Improving the Processability of a One-Step Hydrophobic Coating for Hot-Dipped Galvanised Steel for Industrial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Coating Production
2.2.2. Characterization Techniques
2.2.3. Initial Studies
3. Results and Discussion
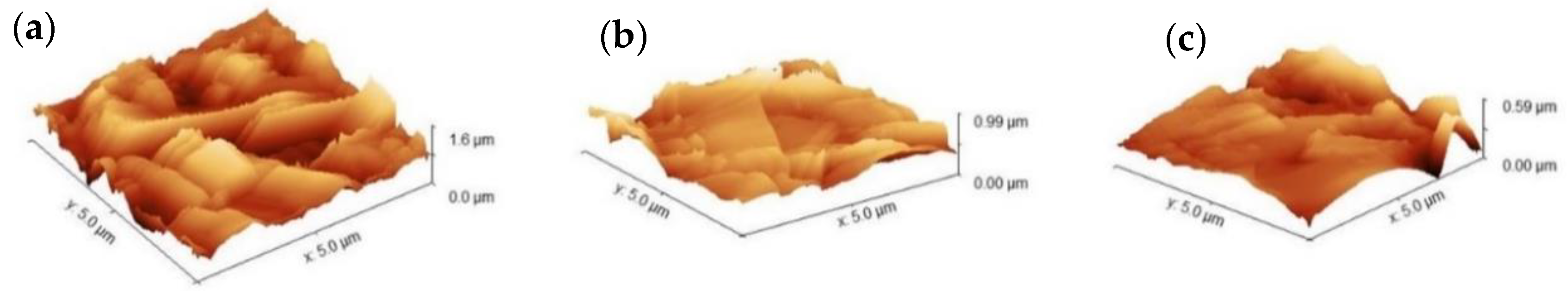
3.1. Surface Morphology and Wettability
3.2. FTIR Analysis
3.3. Optimal Coating Parameters
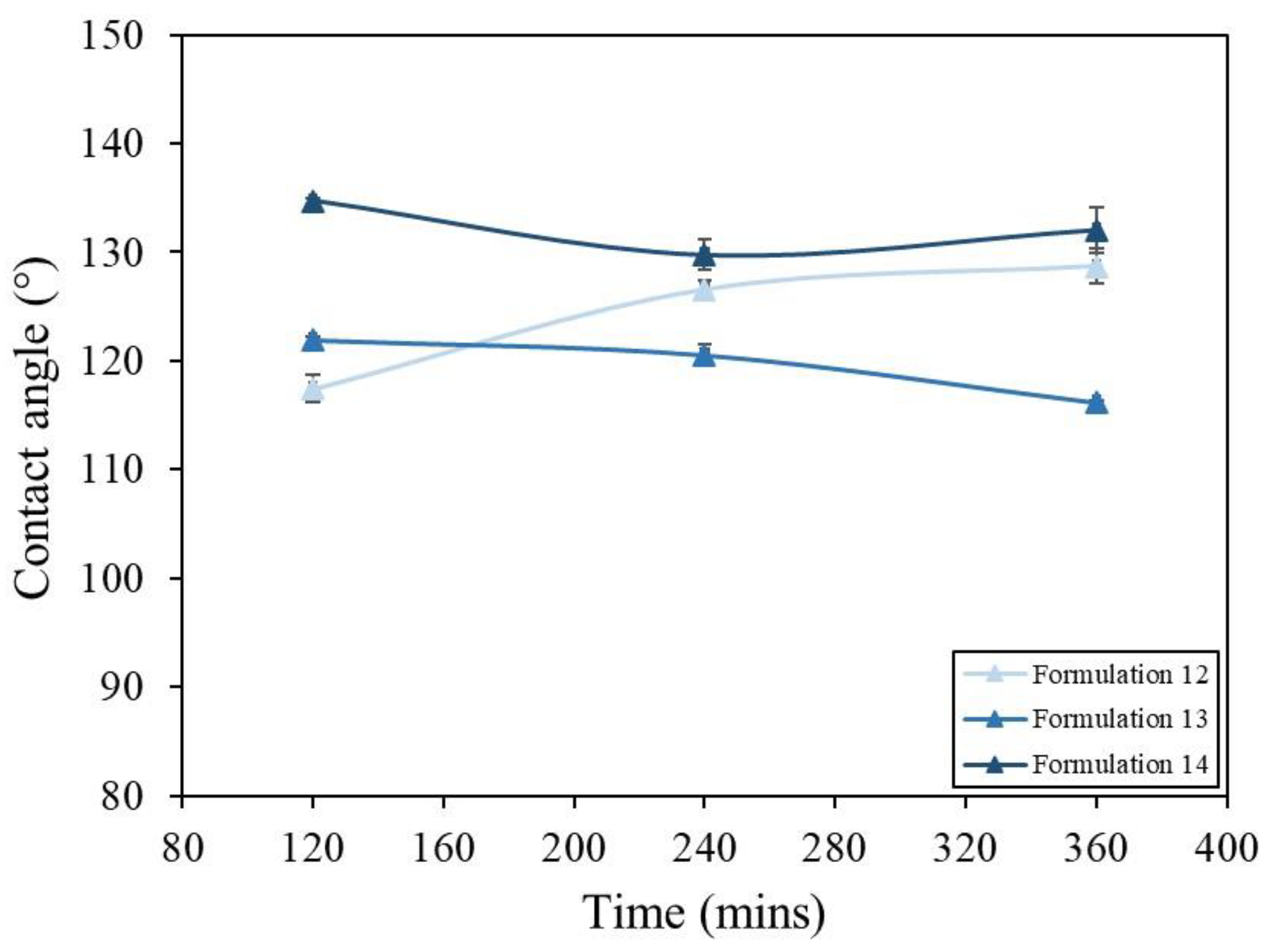
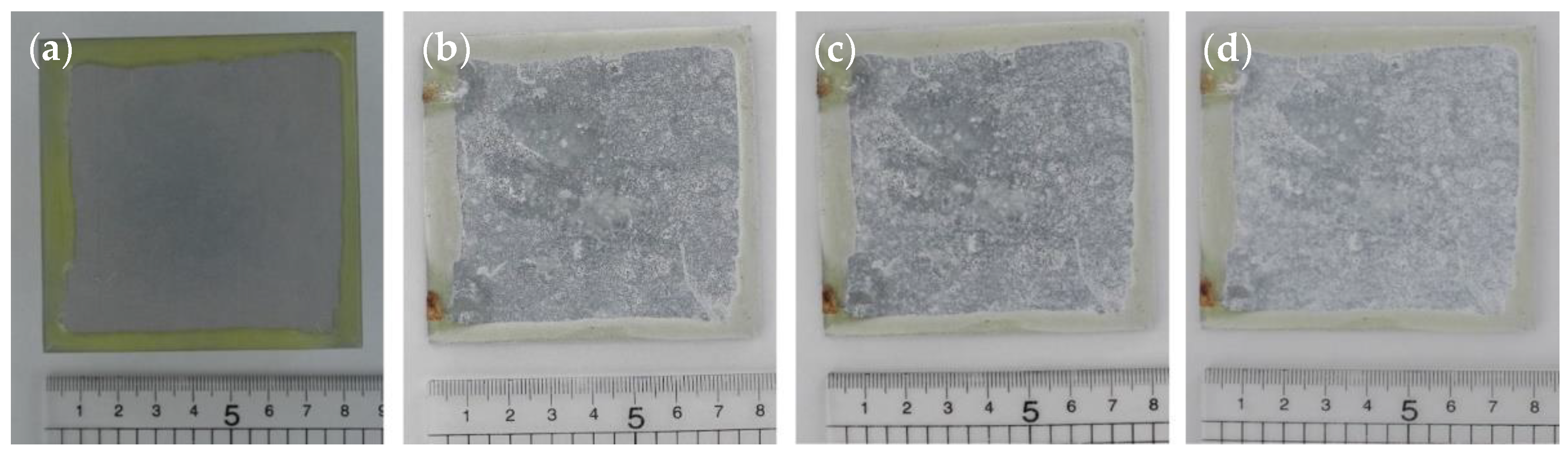
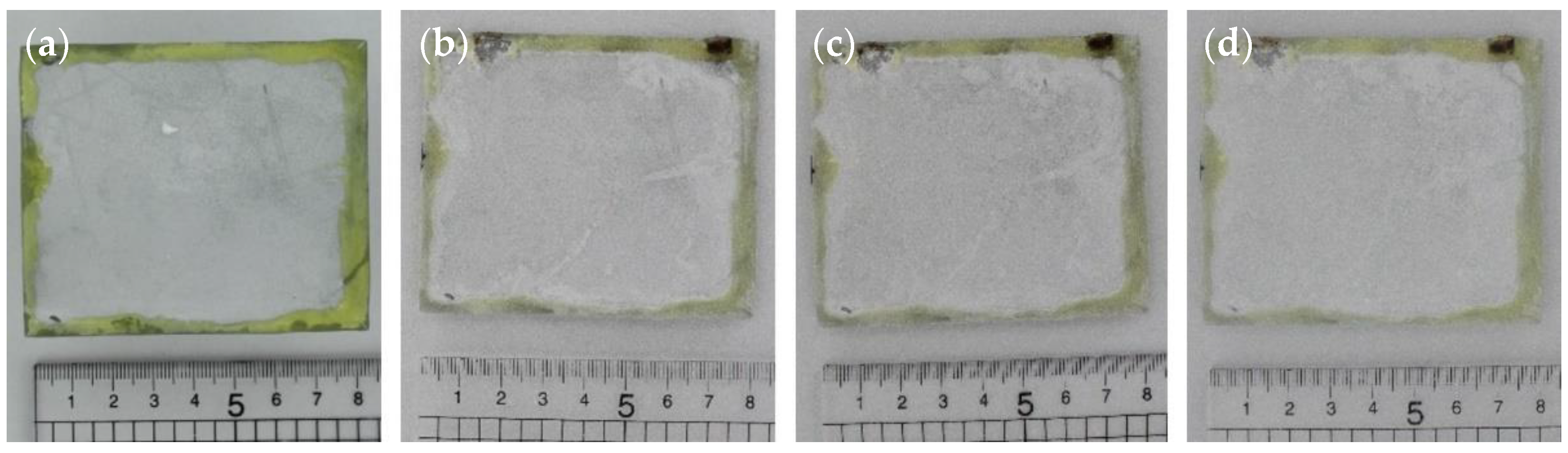
3.4. Humidity Testing
3.4.1. Method and Results
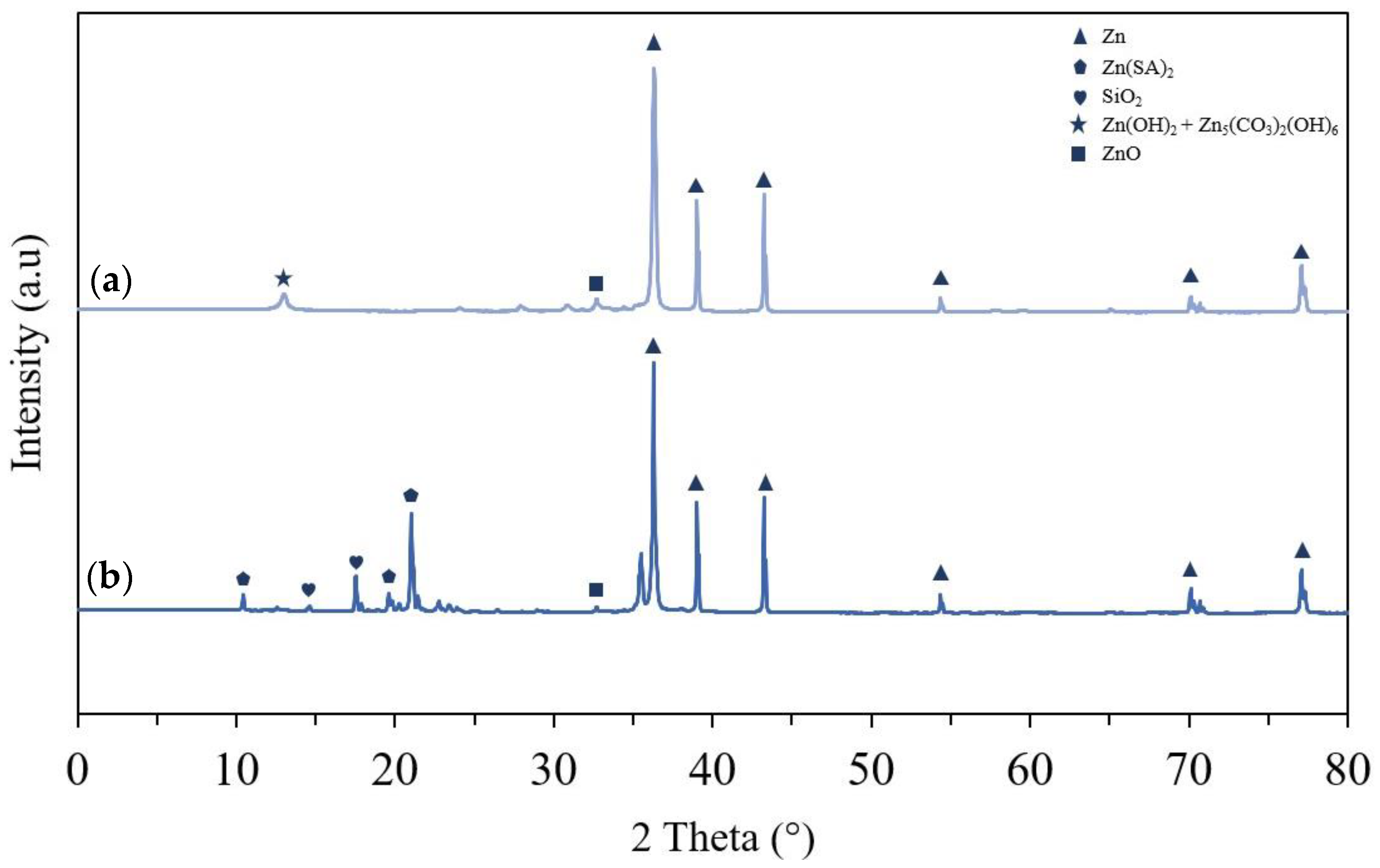
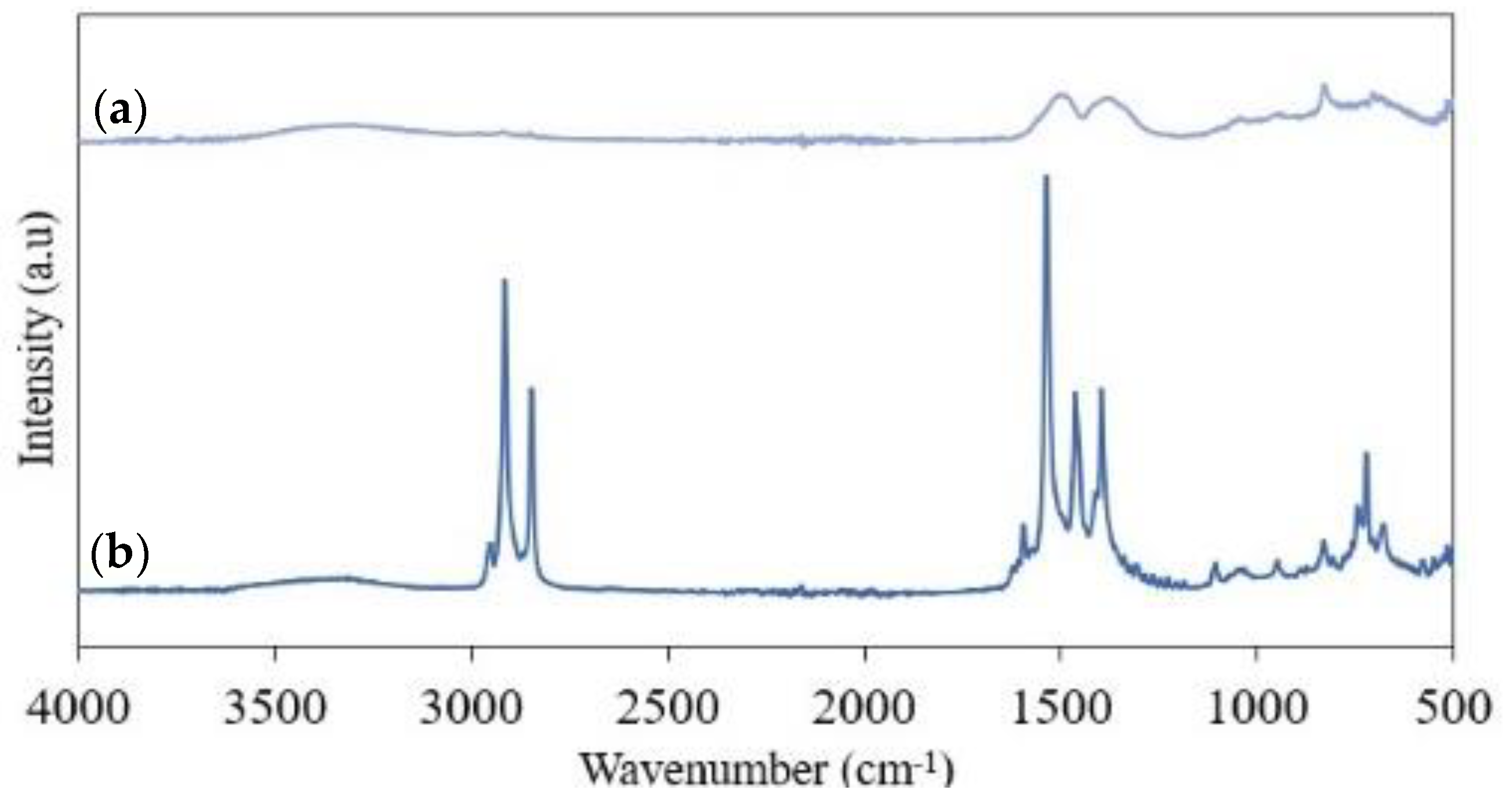
3.4.2. XRD and FTIR Analysis of Humidity Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forget, L.; Delhalle, J.; Mekhalif, Z. Application of scanning Kelvin probe to study the corrosion protection of chromated hot-dip galvanized steel. Mater. Corros. 2001, 52, 181–184. [Google Scholar] [CrossRef]
- Klimow, G.; Fink, N.; Grundmeier, G. Electrochemical studies of the inhibition of the cathodic delamination of organically coated galvanised steel by thin conversion films. Electrochim. Acta 2007, 53, 1290–1299. [Google Scholar] [CrossRef]
- Long, Y.; Liu, C.; Peng, S. Enhanced performance of a green inorganic-based passive film on the batch hot-dip galvanized steel by organic additives. Int. J. Electrochem. Sci. 2020, 15, 2568–2580. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Li, C.