Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials
Abstract
:1. Introduction
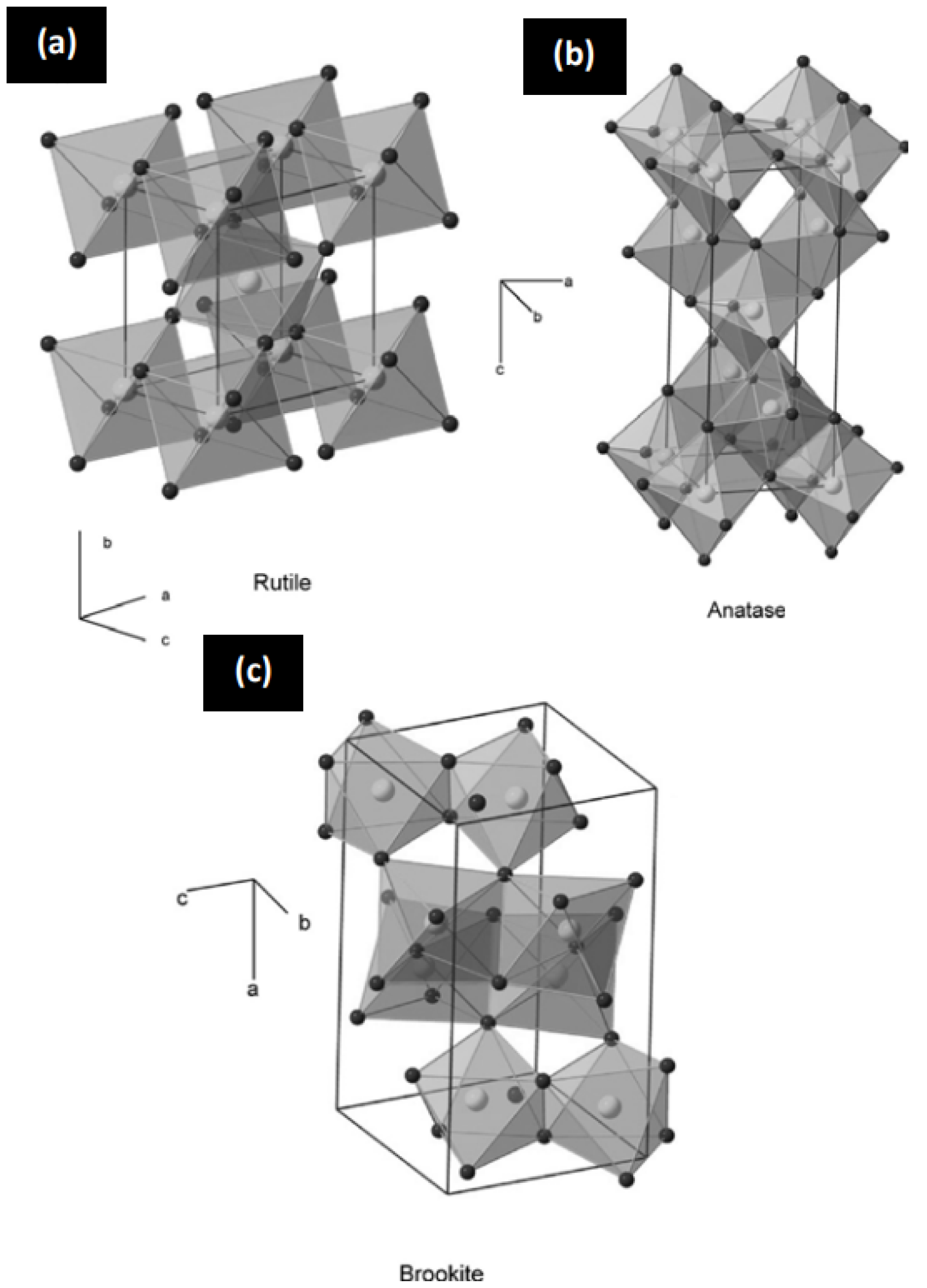
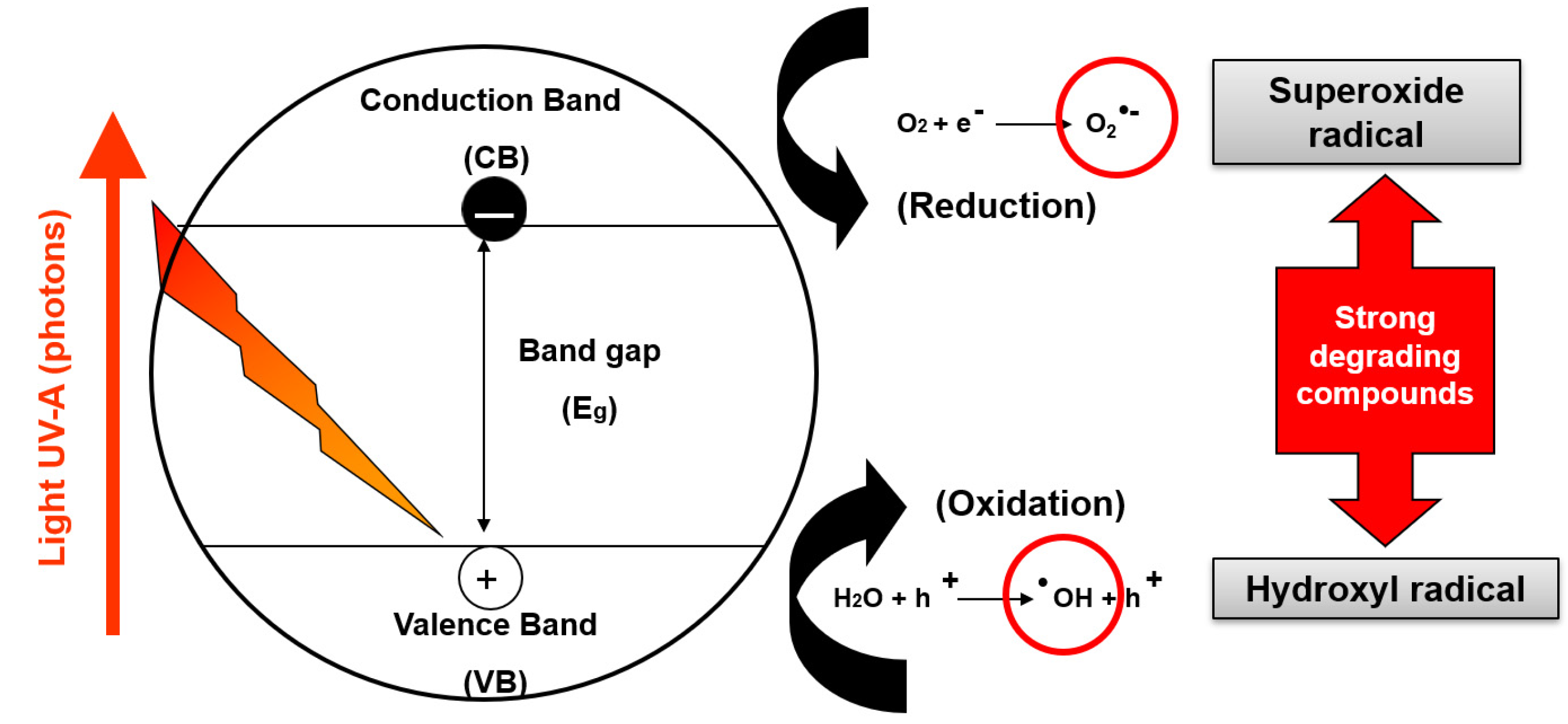
2. TiO2 Fundamentals
3. Cement-Based Materials Loaded with TiO2
4. Multifunctional Properties
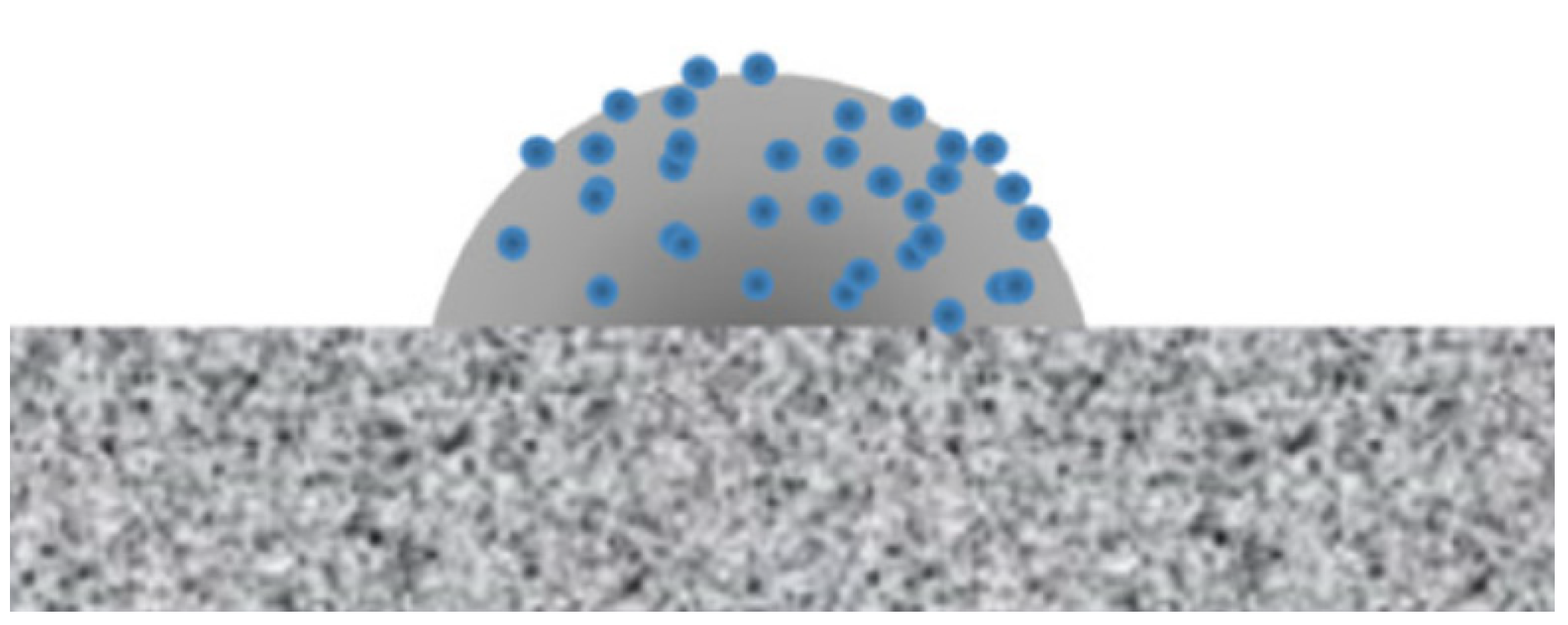
4.1. Self-Cleaning Properties
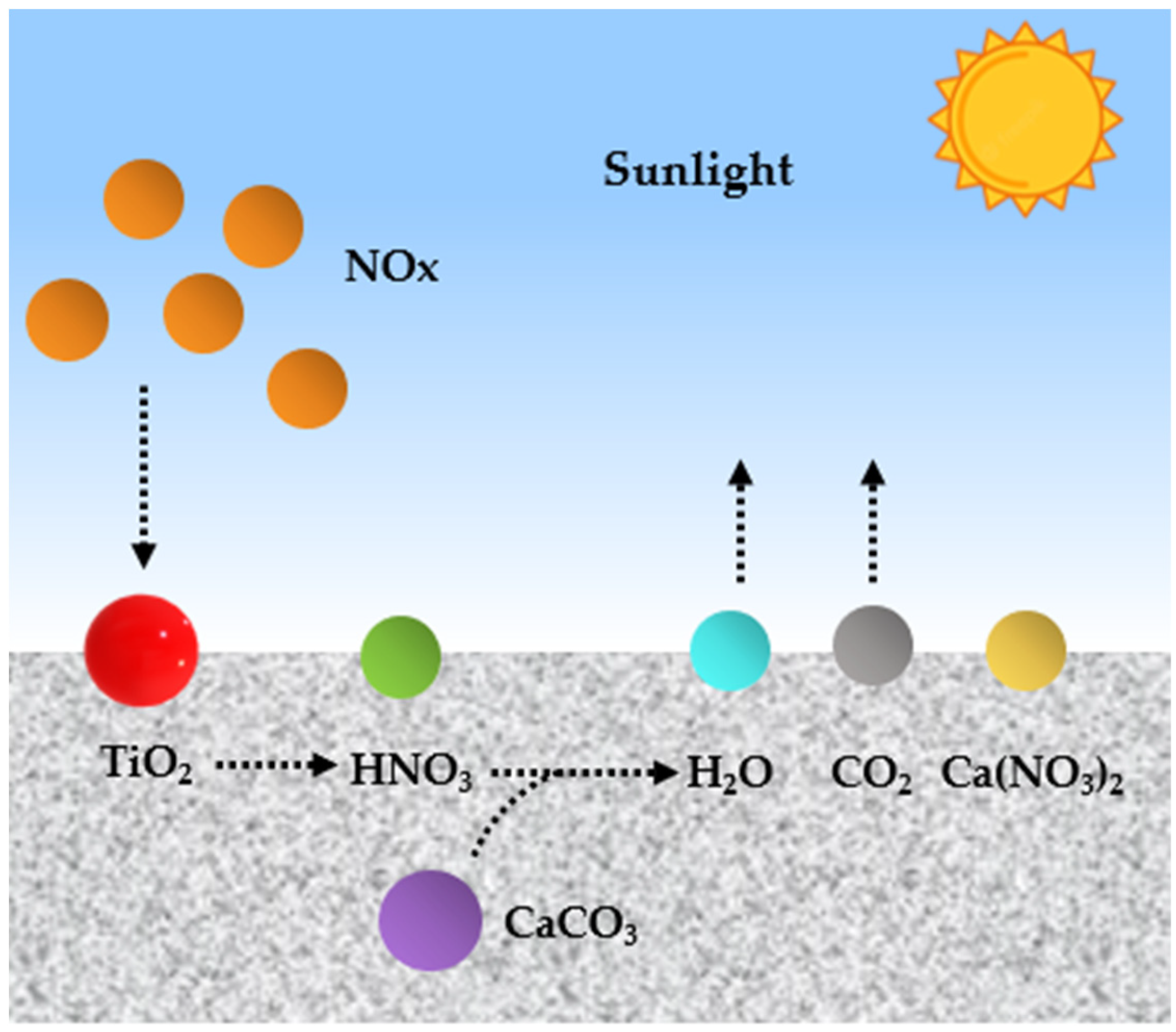
4.2. Air-Purifying Properties
4.3. Antimicrobial Properties
5. Conclusions
- First, at the laboratory scale, nano titanium dioxide (mainly anatase, rutile and its combination P25) has shown ability, under UV-A exposure, to degrade organic and inorganic substances in solid, gas and liquid phases. However, the promising efficiencies obtained at laboratory scale are not enough for in situ application in cement-based materials towards the development of air-purifying, self-cleaning and antiseptic buildings and infrastructure. This situation is mainly