Reactive Sputtered Ytterbium Silicate Environmental Barrier Coatings for Protection of Mo-Si-Based Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
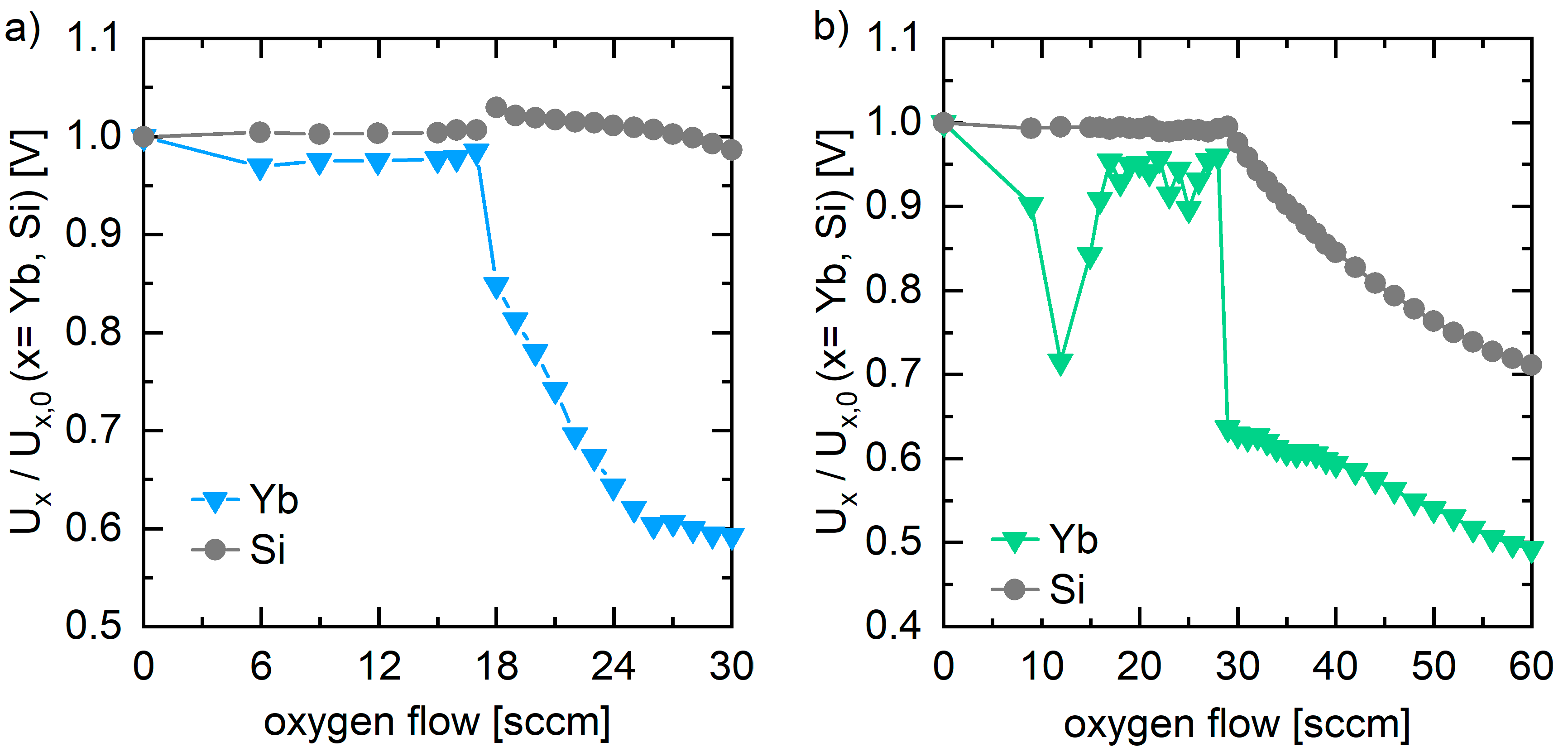
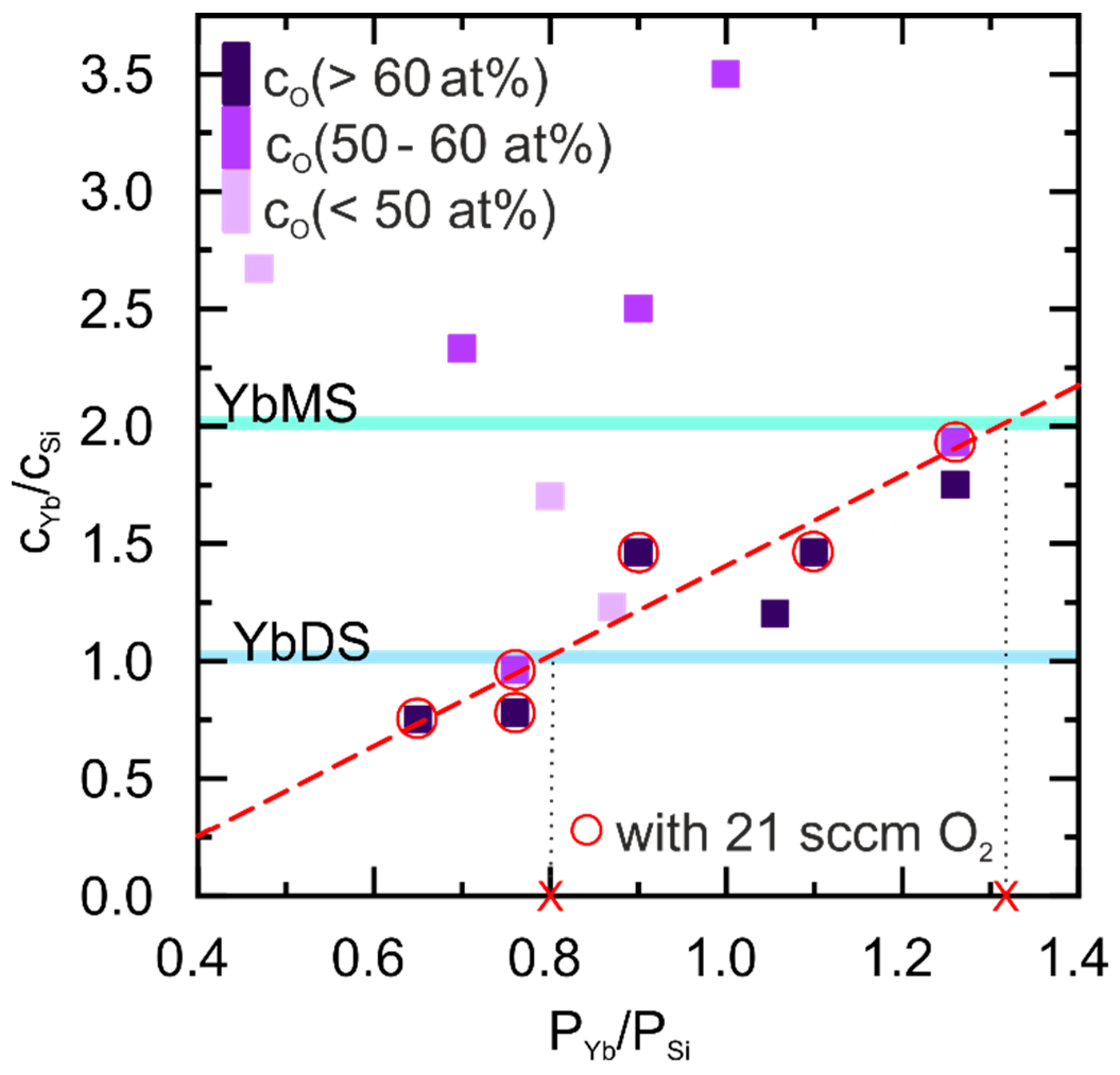
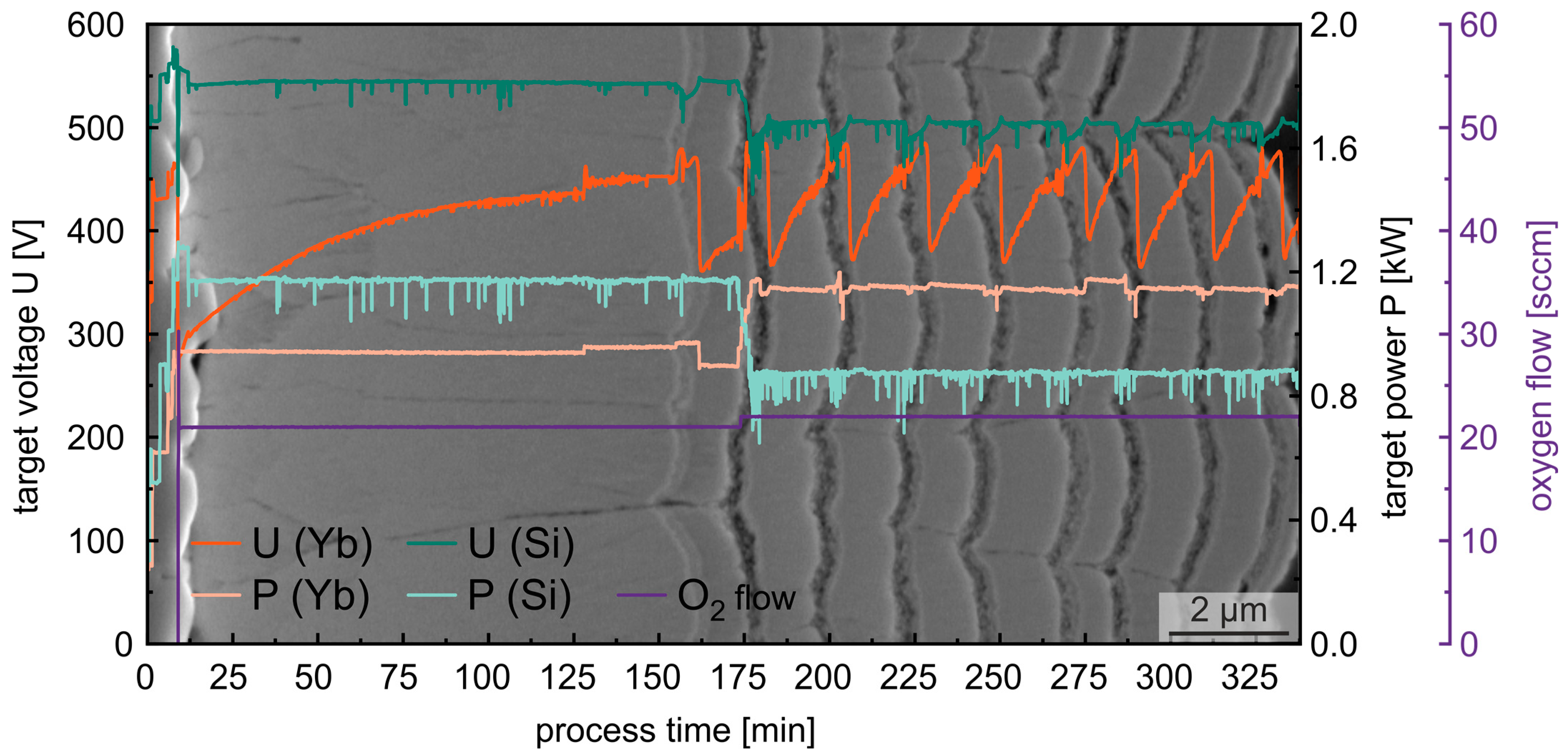
3.1. Reactive Pulsed DC Co-Sputtering
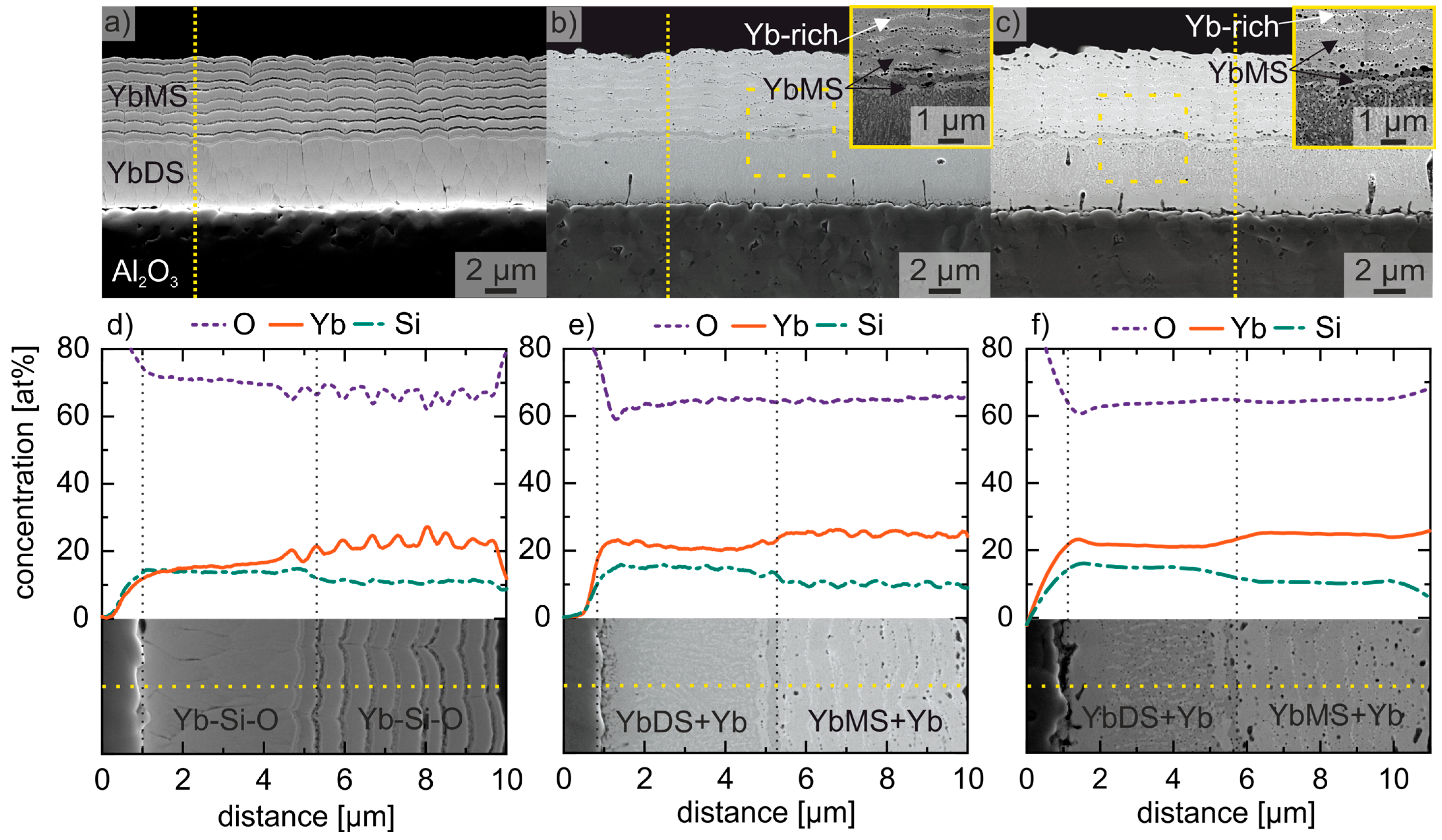
3.2. Analyses of Yb2SiO5 and Yb2Si2O7 Deposited Layers
3.3. Implementation of an Yb-Silicate Based EBC System on Mo-Si-Based Alloys
4. Discussion
4.1. Influence of the PVD Process Parameter on the Yb-Silicate Coatings
4.2. Influence of the Crystallisation, Morphology and Oxidation Process of an EBC Coating System on an Inert Al2O3 Substrate and Different Mo-Si-Based Alloys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.N. Current status of environmental barrier coatings for Si-Based ceramics. Surf. Coat. Technol. 2000, 133–134, 1–7. [Google Scholar] [CrossRef]
- Padture, N.P. Environmental degradation of high-temperature protective coatings for ceramic-matrix composites in gas-turbine engines. npj Mater. Degrad. 2019, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Tejero-Martin, D.; Bennett, C.; Hussain, T. A review on environmental barrier coatings: History, current state of the art and future developments. J. Eur. Ceram. Soc. 2021, 41, 1747–1768. [Google Scholar] [CrossRef]
- Bakan, E.; Marcano, D.; Zhou, D.; Sohn, Y.J.; Mauer, G.; Vaßen, R. Yb2Si2O7 Environmental Barrier Coatings Deposited by Various Thermal Spray Techniques: A Preliminary Comparative Study. J. Therm. Spray Technol. 2017, 26, 1011–1024. [Google Scholar] [CrossRef]
