Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
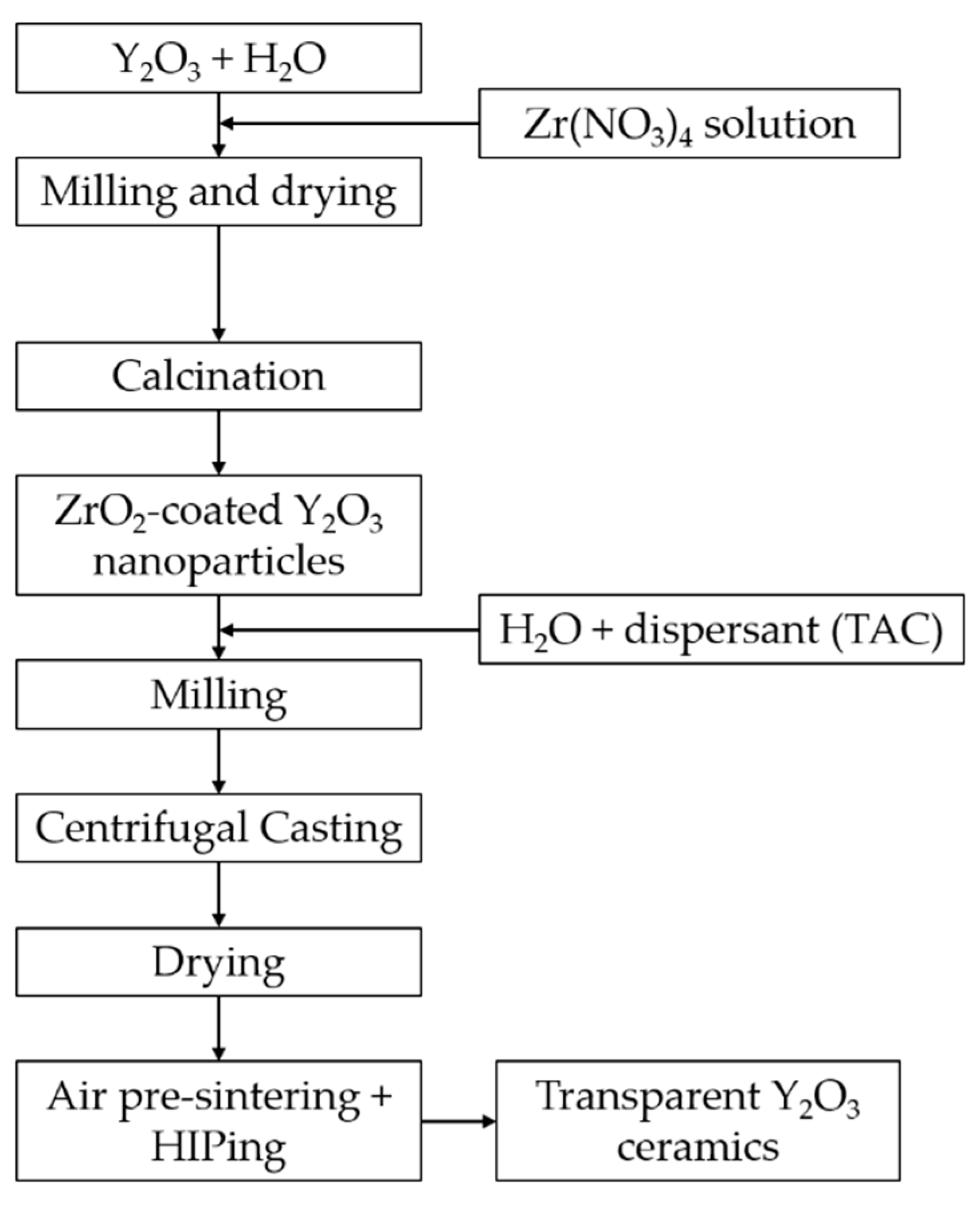
2.2. Coating Procedure and Suspension Preparation
2.3. Consolidation and Sintering
2.4. Characterization
3. Results and Discussion
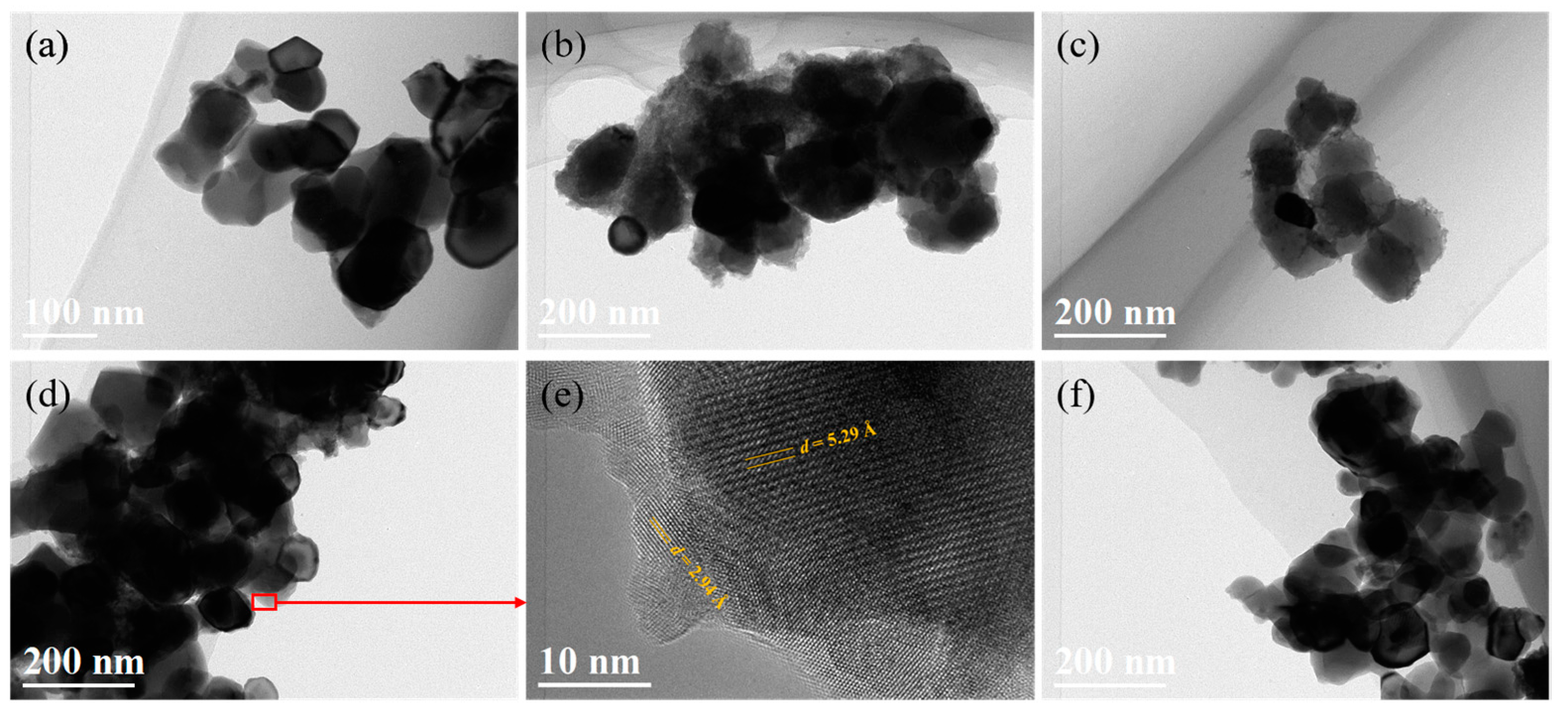
3.1. Coating Y2O3 Particle with Zirconium Nitrate via Thermal Decomposition Method
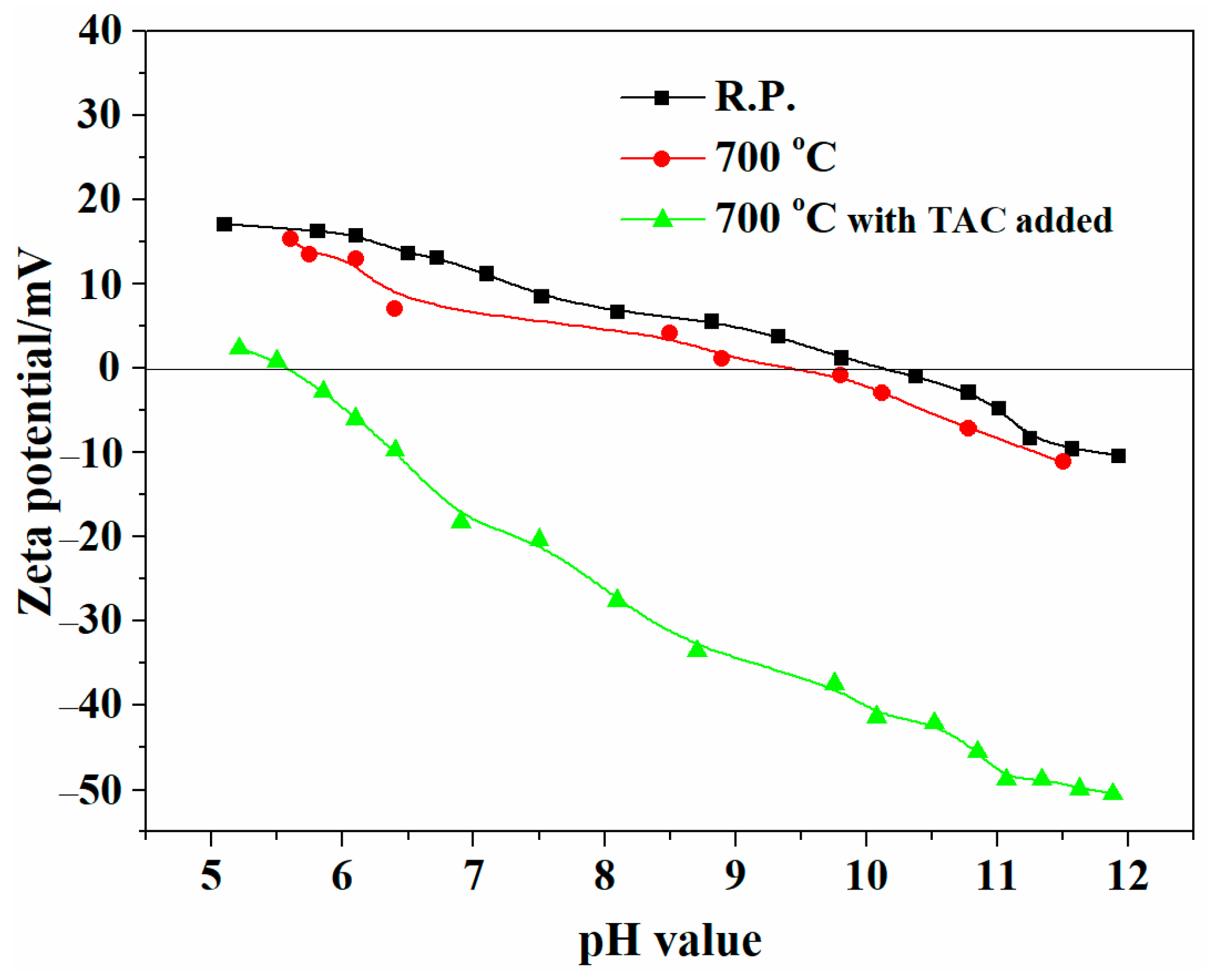
3.2. Dispersion Properties of ZrO2-Coated Y2O3 Powders
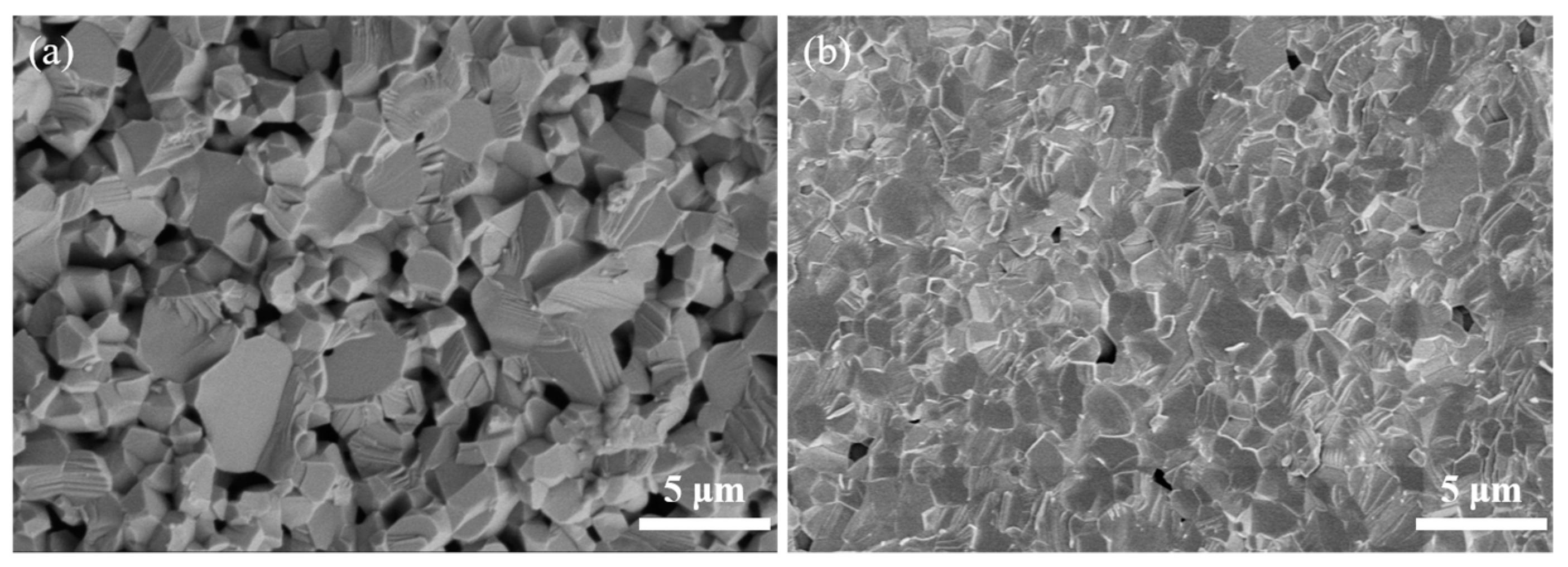
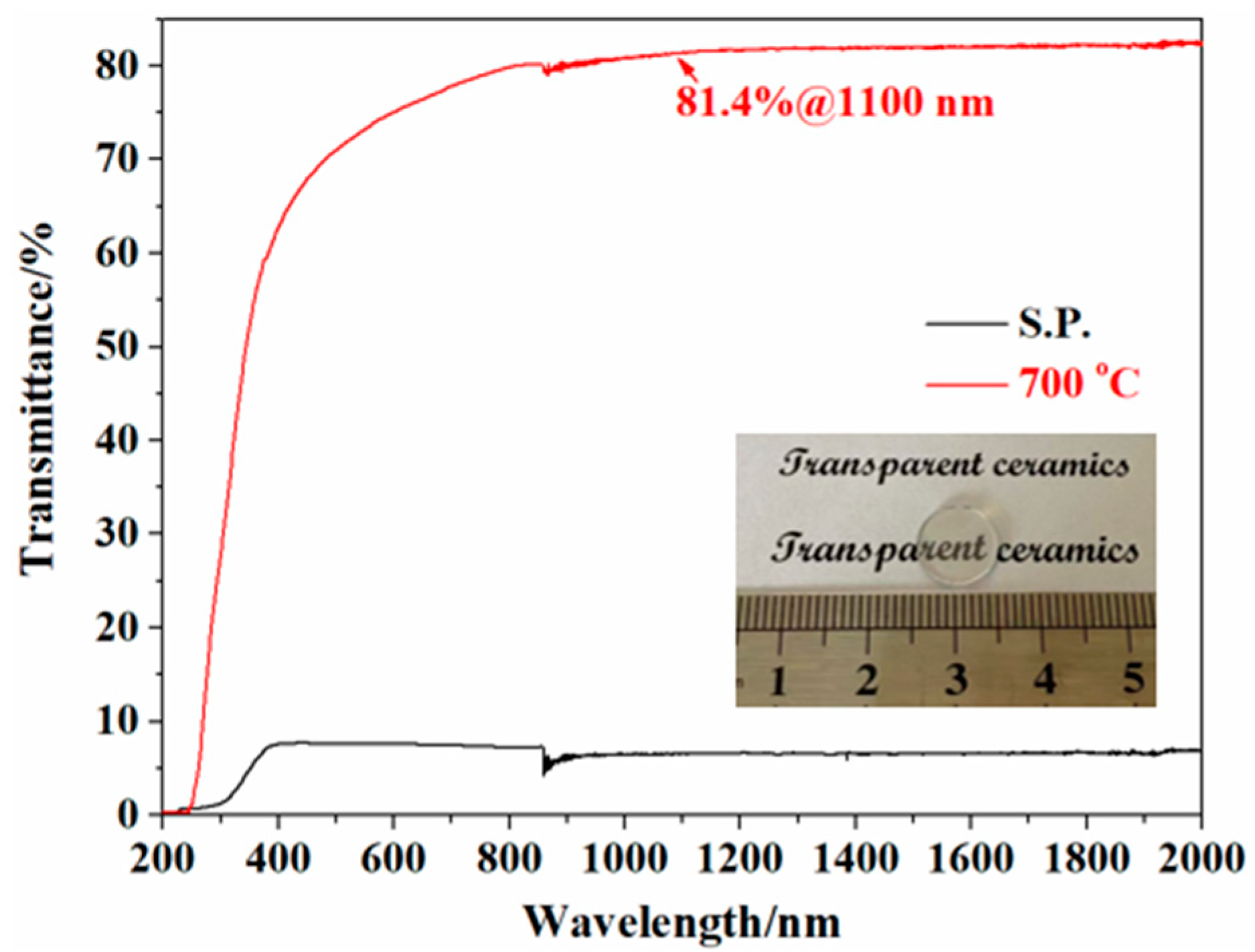
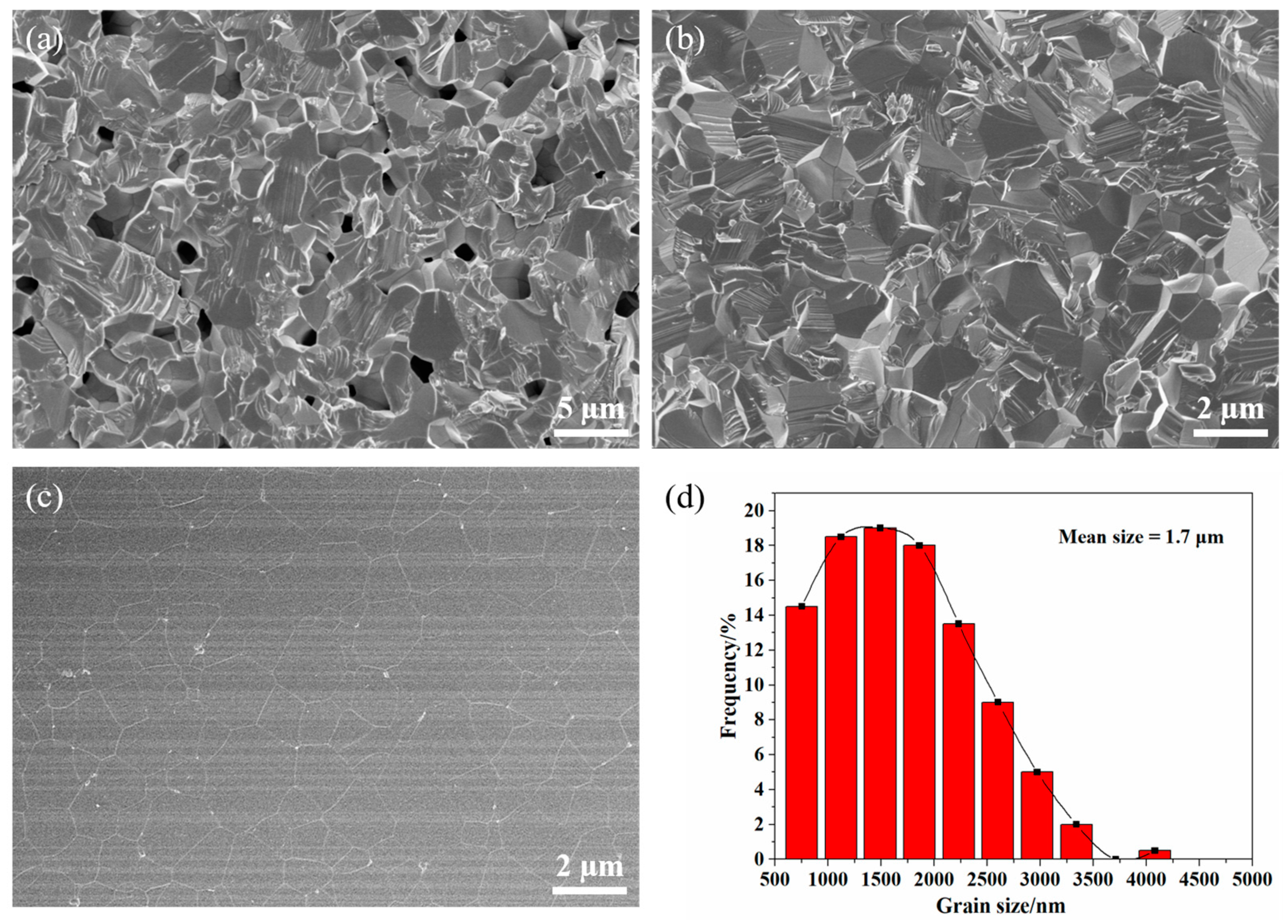
3.3. Consolidation and Sintering of Y2O3 Transparent Ceramics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Wang, J.; Ma, J.; Ni, M.; Yang, F.; Liu, P.; Lee, K.Y.; Hsiang, H.; Shen, D.; Tang, D. Fabrication of, high-efficiency Yb:Y2O3 laser ceramics without photodarkening. J. Am. Ceram. Soc. 2022, 105, 3375–3381. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, S.; Li, Y.; Ruan, S.; Kong, L.B.; Huang, Q.; Huang, Z.; Zhou, K.; Su, H.; Yao, Z.; et al. Materials development and potential applications of transparent ceramics: A review. Mater. Sci. Eng. R 2020, 139, 14–19. [Google Scholar] [CrossRef]