associated with the limited capacity of TiO2 to be photoactivated exclusively with UV-A. Therefore, more research about doped TiO2 and novel semiconductors should be developed to obtain higher efficiencies. Semiconductors such as ZnO, ZnS, CdS, CdSe and CdTe seem to be promising [65]. Similarly, other complementary nanotechnologies should be researched; recently promising results have been obtained with photocatalytic polyurethane coatings containing modified C60 fullerene additives [66] or using synthetized SiO2@TiO2 coatings [50].
- Second, although there has been wide discussion about the best method to load cement-based materials with TiO2, it is now clear that coatings and mixing technologies have different advantages/disadvantages regarding their application. For example, coatings use less catalyst material and expose more efficiently the nanoparticles to UV-A and polluting substances. However, coatings might have durability problems when exposed to high weathering or aggressive environmental conditions. Thus, coatings result as useful for application on tunnels and buildings, while TiO2 mixing technology is very useful for pavements and roads, where there are high weathering and aggressive environmental conditions. Loading TiO2 during concrete or mortar mixing has been normally done in amounts around 5 to 10% of the cement or binder content (weight basis). In this addition range, generally the mechanical and durability properties have not been affected. On the contrary, most research results indicate an increase in the compressive and flexural strengths. This is mainly associated with a denser material structure, evidenced by SEM. Still, resistance to sulphate and leachate attack seem to be reduced when adding TiO2 to cement-based materials. Therefore, it is important to adjust design methods to determine optimal TiO2 dosage.
