Polymer-Assisted Metal Deposited Wood-Based Composites with Antibacterial and Conductive Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of PW Substrates
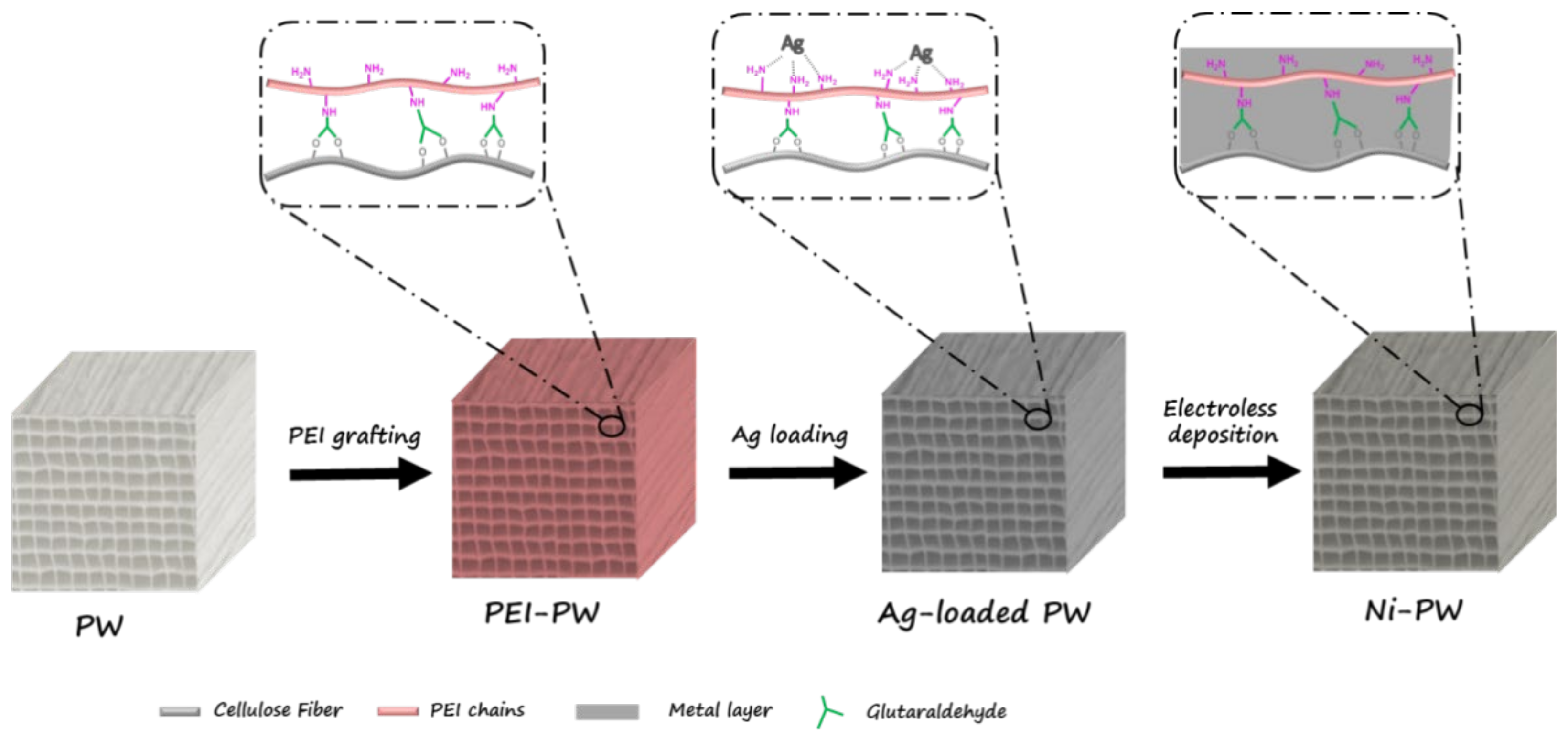
2.3. Preparation of PEI Grafted on PW Substrates
2.4. Preparation of Metallic PW (Ni-PW)
2.5. Antibacterial Test
2.6. Conductive Test
3. Results and Discussion
3.1. The Fabrication of Metallic Porous Woods
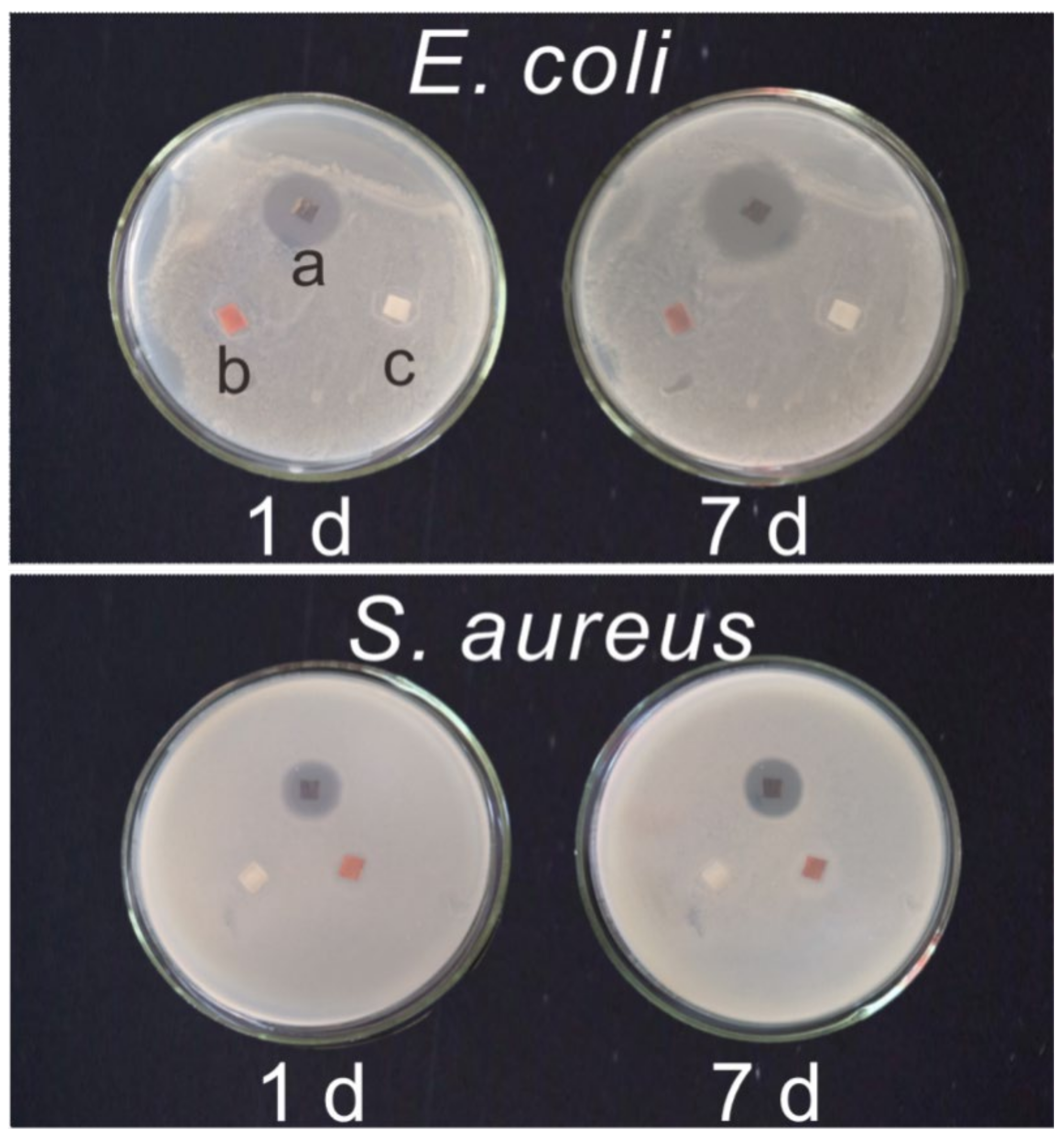
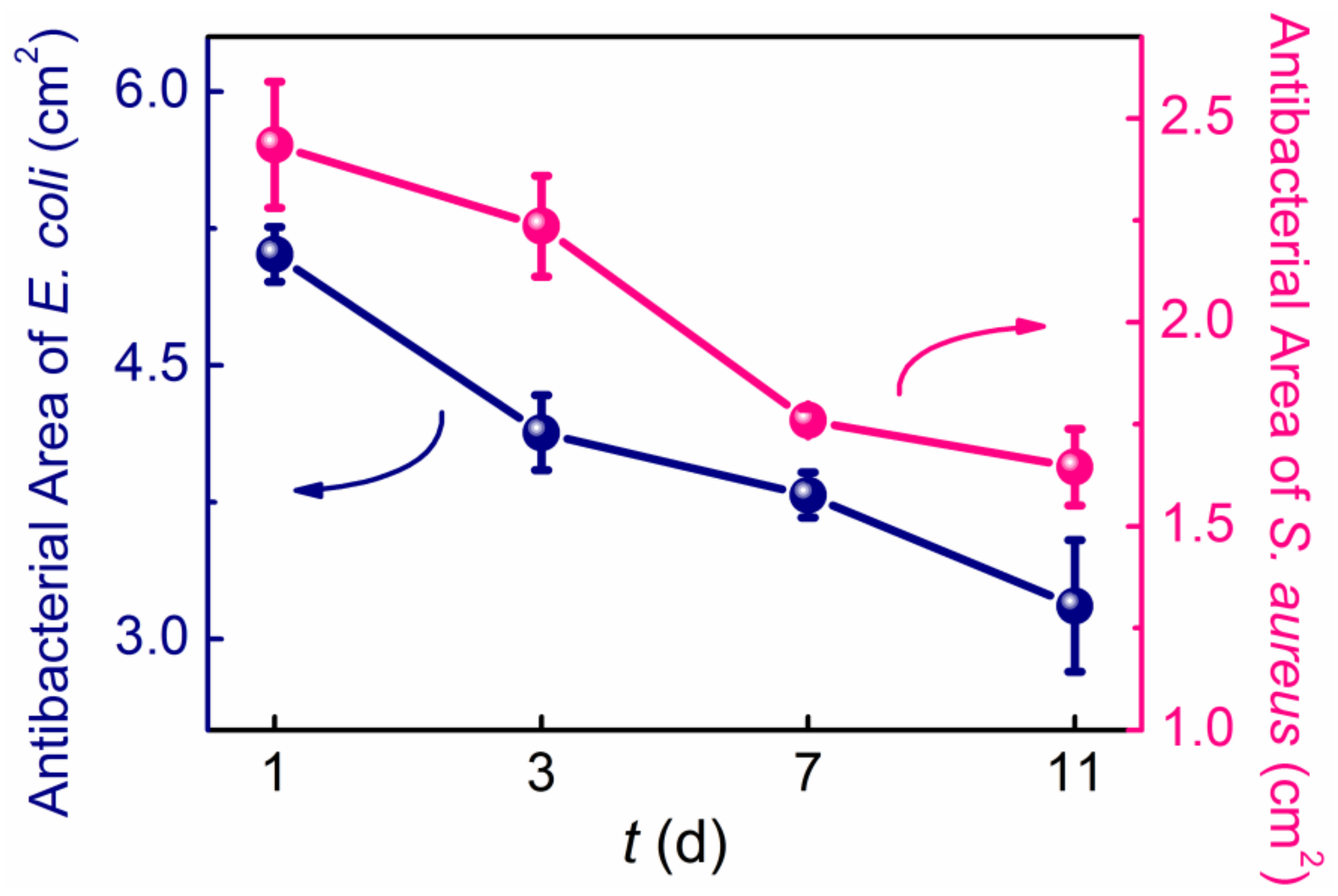
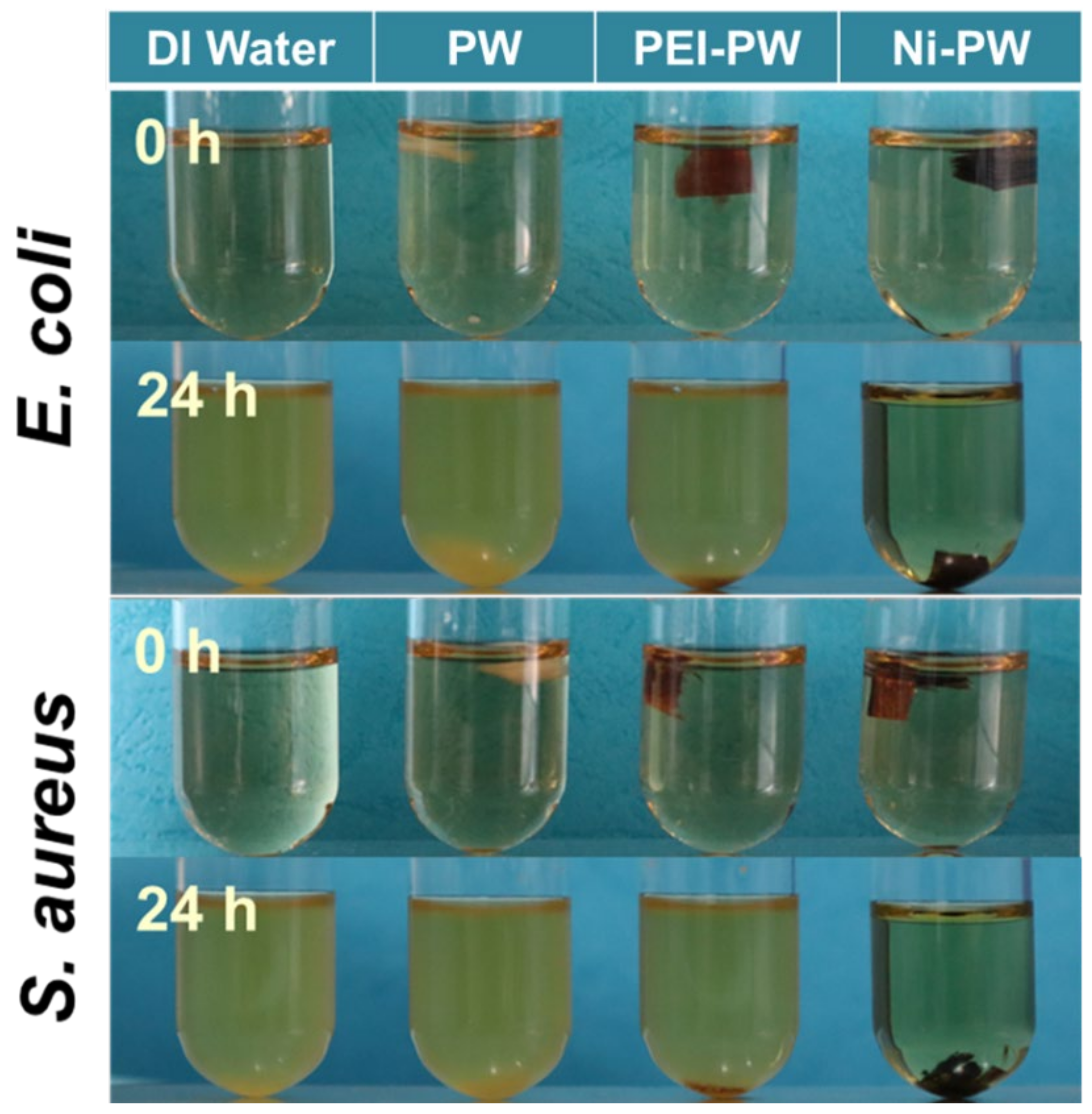
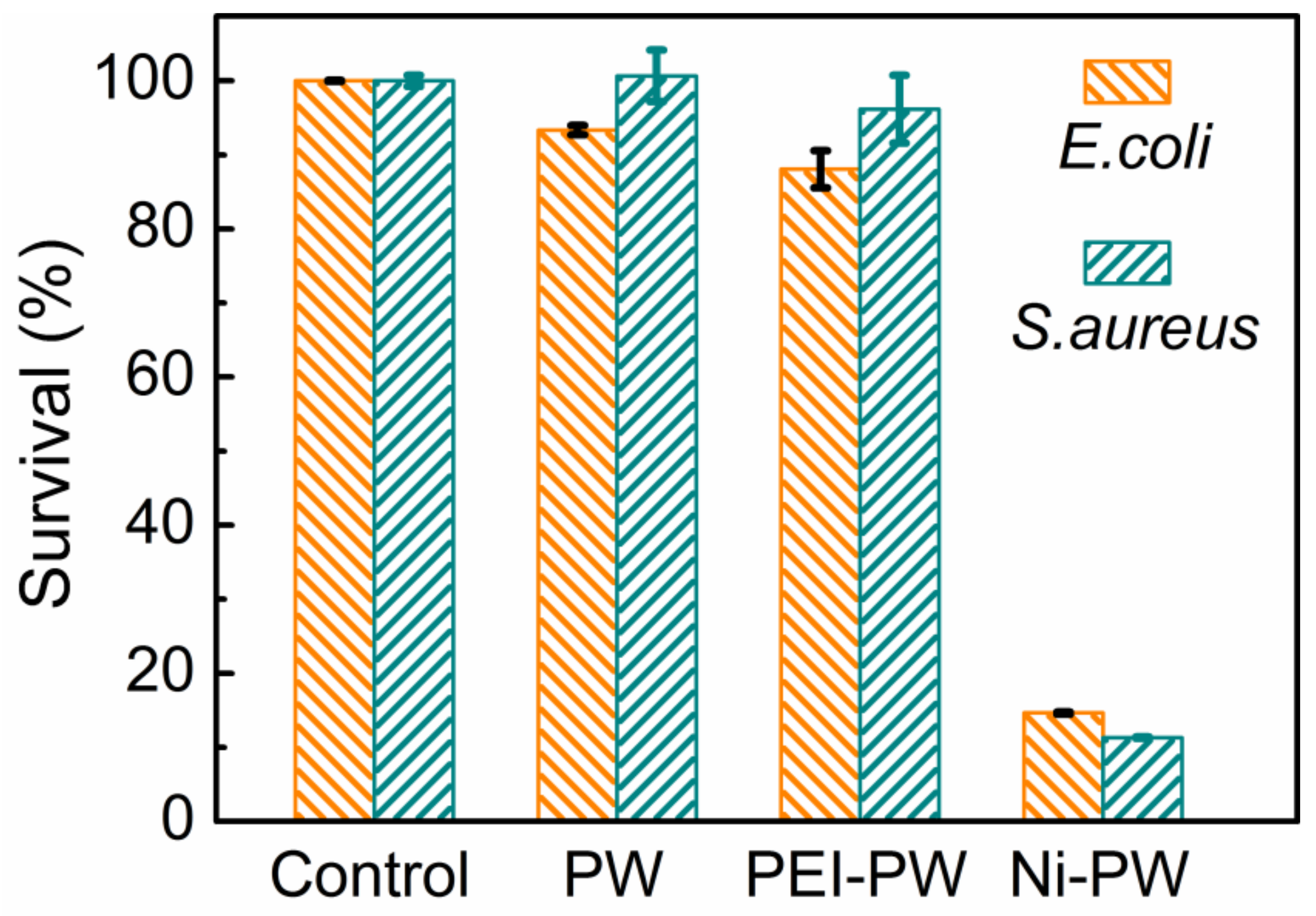
3.2. The Antibacterial Properties of the Ni-PW
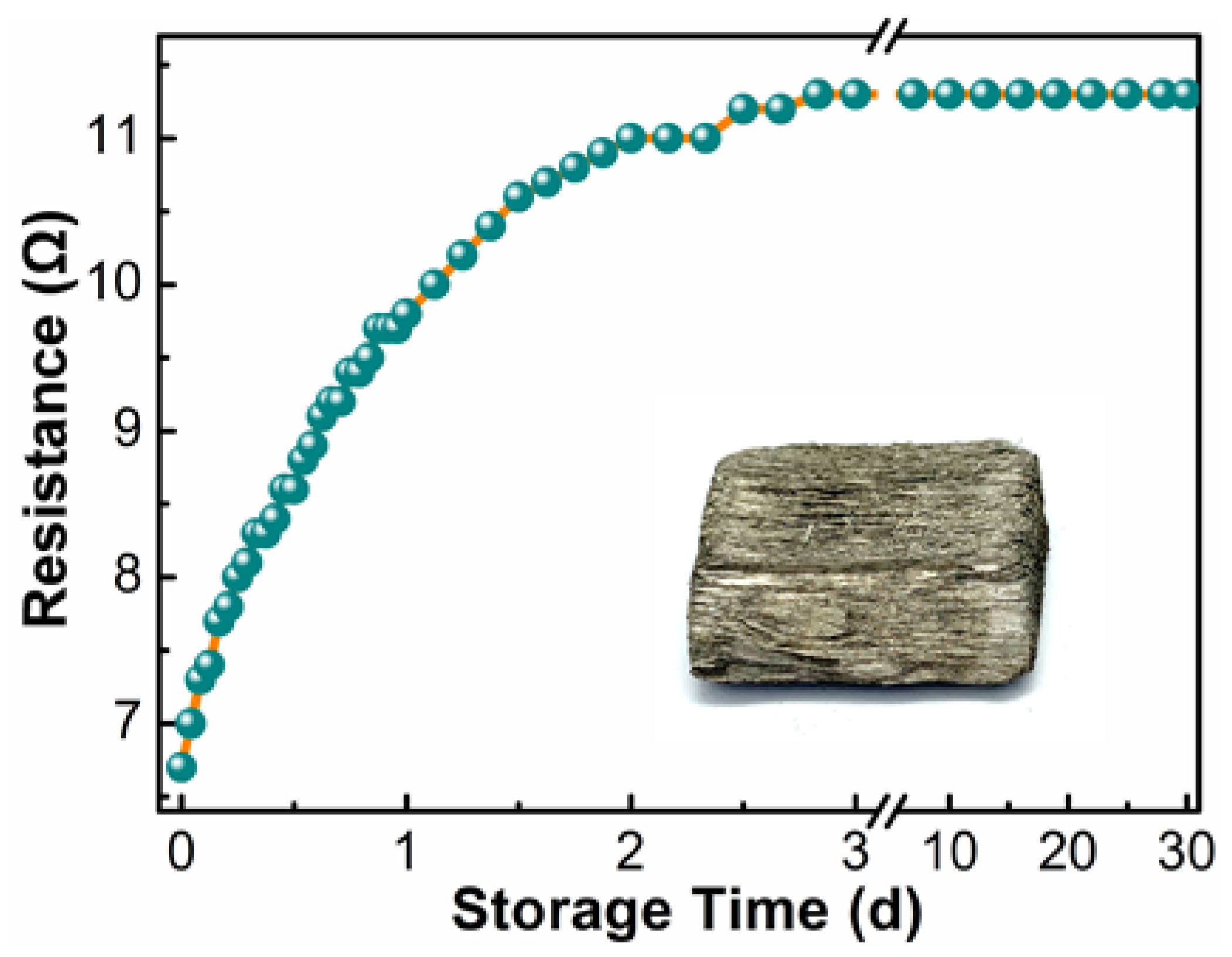
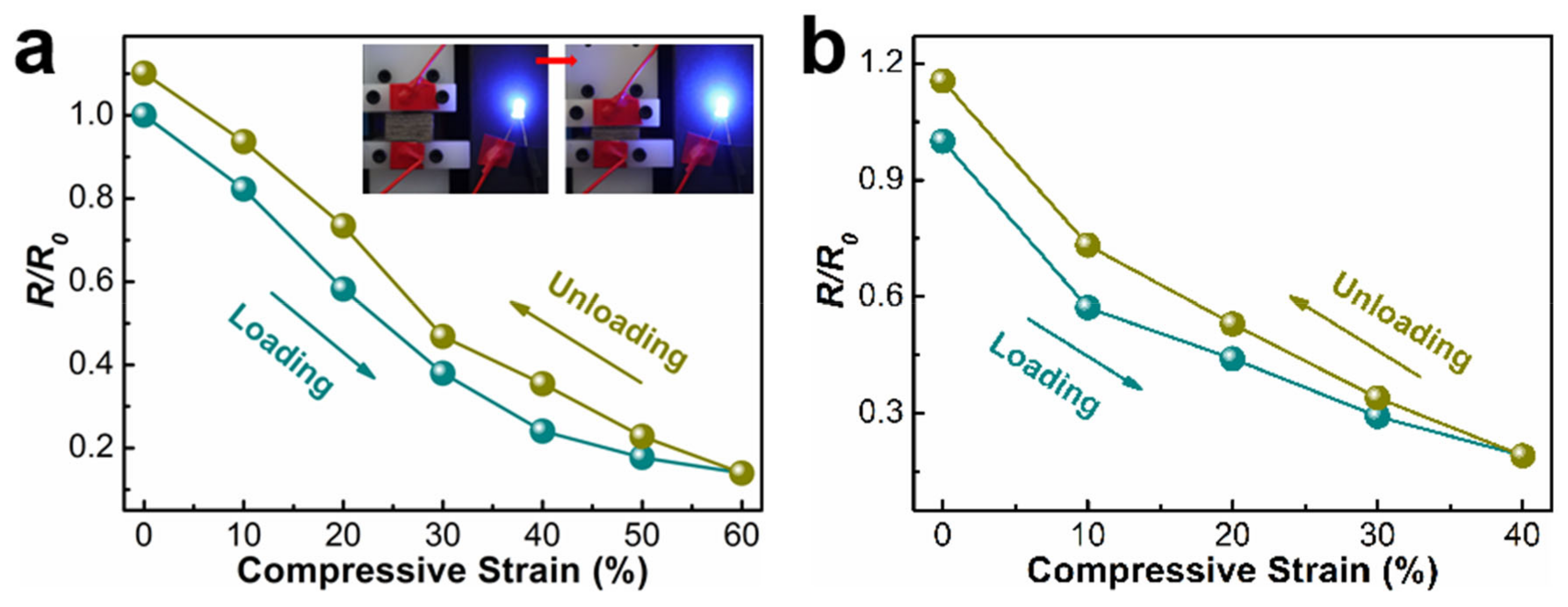
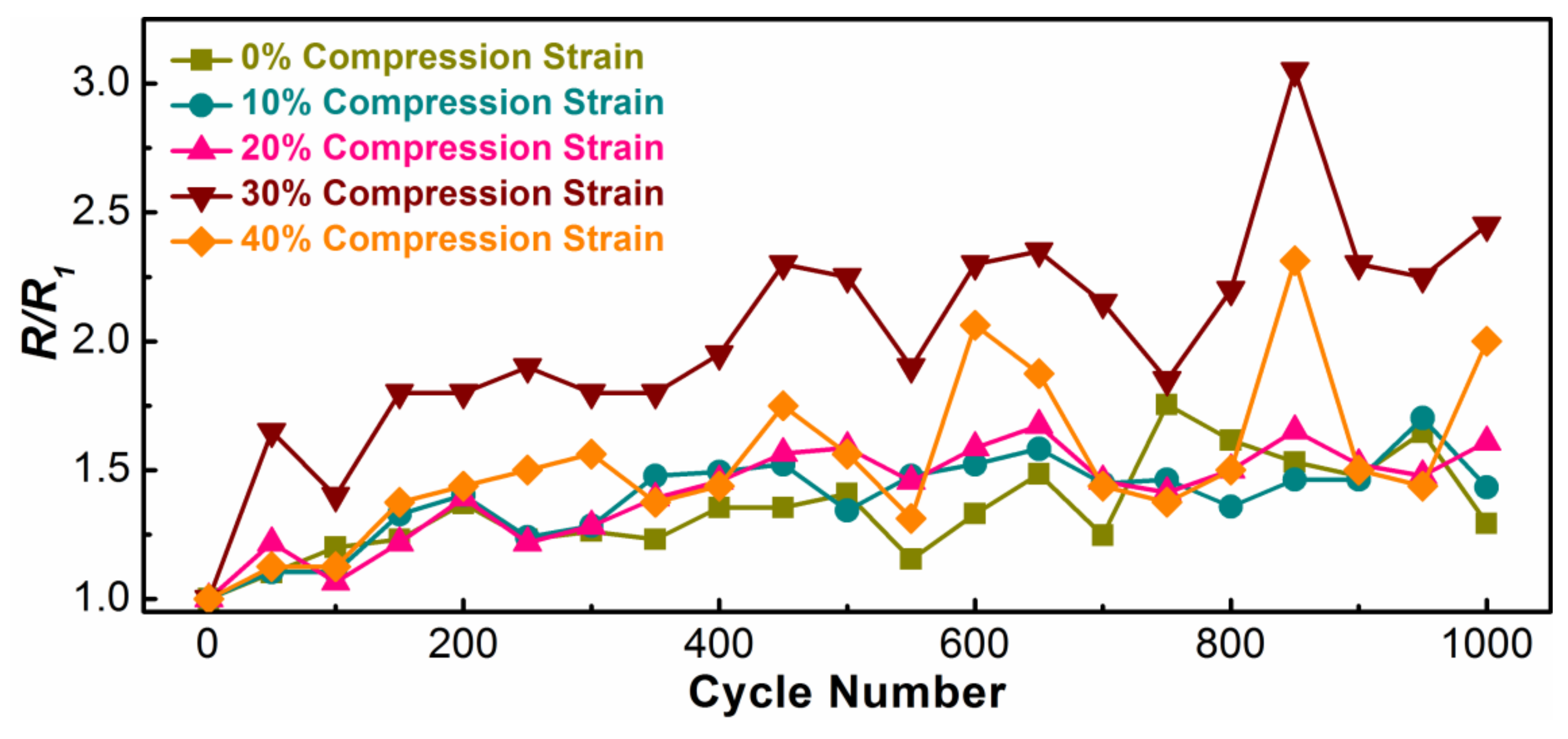
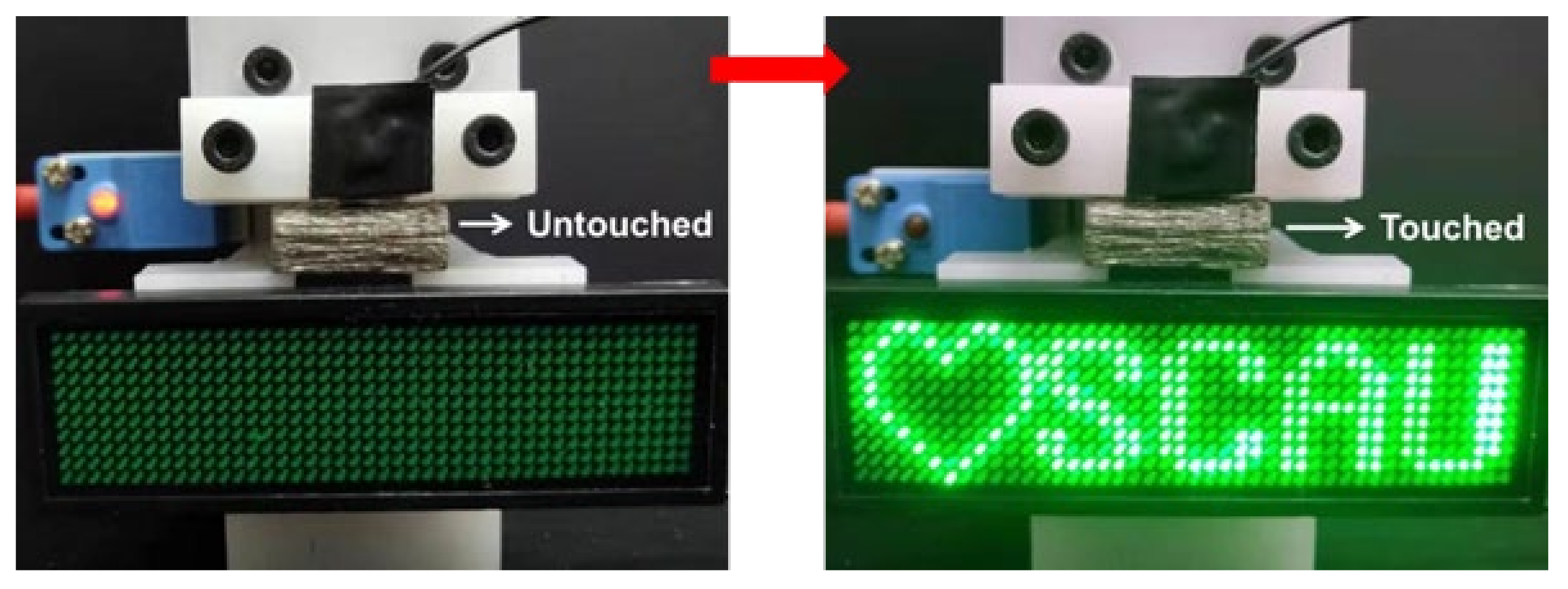
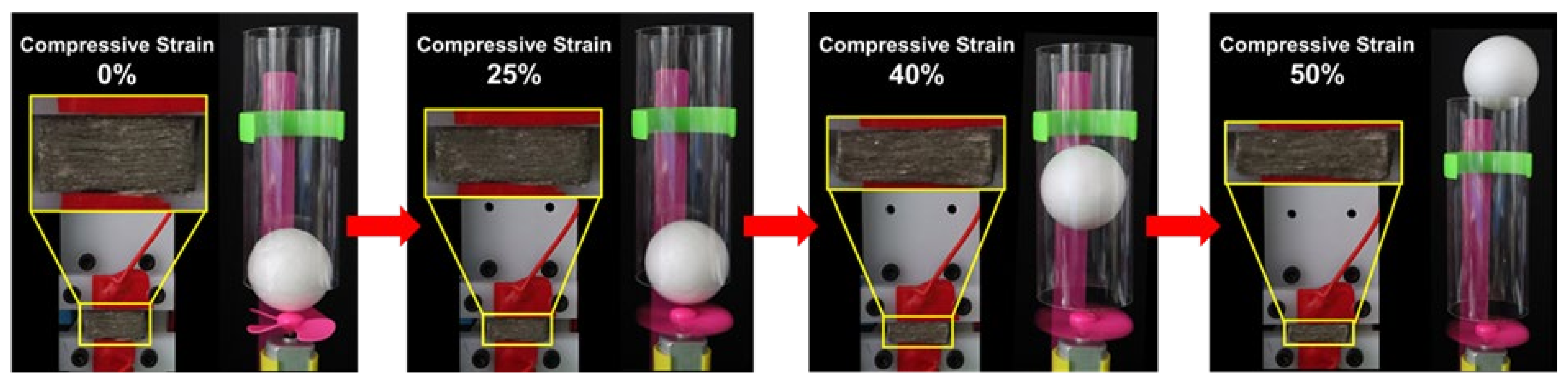
3.3. The Electrical Conductivity of the Ni-PW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal-organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent progress in fabrication and application of polydimethylsiloxane sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Chalmers, E.; Lee, H.; Zhu, C.; Liu, X. Increasing the conductivity and adhesion of polypyrrole hydrogels with electropolymerized polydopamine. Chem. Mater. 2020, 32, 234–244. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, L.; Zhang, J.; Gong, L.; Gao, M.; Zheng, P.; Xiang, L.; Wang, W.; Hu, W.; Xu, Q.; et al. A novel metal-organic layered material with superior supercapacitive performance through ultrafast and reversible tetraethylammonium intercalation. Nano Energy 2019, 59, 102–109. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, P.; Zhang, H.; Li, X.; Lei, L.; Chen, L.; Zheng, Z.; Yu, Y. Universal nature-inspired and amine-promoted metallization for flexible electronics and supercapacitors. ACS Appl. Mater. Inter. 2018, 10, 28963–28970. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, W.; Zhou, T.; Zhang, P.; Yan, C.; Zheng, Z. Monolithic hierarchical gold sponges for efficient and stable catalysis in a continuous-flow microreactor. Mater. Chem. Front. 2017, 1, 482–486. [Google Scholar] [CrossRef]
- Huang, B.; Xie, Q.; Yang, Z.; Lei, C.; Chen, W.; Tang, X.; Maran, F. Surfactant-directed Pd-nanoparticle assemblies as efficient nanoreactors for water remediation. EcoMat 2020, 2, e12046. [Google Scholar] [CrossRef]
- Gross, T.M.; Lahiri, J.; Golas, A.; Luo, J.; Verrier, F.; Kurzejewski, J.L.; Baker, D.E.; Wang, J.; Novak, P.F.; Snyder, M.J. Copper-containing glass ceramic with high antimicrobial efficacy. Nat. Commun. 2019, 10, 1979. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Chen, Y.; Jones, M.; Vanyo, S.; Li, C.; Visser, M.; Mahajan, S.; Sharma, R.; Swihart, M. Copper@ZIF-8 core-shell nanowires for reusable antimicrobial face masks. Adv. Funct. Mater. 2021, 31, 2008054. [Google Scholar] [CrossRef]