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. Superhydrophobic Film on Hot-Dip Galvanized Steel with Corrosion Resistance and Self-Cleaning Properties. Metals 2018, 8, 687. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Chen, R.-J.; Hu, J.-M. Superhydrophobic surface constructed on electrodeposited silica films by two-step method for corrosion protection of mild steel. Corros. Sci. 2016, 104, 336–343. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Naing, T.H.; Rachpech, V.; Janudom, S.; Mahathaninwong, N. Characterization of water-repellent and corrosion-resistant superhydrophobic surfaces on galvanized steel. J. Coat. Technol. Res. 2020, 17, 1537–1548. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Bahgat Radwan, A.; Abdullah, A.M.; Alnuaimi, N.A. Recent advances in corrosion resistant superhydrophobic coatings. Corros. Rev. 2018, 36, 127–153. [Google Scholar] [CrossRef]
- Zhang, F.; Ju, P.; Pan, M.; Zhang, D.; Huang, Y.; Li, G.; Li, X. Self-healing mechanisms in smart protective coatings: A review. Corros. Sci. 2018, 144, 74–88. [Google Scholar] [CrossRef]
- Liang, T.; Yuan, H.; Li, C.; Dong, S.; Zhang, C.; Cao, G.; Fan, Y.; Zhao, X.; Cao, X. Corrosion inhibition effect of nano–SiO2 for galvanized steel superhydrophobic surface. Surf. Coat. Technol. 2021, 406, 126673. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sudhagar, P.; Devadoss, A.; Kumar, A.M.; Liu, S.; Terashima, C.; Nakata, K.; Fujishima, A. A mechanically bendable superhydrophobic steel surface with self-cleaning and corrosion-resistant properties. J. Mater. Chem. A 2015, 3, 14263–14271. [Google Scholar] [CrossRef]