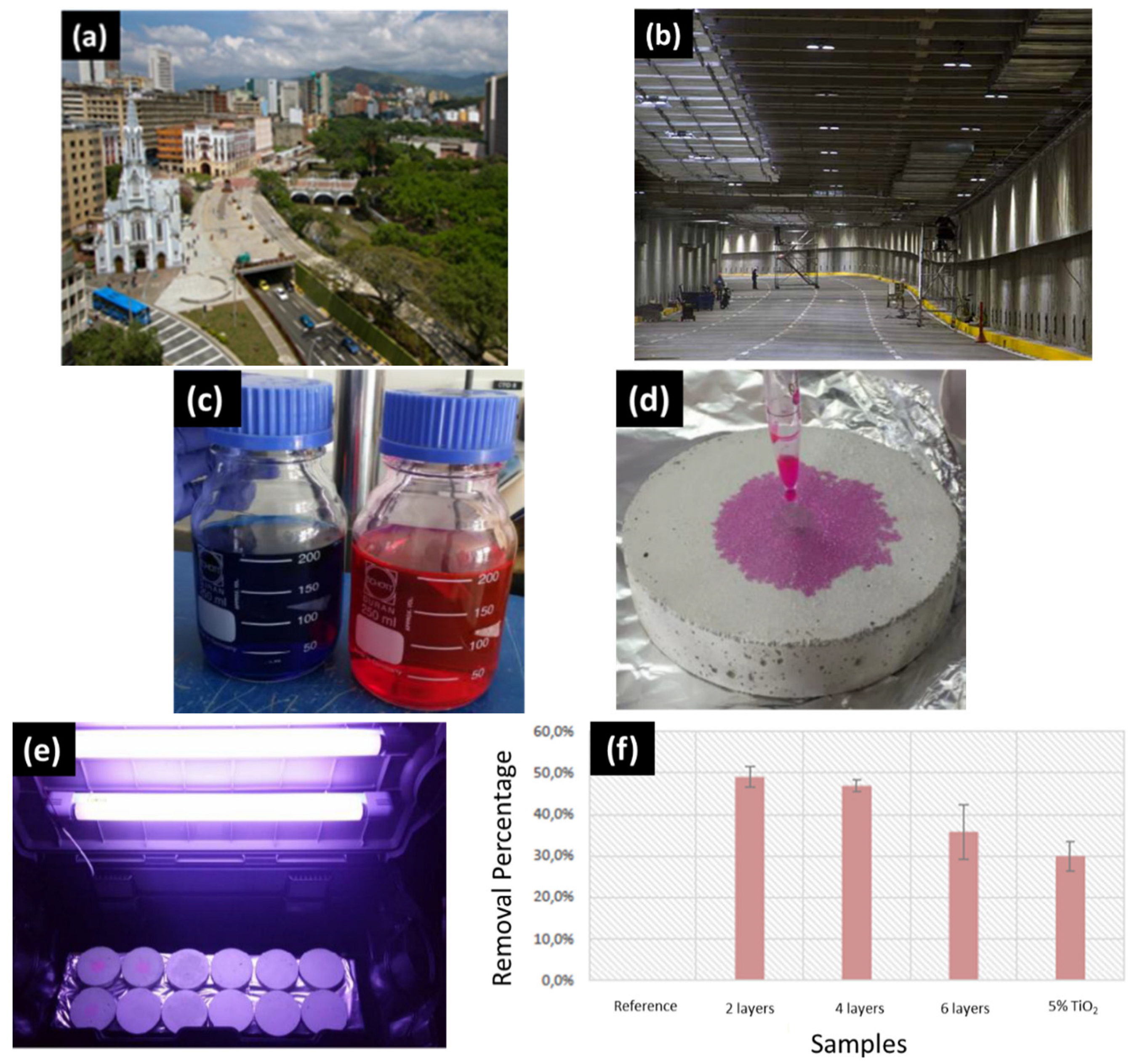
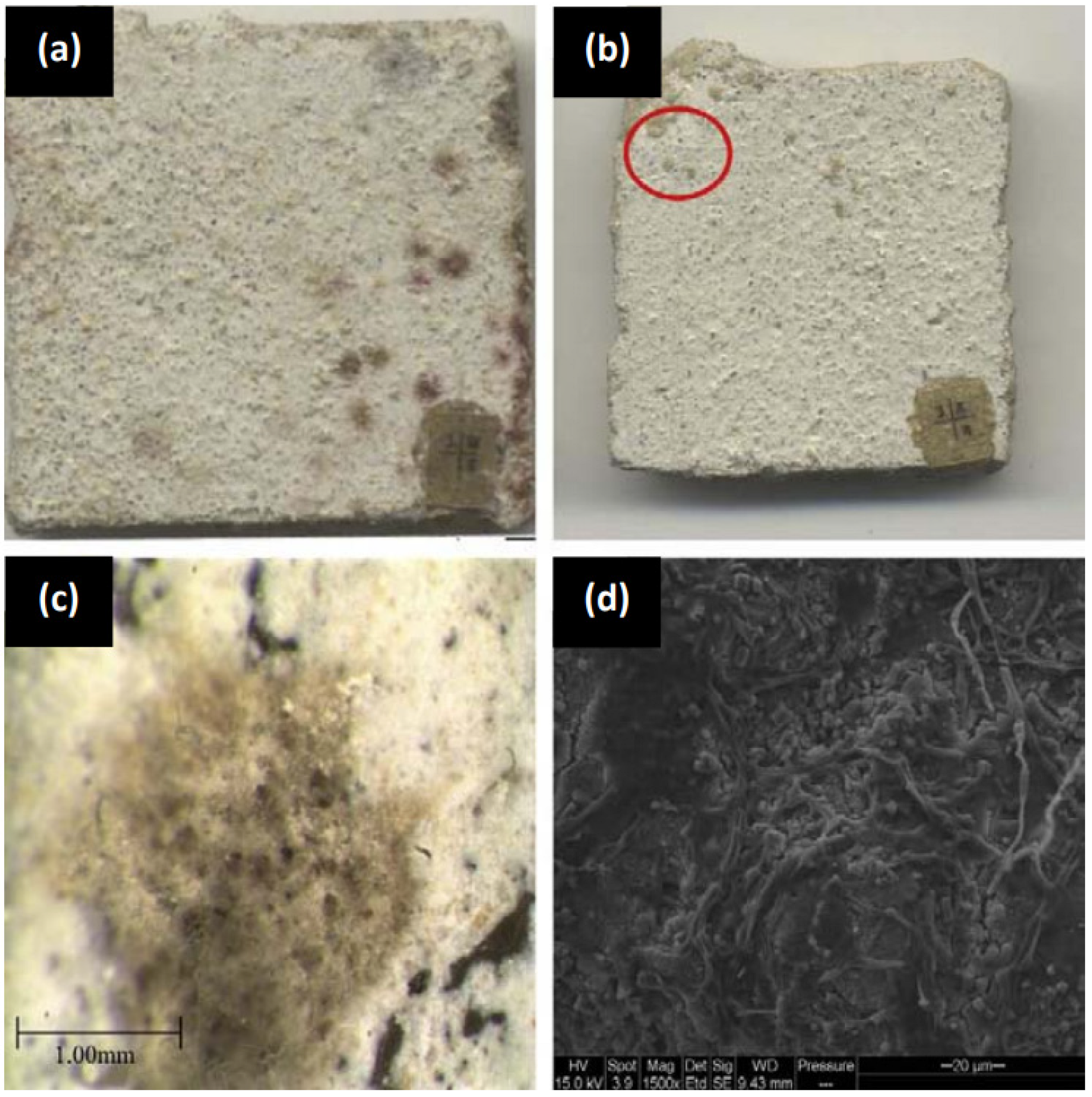
- Third, although countries such as Japan, China, Italy, Belgium, France, United Kingdom and the Netherlands have constructed buildings and infrastructure using TiO2-loaded cementitious materials [67], the obtained self-cleaning, air-purifying and antimicrobial properties still need to be improved. Regarding self-cleaning buildings, particularly when using white cement and TiO2, dirty surfaces, different white intensities and even cracks have been reported. Similarly, although high removal efficiencies towards organic and inorganic air pollutants have been reported, the environmental conditions, particularly rain and high humidity, significantly reduced TiO2 photocatalytic efficiency. With respect to antimicrobial properties, although there has been microbial growth inhibition using TiO2-loaded cementitious materials at laboratory scale tests, enhancing the TiO2 photocatalytic activity to all fouling microorganisms and proving the long-lasting effect are still major concerns for developing the so-called antimicrobial cement-based materials.
- In general, although intensive research on photocatalytic cement-based materials has developed worldwide during the last 20 years, more innovative and strategic research should be done towards the massive application at real scale. Environmental and weathering conditions are demanding very efficient photocatalyst materials or even complementary technologies, e.g., coming from the architectural design, to develop self-cleaning, air-purifying and antimicrobial properties on buildings and infrastructure. In order to achieve this goal, more realistic laboratory tests, modelling prior to real application, Life Cycle Assessments (LCA) and Social Life Cycle Assessments (S-LCA) should be performed; particularly the latter two, as self-cleaning, antimicrobial and air-purifying properties have a positive potential impact by increasing building and infrastructure durability and reducing air pollution, while nanomaterial production has a potential negative environmental impact, a life cycle thinking approach, which is about going beyond the traditional focus on production site and manufacturing processes to include environmental, social and economic impacts of a product over its entire life cycle, is required to develop truly circular economy models in the construction sector [68,69].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ng, D.S.; Paul, S.C.; Anggraini, V.; Kong, S.Y.; Qureshi, T.S.; Rodriguez, C.R.; Liu, Q.-F.; Šavija, B. Influence of SiO2, TiO2 and Fe2O3 nanoparticles on the properties of fly ash blended cement mortars. Constr. Build. Mater. 2020, 258, 119627. [Google Scholar] [CrossRef]
- Saeli, M.; Tobaldi, D.M.; Rozman, N.; Škapin, A.S.; Labrincha, J.A.; Pullar, R.C. Photocatalytic nano-composite architectural lime mortar for degradation of urban pollutants under solar and visible (interior) light. Constr. Build. Mater. 2017, 152, 206–213. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, W.; Jin, R.; Shen, J.; Smallbone, K.; Yan, C.; Hu, L. Experimental investigation of photocatalytic effects of concrete in air purification adopting entire concrete waste reuse model. J. Hazard. Mater. 2018, 353, 421–430. [Google Scholar] [CrossRef]