- Lee, K.N. Environmental Barrier Coatings for SiCf/SiC. In Ceramic Matrix Composites; Bansal, N.P., Lamon, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 430–451. [Google Scholar]
- Costa, G.C.C.; Jacobson, N.S. Mass spectrometric measurements of the silica activity in the Yb2O3–SiO2 system and implications to assess the degradation of silicate-based coatings in combustion environments. J. Eur. Ceram. Soc. 2015, 35, 4259–4267. [Google Scholar] [CrossRef]
- Fritsch, M. Heißgaskorrosion Keramischer Werkstoffe in H2O-Haltigen Rauchgasatmosphären; Fraunhofer IrB Verlag: Stuttgart, Germany, 2016; Volume 2. [Google Scholar]
- Deal, B.E.; Grove, A.S. General Relationship for the Thermal Oxidation of Silicon. J. Appl. Phys. 1965, 36, 3770–3778. [Google Scholar] [CrossRef] [Green Version]
- Opila, E.J. Oxidation Kinetics of Chemically Vapor-Deposited Silicon Carbide in Wet Oxygen. J. Am. Ceram. Soc. 1994, 77, 730–736. [Google Scholar] [CrossRef]
- Ogawa, T.; Otani, N.; Yokoi, T.; Fisher, C.A.J.; Kuwabara, A.; Moriwake, H.; Yoshiya, M.; Kitaoka, S.; Takata, M. Density functional study of the phase stability and Raman spectra of Yb2O3, Yb2SiO5 and Yb2Si2O7 under pressure. Phys. Chem. Chem. Phys. 2018, 20, 16518–16527. [Google Scholar] [CrossRef]
- Richards, B.T.; Wadley, H.N.G. Plasma spray deposition of tri-layer environmental barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 3069–3083. [Google Scholar] [CrossRef]