- Boinovich, L.; Gnedenkov, S.; Alpysbaeva, D.; Egorkin, V.; Emelyanenko, A.; Sinebryukhov, S.; Zaretskaya, A. Corrosion resistance of composite coatings on low-carbon steel containing hydrophobic and superhydrophobic layers in combination with oxide sublayers. Corros. Sci. 2012, 55, 238–245. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Hu, J.; Tang, A.-Q.; Wei, W.-H.; He, J. Preparation and properties of super-hydrophobic coating on magnesium alloy. Appl. Surf. Sci. 2010, 257, 1666–1671. [Google Scholar] [CrossRef]
- Brassard, J.; Sarkar, D.; Perron, J.; Audibert-Hayet, A.; Melot, D. Nano-micro structured superhydrophobic zinc coating on steel for prevention of corrosion and ice adhesion. J. Colloid Interface Sci. 2015, 447, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Qian, B.; Shen, Z. Fabrication of Superhydrophobic Surfaces by Dislocation-Selective Chemical Etching on Aluminum, Copper, and Zinc Substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, J.; Liu, B.; Peng, Z. Preparation of superhydrophobic zinc coating for corrosion protection. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 113–118. [Google Scholar] [CrossRef]
- Xu, W.; Ning, T.; Yang, X.; Lu, S. Fabrication of superhydrophobic surfaces on zinc substrates. Appl. Surf. Sci. 2011, 257, 4801–4806. [Google Scholar] [CrossRef]
