Room-Temperature Synthesis of Titanium Nitride Using Metastable Nitrogen
Abstract
:1. Introduction
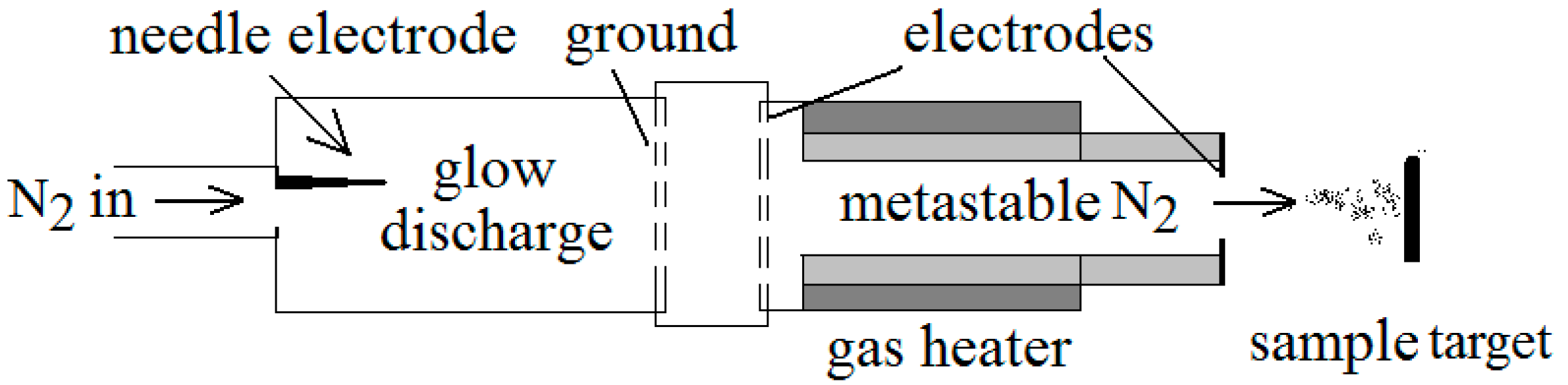
2. Materials and Methods
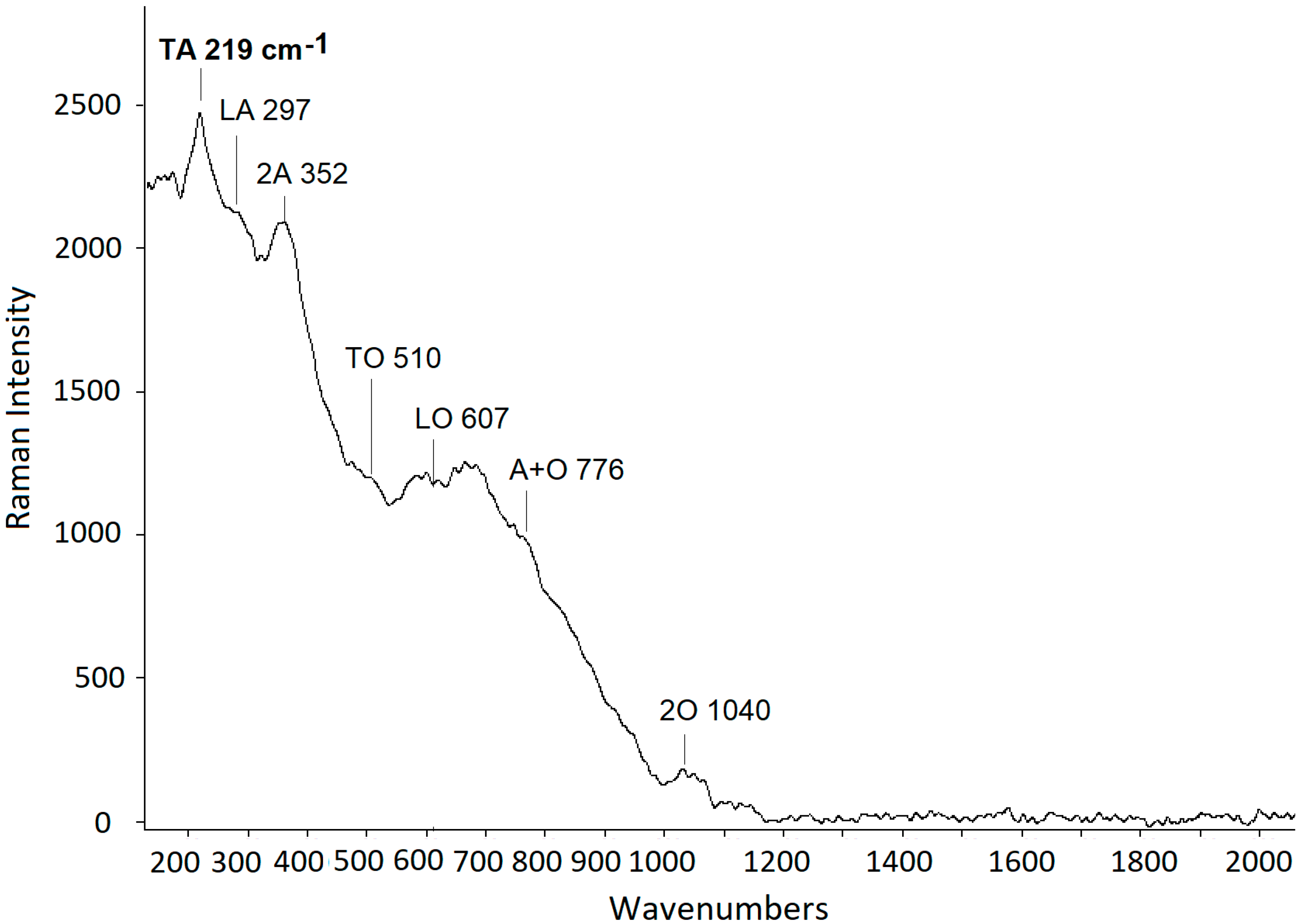
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gopi, R.; Saravanan, I.; Devaraju, A. A Review on Nitride-Based Coating Techniques. In Recent Advances in Materials and Modern Manufacturing; Springer: Singapore, 2022; pp. 803–811. [Google Scholar] [CrossRef]
- Bell, T. Source Book on Nitriding; American Society for Metals: Metals Park, OH, USA, 1977. [Google Scholar]
- Mongis, J.; Peyre, J.P.; Tournier, C. Heat Treat. Metals 1984, 3, 71. [Google Scholar]
- Yan, M.F.; Chen, B.F.; Li, B. Microstructure and mechanical properties from an attractive combination of plasma nitriding and secondary hardening of M50 steel. Appl. Surf. Sci. 2018, 455, 1–7. [Google Scholar] [CrossRef]
- Tong, W.P.; Tao, N.R.; Wang, Z.B.; Lu, J.; Lu, K. Nitriding iron at lower temperatures. Science 2003, 299, 686–688. [Google Scholar] [CrossRef]
- Gobbi, S.J.; Gobbi, V.J.; Reinke, G.; Rocha, Y. Orthopedic Implants: Coating with TiN. Biomed. J. Sci. Tech. Res. 2019, 16, 1–3. [Google Scholar] [CrossRef]
- Katz, I.; Anderson, M.S.; White, L.M.; Boeder, P.A.; Hoey, W.A. Mars 2020 sample cleanliness molecular transport model. In Systems Contamination: Prediction, Control, and Performance 2018; International Society for Optics and Photonics: Washington, DC, USA, 2018; Volume 10748, p. 107480A. [Google Scholar]
- Gui, L.; Bagheri, S.; Strohfeldt, N.; Hentschel, M.; Zgrabik, C.M.; Metzger, B.; Linnenbank, H.; Hu, E.L.; Giessen, H. Nonlinear refractory plasmonics with titanium nitride nanoantennas. Nano Lett. 2016, 16, 5708–5713. [Google Scholar] [CrossRef] [PubMed]
- Butet, J.; Duboisset, J.; Bachelier, G.; Russier-Antoine, I.; Benichou, E.; Jonin, C.; Brevet, P.F. Optical second harmonic generation of single metallic nanoparticles embedded in a homogeneous medium. Nano Lett. 2010, 10, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, M.; Novotny, L. Optical frequency mixing at coupled gold nanoparticles. Phys. Rev. Lett. 2007, 98, 026104. [Google Scholar] [CrossRef]
