Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development
Abstract
:1. Introduction
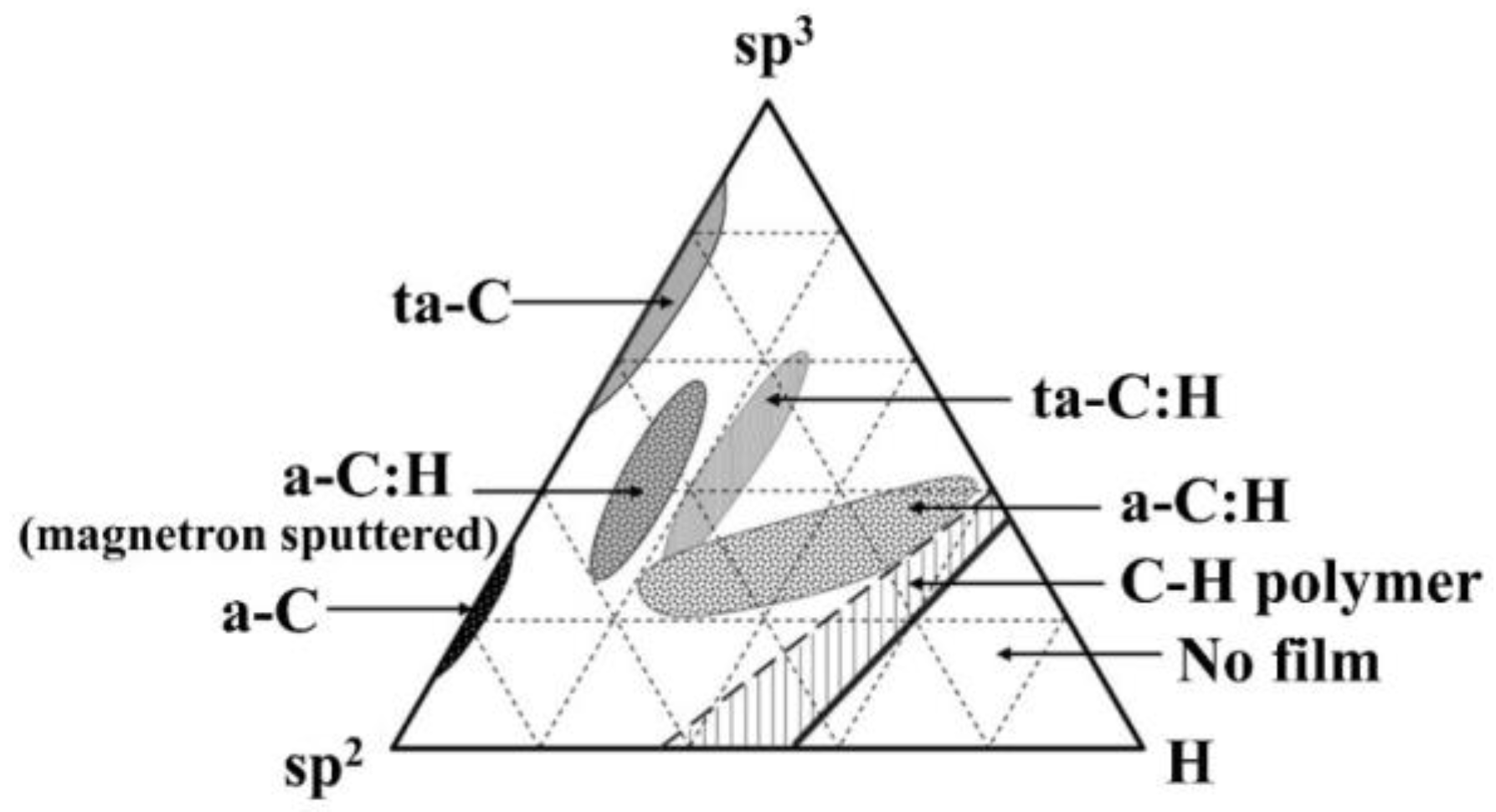
2. DLC Coatings and Their Characteristics
3. Application Requirements of Biomedical Materials
4. Application of DLCs in Biomedical Devices
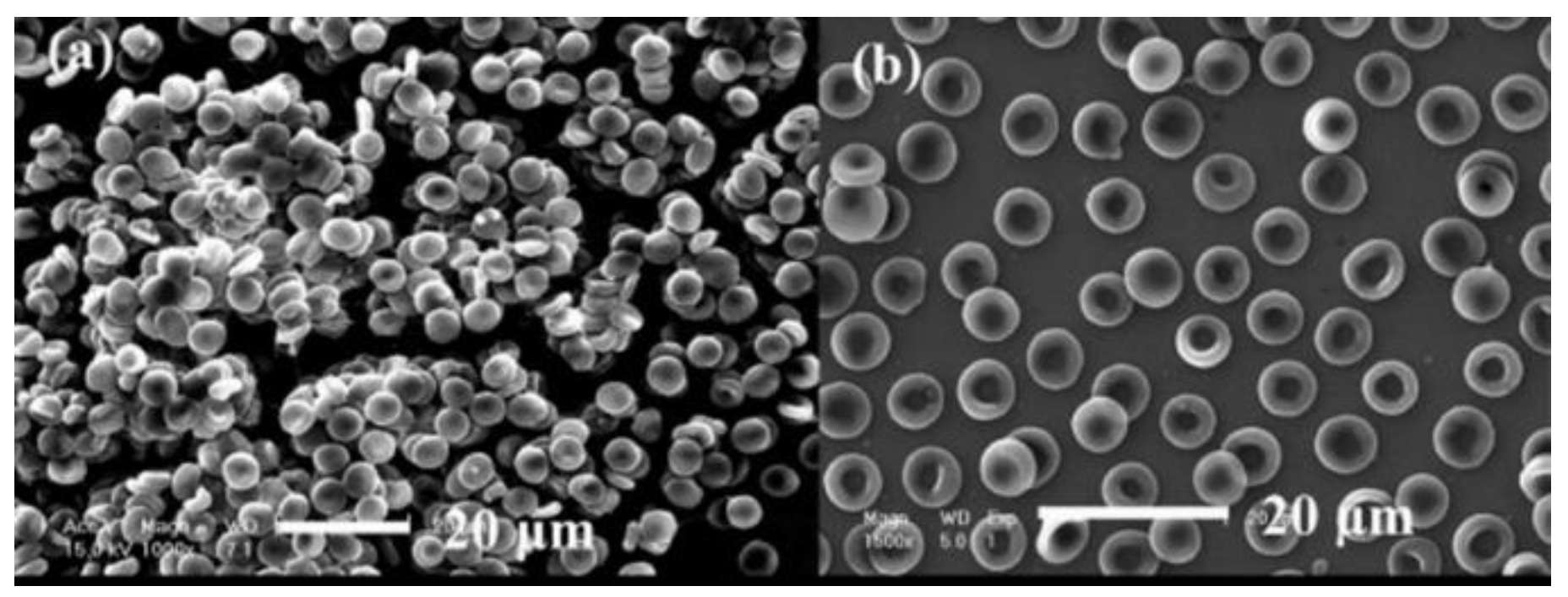
4.1. Vascular Stent
4.2. Prosthetic Heart Valve
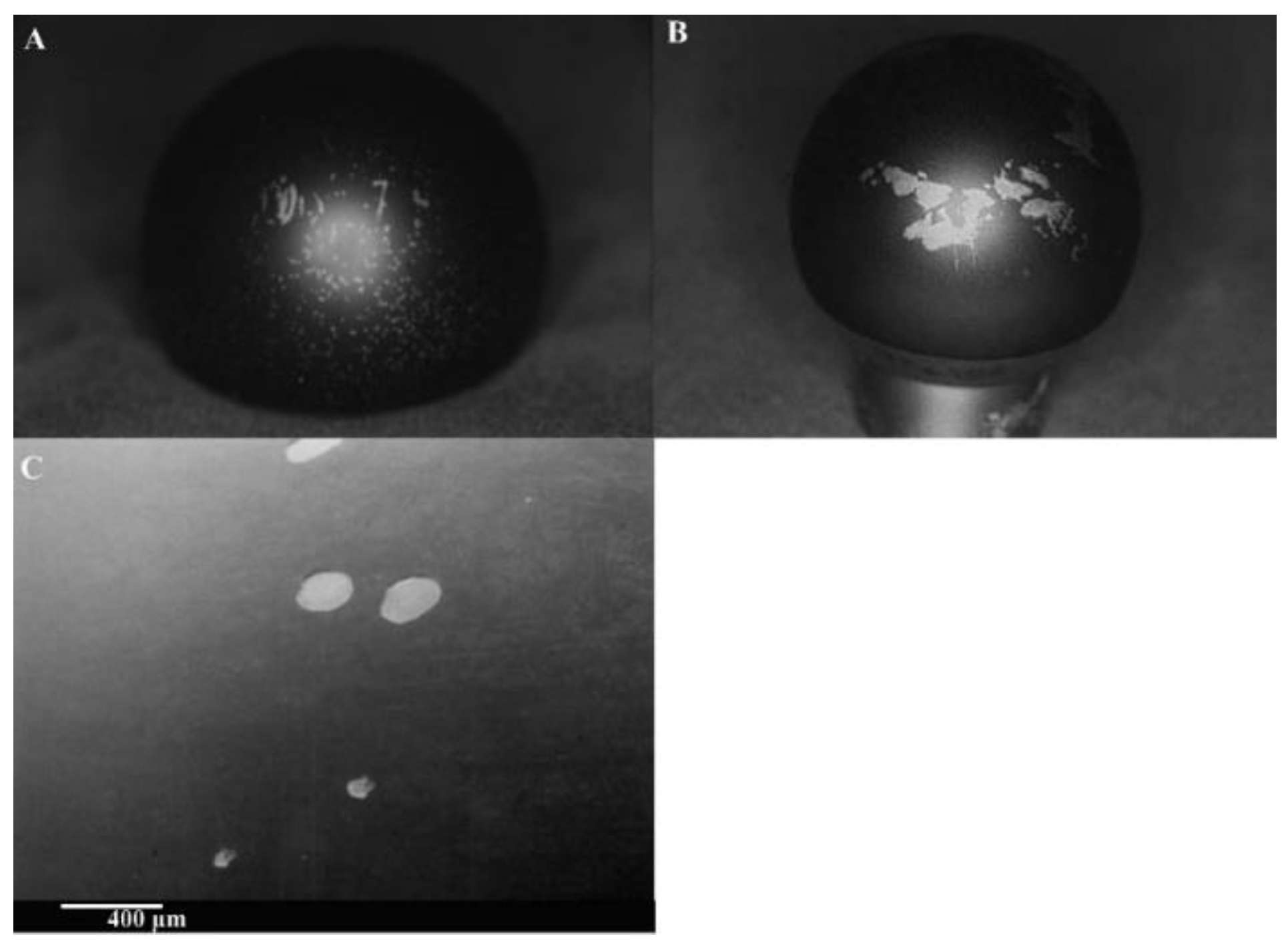
4.3. Joint Prosthesis
4.4. Surgical Instruments
5. Application of DLCs in Information Sensing in the Biomedical Field
5.1. Biochemical Sensors
5.2. Pressure Sensor
6. Future Outlooks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations in This Review
| BP | Blood pressure |
| CVD | Chemical vapor deposition |
| CSF | Cerebrospinal fluid |
| DLC | Diamond-like carbon |
| ERDA | Elastic recoil detection analysis |
| EVD | External ventricular drain |
| GF | Gage factor |
| GLC | Graphite-like carbon |
| HFEK | High frequency electric knife |
| HVD | Heart valve disease |
| ICP | Intracranial pressure |
| ISR | In-stent restenosis |
| LDL | Low-density lipoprotein |
| MRI | Magnetic resonance imaging |
| MWCNT | Multiwalled carbon nanotubes |
| NEXAFS | Near-edge X-ray absorption fine structure |
| NMR | Nuclear magnetic resonance |
| PHV | Prosthetic heart valve |
| PLC | Polymer-like carbon |
| PVD | Plasma-enhanced physical vapor deposition |
| RBS | Rutherford backscattering spectroscopy |
| R&D | Research and development |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Anene, F.A.; Jaafar, C.N.A.; Zainol, I.; Hanim, M.A.A.; Suraya, M.T. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. C-J Mech. 2021, 235, 3792–3805. [Google Scholar] [CrossRef]
- Wu, T.; Chen, X.; Fan, D.Z.; Pang, X.L. Development and application of metal materials in terms of vascular stents. Bio-Med. Mater. Eng. 2015, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Binyamin, G.; Shafi, B.M.; Mery, C.M. Biomaterials: A primer for surgeons. Semin. Pediatr. Surg. 2006, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C-Mater. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Fernandes, R.M.; Correa, M.G.; Aragao, W.A.B.; Nascimento, P.C.; Cartagenes, S.C.; Rodrigues, C.A.; Sarmiento, L.F.; Monteiro, M.C.; Maia, C.D.F.; Crespo-Lopez, M.E.; et al. Preclinical evidences of aluminum-induced neurotoxicity in hippocampus and pre-frontal cortex of rats exposed to low doses. Ecotoxicol. Environ. Saf. 2020, 206, 111139. [Google Scholar] [CrossRef] [PubMed]
- Bordjih, K.J.; Jouzeau, Y.J.; Mainard, D.; Payan, E.; Delagoutte, J.; Netter, P. Evaluation of the effect of three surface treatments on the biocompatibility of 316L stainless steel using human differentiated cells. Biomaterials 1996, 17, 491–500. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, K.R.; Ahn, S.H.; Kim, J.G. Instability of diamond-like carbon (DLC) films during sliding in aqueous environment. Diam. Relat. Mater. 2008, 17, 247–251. [Google Scholar] [CrossRef]
- Lepicka, M.; Gradzka-Dahlke, M.; Pieniak, D.; Pasierbiewicz, K.; Niewczas, A. Effect of mechanical properties of substrate and coating on wear performance of TiN- or DLC-coated 316LVM stainless steel. Wear 2017, 382, 62–70. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, D.R. Tetrahedral bonding in amorphous carbon. Rep. Prog. Phys. 1996, 59, 1611–1664. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.R. Biomedical applications of diamond-like carbon coatings: A review. J. Biomed. Mater. Res. B 2007, 83, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Peng, J.; Yang, M.; Bi, J.; Sheng, R.; Li, L. Hydrogen existence state of a hydrogenated amorphous carbon coating and its thermal stability. Diam. Relat. Mater. 2019, 99, 107535. [Google Scholar] [CrossRef]
- Petersen, M.; Bandorf, R.; Brauer, G.; Klages, C.P. Diamond-like carbon films as piezoresistors in highly sensitive force sensors. Diam. Relat. Mater. 2012, 26, 50–54. [Google Scholar] [CrossRef]