- Hao, Y.; Soolaman, D.M.; Yu, H.-Z. Controlled Wetting on Electrodeposited Oxide Thin Films: From Hydrophilic to Superhydrophobic. J. Phys. Chem. C 2013, 117, 7736–7743. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-Step Electrodeposition Process to Fabricate Corrosion-Resistant Superhydrophobic Surface on Magnesium Alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Mohapatra, S.S.; Kumar, A. Superhydrophobic coatings for aluminium surfaces synthesized by chemical etching process. Int. J. Smart Nano Mater. 2016, 7, 248–264. [Google Scholar] [CrossRef] [Green Version]
- Jurak, S.F.; Jurak, E.F.; Uddin, N.; Asmatulu, R. Functional Superhydrophobic Coating Systems for Possible Corrosion Mitigation. Int. J. Autom. Technol. 2020, 14, 148–158. [Google Scholar] [CrossRef]
- Li, C.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. One-step fabrication of bionic superhydrophobic coating on galvanised steel with excellent corrosion resistance. J. Alloys Compd. 2019, 786, 272–283. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, L.; Chen, A.; Song, Q.; Chen, C. A rapid one-step process for fabrication of superhydrophobic surface by electrodeposition method. Electrochim. Acta 2012, 59, 168–171. [Google Scholar] [CrossRef]
- Knudsen, O.Ø.; Forsgren, A. Corrosion Control Through Organic Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Peißker, P. Surface-preparation technology. In Handbook of Hot-Dip Galvanization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 29–90. [Google Scholar]
- Calvo, M.B.; Cepeda, E.A. Solubilities of Stearic Acid in Organic Solvents and in Azeotropic Solvent Mixtures. Available online: https://pubs.acs.org/sharingguidelines (accessed on 25 January 2022).