- Atta-ur-Rehman; Qudoos, A.; Kim, H.G.; Ryou, J.-S. Influence of titanium dioxide nanoparticles on the sulfate attack upon ordinary Portland cement and slag-blended mortars. Materials 2018, 11, 356. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Cui, S.; Li, H.; Abdelhady, A.; Wang, H.; Zhou, H. Removal effect on stormwater runoff pollution of porous concrete treated with nanometer titanium dioxide. Transp. Res. Part D Transp. Environ. 2019, 73, 34–45. [Google Scholar] [CrossRef]
- Yuenyongsuwan, J.; Sinthupinyo, S.; O’Rear, E.A.; Pongprayoon, T. Hydration accelerator and photocatalyst of nanotitanium dioxide synthesized via surfactant-assisted method in cement mortar. Cem. Concr. Compos. 2018, 96, 182–193. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Rehman, A.U.; Kim, J.H.; Kim, H.G.; Qudoos, A.; Ryou, J.-S. Effect of leaching on the hardened, microstructural and self-cleaning characteristics of titanium dioxide containing cement mortars. Constr. Build. Mater. 2019, 207, 640–650. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Li, J.-S.; Poon, C.S. Improved photocatalytic nitrogen oxides removal using recycled glass-nano-TiO2 composites with NaOH pre-treatment. J. Clean. Prod. 2018, 209, 1095–1104. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Obregón, S.; Patrón-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B Environ. 2020, 270, 118853. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Verran, J.; Stratton, J.; Maltby, J.; Bygott, C. Photocatalytic surfaces: Antipollution and antimicrobial effects. In Nanotechnology; Volume 2: Environmental Aspects; Krug, H.F., Ed.; WILEY: Hoboken, NJ, USA, 2008. [Google Scholar]
- Maury, A.; De Belie, N. State of the art of TiO2 containing cementitious materials: Self-cleaning properties. Mater. Constr. 2010, 60, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-G.; Ishigaki, T. Brookite → rutile phase transformation of TiO2 studied with monodispersed particles. Acta Mater. 2004, 52, 5143–5150. [Google Scholar] [CrossRef]
- Feng, S.; Song, J.; Liu, F.; Fu, X.; Guo, H.; Zhu, J.; Zeng, Q.; Peng, X.; Wang, X.; Ouyang, Y.; et al. Photocatalytic properties, mechanical strength and durability of TiO2/cement composites prepared by a spraying method for removal of organic pollutants. Chemosphere 2020, 254, 126813. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Jafari, H.; Afshar, S. Improved Photodegradation of Organic Contaminants Using Nano-TiO2 and TiO2-SiO2 Deposited on Portland Cement Concrete Blocks. Photochem. Photobiol. 2015, 92, 87–101. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.; Dionísio, A. Self-cleaning property of mortars with TiO2 addition using real diesel exhaust soot. J. Clean. Prod. 2017, 161, 850–859. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Lucas, S. Influence of operating parameters and ion doping on the photocatalytic activity of mortars containing titanium dioxide nanoparticles. Mater. Today Proc. 2017, 4, 11588–11593. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Self-cleaning ability of titanium dioxide clear paint coated architectural mortar and its potential in field application. J. Clean. Prod. 2016, 112, 3583–3588. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Zheng, L.; Jones, R.; Wang, F.; Macphee, D.E. Photocatalytic concrete for NOx abatement: Supported TiO2 efficiencies and impacts. Cem. Concr. Res. 2018, 116, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Constantino, J.C.; Garcia, D.C.S.; Palhares, H.G.; Houmard, M.; Figueiredo, R.B. Development of functional TiO2 coatings deposited on cementitious materials. Constr. Build. Mater. 2020, 250, 118732. [Google Scholar] [CrossRef]