- Horiuchi, T.; Yoshida, K.; Kano, M.; Kumagai, M.; Suzuki, T. Evaluation of Adhesion and Wear Resistance of DLC Films Deposited by Various Methods. Plasma Process. Polym. 2009, 6, 410–416. [Google Scholar] [CrossRef]
- Drees, D.C.; Celis, J.P.; Dekempeneer, E.; Meneve, J. The electrochemical and wear behaviour of amorphous diamond-like carbon coatings and multilayered coatings in aqueous environments. Surf. Coat. Technol. 1996, 86–87, 575–580. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, S.Y.; Luque, R.; Han, S.; Hu, L.Z.; Xu, G.B. Recent development of carbon electrode materials and their bioanalytical and environmental applications. Chem. Soc. Rev. 2016, 45, 715–752. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Vetter, J. 60 years of DLC coatings: Historical highlights and technical review of cathodic arc processes to synthesize various DLC types, and their evolution for industrial applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Konyashin, I.Y. PVD/CVD technology for coating cemented carbides. Surf. Coat. Technol. 1995, 71, 277–283. [Google Scholar] [CrossRef]
- Sheng, H.X.; Xiong, W.W.; Zheng, S.S.; Chen, C.; He, S.; Cheng, Q.J. Evaluation of the sp(3)/sp(2) ratio of DLC films by RF-PECVD and its quantitative relationship with optical band gap. Carbon Lett. 2021, 31, 929–939. [Google Scholar] [CrossRef]
- Zhou, X.L.; Tunmee, S.; Suzuki, T.; Phothongkam, P.; Kanda, K.; Komatsu, K.; Kawahara, S.; Ito, H.; Saitoh, H. Quantitative NEXAFS and solid-state NMR studies of sp(3)/(sp(2) + sp(3)) ratio in the hydrogenated DLC films. Diam. Relat. Mater. 2017, 73, 232–240. [Google Scholar] [CrossRef]
- Merlen, A.; Buijnsters, J.G.; Pardanaud, C. A Guide to and Review of the Use of Multiwavelength Raman Spectroscopy for Characterizing Defective Aromatic Carbon Solids: From Graphene to Amorphous Carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Higuchi, T.; Weihnacht, V. Effect of sp(2)/sp(3) bonding ratio and nitrogen content on friction properties of hydrogen-free DLC coatings. Tribol. Int. 2013, 62, 130–140. [Google Scholar] [CrossRef]
- Paul, R.; Das, S.N.; Dalui, S.; Gayen, R.N.; Roy, R.K.; Bhar, R.; Pal, A.K. Synthesis of DLC films with different sp(2)/sp(3) ratios and their hydrophobic behaviour. J. Phys. D Appl. Phys. 2008, 41, 5309. [Google Scholar] [CrossRef]
- Erdemir, A. The role of hydrogen in tribological properties of diamond-like carbon films. Surf. Coat. Technol. 2001, 146, 292–297. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.S.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Lu, Y.M.; Wang, S.; Huang, G.J.; Xi, L.; Qin, G.H.; Zhu, M.Z.; Chu, H. Fabrication and applications of the optical diamond-like carbon films: A review. J. Mater. Sci. 2022, 57, 3971–3992. [Google Scholar] [CrossRef]
- Liu, G.; Wen, Z.; Chen, K.; Dong, L.; Wang, Z.; Zhang, B.; Qiang, L. Optimizing the Microstructure, Mechanical, and Tribological Properties of Si-DLC Coatings on NBR Rubber for Its Potential Applications. Coatings 2020, 10, 671. [Google Scholar] [CrossRef]
- Tyan, Y.C.; Yang, M.H.; Chang, C.C.; Chung, T.W. Biocompatibility of Materials for Biomedical Engineering. Adv. Exp. Med. Biol. 2020, 1250, 125–140. [Google Scholar] [CrossRef]
- Du, C.; Su, X.W.; Cui, F.Z.; Zhu, X.D. Morphological behaviour of osteoblasts on diamond-like carbon coating and amorphous C-N film in organ culture. Biomaterials 1998, 19, 651–658. [Google Scholar] [CrossRef]
- Wickramasinghe, M.L.; Dias, G.J.; Premadasa, K.M.G.P. A novel classification of bone graft materials. J. Biomed. Mater. Res. B 2022, 110, 1724–1749. [Google Scholar] [CrossRef]
- Tallant, D.R.P.; Parmeter, J.E.; Siegal, M.P.; Simpson, R.L. The thermal stability of diamond-like carbon. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef]
- Serrano-Aroca, A.; Pous-Serrano, S. Prosthetic meshes for hernia repair: State of art, classification, biomaterials, antimicrobial approaches, and fabrication methods. J. Biomed. Mater. Res. A 2021, 109, 2695–2719. [Google Scholar] [CrossRef]