- Heryanto, R.; Hasan, M.; Abdullah, E.C.; Kumoro, A.C. Solubility of stearic acid in various organic solvents and its prediction using non-ideal solution models. ScienceAsia 2007, 33, 469–472. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- De Leon, A.; Advincula, R.C. Conducting polymers with superhydrophobic effects as anticorrosion coating. In Intelligent Coatings for Corrosion Control; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 409–430. Available online: https://app.knovel.com/web/view/khtml/show.v/rcid:kpICCC0001/cid:kt00U8P2E2/viewerType:khtml/root_slug:intelligent-coatings/url_slug:conducting-polymers-with?&b-toc-cid=kpICCC0001&b-toc-root-slug=intelligent-coatings&b-toc-title=IntelligentCoatingsforC (accessed on 11 May 2022).
- Zouboy, N.D.E.; Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pourbaix, M., Ed.; Pergamon Press: Oxford, UK, 1966; 406p. [Google Scholar]
- Zhao, W.; Zhu, R.; Jiang, J.; Wang, Z. Environmentally-friendly superhydrophobic surface based on Al2O3@KH560@SiO2 electrokinetic nanoparticle for long-term anti-corrosion in sea water. Appl. Surf. Sci. 2019, 484, 307–316. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Pedraza, F.; Vhatkar, R.S. Silica based superhydrophobic coating for long-term industrial and domestic applications. J. Alloys Compd. 2016, 663, 487–493. [Google Scholar] [CrossRef]