- Garcia, E.; Sotelo-Mazon, O.; Poblano-Salas, C.A.; Trapaga, G.; Sampath, S. Characterization of Yb2Si2O7–Yb2SiO5 composite environmental barrier coatings resultant from in situ plasma spray processing. Ceram. Int. 2020, 46, 21328–21335. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Liu, Y.; Luo, H. Hydrothermal synthesis of ytterbium silicate nanoparticles. Inorg. Chem. 2010, 49, 1942–1946. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Niu, Y.R.; Li, H.; Zhou, H.J.; Dong, S.M.; Zheng, X.B.; Ding, C.X.; Sun, J.L. Thermal shock resistance of tri-layer Yb2SiO5/Yb2Si2O7/Si coating for SiC and SiC-matrix composites. J. Am. Ceram. Soc. 2018, 101, 4743–4752. [Google Scholar] [CrossRef]
- Richards, B.T.; Young, K.A.; de Francqueville, F.; Sehr, S.; Begley, M.R.; Wadley, H.N.G. Response of ytterbium disilicate–silicon environmental barrier coatings to thermal cycling in water vapor. Acta Mater. 2016, 106, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bakan, E.; Sohn, Y.J.; Kunz, W.; Klemm, H.; Vaßen, R. Effect of processing on high-velocity water vapor recession behavior of Yb-silicate environmental barrier coatings. J. Eur. Ceram. Soc. 2019, 39, 1507–1513. [Google Scholar] [CrossRef]
- Leisner, V. Neue Environmental Barrier Coatings für SiC/SiC-Faserverbundwerkstoffe durch PVD-Technologien. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2020. [Google Scholar]
- Leisner, V.; Kelm, K.; Schulz, U. Thin single-phase yttrium-based environmental barrier coating systems for SiC/SiC CMCs. J. Eur. Ceram. Soc. 2022. (submitted and under review). [Google Scholar]
- Obert, S. Characterisation of the Oxidation and Creep Behaviour of Novel Mo-Si-Ti Alloys. Ph.D. Thesis, Karlsruher Institut für Technologie (KIT), Karlsruhe, Germany, 2021. [Google Scholar]