- Koya, A.N.; Zhu, X.; Ohannesian, N.; Yanik, A.A.; Alabastri, A.; Proietti Zaccaria, R.; Krahne, R.; Shih, W.C.; Garoli, D. Nanoporous metals: From plasmonic properties to applications in enhanced spectroscopy and photocatalysis. ACS Nano 2021, 15, 6038–6060. [Google Scholar] [CrossRef]
- Kauranen, M.; Zayats, A.V. Nonlinear plasmonics. Nat. Photonics 2012, 6, 737–748. [Google Scholar] [CrossRef]
- Anderson, M.S. Locally enhanced Raman spectroscopy with an atomic force microscope. Appl. Phys. Lett. 2000, 76, 3130–3132. [Google Scholar] [CrossRef]
- Lorite, I.; Serrano, A.; Schwartzberg, A.; Bueno, J.; Costa-Krämer, J.L. Surface enhanced Raman spectroscopy by titanium nitride non-continuous thin films. Thin Solid Films 2013, 531, 144–146. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical properties and plasmonic performance of titanium nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef]
- Musselman, B.; Tice, J.; Crawford, E. Enabling automated sample analysis by direct analysis in real time (DART) mass spectrometry. In Ambient Ionization Mass Spectrometry; Royal Society of Chemistry: London, UK, 2014; pp. 445–461. [Google Scholar]
- Monge, M.E.; Harris, G.A.; Dwivedi, P.; Fernandez, F.M. Mass spectrometry: Recent advances in direct open-air surface sampling/ionization. Chem. Rev. 2013, 113, 2269–2308. [Google Scholar] [CrossRef]
- Gross, J.H. Direct analysis in real time–a critical review on DART-MS. Anal. Bioanal. Chem. 2014, 406, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chuah, W.C.; Lu, X.; Remsen, E.; Bartmess, J.E. Ionization mechanism of positive-ion nitrogen direct analysis in real time. J. Am. Soc. Mass Spectrom. 2018, 29, 640–650. [Google Scholar] [CrossRef]
- Gilmore, F.R. Potential energy curves for N2, NO, O2,and corresponding ions. J. Quant. Spectrosc. Rad. Trans. 1965, 5, 369–390. [Google Scholar] [CrossRef]
- Slanger, T.G. Reactions of electronically excited diatomic molecules. In Reactions of Small Transient Species, Kinetics, and Energetics; Fontijn, A., Clyne, M.A.A., Eds.; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Cernogora, G.; Hochard, L.; Touzeau, M.; Matos Ferreira, C. Population of N2 (A 3Σ+ u) metastable states in a pure nitrogen glow discharge. J. Phys. B At. Mol. Phys. 1981, 14, 2977–2987. [Google Scholar] [CrossRef]