- Zhang, J.; Brown, G.; Fu, J.; James, P.; Mukandiwa, L.; Huang, X.; Yu, C. Nanobiopesticides: Silica nanoparticles with spiky surfaces enable dual adhesion and enhanced performance. EcoMat 2020, 2, e12028. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron transfer directed antibacterial properties of graphene oxide on metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, L.; Duan, Y.; An, J.; Luo, Q.; Zhang, Y.; Tang, Y.; Huang, J.; Zhang, B.; Liu, J.; et al. A novel strategy to construct supported silver nanocomposite as an ultra-high efficient catalyst. Appl. Catal. B Environ. 2016, 283, 119592. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, X.; Shi, F.; Deng, Y. Nano-gold catalysis in fine chemical synthesis. Chem. Rev. 2012, 112, 2467–2505. [Google Scholar] [CrossRef]
- Kankala, R.; Han, Y.; Na, J.; Lee, C.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.; Yamauchi, Y.; Chen, A.; et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Feng, Z.; Lin, Y.; Zhang, L.; Su, D. Facile synthesis of Au nanoparticles embedded in an ultrathin hollow graphene nanoshell with robust catalytic performance. Small 2015, 11, 5059–5064. [Google Scholar] [CrossRef]
- Dai, S.; Nouar, F.; Zhang, S.; Tissot, A.; Serre, C. One-step room-temperature synthesis of metal (IV) carboxylate metal-organic frameworks. Angew. Chem. Int. Ed. 2021, 60, 4283. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-derived heteroatom-doped porous carbon materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Q.P.; Zhang, D.Q.; Tang, Y.Q.; Shu, L.; Zhu, Y.Y.; Fan, Z.Y. Scalable all-evaporation fabrication of efficient light-emitting diodes with hybrid 2D-3D perovskite nanostructures. Adv. Funct. Mater. 2020, 30, 2002913. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Alghoraibi, N.; Henckel, D.; Zhang, R.; Nwabara, U.; Madsen, K.; Kenis, P.; Zimmerman, S.; Gewirth, A. Electrochemical CO2-to-ethylene conversion on polyamine-incorporated Cu electrodes. Nat. Catal. 2021, 4, 20–27. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, J.; Chen, C.; Xie, Z.; Guo, R.; Liu, Z.; Zhou, X.; Yang, Y.; Zheng, Z. Three-dimensional compressible and stretchable conductive composites. Adv. Mater. 2014, 26, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yu, Y.; Xie, Z.; Liu, X.; Zhou, X.; Gao, Y.; Liu, Z.; Zhou, F.; Yang, Y.; Zheng, Z. Matrix-assisted catalytic printing for the fabrication of multiscale, flexible, foldable, and stretchable metal conductors. Adv. Mater. 2013, 25, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, R.; Chen, X.; Chalmers, E.; Liu, X.; Wang, Y.; Xu, B.; Liu, X. Ultraelastic yarns from curcumin-assisted ELD toward wearable human-machine interface textiles. Adv. Sci. 2020, 7, 2002009. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, C.; Cheng, S.; Xu, Z.; Sun, X.; Xu, Y.; Chen, J.; Jiang, Z.; Liang, K.; Feng, Z. Flexible RFID tag metal antenna on paper-based substrate by inkjet printing technology. Adv. Funct. Mater. 2019, 29, 1902579. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 2015, 6, 7260. [Google Scholar] [CrossRef]
- Chu, Z.; Zheng, P.; Yang, Y.; Wang, C.; Yang, Z. Compressible nanowood/polymer composite adsorbents for wastewater purification applications. Compos. Sci. Technol. 2020, 198, 108320. [Google Scholar] [CrossRef]
- Chu, Z.; Li, Y.; Zhou, A.; Zhang, L.; Zhang, X.; Yang, Y.; Yang, Z. Polydimethylsiloxane-decorated magnetic cellulose nanofiber composite for highly efficient oil-water separation. Carbohydr. Polym. 2022, 277, 118787. [Google Scholar] [CrossRef]
- Chu, Z.; Zheng, B.; Wang, W.; Li, Y.; Yang, Y.; Yang, Z. Magnetic nitrogen-doped biochar for adsorptive and oxidative removal of antibiotics in aqueous solutions. Sep. Purif. Technol. 2022, 297, 121508. [Google Scholar] [CrossRef]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Xiang, P.; Liu, T.; Hou, Y.; Yang, Y.; Zhu, H. Biopolymers derived from trees as sustainable multifunctional materials: A review. Adv. Mater. 2021, 33, 2001654. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiyah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-derived materials for green electronics, biological devices, and energy applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, H.; Tao, X.; Liang, Y.; Yang, S.J.; Huang, J.Q.; Yuan, T.Q.; Titirici, M.M.; Zhang, Q. Recent progress on biomass-derived ecomaterials toward advanced rechargeable lithium batteries. EcoMat 2020, 2, e12019. [Google Scholar] [CrossRef]
- Li, T.; Song, J.; Zhao, X.; Yang, Z.; Pastel, G.; Xu, S.; Jia, C.; Dai, J.; Chen, C.; Gong, A. Anisotropic, lightweight, strong, and super thermally insulating nanowood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, eaar3724. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Li, B.; Wang, X.; Yu, B.; Pei, X.; Huck, W.T.; Zhou, F. Bio-inspired renewable surface-initiated polymerization from permanently embedded initiators. Angew. Chem. Int. Ed. 2016, 55, 4260–4264. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xiao, X.; Zhang, Y.; Li, K.; Yan, C.; Wei, X.; Chen, L.; Zhen, H.; Zhou, H.; Zhang, S. Photoreactive and metal-platable copolymer inks for high-throughput, room-temperature printing of flexible metal electrodes for thin-film electronics. Adv. Mater. 2016, 28, 4926–4934. [Google Scholar] [CrossRef]
- Oh, Y.J.; Khan, E.S.; Del Campo, A.; Hinterdorfer, P.; Li, B. Nanoscale characteristics and antimicrobial properties of (SI-ATRP)-seeded polymer brush surfaces. ACS Appl. Mater. Inter. 2019, 11, 29312–29319. [Google Scholar] [CrossRef]