- Obert, S.; Kauffmann, A.; Seils, S.; Boll, T.; Kauffmann-Weiss, S.; Chen, H.; Anton, R.; Heilmaier, M. Microstructural and chemical constitution of the oxide scale formed on a pesting-resistant Mo-Si-Ti alloy. Corros. Sci. 2021, 178, 109081. [Google Scholar] [CrossRef]
- Lange, A.; Braun, R.; Heilmaier, M. Oxidation behavior of magnetron sputtered double layer coatings containing molybdenum, silicon and boron. Intermetallics 2014, 48, 19–27. [Google Scholar] [CrossRef]
- Anton, R.; Hüning, S.; Laska, N.; Weber, M.; Schellert, S.; Gorr, B.; Christ, H.-J.; Heilmaier, M.; Schulz, U. Interface reactions of magnetron sputtered Si-based dual layer coating systems as oxidation protection for Mo-Si-Ti alloys. Surf. Coat. Technol. 2022, 444, 128620. [Google Scholar] [CrossRef]
- Anton, R.; Laska, N.; Schulz, U.; Obert, S.; Heilmaier, M. Magnetron Sputtered Silicon Coatings as Oxidation Protection for Mo-Based Alloys. Adv. Eng. Mater. 2020, 22, 2000218. [Google Scholar] [CrossRef]
- Anton, R.; Hüning, S.; Laska, N.; Weber, M.; Schellert, S.; Gorr, B.; Christ, H.-J.; Schulz, U. Graded PVD Mo-Si interlayer between Si coating and Mo-Si-B alloys: Investigation of oxidation behaviour. Corros. Sci. 2021, 192, 109843. [Google Scholar] [CrossRef]
- Berg, S.; Nyberg, T. Fundamental understanding and modeling of reactive sputtering processes. Thin Solid Film 2005, 476, 215–230. [Google Scholar] [CrossRef]
- Spencer, S. Table of Argon Sputtering Yields at 0°; National Physical Laboratory: London, UK, 2005. [Google Scholar]
- Martin, N.; Rousselot, C. Modelling of reactive sputtering processes involving two separated metallic targets. Surf. Coat. Technol. 1999, 114, 235–249. [Google Scholar] [CrossRef]