- Chethan, K.N.; Satish Shenoy, B.; Shyamasunder Bhat, N. Role of different orthopedic biomaterials on wear of hip joint prosthesis: A review. Mater. Today Proc. 2018, 5, 20827–20836. [Google Scholar] [CrossRef]
- Shores, J.T.; Hiersche, M.; Gabriel, A.; Gupta, S. Tendon coverage using an artificial skin substitute. J. Plast. Reconstruct. Aesthet. Surg. 2012, 65, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Han, Y.; Lu, J. Structural Design of Vascular Stents: A Review. Micromachines 2021, 12, 770. [Google Scholar] [CrossRef]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H.; Kobayashi, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood Purif. 2015, 40, 293–297. [Google Scholar] [CrossRef]
- Greenberg, J.A. The use of barbed sutures in obstetrics and gynecology. Rev. Obstet. Gynecol. 2010, 3, 82–91. [Google Scholar]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, S.; Orris, P.; Karliner, J. Alternatives to the mercury sphygmomanometer. J. Public Health Policy 2011, 32, 107–120. [Google Scholar] [CrossRef]
- Klinkmann, H.; Vienken, J. Membranes for dialysis. Nephrol. Dial. Transpl. 1995, 10 (Supp. 3), 39–45. [Google Scholar] [CrossRef]
- Thomson, L.A.L.; Law, F.C.; Rushton, N.; Franks, J. Biocompatibility of diamond-like carbon coating. Biomaterials 1991, 12, 37–40. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Nicolais, C.; Lakhter, V.; Virk, H.U.H.; Sardar, P.; Bavishi, C.; O’Murchu, B.; Chatterjee, S. Therapeutic Options for In-Stent Restenosis. Curr. Cardiol. Rep. 2018, 20, 7. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr. In-stent restenosis. Rev. Cardiovasc. Med. 2001, 2, 115–119. [Google Scholar]
- Inoue, T.; Croce, K.; Morooka, T.; Sakuma, M.; Node, K.; Simon, D.I. Vascular inflammation and repair: Implications for re-endothelialization, restenosis, and stent thrombosis. JACC Cardiovasc. Interv. 2011, 4, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Micheletti, P.L.; Carla-da-Silva, J.; Scandolara, T.B.; Kern, R.; Alves, V.D.; Malanowski, J.; Victorino, V.J.; Herrera, A.; Rech, D.; Souza, J.A.O.; et al. Proinflammatory circulating markers: New players for evaluating asymptomatic acute cardiovascular toxicity in breast cancer treatment. J. Chemother. 2021, 33, 106–115. [Google Scholar] [CrossRef]
- Senkus, E.; Jassem, J. Cardiovascular effects of systemic cancer treatment. Cancer Treat. Rev. 2011, 37, 300–311. [Google Scholar] [CrossRef]
- Salahas, A.; Vrahatis, A.; Karabinos, I.; Antonellis, I.; Ifantis, G.; Gavaliatsis, I.; Anthopoulos, P.; Tavernarakis, A. Success, safety, and efficacy of implantation of diamond-like carbon-coated stents. Angiology 2007, 58, 203–210. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, J.H.; Shin, D.H.; Moon, M.W.; Park, K.; Kim, T.H.; Shin, K.M.; Won, Y.H.; Han, D.K.; Lee, K.R. Comparison of diamond-like carbon-coated nitinol stents with or without polyethylene glycol grafting and uncoated nitinol stents in a canine iliac artery model. Br. J. Radiol. 2011, 84, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Ando, K.; Ishii, K.; Tada, E.; Kataoka, K.; Hirohata, A.; Goto, K.; Kobayashi, K.; Tsutsui, H.; Nakahama, M.; Nakashima, H.; et al. Prospective multi-center registry to evaluate efficacy and safety of the newly developed diamond-like carbon-coated cobalt-chromium coronary stent system. Cardiovasc. Interv. Ther. 2017, 32, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Gutensohn, K.; Beythien, C.; Bau, J.; Fenner, T.; Grewe, P.; Koester, R.; Padmanaban, K.; Kuehnl, P. In vitro analyses of diamond-like carbon coated stents: Reduction of metal ion release, platelet activation, and thrombogenicity. Thromb. Res. 2000, 99, 577–585. [Google Scholar] [CrossRef]