- Iwasawa, J.; Nishimizu, R.; Tokita, M. Plasma-resistant dense yttrium oxide film prepared by aerosol deposition process. J. Am. Ceram. Soc. 2007, 90, 2327–2332. [Google Scholar] [CrossRef]
- Kirm, M.; Feldbach, E.; Kink, R.; Lushchik, A.; Lushchik, C.; Maaroos, A.; Martinson, I. Mechanisms of intrinsic and impurity luminescence excitation by synchrotron radiation in wide-gap oxides. J. Electron Spectros. Relat. Phenom. 1996, 79, 91–94. [Google Scholar] [CrossRef]
- Furuse, H.; Nakasawa, S.; Yoshida, H.; Morita, K.; Suzuki, T.S.; Kim, B.-N.; Sakka, Y.; Hiraga, K. Transparent ultrafine Yb3+:Y2O3 laser ceramics fabricated by spark plasma sintering. J. Am. Ceram. Soc. 2018, 101, 694–702. [Google Scholar] [CrossRef]
- Gan, L.; Park, Y.-J.; Park, M.-J.; Kim, H.; Kim, J.-M.; Ko, J.-W.; Lee, J.-W. Facile fabrication of highly transparent yttria ceramics with fine microstructures by a hot-pressing method. J. Am. Ceram. Soc. 2015, 989, 2002–2004. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, L.; Yang, H.; Zhang, J.; Wang, L.X.; Zhang, Q.T. High optical quality Y2O3 transparent ceramics with fine grain size fabricated by low temperature air pre-sintering and post-HIP treatment. Ceram. Int. 2016, 42, 4238–4245. [Google Scholar] [CrossRef]
- Zhang, L.; Ben, Y.; Chen, H.; Tang, D.; Fu, X.; Sun, R.; Song, B.; Wong, C. Low temperature-sintering and microstructure of highly transparent yttria ceramics. J. Alloys Compd. 2017, 695, 2580–2586. [Google Scholar] [CrossRef]
- Liu, L.K.; Zhu, Q.H.; Zhu, Q.Q.; Jiang, B.X.; Feng, M.H.; Zhang, L. Fabrication of fine-grained undoped Y2O3 transparent ceramic using nitrate pyrogenation synthesized nanopowders. Ceram. Int. 2019, 45, 5339–5345. [Google Scholar] [CrossRef]
- Fu, Z.C.; Li, X.D.; Ren, Y.; Zhang, M.; Geng, X.T.; Zhu, Q.; Li, J.-G.; Sun, X.D. Coating Y2O3 nano-particles with ZrO2-additive via precipitation method for colloidal processing of highly transparent Y2O3 ceramics. J. Eur. Ceram. Soc. 2019, 39, 4996–5004. [Google Scholar] [CrossRef]