- Liang, J.-J.; Navrotsky, A.; Ludwig, T.; Seifert, H.J.; Aldinger, F. Enthalpy of Formation of Rare-earth Silicates Y2SiO5 and Yb2SiO5 and N-containing Silicate Y10(SiO4)6N2. J. Mater. Res. 1999, 14, 1181–1185. [Google Scholar] [CrossRef]
- Miyazaki, T.; Usami, S.; Inoue, R.; Kogo, Y.; Arai, Y. Oxidation behavior of ytterbium silicide. Ceram. Int. 2019, 45, 9560–9566. [Google Scholar] [CrossRef]
- Depla, D.; Heirwegh, S.; Mahieu, S.; Haemers, J.; Gryse, R.D. Understanding the discharge voltage behavior during reactive sputtering of oxides. J. Appl. Phys. 2007, 101, 013301. [Google Scholar] [CrossRef]
- Strijckmans, K.; Schelfhout, R.; Depla, D. Tutorial: Hysteresis during the reactive magnetron sputtering process. J. Appl. Phys. 2018, 124, 241101. [Google Scholar] [CrossRef]
- Depla, D.; De Gryse, R. Target poisoning during reactive magnetron sputtering: Part III: The prediction of the critical reactive gas mole fraction. Surf. Coat. Technol. 2004, 183, 196–203. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S.; Greene, J.E. Chapter 5—Sputter Deposition Processes. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Boston, MA, USA, 2010; pp. 253–296. [Google Scholar]
- Xia, X.; Qiu, P.; Shi, X.; Li, X.; Huang, X.; Chen, L. High-Temperature Oxidation Behavior of Filled Skutterudites YbyCo4Sb12. J. Electron. Mater. 2012, 41, 2225–2231. [Google Scholar] [CrossRef]
- Toberer, E.S.; May, A.F.; Melot, B.C.; Flage-Larsen, E.; Snyder, G.J. Electronic structure and transport in thermoelectric compounds AZn2Sb2 (A = Sr, Ca, Yb, Eu). Dalton Trans. 2010, 39, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Mattox, D.M. Handbook of Physical Vapor Deposition (PVD) Processing, 2nd ed.; William Andrew Publishing: Boston, MA, USA, 2010. [Google Scholar]