- Maury-Ramirez, A.; Nikkanen, J.-P.; Honkanen, M.; Demeestere, K.; Levänen, E.; De Belie, N. TiO2 coatings synthesized by liquid flame spray and low temperature sol–gel technologies on autoclaved aerated concrete for air-purifying purposes. Mater. Charact. 2014, 87, 74–85. [Google Scholar] [CrossRef]
- Daniyal, M.; Akhtar, S.; Azam, A. Effect of nano-TiO2 on the properties of cementitious composites under different exposure environments. J. Mater. Res. Technol. 2019, 8, 6158–6172. [Google Scholar] [CrossRef]
- De Melo, J.V.S.; Trichês, G. Study of the influence of nano-TiO2 on the properties of Portland cement concrete for application on road surfaces. Road Mater. Pavement Des. 2017, 19, 1011–1026. [Google Scholar] [CrossRef]
- Guzmán-Aponte, L.; De Gutiérrez, R.M.; Maury-Ramírez, A. Metakaolin-Based Geopolymer with Added TiO2 Particles: Physicomechanical Characteristics. Coatings 2017, 7, 233. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Li, H.; Li, X.; Mei, J.; Lv, Y. Influence of nano-TiO2 on physical and hydration characteristics of fly ash–cement systems. Constr. Build. Mater. 2016, 122, 242–253. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Ling, T.-C.; Poon, C.S. Photocatalytic NO x degradation of concrete surface layers intermixed and spray-coated with nano-TiO2: Influence of experimental factors. Cem. Concr. Compos. 2017, 83, 279–289. [Google Scholar] [CrossRef]
- Maury-Ramírez, A. Cementitious Materials with Air-Purifying and Self-Cleaning Properties Using Titanium Dioxide Photocatalysis. Ph.D. Dissertation, Ghent University, Ghent, Belgium, 2011. [Google Scholar]
- Jafari, H.; Afshar, S.; Zabihi, O.; Naebe, M. Enhanced photocatalytic activities of TiO2–SiO2 nanohybrids immobilized on cement-based materials for dye degradation. Res. Chem. Intermed. 2015, 42, 2963–2978. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Chen, J.; Xia, M.; Wang, T.; Poon, C.S. Pathways of conversion of nitrogen oxides by nano TiO2 incorporated in cement-based materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Maury-Ramirez, A.; Demeestere, K.; De Belie, N. Photocatalytic activity of titanium dioxide nanoparticle coatings applied on autoclaved aerated concrete: Effect of weathering on coating physical characteristics and gaseous toluene removal. J. Hazard. Mater. 2012, 211–212, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Horszczaruk, E.; Rucinska, T. The Effect of nanosilica and titanium dioxide on the mechanical and self-cleaning properties of waste-glass cement mortar. Procedia Eng. 2015, 108, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.C.-M. Deactivation and Regeneration of Environmentally Exposed Titanium Dioxide (TiO2) Based Products. Testing Report Prepared for the Environmental Protection Department of Hong Kong City. 2003. Available online: https://www.epd.gov.hk/epd/sites/default/files/epd/english/environmentinhk/air/studyrpts/files/report-updatedcb_paving_blocks_2003.pdf (accessed on 15 May 2022).