- Zhang, J.; An, L.-Q.; Liu, M.; Shimai, S.; Wang, S.-W. Sintering of Yb3+:Y2O3 transparent ceramics in hydrogen atmosphere. J. Eur. Ceram. Soc. 2009, 29, 305–309. [Google Scholar] [CrossRef]
- Fu, Z.C.; Li, X.D.; Zhang, M.; Zhu, Q.; Li, J.-G.; He, J.; Wang, X.-A.; Sun, X.D. Achieving fabrication of highly transparent Y2O3 ceramics via air pre-sintering by deionization treatment of suspension. J. Am. Ceram. Soc. 2021, 104, 2689–2701. [Google Scholar] [CrossRef]
- Ning, K.J.; Wang, J.; Ma, J.; Dong, Z.L.; Kong, L.B.; Tang, D.Y. Fabrication of laser grade Yb: Y2O3 transparent ceramics with ZrO2 additive through hot isostatic pressing. Mater. Today Commun. 2020, 24, 2352–4928. [Google Scholar] [CrossRef]
- Lange, F.F. Powder processing science and technology for increased reliability. J. Am. Ceram. Soc. 1989, 72, 3–15. [Google Scholar] [CrossRef]
- Olhero, S.M.; Ganesh, I.; Torres, P.M.C.; Alves, F.J.; Ferreira, J.M.F. Aqueous colloidal processing of ZTA composites. J. Am. Ceram. Soc. 2009, 92, 9–16. [Google Scholar] [CrossRef]