- Yu, F.; Gao, J.; Liu, C.; Chen, Y.; Zhong, G.; Hodges, C.; Chen, M.; Zhang, H. Preparation and UV aging of nano-SiO2/fluorinated polyacrylate polyurethane hydrophobic composite coating. Prog. Org. Coat. 2020, 141, 105556. [Google Scholar] [CrossRef]
- Zeng, Y.; Qin, Z.; Hua, Q.; Min, Y.; Xu, Q. Sheet-like superhydrophobic surfaces fabricated on copper as a barrier to corrosion in a simulated marine system. Surf. Coat. Technol. 2019, 362, 62–71. [Google Scholar] [CrossRef]
- Cao, L.; Wan, Y.; Li, Y.; Yang, S. Corrosion-resistant and friction-reducing performance of super-hydrophobic coating on hot-dip galvanised steel in a 3.5% NaCl solution. Lubr. Sci. 2021, 33, 325–334. [Google Scholar] [CrossRef]
- Wahab, R.; Ansari, S.G.; Kim, Y.S.; Dar, M.A.; Shin, H.S. Synthesis and characterization of hydrozincite and its conversion into zinc oxide nanoparticles. J. Alloys Compd. 2008, 461, 66–71. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Sun, L. A comparative study on the corrosion of galvanized steel under simulated rust layer solution with and without 3.5wt% NaCl. Int. J. Electrochem. Sci. 2013, 8, 3494–3509. [Google Scholar]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygiel, B. XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018, 2018, 2079278. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.J.; Sun, C.; Zhao, P.X.; Wang, S.F. Solubility of Stearic Acid in Ethanol, 1-Propanol, 2-Propanol, L-Butanol, Acetone, Methylene Chloride, Ethyl Acetate and 95% Ethanol from (293 to 315) K. Adv. Mater. Res. 2012, 550–553, 71–74. [Google Scholar] [CrossRef]

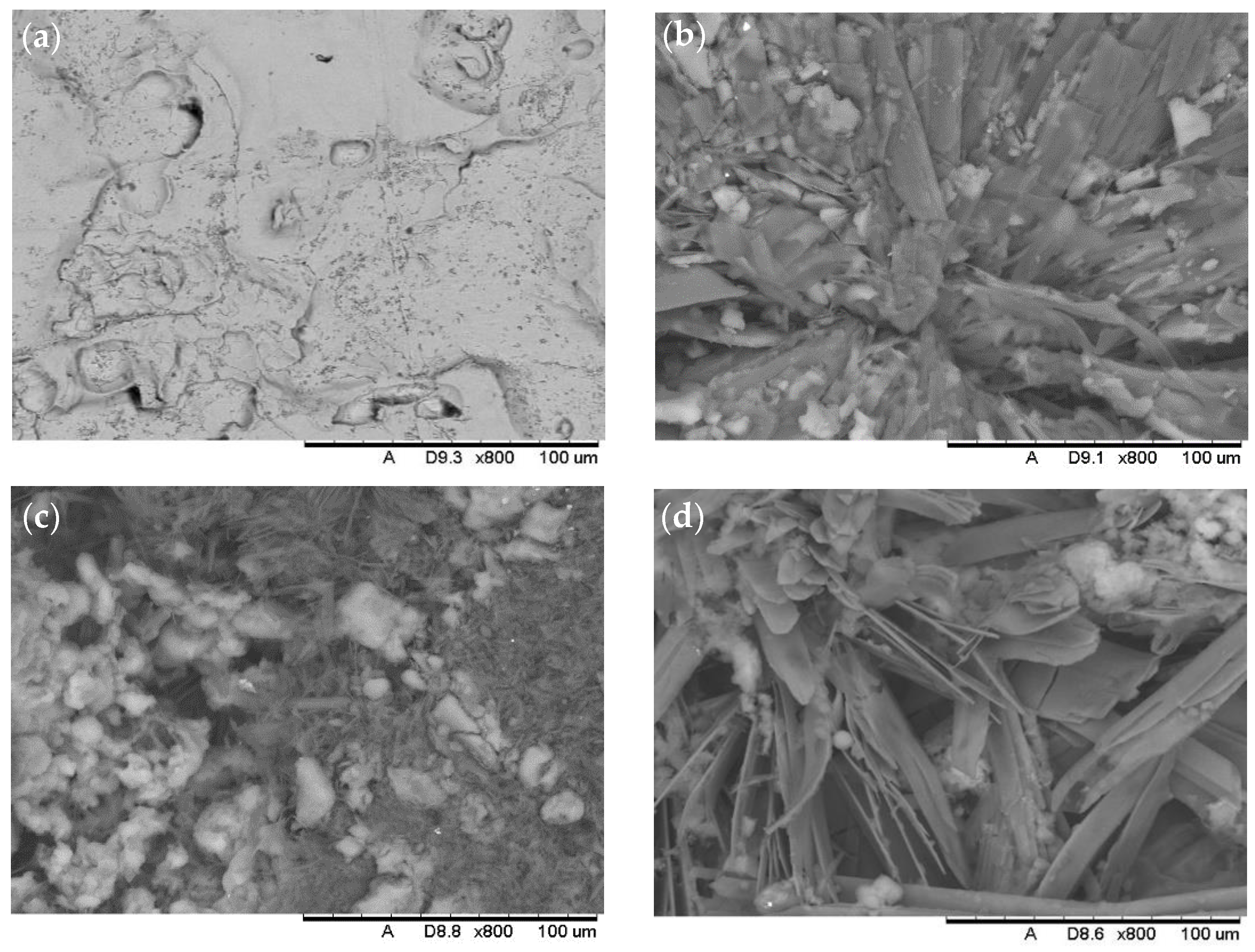
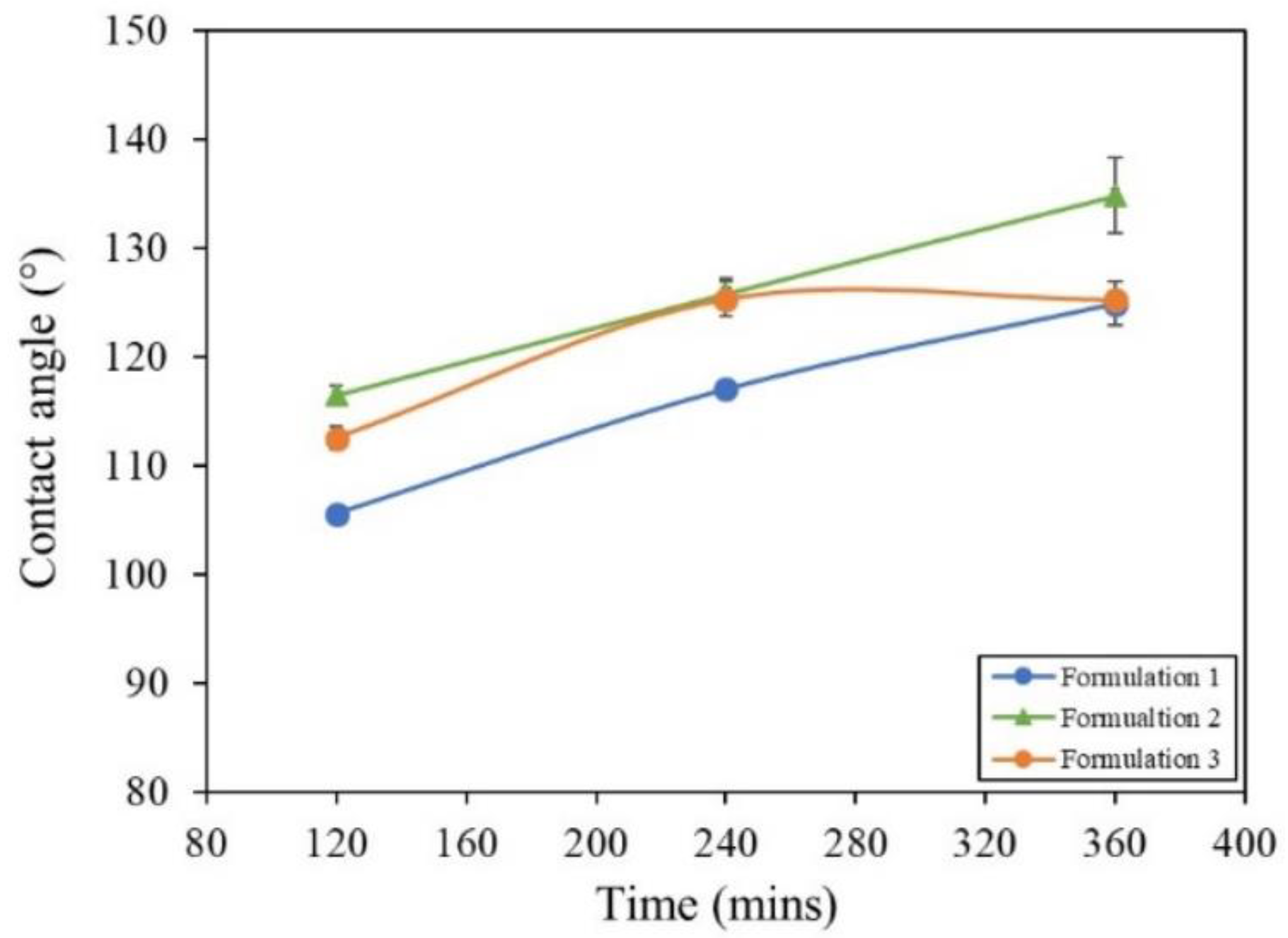

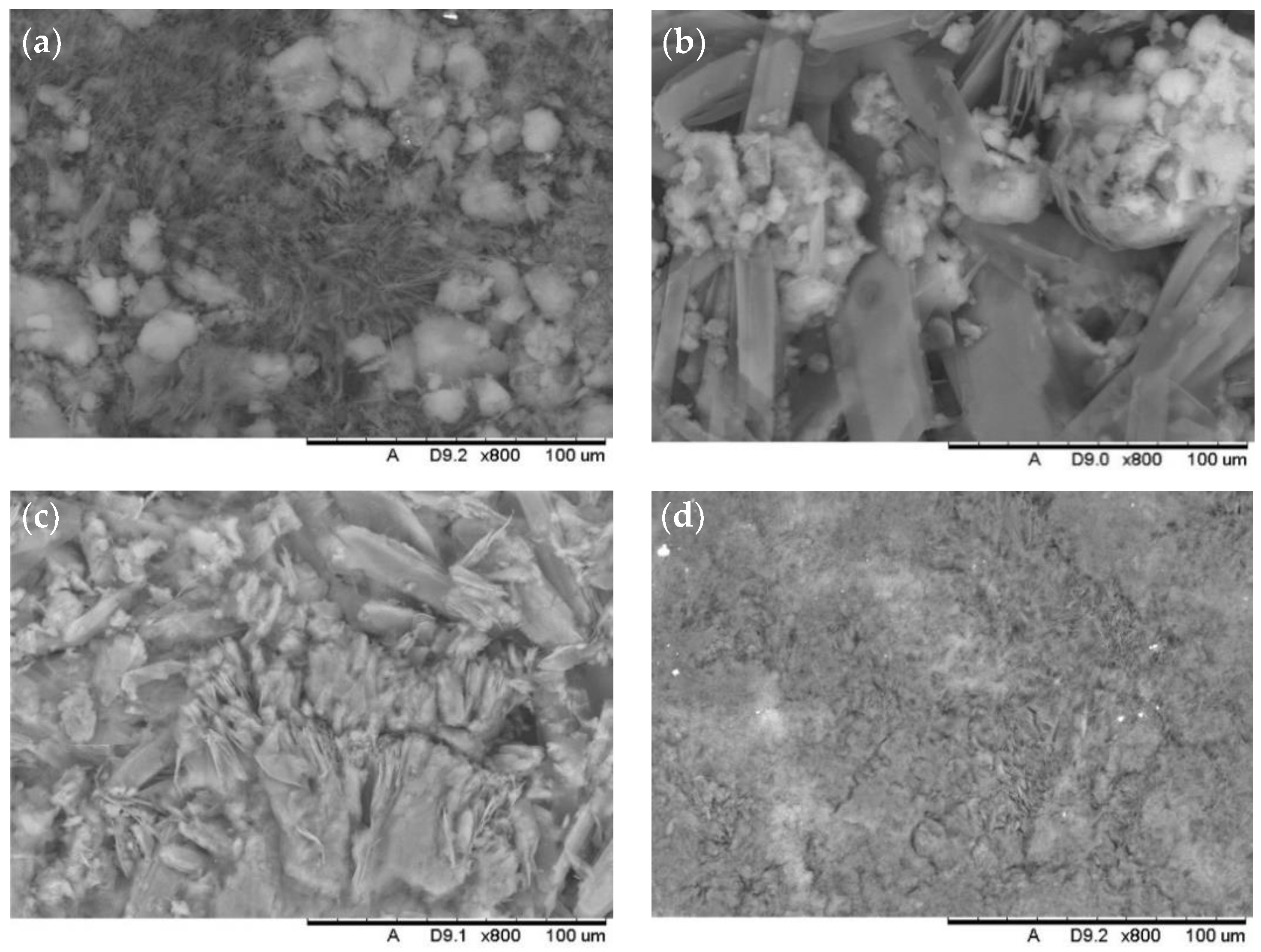
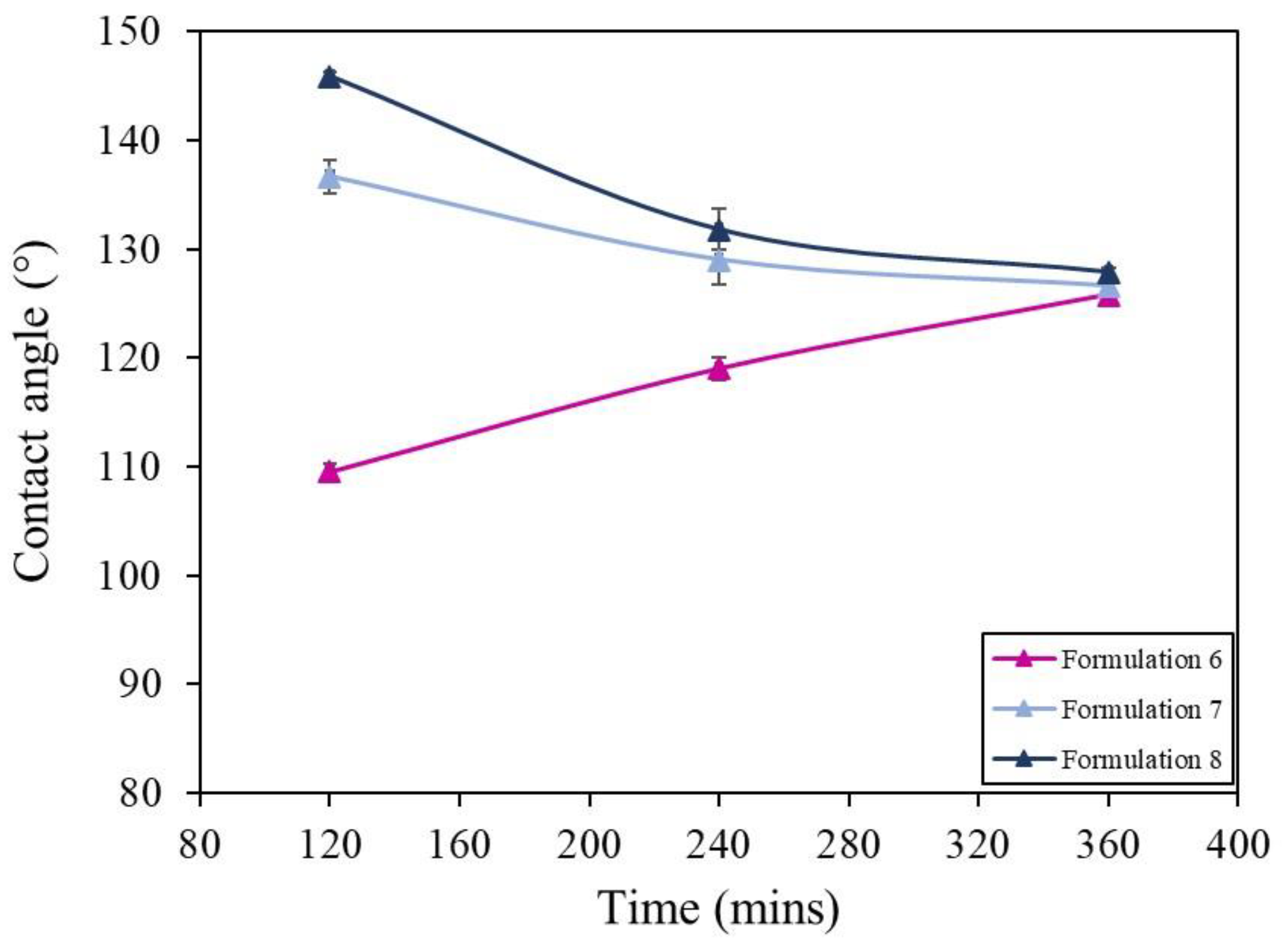



| Formulation | Solvent | Molarity of Stearic Acid (M) | SiO2 Size (nm) | NaOH Addition |
|---|---|---|---|---|
| 1 | Ethanol | 0.05 | 200–300 | ✗ |
| 2 | IPA | 0.05 | 200–300 | ✗ |
| 3 | Ethanol | 0.05 | 200–300 | ✓ |
| 4 | Ethanol | 0.1 | 200–300 | ✗ |
| 5 | Ethanol | 0.2 | 200–300 | ✗ |
| 6 | IPA | 0.05 | 200–300 | ✓ |
| 7 | IPA | 0.1 | 200–300 | ✓ |
| 8 | IPA | 0.2 | 200–300 | ✓ |
| 9 | Ethanol | 0.05 | 9–20 | ✓ |
| 10 | Ethanol | 0.1 | 9–20 | ✓ |
| 11 | Ethanol | 0.2 | 9–20 | ✓ |
| 12 | IPA | 0.05 | 9–20 | ✓ |
| 13 | IPA | 0.1 | 9–20 | ✓ |
| 14 | IPA | 0.2 | 9–20 | ✓ |