- Karagkiozaki, V.C.; Logothetidis, S.D.; Kassavetis, S.N.; Giannoglou, G.D. Nanomedicine for the reduction of the thrombogenicity of stent coatings. Int. J. Nanomed. 2010, 5, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.L.; Barber, Z.H.; Clyne, T.W. Surface roughness of diamond-like carbon films prepared using various techniques. Surf. Coat. Technol. 2001, 138, 23–32. [Google Scholar] [CrossRef]
- Hasebe, T.; Yohena, S.; Kamijo, A.; Okazaki, Y.; Hotta, A.; Takahashi, K.; Suzuki, T. Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J. Biomed. Mater. Res. A 2007, 83, 1192–1199. [Google Scholar] [CrossRef]
- Maegawa, S.; Hasebe, T.; Yamato, Y.; Bito, K.; Nagashima, S.; Hayashi, T.; Mine, T.; Matsumoto, T.; Hotta, A.; Suzuki, T. Time course analysis of antithrombogenic properties of fluorinated diamond-like carbon coating determined via accelerated aging tests: Quality control for medical device commercialization. Diam. Relat. Mater. 2016, 70, 33–38. [Google Scholar] [CrossRef]
- Saito, T.; Hasebe, T.; Yohena, S.; Matsuoka, Y.; Kamijo, A.; Takahashi, K.; Suzuki, T. Antithrombogenicity of fluorinated diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 1116–1119. [Google Scholar] [CrossRef]
- Thomas, V.; Halloran, B.A.; Ambalavanan, N.; Catledge, S.A.; Vohra, Y.K. In vitro studies on the effect of particle size on macrophage responses to nanodiamond wear debris. Acta Biomater. 2012, 8, 1939–1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turpie, A.G.; Gent, M.; Laupacis, A.; Latour, Y.; Gunstensen, J.; Basile, F.; Klimek, M.; Hirsh, J. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N. Engl. J. Med. 1993, 329, 524–529. [Google Scholar] [CrossRef]
- Antonowicz, M.; Kurpanik, R.; Walke, W.; Basiaga, M.; Sondor, J.; Paszenda, Z. Selected Physicochemical Properties of Diamond Like Carbon (DLC) Coating on Ti-13Nb-13Zr Alloy Used for Blood Contacting Implants. Materials 2020, 13, 5077. [Google Scholar] [CrossRef]
- Kwok, S.C.H.; Yang, P.; Wang, J.; Liu, X.Y.; Chu, P.K. Hemocompatibility of nitrogen-doped, hydrogen-free diamond-like carbon prepared by nitrogen plasma immersion ion implantation-deposition. J. Biomed. Mater. Res. A 2004, 70, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Andara, M.; Agarwal, A.; Scholvin, D.; Gerhardt, R.A.; Doraiswamy, A.; Jin, C.M.; Narayan, R.J.; Shih, C.C.; Shih, C.M.; Lin, S.J.; et al. Hemocompatibility of diamondlike carbon-metal composite thin films. Diam. Relat. Mater. 2006, 15, 1941–1948. [Google Scholar] [CrossRef]
- Chou, C.C.; Wu, Y.Y.; Lee, J.W.; Yeh, C.H.; Huang, J.C. Characterization and haemocompatibility of fluorinated DLC and Si interlayer on Ti6Al4V. Surf. Coat. Technol. 2013, 231, 418–422. [Google Scholar] [CrossRef]
- Tang, X.S.; Wang, H.J.; Feng, L.; Shao, L.X.; Zou, C.W. Mo doped DLC nanocomposite coatings with improved mechanical and blood compatibility properties. Appl. Surf. Sci. 2014, 311, 758–762. [Google Scholar] [CrossRef]
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Porrino, J.; Wang, A.N.; Moats, A.; Mulcahy, H.; Kani, K. Prosthetic joint infections: Diagnosis, management, and complications of the two-stage replacement arthroplasty. Skelet. Radiol. 2020, 49, 847–859. [Google Scholar] [CrossRef]
- Matz, J.; Lanting, B.A.; Howard, J.L. Understanding the patellofemoral joint in total knee arthroplasty. Can. J. Surg. 2019, 62, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef]
- Paul, J.P. Strength requirements for internal and external prostheses. J. Biomech. 1999, 32, 381–393. [Google Scholar] [CrossRef]
- Allen, M.; Myer, B.; Rushton, N. In vitro and in vivo investigations into the biocompatibility of diamond-like carbon (DLC) coatings for orthopedic applications. J. Biomed. Mater. Res. 2001, 58, 319–328. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Wu, B.J.; Zhang, T.F.; Leng, Y.X.; Huang, N. Tribocorrosion behavior of DLC-coated CoCrMo alloy in simulated biological environment. Vacuum 2013, 92, 39–43. [Google Scholar] [CrossRef]