- Berg, S.; Särhammar, E.; Nyberg, T. Upgrading the “Berg-model” for reactive sputtering processes. Thin Solid Films 2014, 565, 186–192. [Google Scholar] [CrossRef]
- Lei, P.; Leroy, W.; Dai, B.; Zhu, J.; Chen, X.; Han, J.; Depla, D. Study on reactive sputtering of yttrium oxide: Process and thin film properties. Surf. Coat. Technol. 2015, 276, 39–46. [Google Scholar] [CrossRef]
- Kelly, P.J.; Henderson, P.S.; Arnell, R.D.; Roche, G.A.; Carter, D. Reactive pulsed magnetron sputtering process for alumina films. J. Vac. Sci. Technol. A 2000, 18, 2890–2896. [Google Scholar] [CrossRef]
- Carter, D.; Walde, H.; McDonough, G.; Roche, G. Parameter Optimization in Pulsed DC Reactive Sputter Deposition of Aluminum Oxide. In Proceedings of the 45th Annual Technical Conference of the Society of Vauum Coaters, Lake Buena Vista, FL, USA, 15–16 April 2002; pp. 570–577. [Google Scholar]
- Sproul, W.D.; Christie, D.J.; Carter, D.C. Control of reactive sputtering processes. Thin Solid Films 2005, 491, 1–17. [Google Scholar] [CrossRef]
- Sellers, J. Asymmetric bipolar pulsed DC: The enabling technology for reactive PVD. Surf. Coat. Tech. 1998, 98, 1245–1250. [Google Scholar] [CrossRef]
- Belkind, A.; Zhao, Z.; Carter, D.; Mahoney, L.; McDonough, G.; Roche, G.; Scholl, R.; Walde, H. Pulsed-DC Reactive Sputtering of Dielectrics: Pulsing Parameter Effects. In Proceedings of the 43rd Annual Technical Conference Proceedings, Denver, CO, USA, 15–20 April 2000; pp. 86–90. [Google Scholar]
