49, 149–154. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, H.; Zhang, Y.; Li, Y.; Feng, Q.; Chen, K. Meso-damage evolution analysis of magnesium oxychloride cement concrete based on X-CT and grey-level co-occurrence matrix. Constr. Build. Mater. 2020, 255, 119373. [Google Scholar] [CrossRef]
- Patel, D.R.; Kiran, M.B. Vision based prediction of surface roughness for end milling. Mater. Today Proc. 2021, 44, 792–796. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhao, Z.; Bai, P.; Liu, B.; Tan, J.; Wu, X. In-situ monitoring of pitting corrosion of Q235 carbon steel by electrochemical noise: Wavelet and recurrence quantification analysis. J. Electroanal. Chem. 2020, 879, 114776. [Google Scholar] [CrossRef]
- Li, J.; Du, C.W.; Liu, Z.Y.; Li, X.G.; Liu, M. Effect of microstructure on the corrosion resistance of 2205 duplex stainless steel. Part 2: Electrochemical noise analysis of corrosion behaviors of different microstructures based on wavelet transform. Constr. Build. Mater. 2018, 189, 1294–1302. [Google Scholar]
- Zhu, Y.; Wang, L.; Behnamian, Y.; Song, S.; Wang, R.; Gao, Z.; Hu, W.; Xia, D.-H. Metal pitting corrosion characterized by scanning acoustic microscopy and binary image processing. Corros. Sci. 2020, 170, 108685. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Wang, J.Q.; Behnamian, Y.; Gao, Z.M.; Wang, J.H.; Xia, D.H. Pitting growth rate on alloy 800 in chloride solutions containing thiosulphate: Image analysis assessment. Corros. Eng. Sci. Tec. 2018, 53, 206–213. [Google Scholar] [CrossRef]
- Jing, J.T.; Feng, P.F.; Wei, S.L.; Zhao, H.; Liu, Y.F. Investigation on the surface morphology of Si3N4 ceramics by a new fractal dimension calculation method. Appl. Surf. Sci. 2016, 387, 812–821. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Wu, W.; Yang, S.; Sheng, L. Corrosion Assessment of Carbon Steel Using Texture and Color Features. In Proceedings of the 2020 3rd International Conference on Advanced Electronic Materials, Computers and Software Engineering (AEMCSE), Shenzhen, China, 24–26 April 2020. [Google Scholar]
- Benco, M.; Hudec, R.; Kamencay, P.; Zachariasova, M.; Matuska, S. An advanced approach to extraction of colour texture features based on GLCM. Int. J. Adv. Robot. Syst. 2014, 11, 104. [Google Scholar] [CrossRef]
- Irarrazaval, G.P. A correction algorithm for undersampled images using dynamic segmentation and entropy based focus criterion. Magn. Reson. Imaging 2002, 20, 659–666. [Google Scholar]
- Bondada, V.; Pratihar, D.K.; Kumar, C.S. Detection and quantitative assessment of corrosion on pipelines through image analysis. Procedia Comput. Sci. 2018, 133, 804–811. [Google Scholar] [CrossRef]
- Sarkar, N.; Chaudhuri, B.B. An efficient approach to estimate fractal dimension of textural images. Pattern Recognit. 1992, 25, 1035–1041. [Google Scholar] [CrossRef]

| Element | C | Mn | Si | P | S | Al | Ni + Cu + Cr |
|---|---|---|---|---|---|---|---|
| Content (wt%) | 0.05 | 1.68 | 0.44 | 0.01 | 0.003 | 0.037 | 1.1 |
| Species | NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 |
|---|---|---|---|---|---|---|
| Concentration (g/L) | 24.53 | 5.2 | 4.09 | 1.16 | 0.69 | 0.20 |
| [Cl−]Std | 50% | 100% | 150% | 200% |
|---|---|---|---|---|
| Ecorr (V) | −0.81 | −0.92 | −0.94 | −0.94 |
| Icorr (µA·cm−2) | 2.35 | 72.68 | 93.37 | 187.01 |
| Cl− Concentration | Average Gray Value | Standard Deviation | Energy | Entropy |
|---|---|---|---|---|
| 50% [Cl−]Std | 129.43 | 50.32 | 0.045 | 5.34 |
| 100% [Cl−]Std | 118.86 | 56.59 | 0.02 | 5.7 |
| 150% [Cl−]Std | 115.55 | 57.93 | 0.02 | 5.72 |
| 200% [Cl−]Std | 92.38 | 59.65 | 0.02 | 5.74 |
| Cl− Concentration | Number of Corrosion Pits | Total Number of Corroded Pixels | Pitting Ratio (%) |
|---|---|---|---|
| 50% [Cl−]Std | 298 | 18,171 | 20.19 |
| 100% [Cl−]Std | 336 | 24,948 | 27.72 |
| 150% [Cl−]Std | 344 | 32,688 | 36.32 |
| 200% [Cl−]Std | 209 | 46,476 | 51.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, C.; Lu, J.; Nan, C.; Zhang, Q.; Zhang, H. Intelligent Evaluation of Marine Corrosion of Q420 Steel Based on Image Recognition Method. Coatings 2022, 12, 881. https://doi.org/10.3390/coatings12070881
Wang K, Li C, Lu J, Nan C, Zhang Q, Zhang H. Intelligent Evaluation of Marine Corrosion of Q420 Steel Based on Image Recognition Method. Coatings. 2022; 12(7):881. https://doi.org/10.3390/coatings12070881
Chicago/Turabian StyleWang, Kai, Chenpei Li, Jinling Lu, Cuihong Nan, Qiaoling Zhang, and Hao Zhang. 2022. "Intelligent Evaluation of Marine Corrosion of Q420 Steel Based on Image Recognition Method" Coatings 12, no. 7: 881. https://doi.org/10.3390/coatings12070881
APA StyleWang, K., Li, C., Lu, J., Nan, C., Zhang, Q., & Zhang, H. (2022). Intelligent Evaluation of Marine Corrosion of Q420 Steel Based on Image Recognition Method. Coatings, 12(7), 881. https://doi.org/10.3390/coatings12070881