- Etxeberria, M.; Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Influence of dust and oil accumulation on effectiveness of photocatalytic concrete surfaces. J. Environ. Eng. 2017, 143, 04017040. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Yang, J.; Yang, E.-H. Self-cleaning engineered cementitious composites. Cem. Concr. Compos. 2015, 64, 74–83. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef] [Green Version]
- Cassar, L.; Pepe, C.; Tognon, G.; Guerrini, G.L.; Amadelli, R. White cement for architectural concrete, possessing photocatalytic properties. In Proceedings of the 11th International Congress on the Chemistry of Cement, Durban, South Africa, 1–11 May 2003. [Google Scholar]
- Wang, D.; Hou, P.; Zhang, L.; Yang, P.; Cheng, X. Photocatalytic and hydrophobic activity of cement-based materials from benzyl-terminated-TiO2 spheres with core-shell structures. Constr. Build. Mater. 2017, 148, 176–183. [Google Scholar] [CrossRef]
- Faraldos, M.; Kropp, R.; Anderson, M.; Sobolev, K. Photocatalytic hydrophobic concrete coatings to combat air pollution. Catal. Today 2016, 259, 228–236. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Q.; Peng, B.; Chai, L.; Liu, H. Self-cleaning performance of TiO2-coating cement materials prepared based on solidification/stabilization of electrolytic manganese residue. Constr. Build. Mater. 2016, 106, 236–242. [Google Scholar] [CrossRef]
- Suave, J.; Amorim, S.M.; Moreira, R.F. TiO2-graphene nanocomposite supported on floating autoclaved cellular concrete for photocatalytic removal of organic compounds. J. Environ. Chem. Eng. 2017, 5, 3215–3223. [Google Scholar] [CrossRef]
- Yang, J.; Wang, G.; Wang, D.; Liu, C.; Zhang, Z. A self-cleaning coating material of TiO2 porous microspheres/cement composite with high-efficient photocatalytic depollution performance. Mater. Lett. 2017, 200, 1–5. [Google Scholar] [CrossRef]
- Rosales, A.; Maury-Ramírez, A.; Gutiérrez, R.M.-D.; Guzmán, C.; Esquivel, K. SiO2@TiO2 Coating: Synthesis, Physical Characterization and Photocatalytic Evaluation. Coatings 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.-Z.; Maury-Ramirez, A.; Poon, C.S. Versatile photocatalytic functions of self-compacting architectural glass mortars and their inter-relationship. Mater. Des. 2015, 88, 1260–1268. [Google Scholar] [CrossRef]
- Karapati, S.; Giannakopoulou, T.; Todorova, N.; Boukos, N.; Antiohos, S.; Papageorgiou, D.; Chaniotakis, E.; Dimotikali, D.; Trapalis, C. TiO2 functionalization for efficient NOx removal in photoactive cement. Appl. Surf. Sci. 2014, 319, 29–36. [Google Scholar] [CrossRef]
- Wang, D.; Leng, Z.; Hüben, M.; Oeser, M.; Steinauer, B. Photocatalytic pavements with epoxy-bonded TiO2-containing spreading material. Constr. Build. Mater. 2016, 107, 44–51. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Medina Medina, A.F.; Torres Rojas, D.F.; Meza Girón, G.; Villota Grisales, R.A. Design of a System to Generate Air Purification and Self-Cleaning in the Surfaces of the Colombia Avenue Tunnel; Pontificia Universidad Javeriana Cali: Cali, Colombia, 2016. [Google Scholar]
- The Hong Kong Polytechnique University. Green Deck—A Dream of Turning Grey to Green. Available online: https://www.greendeck.hk/ (accessed on 9 June 2022).
- Naranjo, A.; Colonia, A.; Mesa, J.; Maury, H.; Maury-Ramírez, A. State-of-the-Art Green Roofs: Technical Performance and Certifications for Sustainable Construction. Coatings 2020, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, A.; Colonia, A.; Mesa, J.; Maury-Ramírez, A. Evaluation of Semi-Intensive Green Roofs with Drainage Layers Made Out of Recycled and Reused Materials. Coatings 2020, 10, 525. [Google Scholar] [CrossRef]
- Maury-Ramirez, A.; De Muynck, W.; Stevens, R.; Demeestere, K.; De Belie, N. Titanium dioxide based strategies to prevent algal fouling on cementitious materials. Cem. Concr. Compos. 2013, 36, 93–100. [Google Scholar] [CrossRef]
- Linkous, C.A.; Carter, G.J.; Locuson, D.B.; Ouellete, A.J.; Slattery, D.K.; Smitha, L.A. Photocatalytic inhibition of algae growth using TiO2, WO3, and cocatalyts modifications. Environ. Sci. Technol. 2000, 34, 4754–4758. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef] [Green Version]
- Gladis, F.; Schumann, R. Influence of material properties and photocatalysis on phototrophic growth in multi-year roof weathering. Int. Biodeterior. Biodegrad. 2011, 65, 36–44. [Google Scholar] [CrossRef]
- Giannantonio, D.J.; Kurth, J.C.; Kurtis, K.E.; Sobecky, P.A. Effects of concrete properties and nutrients on fungal colonization and fouling. Int. Biodeterior. Biodegrad. 2009, 63, 252–259. [Google Scholar] [CrossRef]