- Wongpanya, P.; Pintitraratibodee, N.; Thumanu, K.; Euaruksakul, C. Improvement of corrosion resistance and biocompatibility of 316L stainless steel for joint replacement application by Ti-doped and Ti-interlayered DLC films. Surf. Coat. Technol. 2021, 425, 127734. [Google Scholar] [CrossRef]
- Platon, F.; Fournier, P.; Rouxel, S. Tribological behaviour of DLC coatings compared to different materials used in hip joint prostheses. Wear 2001, 250, 227–236. [Google Scholar] [CrossRef]
- Tan, D.; Dai, M.J.; Fu, W.B.; Lin, S.S.; Wei, C.B.; Zhao, M.C. Performance of CoCrMo Alloy with Me-Doped DLC Coatings Prepared by a Magnetron Sputtering Method. Rare Met. Mater. Eng. 2015, 44, 2982–2986. [Google Scholar] [CrossRef]
- Jing, P.P.; Su, Y.H.; Li, Y.X.; Liang, W.L.; Leng, Y.X. Mechanism of protein biofilm formation on Ag-DLC films prepared for application in joint implants. Surf. Coat. Technol. 2021, 422, 7553. [Google Scholar] [CrossRef]
- Taeger, G.; Podleska, L.E.; Schmidt, B.; Ziegler, M.; Nast-Kolb, D. Comparison of diamond-like-carbon and alumina-oxide articulating with polyethylene in total hip arthroplasty. Mater. Werkst 2003, 34, 1094–1100. [Google Scholar] [CrossRef]
- Chen, Y.; Nie, X.Y.; Leyland, A.; Housden, J.; Matthews, A. Substrate and bonding layer effects on performance of DLC and TiN biomedical coatings in Hank’s solution under cyclic impact-sliding loads. Surf. Coat. Technol. 2013, 237, 219–229. [Google Scholar] [CrossRef]
- Ortega-Saenz, J.A.; Alvarez-Vera, M.; Hernandez-Rodriguez, M.A.L. Biotribological study of multilayer coated metal-on-metal hip prostheses in a hip joint simulator. Wear 2013, 301, 234–242. [Google Scholar] [CrossRef]
- Gaines, S.; Luo, J.N.; Gilbert, J.; Zaborina, O.; Alverdy, J.C. Optimum Operating Room Environment for the Prevention of Surgical Site Infections. Surg. Infect. 2017, 18, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Rodelo, C.; Salinas, R.A.; Armenta Jaime, E.; Armenta, S.; Galdámez-Martínez, A.; Castillo-Blum, S.E.; Astudillo-de la Vega, H.; Nirmala Grace, A.; Aguilar-Salinas, C.A.; Gutiérrez Rodelo, J.; et al. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Coord. Chem. Rev. 2022, 457, 214402. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Q. The CQ ratio of surface energy components influences adhesion and removal of fouling bacteria. Biofouling 2011, 27, 275–285. [Google Scholar] [CrossRef]
- Del Prado, G.; Terriza, A.; Ortiz-Perez, A.; Molina-Manso, D.; Mahillo, I.; Yubero, F.; Puertolas, J.A.; Manrubia-Cobo, M.; Barrena, E.G.; Esteban, J. DLC coatings for UHMWPE: Relationship between bacterial adherence and surface properties. J. Biomed. Mater. Res. A 2012, 100, 2813–2820. [Google Scholar] [CrossRef]
- Wei, C.H.; Peng, K.S.; Hung, M.S. The effect of hydrogen and acetylene mixing ratios on the surface, mechanical and biocompatible properties of diamond-like carbon films. Diam. Relat. Mater. 2016, 63, 108–114. [Google Scholar] [CrossRef]
- Ren, D.W.; Zhao, Q.; Bendavid, A. Anti-bacterial property of Si and F doped diamond-like carbon coatings. Surf. Coat. Technol. 2013, 226, 1–6. [Google Scholar] [CrossRef]
- Tang, S.H.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1503. [Google Scholar] [CrossRef]
- Schwarz, F.P.; Hauser-Gerspach, I.; Waltimo, T.; Stritzker, B. Antibacterial properties of silver containing diamond like carbon coatings produced by ion induced polymer densification. Surf. Coat. Technol. 2011, 205, 4850–4854. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Hsu Lillian, C.; Fang, J.; Borca-Tasciuc Diana, A.; Worobo Randy, W.; Moraru Carmen, I. Effect of Micro- and Nanoscale Topography on the Adhesion of Bacterial Cells to Solid Surfaces. Appl. Environ. Microbiol. 2013, 79, 2703–2712. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.S.; Garvin, C.P.; Dowling, D.; Donnelly, K.; Gorman, S.P. Examination of surface properties and in vitro biological performance of amorphous diamond-like carbon-coated polyurethane. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.B.; Vieira, A.A.; Paula, L.O.; Santos, E.D.; Radi, P.A.; Khouri, S.; Maciel, H.S.; Pessoa, R.S.; Vieira, L. Flexible camphor diamond-like carbon coating on polyurethane to prevent Candida albicans biofilm growth. J. Mech. Behav. Biomed. Mater. 2017, 68, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Watari, S.; Wada, K.; Araki, M.; Sadahira, T.; Ousaka, D.; Oozawa, S.; Nakatani, T.; Imai, Y.; Kato, J.; Kariyama, R.; et al. Intraluminal diamond-like carbon coating with anti-adhesion and anti-biofilm effects for uropathogens: A novel technology applicable to urinary catheters. Int. J. Urol. 2021, 28, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Myllymaa, K.; Levon, J.; Tiainen, V.-M.; Myllymaa, S.; Soininen, A.; Korhonen, H.; Kaivosoja, E.; Lappalainen, R.; Konttinen, Y.T. Formation and retention of staphylococcal biofilms on DLC and its hybrids compared to metals used as biomaterials. Colloids Surf. B Biointerfaces 2013, 101, 290–297. [Google Scholar] [CrossRef]
- Cazalini, E.M.; Miyakawa, W.; Teodoro, G.R.; Sobrinho, A.S.S.; Matieli, J.E.; Massi, M.; Koga-Ito, C.Y. Antimicrobial and anti-biofilm properties of polypropylene meshes coated with metal-containing DLC thin films. J. Mater. Sci. Mater. Med. 2017, 28, 97. [Google Scholar] [CrossRef] [Green Version]
- Weissmann, C. The state of the prion. Nat. Rev. Microbiol. 2004, 2, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.C. Environmental and host factors that contribute to prion strain evolution. Acta Neuropathol. 2021, 142, 5–16. [Google Scholar] [CrossRef]
- Sakudo, A.; Ano, Y.; Onodera, T.; Nitta, K.; Shintani, H.; Ikuta, K.; Tanaka, Y. Fundamentals of prions and their inactivation (review). Int. J. Mol. Med. 2011, 27, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secker, T.J.; Herve, R.; Zhao, Q.; Borisenko, K.B.; Abel, E.W.; Keevil, C.W. Doped diamond-like carbon coatings for surgical instruments reduce protein and prion-amyloid biofouling and improve subsequent cleaning. Biofouling 2012, 28, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Ou, K.L.; Weng, C.C.; Sugiatno, E.; Ruslin, M.; Lin, Y.H.; Cheng, H.Y. Effect of nanostructured thin film on minimally invasive surgery devices applications: Characterization, cell cytotoxicity evaluation and an animal study in rat. Surg. Endosc. 2016, 30, 3035–3049. [Google Scholar] [CrossRef]
- Su, C.H.; Lin, C.R.; Chang, C.Y.; Hung, H.C.; Lin, T.Y. Mechanical and optical properties of diamond-like carbon thin films deposited by low temperature process. Thin Solid Films 2006, 498, 220–223. [Google Scholar] [CrossRef]
- Meskinis, S.; Vasiliauskas, A.; Andrulevicius, M.; Peckus, D.; Tamulevicius, S.; Viskontas, K. Diamond Like Carbon Films Containing Si: Structure and Nonlinear Optical Properties. Materials 2020, 13, 1003. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, T.; Li, X.; Wang, X.; Zhang, W.; Yang, Y.; Tang, Y. UV-to-IR highly transparent ultrathin diamond nanofilms with intriguing performances: Anti-fogging, self-cleaning and self-lubricating. Appl. Surf. Sci. 2020, 527, 146733. [Google Scholar] [CrossRef]
- Sakurai, K.; Hiratsuka, M.; Nakamori, H.; Namiki, K.; Hirakuri, K. Evaluation of sliding properties and durability of DLC coating for medical devices. Diam. Relat. Mater. 2019, 96, 97–103. [Google Scholar] [CrossRef]
- Peng, J.; Xiao, Y.; Peng, Y.; Zeng, J. Effect of traceable nitrogen from low-pressure plasma nitriding on diamond growth over WC-co cemented carbides. Diam. Relat. Mater. 2021, 120, 108717. [Google Scholar] [CrossRef]
- Al Mahmud, K.A.H.; Kalam, M.A.; Masjuki, H.H.; Mobarak, H.M.; Zulkifli, N.W.M. An updated overview of diamond-like carbon coating in tribology. Crit. Rev. Solid State 2015, 40, 90–118. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.W.; Wu, Y.Z.; Wang, X.; Pan, H.X.; Li, P.; Du, W.Y.; Qi, Z.; Huang, A.; Zhang, L.W.; Zhang, L.; et al. Experimental and clinical study of influence of high-frequency electric surgical knives on healing of abdominal incision. World J. Gastroenterol. 2006, 12, 4082–4085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.F.; Wan, Z.X.; Ding, J.C.; Zhang, S.; Wang, Q.M.; Kim, K.H. Microstructure and high-temperature tribological properties of Si-doped hydrogenated diamond-like carbon films. Appl. Surf. Sci. 2018, 435, 963–973. [Google Scholar] [CrossRef]