- Wu, M.-L.; Wu, Y.-H.; Chao, C.-Y.; Lin, C.-C.; Wu, C.-Y. Crystalline ZrTiO4-Gated Ge Metal–Oxide–Semiconductor Devices With Amorphous Yb2O3 as a Passivation Layer. IEEE Trans. Nanotechnol. 2013, 12, 1018–1021. [Google Scholar] [CrossRef]
- Pan, T.-M.; Cheng, C.-H.; Lee, C.-D. Yb2O3 Thin Films as a Sensing Membrane for pH-ISFET Application. J. Electrochem. Soc. 2009, 156, J108. [Google Scholar] [CrossRef]
- Garcia, E.; Lee, H.; Sampath, S. Phase and microstructure evolution in plasma sprayed Yb2Si2O7 coatings. J. Eur. Ceram. Soc. 2019, 39, 1477–1486. [Google Scholar] [CrossRef]
- Garcia, E.; Garces, H.F.; Turcer, L.R.; Bale, H.; Padture, N.P.; Sampath, S. Crystallization behavior of air-plasma-sprayed ytterbium-silicate-based environmental barrier coatings. J. Eur. Ceram. Soc. 2021, 41, 3696–3705. [Google Scholar] [CrossRef]
- Ridley, M.; Opila, E. Thermochemical stability and microstructural evolution of Yb2Si2O7 in high-velocity high-temperature water vapor. J. Eur. Ceram. Soc. 2021, 41, 3141–3149. [Google Scholar] [CrossRef]
- Schliephake, D.; Kauffmann, A.; Cong, X.; Gombola, C.; Azim, M.; Gorr, B.; Christ, H.-J.; Heilmaier, M. Constitution, oxidation and creep of eutectic and eutectoid Mo-Si-Ti alloys. Intermetallics 2019, 104, 133–142. [Google Scholar] [CrossRef]

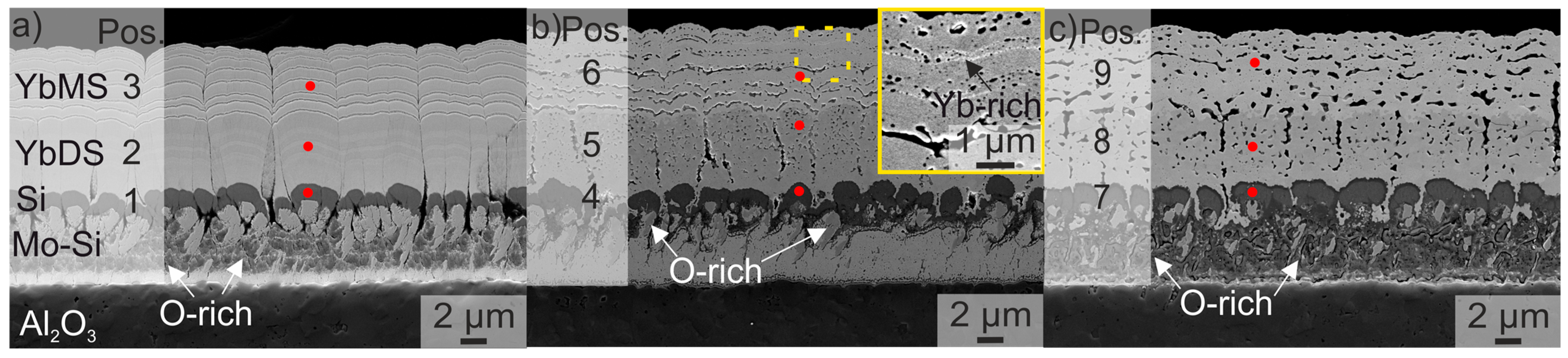
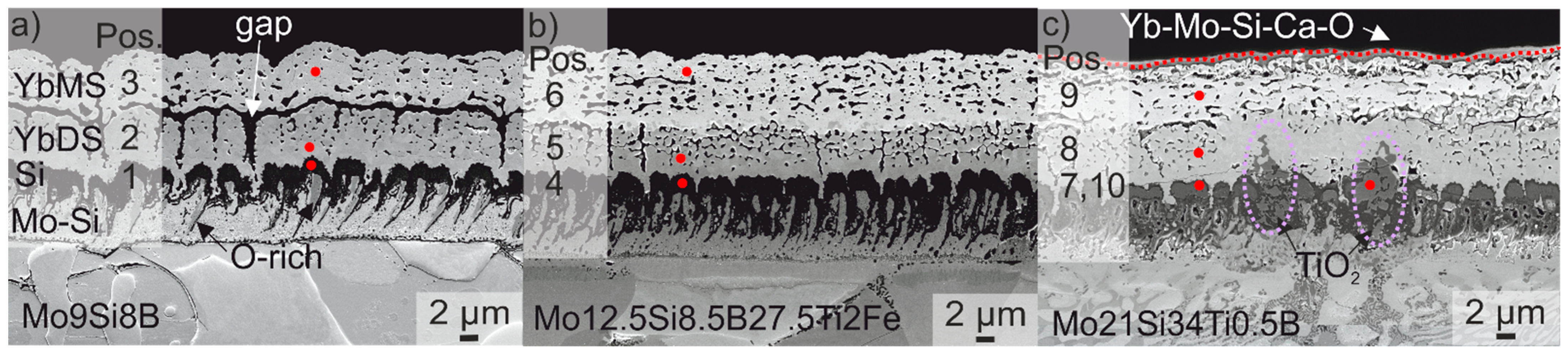


| as-Coated | Crystallised | 10 h at 1200 °C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Yb (at %) | 1.4 | 16.5 | 22.2 | 2.0 | 18.1 | 23.2 | 1.0 | 15.5 | 21.8 |
| Si (at %) | 82.6 | 14.8 | 10.6 | 79.5 | 15.1 | 9.6 | 61.0 | 14.3 | 10.7 |