- Bae, S.K.; Choo, D.C.; Kang, H.S.; Yoo, K.; Kim, T.W. Transparent ultra-thin silver electrodes formed via a maskless evaporation process for applications in flexible organic light-emitting devices. Nano Energy 2020, 71, 104649. [Google Scholar] [CrossRef]
- Zhao, R.T.; Kong, W.; Sun, M.X.; Yang, Y.; Liu, W.Y.; Lv, M.; Song, S.P.; Wang, L.H.; Song, H.B.; Hao, R.Z. Highly stable graphene-based nanocomposite (GO-PEI-Ag) with broad-spectrum, long-term antimicrobial activity and antibiofilm effects. ACS Appl. Mater. Inter. 2018, 10, 17617–17629. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, D.; Huang, Q.; Li, Y.; Wu, Z.; Yang, Y.; Yang, Z. Polymer-assisted preparation of porous wood-based metallic composites for efficient catalytic reduction of organic pollutants. Ind. Crop. Prod. 2022, 187, 115387. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar]
- Peng, B.; Zhang, X.; Aarts, D.; Dullents, R. Superparamagnetic nickel colloidal nanocrystal clusters with antibacterial activity and bacteria binding ability. Nat. Nanotechnol. 2018, 13, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.L.; Xing, W.S.; Jiang, L.L.; Fang, J.; Zhao, C.C.; Ren, F.Z.; Fang, L.M.; Wang, K.F.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Hoop, M.; Shen, Y.; Chen, X.; Mushtaq, F.; Iuliano, L.M.; Sakar, M.S.; Petruska, A.J.; Loessner, M.J.; Nelson, B.J.; Pané, S. Magnetically driven silver-coated nanocoils for efficient bacterial contact killing. Adv. Funct. Mater. 