| Surface Roughness | Formulation 8 | Formulation 14 | |
|---|---|---|---|
| 120 min | 120 min | 360 min | |
| Average, Sa | 194 nm | 77 nm | 47 nm |
| RMS, Sq | 243 nm | 101 nm | 64 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, J.; Griffiths, C.; Dunlop, T.; Jewell, E. Improving the Processability of a One-Step Hydrophobic Coating for Hot-Dipped Galvanised Steel for Industrial Applications. Coatings 2022, 12, 895. https://doi.org/10.3390/coatings12070895
Williams J, Griffiths C, Dunlop T, Jewell E. Improving the Processability of a One-Step Hydrophobic Coating for Hot-Dipped Galvanised Steel for Industrial Applications. Coatings. 2022; 12(7):895. https://doi.org/10.3390/coatings12070895
Chicago/Turabian StyleWilliams, Jamie, Christian Griffiths, Tom Dunlop, and Eifion Jewell. 2022. "Improving the Processability of a One-Step Hydrophobic Coating for Hot-Dipped Galvanised Steel for Industrial Applications" Coatings 12, no. 7: 895. https://doi.org/10.3390/coatings12070895
APA StyleWilliams, J., Griffiths, C., Dunlop, T., & Jewell, E. (2022). Improving the Processability of a One-Step Hydrophobic Coating for Hot-Dipped Galvanised Steel for Industrial Applications. Coatings, 12(7), 895. https://doi.org/10.3390/coatings12070895