| Mo (at %) | 2.7 | 0 | 0 | 2.0 | 0 | 0 | 1.6 | 0 | 0 |
| O (at %) | 13.3 | 68.6 | 67.2 | 16.5 | 65.8 | 66.2 | 36.4 | 70.2 | 67.5 |
| Mo9Si8B | Mo12.5Si8.5B27.5Ti2Fe | Mo21Si34Ti0.5B | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Yb (at %) | 0.9 | 16.0 | 21.4 | 1.3 | 19.0 | 22.0 | 1.8 | 19.5 | 24.0 | 0 |
| Si (at %) | 72.2 | 16.0 | 10.5 | 65.2 | 16.1 | 11.0 | 72.9 | 15.4 | 11.0 | 1.8 |
| Mo (at %) | 2.3 | 0 | 0 | 1.5 | 0 | 0 | 1.1 | 0 | 0 | 0.4 |
| Ti (at %) | - | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 32.3 |
| O (at %) | 24.6 | 68.0 | 68.1 | 32.0 | 64.9 | 67.0 | 24.2 | 65.1 | 65.0 | 65.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anton, R.; Leisner, V.; Laska, N.; Schulz, U. Reactive Sputtered Ytterbium Silicate Environmental Barrier Coatings for Protection of Mo-Si-Based Alloys. Coatings 2022, 12, 1086. https://doi.org/10.3390/coatings12081086
Anton R, Leisner V, Laska N, Schulz U. Reactive Sputtered Ytterbium Silicate Environmental Barrier Coatings for Protection of Mo-Si-Based Alloys. Coatings. 2022; 12(8):1086. https://doi.org/10.3390/coatings12081086
Chicago/Turabian StyleAnton, Ronja, Vito Leisner, Nadine Laska, and Uwe Schulz. 2022. "Reactive Sputtered Ytterbium Silicate Environmental Barrier Coatings for Protection of Mo-Si-Based Alloys" Coatings 12, no. 8: 1086. https://doi.org/10.3390/coatings12081086
APA StyleAnton, R., Leisner, V., Laska, N., & Schulz, U. (2022). Reactive Sputtered Ytterbium Silicate Environmental Barrier Coatings for Protection of Mo-Si-Based Alloys. Coatings, 12(8), 1086. https://doi.org/10.3390/coatings12081086