2016, 26, 1063–1069. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, injectable, and self-healing conductive hydrogel enabled by multiple hydrogen bonding toward wearable electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Ko, H.C.; Stoykovich, M.P.; Song, J.; Malyarchuk, V.; Choi, W.M.; Yu, C.J.; Geddes, J.B.; Xiao, J.; Wang, S.; Huang, Y. A hemispherical electronic eye camera based on compressible silicon optoelectronics. Nature 2008, 454, 748–753. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, F.; Yang, Y.; Chu, Z.; Yang, Z. Polymer-Assisted Metal Deposited Wood-Based Composites with Antibacterial and Conductive Properties. Coatings 2022, 12, 1161. https://doi.org/10.3390/coatings12081161
Shen F, Yang Y, Chu Z, Yang Z. Polymer-Assisted Metal Deposited Wood-Based Composites with Antibacterial and Conductive Properties. Coatings. 2022; 12(8):1161. https://doi.org/10.3390/coatings12081161
Chicago/Turabian StyleShen, Fangning, Yu Yang, Zhuangzhuang Chu, and Zhuohong Yang. 2022. "Polymer-Assisted Metal Deposited Wood-Based Composites with Antibacterial and Conductive Properties" Coatings 12, no. 8: 1161. https://doi.org/10.3390/coatings12081161
APA StyleShen, F., Yang, Y., Chu, Z., & Yang, Z. (2022). Polymer-Assisted Metal Deposited Wood-Based Composites with Antibacterial and Conductive Properties. Coatings, 12(8), 1161. https://doi.org/10.3390/coatings12081161