- Lewis, J.A. Colloidal processing of ceramics. J. Am. Ceram. Soc. 2000, 83, 2341–2359. [Google Scholar] [CrossRef]
- Yasrebi, M.; Zimek-Moroz, M.; Kemp, W.; Sturgis, D.H. Role of particle dissolution in stability of binary yttria–silica colloidal suspensions. J. Am. Ceram. Soc. 1996, 79, 1223–1227. [Google Scholar] [CrossRef]
- Kuroda, Y.; Hamano, H.; Mori, T.; Yoshikawa, Y.; Nagao, M. Specific adsorption behavior of water on a Y2O3 surface. Langmuir 2000, 16, 6937–6947. [Google Scholar] [CrossRef]
- Jin, L.L.; Mao, X.J.; Wang, S.W.; Dong, M.J. Optimization of the rheological properties of yttria suspensions. Ceram. Int. 2009, 35, 925–927. [Google Scholar] [CrossRef]
- Sun, Z.Q.; Zhu, X.W.; Li, M.S.; Zhou, Y.C.; Sakka, Y. Hydrolysis and dispersion properties of aqueous Y2Si2O7 suspensions. J. Am. Ceram. Soc. 2009, 92, 54–61. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Mao, X.J.; Fan, J.T.; Li, X.K.; Feng, M.H.; Jiang, B.X.; Leia, F.; Zhang, L. Fabrication of transparent yttria ceramics by alcoholic slip-casting. Ceram. Int. 2017, 43, 8839–8844. [Google Scholar] [CrossRef]