- Aroca, R.F.; Ross, D.J.; Domingo, C. Surface-enhanced infrared spectroscopy. Appl. Spectrosc. 2004, 58, 324A–338A. [Google Scholar] [CrossRef]
- Morozov, I.G.; Belousova, O.V.; Belyakov, O.A.; Parkin, I.P.; Sathasivam, S.; Kuznetcov, M.V. Titanium nitride room-temperature ferromagnetic nanoparticles. J. Alloys Compd. 2016, 675, 266–276. [Google Scholar] [CrossRef]
- Judek, J.; Wróbel, P.; Michałowski, P.P.; Ożga, M.; Witkowski, B.; Seweryn, A.; Struzik, M.; Jastrzębski, C.; Zberecki, K. Titanium Nitride as a Plasmonic Material from Near-Ultraviolet to Very-Long-Wavelength Infrared Range. Materials 2021, 14, 7095. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, M.A.; Hinrichs, R.; Javorsky, C.S.; Giuriatti, G.; Da Costa, J.B. Micro-Raman characterization of plasma nitrided Ti6Al4V-ELI. Surf. Coat. Technol. 2007, 202, 275–279. [Google Scholar] [CrossRef]
- Baldwin, M.J.; Haydon, S.C.; Fewell, M.P. Metastable states and nitriding plasmas. Surf. Coat. Technol. 1997, 97, 97–101. [Google Scholar] [CrossRef]
- Dovbeshko, G.I.; Shirshov, Y.M.; Chegel, V.I.; Fesenko, O.M. Experimental and calculated enhancement factor in the SEIRA method. In XVI International Conference on Spectroscopy of Molecules and Crystals; International Society for Optics and Photonics: Washington, DC, USA, 2004; Volume 5507, pp. 386–396. [Google Scholar]
- Sun, W.; Holder, A.; Orvañanos, B.; Arca, E.; Zakutayev, A.; Lany, S.; Ceder, G. Thermodynamic routes to novel metastable nitrogen-rich nitrides. Chem. Mater. 2017, 29, 6936–6946. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, M.S. Room-Temperature Synthesis of Titanium Nitride Using Metastable Nitrogen. Coatings 2022, 12, 1177. https://doi.org/10.3390/coatings12081177
Anderson MS. Room-Temperature Synthesis of Titanium Nitride Using Metastable Nitrogen. Coatings. 2022; 12(8):1177. https://doi.org/10.3390/coatings12081177
Chicago/Turabian StyleAnderson, Mark S. 2022. "Room-Temperature Synthesis of Titanium Nitride Using Metastable Nitrogen" Coatings 12, no. 8: 1177. https://doi.org/10.3390/coatings12081177
APA StyleAnderson, M. S. (2022). Room-Temperature Synthesis of Titanium Nitride Using Metastable Nitrogen. Coatings, 12(8), 1177. https://doi.org/10.3390/coatings12081177