- Zeng, A.P.; Neto, V.F.; Gracio, J.J.; Fan, Q.H. Diamond-like carbon (DLC) films as electrochemical electrodes. Diam. Relat. Mater. 2014, 43, 12–22. [Google Scholar] [CrossRef]
- Compton, R.G.; Foord, J.S.; Marken, F. Electroanalysis at diamond-like and doped-diamond electrodes. Electroanal 2003, 15, 1349–1363. [Google Scholar] [CrossRef]
- Grill, A. Electrical and optical properties of diamond-like carbon. Thin Solid Films 1999, 355, 189–193. [Google Scholar] [CrossRef]
- Triroj, N.; Saensak, R.; Porntheeraphat, S.; Paosawatyanyong, B.; Amornkitbamrung, V. Diamond-like carbon thin film electrodes for microfluidic bioelectrochemical sensing platforms. Anal. Chem. 2020, 92, 3650–3657. [Google Scholar] [CrossRef] [PubMed]
- Palomaki, T.; Chumillas, S.; Sainio, S.; Protopopova, V.; Kauppila, M.; Koskinen, J.; Climent, V.; Feliu, J.M.; Laurila, T. Electrochemical reactions of catechol, methylcatechol and dopamine at tetrahedral amorphous carbon (ta-C) thin film electrodes. Diam. Relat. Mater. 2015, 59, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.A.; Zanin, H.; May, P.W.; Corat, E.J.; Fatibello, O. Electrochemical Performance of Porous Diamond-like Carbon Electrodes for Sensing Hormones, Neurotransmitters, and Endocrine Disruptors. ACS Appl. Mater. Interface 2014, 6, 21086–21092. [Google Scholar] [CrossRef] [PubMed]
- Sainio, S.; Palomaki, T.; Rhode, S.; Kauppila, M.; Pitkanen, O.; Selkala, T.; Toth, G.; Moram, M.; Kordas, K.; Koskinen, J.; et al. Carbon nanotube (CNT) forest grown on diamond-like carbon (DLC) thin films significantly improves electrochemical sensitivity and selectivity towards dopamine. Sens. Actuators B-Chem. 2015, 211, 177–186. [Google Scholar] [CrossRef]
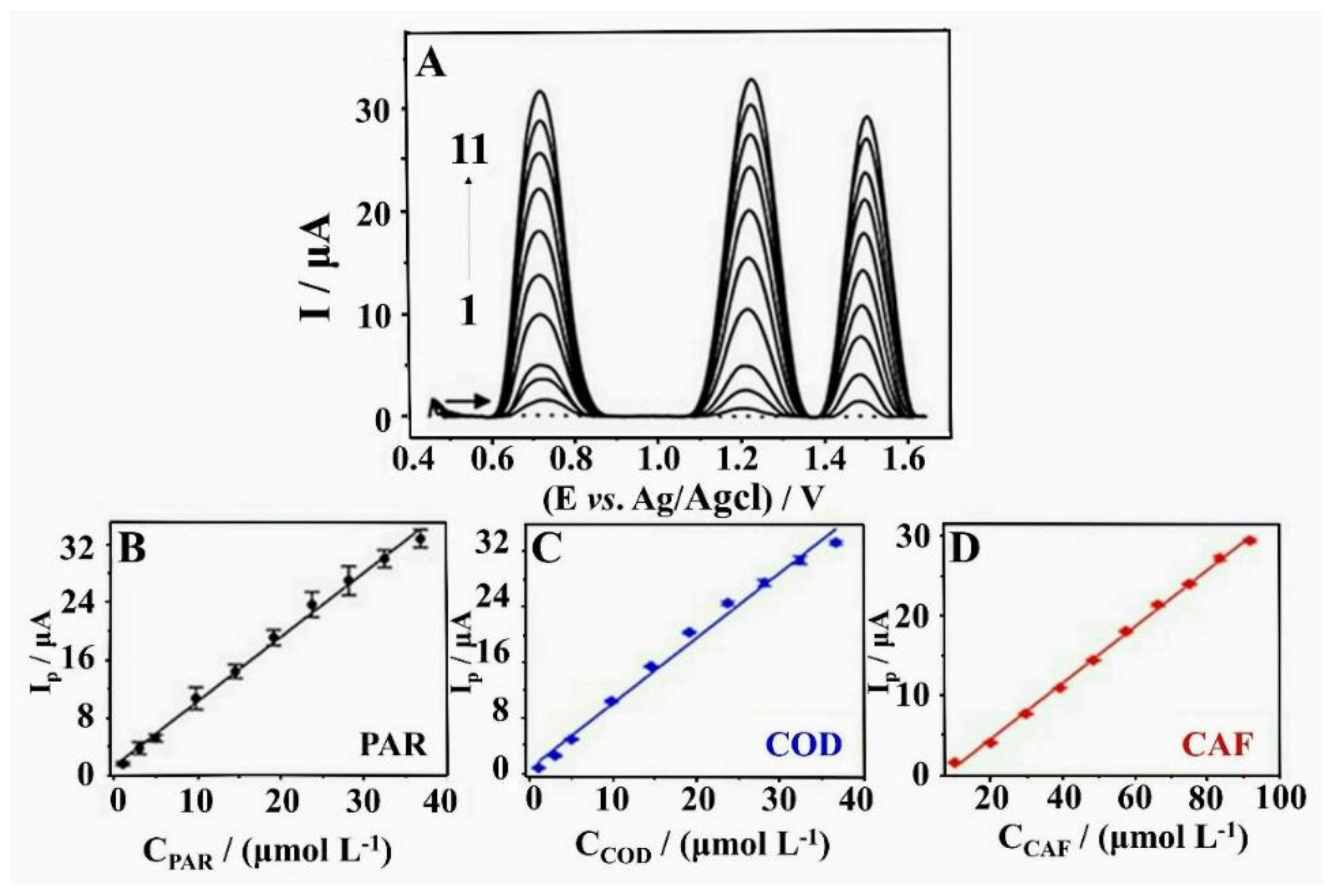
- Silva, T.A.; Zanin, H.; Corat, E.J.; Fatibello, O. Simultaneous Voltammetric Determination of Paracetamol, Codeine and Caffeine on Diamond-like Carbon Porous Electrodes. Electroanalysis 2017, 29, 907–916. [Google Scholar] [CrossRef]
- O’Brien, E.; White, W.B.; Parati, G.; Dolan, E. Ambulatory blood pressure monitoring in the 21st century. J. Clin. Hypertens. 2018, 20, 1108–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, C.E.; Dempsey, E.M. Management of Neonatal Hypotension and Shock. Semin. Fetal Neonatal. Med. 2020, 25, 101121. [Google Scholar] [CrossRef] [PubMed]
- Kalkwarf, K.J.; Cotton, B.A. Resuscitation for Hypovolemic Shock. Surg. Clin. N. Am. 2017, 97, 1307–1321. [Google Scholar] [CrossRef]
- Raamat, R.; Jagomagi, K.; Talts, J.; Kivastik, J. A Model-Based Retrospective Analysis of the Fixed-Ratio Oscillometric Blood Pressure Measurement. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 10–13 November 2013; pp. 632–636. [Google Scholar] [CrossRef]
- Benmira, A.; Perez-Martin, A.; Schuster, I.; Aichoun, I.; Coudray, S.; Bereksi-Reguig, F.; Dauzat, M. From Korotkoff and Marey to automatic non-invasive oscillometric blood pressure measurement: Does easiness come with reliability? Expert Rev. Med. Devices 2016, 13, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Qatatsheh, A.; Morsi, Y.; Zavabeti, A.; Zolfagharian, A.; Salim, N.A.; Mosadegh, Z.K.B.; Gharaie, S. Blood Pressure Sensors: Materials, Fabrication Methods, Performance Evaluations and Future Perspectives. Sensors 2020, 20, 4484. [Google Scholar] [CrossRef] [PubMed]
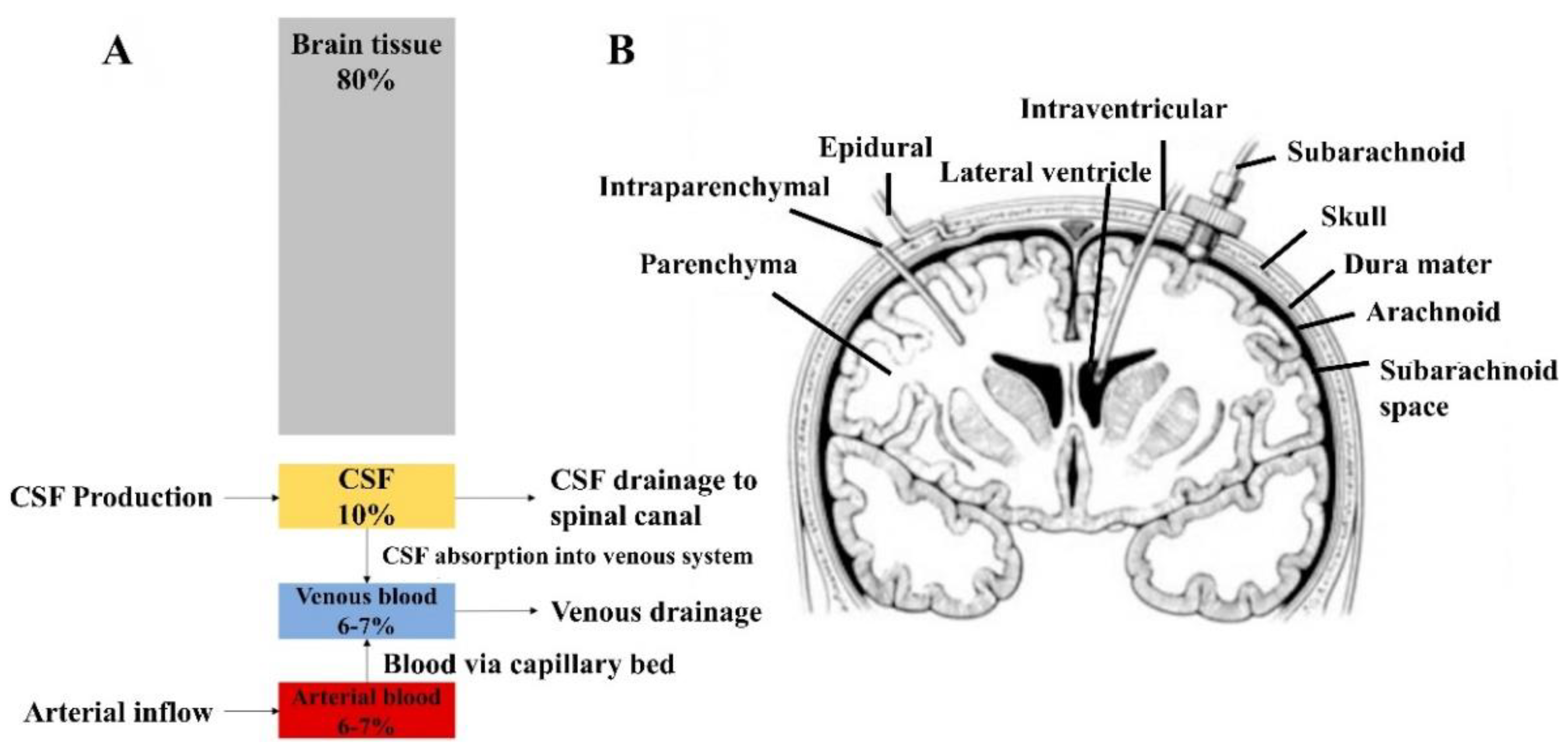
- Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Harary, M.; Dolmans, R.G.F.; Gormley, W.B. Intracranial Pressure Monitoring-Review and Avenues for Development. Sensors 2018, 18, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raboel, P.H.; Bartek, J., Jr.; Andresen, M.; Bellander, B.M.; Romner, B. Intracranial Pressure Monitoring: Invasive versus Non-Invasive Methods-A Review. Crit. Care Res. Pract. 2012, 2012, 950393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinera, E.T.; Tibrewala, A.; Bandorf, R.; Biehl, S.; Lüthje, H.; Doering, L. Micro force sensor with piezoresistive amorphous carbon strain gauge. Sens. Actuators A Phys. 2006, 130–131, 75–82. [Google Scholar] [CrossRef]
- Tibrewala, A.P.; Peiner, E.; Bandorf, R.; Biehl, S.; Lüthje, H. Transport and optical properties of amorphous carbon and hydrogenated amorphous carbon films. Appl. Surf. Sci. 2006, 252, 5387–5390. [Google Scholar] [CrossRef]
- Fraga, M.A.F.; Furlan, H.; Pessoa, R.S.; Rasia, L.A.; Mateus, C.F.R. Studies on SiC, DLC and TiO2 thin films as piezoresistive sensor materials for high temperature application. Microsyst. Technol. 2012, 18, 1027–1033. [Google Scholar] [CrossRef]
- Fraga, M.A.; Furlan, H.; Pessoa, R.S.; Massi, M. Wide bandgap semiconductor thin films for piezoelectric and piezoresistive MEMS sensors applied at high temperatures: An overview. Microsyst. Technol. 2014, 20, 9–21. [Google Scholar] [CrossRef]
- Rasia, L.A.; Leal, G.; Koberstein, L.L.; Furlan, H.; Massi, M.; Fraga, M.A. Design and Analytical Studies of a DLC Thin-Film Piezoresistive Pressure Microsensor. Comm. Comput. Inform. Sci. 2017, 742, 433–443. [Google Scholar] [CrossRef]
- Meng, K.Y.; Chen, J.; Li, X.S.; Wu, Y.F.; Fan, W.J.; Zhou, Z.H.; He, Q.; Wang, X.; Fan, X.; Zhang, Y.X.; et al. Flexible Weaving Constructed Self-Powered Pressure Sensor Enabling Continuous Diagnosis of Cardiovascular Disease and Measurement of Cuffless Blood Pressure. Adv. Funct. Mater. 2019, 29, 388. [Google Scholar] [CrossRef]
- Ion, M.; Dinulescu, S.; Firtat, B.; Savin, M.; Ionescu, O.N.; Moldovan, C. Design and Fabrication of a New Wearable Pressure Sensor for Blood Pressure Monitoring. Sensors 2021, 21, 2075. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Park, Y.; Luan, H.; Wang, H.; Kwon, K.; Rogers, J.A.; Huang, Y. Measurement of Blood Pressure via a Skin-Mounted, Non-Invasive Pressure Sensor. J. Appl. Mech. 2021, 88, 1183. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Q.; Guo, P.; Zhao, Y.; Wang, A. Diamond-like carbon based micro-pressure sensor with ultra-thin sensitive membrane. In Proceedings of the 2021 IEEE Sensors, Sydney, Australia, 31 October–3 November 2021. [Google Scholar] [CrossRef]


| Classification | Uses | Cites |
|---|---|---|
| Musculoskeletal repair materials | Joint prosthesis and artificial dentures | [37] |
| Soft-tissue repair materials | Artificial skin | [38] |
| Cardiovascular system materials | Artificial valves and vascular stents | [39] |
| Medical membrane materials | Membranes for dialysis and gas selective permeation | [40] |
| Suture materials | Sutures | [41] |
| Drug-release carrier materials | Drug-delivery systems | [42] |
| Clinical diagnosis and biosensor materials | Sphygmomanometer and ultrasonic probe | [43] |
| Classification | Features |
|---|---|
| Biologically inert material Bioactive materials Bioabsorbable material | Placed in the human body with little interaction with surrounding tissue Placed in the human body with interaction with tissue Absorbed by the body after being placed in the body |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Peng, J.; Wang, Z.; Xiao, Y.; Qiu, X. Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development. Coatings 2022, 12, 1088. https://doi.org/10.3390/coatings12081088
Peng Y, Peng J, Wang Z, Xiao Y, Qiu X. Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development. Coatings. 2022; 12(8):1088. https://doi.org/10.3390/coatings12081088
Chicago/Turabian StylePeng, Yinglong, Jihua Peng, Ziyan Wang, Yang Xiao, and Xianting Qiu. 2022. "Diamond-like Carbon Coatings in the Biomedical Field: Properties, Applications and Future Development" Coatings 12, no. 8: 1088. https://doi.org/10.3390/coatings12081088