- Sun, Y.; Shimai, S.Z.; Peng, X.; Zhou, G.H.; Kamiya, H.; Wang, S.W. Fabrication of transparent Y2O3 ceramics via aqueous gelcasting. Ceram. Int. 2014, 40, 8841–8845. [Google Scholar] [CrossRef]
- Arshad, M.; Amer, M.; Hayat, Q.; Janik, V.; Zhang, X.; Moradi, M.; Bai, M. High-Entropy Coatings (HEC) for High-Temperature Applications: Materials, Processing, and Properties. Coatings 2022, 12, 691. [Google Scholar] [CrossRef]
- Aiken, B.; Matijević, E. Preparation and properties of uniform coated inorganic colloidal particles. IV. Yttrium basic carbonate and yttrium oxide on hematite. J. Colloid. Interface Sci. 1988, 126, 645–649. [Google Scholar] [CrossRef]
- Shojai, F.; Pettersson, A.B.A.; Mäntylä, T.; Rosenholm, J.B. Electrostatic and electrosteric stabilization of aqueous slips of 3Y–ZrO2 powder. J. Eur. Ceram. Soc. 2000, 20, 277–283. [Google Scholar] [CrossRef]
- Yasrebi, M.; Springgate, M.E.; Nikolas, D.G.; Kemp, W.; Sturgis, D.H. Colloidal stability of zirconia-doped yttria–silica binary aqueous suspensions. J. Am. Ceram. Soc. 1997, 80, 1615–1618. [Google Scholar] [CrossRef]
- Crocombette, J.P.; Jollet, F. Site selectivity in ZrO2-doped Y2O3 evidenced by X-ray absorption spectra calculations. J. Phys. Condens. Matter. 1994, 6, 8341–8348. [Google Scholar] [CrossRef]
- Wakai, F.; Sakaguchi, S.; Matsuno, Y. Superplasticity of yttria-stabilized tetragonal ZrO2 polycrystals. Adv. Ceram. Mater. 1986, 1, 259–263. [Google Scholar] [CrossRef]
- Boniecki, M.; Natanzon, Y.; Łodziana, Z. Effect of cation doping on lattice and grain boundary diffusion in superplastic yttria-stabilized tetragonal zirconia. J. Eur. Ceram. Soc. 2010, 30, 657–668. [Google Scholar] [CrossRef]
- Coble, R.L. Sintering crystalline solids I. Intermediate and final state diffusion models. J. Appl. Phys. 1961, 32, 787–792. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryavtseva, I.; Kuzovkov, V.N.; Popov, A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion-controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and transparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Wu, N.; Long, H.; Wang, J.; Zhang, J.; Hou, Z.; Li, X.; Sun, X. Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles. Coatings 2022, 12, 1077. https://doi.org/10.3390/coatings12081077
Fu Z, Wu N, Long H, Wang J, Zhang J, Hou Z, Li X, Sun X. Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles. Coatings. 2022; 12(8):1077. https://doi.org/10.3390/coatings12081077
Chicago/Turabian StyleFu, Zhongchao, Nan Wu, Haibo Long, Jianming Wang, Jun Zhang, Zhaoxia Hou, Xiaodong Li, and Xudong Sun. 2022. "Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles" Coatings 12, no. 8: 1077. https://doi.org/10.3390/coatings12081077
APA StyleFu, Z., Wu, N., Long, H., Wang, J., Zhang, J., Hou, Z., Li, X., & Sun, X. (2022). Fabrication of Highly Transparent Y2O3 Ceramics via Colloidal Processing Using ZrO2-Coated Y2O3 Nanoparticles. Coatings, 12(8), 1077. https://doi.org/10.3390/coatings12081077