- Maury-Ramírez, A.; Flores-Colen, I.; Kanematsu, H. Advanced Coatings for Buildings. Coatings 2020, 10, 728. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H. Heterogeneous Photocatalysis as an Advanced Oxidation Process for the Abatement of Chlorinated, Monocyclic Aromatic and Sulfurous Volatile Organic Compounds in Air: State of the Art. Crit. Rev. Environ. Sci. Technol. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Lundin, J.G.; Giles, S.L.; Cozzens, R.F.; Wynne, J.H. Self-Cleaning Photocatalytic Polyurethane Coatings Containing Modified C60 Fullerene Additives. Coatings 2014, 4, 614–629. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Hakki, A.; Wang, F.; Macphee, D.E. Photocatalyst efficiencies in concrete technology: The effect of photocatalyst placement. Appl. Catal. B Environ. 2018, 222, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Maury-Ramírez, A.; Illera-Perozo, D.; Mesa, J.A. Circular Economy in the Construction Sector: A Case Study of Santiago de Cali (Colombia). Sustainability 2022, 14, 1923. [Google Scholar] [CrossRef]
- Mesa, J.A.; Fúquene-Retamoso, C.; Maury-Ramírez, A. Life Cycle Assessment on Construction and Demolition Waste: A Systematic Literature Review. Sustainability 2021, 13, 7676. [Google Scholar] [CrossRef]



| Material | Application Process | Quantity | Geometry | Compressive Strength (28 Days) | Flexural Strength (28 Days) | Modulus of Elasticity | Durability | Observations | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Medium | Compressive Strength (360 Days) | |||||||||
| NT (nano TiO2) | Mixing technique | 5% by weight of OPC replacement | 70.6 mm side. | 16.21% higher than the control specimen. | - | - | Tap water. | Higher than the control specimen. | Mild steel bar of 3 mm diameter was used as embedding reinforcement. | Daniyal et al. [28] |
| 26.14% higher than the control specimen. | - | - | Saline water. | Higher than the control specimen. | ||||||
| 25.80% higher than the control specimen. | - | - | Acidic solution. | Higher than the control specimen. | ||||||
| Anatase I | Mixing technique | 10% by weight | 50 mm diameter, 100 mm height. | −3.70% | - | −38.90% | - | - | - | Victor et al. [29] |
| Anatase II | 17.30% | - | −26% | - | - | - | ||||
| Rutile | 10.50% | - | −30.10% | - | - | - | ||||
| NT (nano TiO2) | Mixing technique | 3 wt% of cement. | 50 mm side. | 36% higher than the control specimen. | - | - | - | - | Fly ash was added with a dosage of 30% wt.% of cement. | Siang et al. [1] |
| 40 mm side, 160 mm height. | - | 11% higher than the control specimen. | - | - | - | |||||
| Anatase | Mixing technique | 3% of the binder weight | 25.4 mm × 25.4 mm × 279.4 mm | 10% higher than the control specimen. | 3.80% | - | - | - | - | Atta-ur-Rehman et al. [4] |
| 6% of the binder weight | 15% higher than the control specimen. | −15.10% | - | - | - | - | ||||
| mSiO2/TiO2 | Mixing technique | 3 wt% | 40 mm × 40 mm × 160 mm | 15% higher than the control specimen. | Slightly higher than the control sample. | - | - | - | - | Sikora et al. [13] |
| Material | Application Process | Quantity | Efficiency | Environmental Conditions | Observations | References |
|---|---|---|---|---|---|---|
| Anatase I | Mixing technique | 10% by weight | 44.1 mg × h−1 × m−2 | Flow rate 1.0 L × min−1; UV-A radiation 10 ± 2 W × m−2, relative atmospheric humidity 50 ± 5% and pollutant concentration (NOx) 20 ppmv. | The efficiency is in terms of removal of NOx. | Victor et al. [29] |
| Anatase II | 11.71 mg × h−1 × m−2 | |||||
| Rutile | 37.24 mg × h−1 × m−2 | |||||
| Nano-TiO2–SiO2 | Dip coating | - | 87% of MG, 80% MB and 65% MO | UV irradiation, room temperature. | The photocatalytic material was added to WPC blocks. The efficiency is in terms of percent of decomposition of dyes. | Hoda Jafari et al. [35] |
| P25 (75% anatase and 25%rutile) | Mixing technique | 2% by weight of binders | NOx removal rate: 28 μmol m−2 × h−1. NOx removal ratio: 5.8% | 24 h UV-A irradiation. | The TiO2 was incorporated in self-compacting glass mortar (SCGM). WPC and metakaolin were used as cementitious materials. | M.-Z. Guo et al. [36] |
| Nano-TiO2 | Dip coating and vacuum saturation | TiO2 ethanol suspension of 0.05 g × mL−1 | Toluene removal efficiency: 95 % elimination rate: 60–70 mg × m−2 × h−1 | 24 °C, 52% relative humidity. | Initial toluene concentration: 15 ppmv gas residence time: 3 min. | A. Maury-Ramirez et al. [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Hoyos, A.M.; Rojas Manzano, M.A.; Maury-Ramírez, A. Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings 2022, 12, 968. https://doi.org/10.3390/coatings12070968
Castro-Hoyos AM, Rojas Manzano MA, Maury-Ramírez A. Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings. 2022; 12(7):968. https://doi.org/10.3390/coatings12070968
Chicago/Turabian StyleCastro-Hoyos, Angélica María, Manuel Alejandro Rojas Manzano, and Aníbal Maury-Ramírez. 2022. "Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials" Coatings 12, no. 7: 968. https://doi.org/10.3390/coatings12070968
APA StyleCastro-Hoyos, A. M., Rojas Manzano, M. A., & Maury-Ramírez, A. (2022). Challenges and Opportunities of Using Titanium Dioxide Photocatalysis on Cement-Based Materials. Coatings, 12(7), 968. https://doi.org/10.3390/coatings12070968








