Abstract
The present study aimed to prepare experimental adhesives (EAs): 5 wt.% titanium dioxide (TiO2) adhesive; and 5 wt.% zirconium oxide (ZrO2) adhesive; and analyze their impact on bond integrity of fiber posts to root dentin, and viscosity. The EA was composed of: bisphenol A glycol dimethacrylate (BisGMA); triethylene glycol dimethacrylate (TEGDMA); 2-hydroxyethyl methacrylate (HEMA); and ethyl 4-dimethylamino benzoate and camphorquinone. TiO2 and ZrO2 particles were individually incorporated into the EA at 5 wt.%, to form two groups (5% TiO2 and 5% ZrO2). The adhesives, with particles, were characterized using scanning electron microscopy (SEM) and energy-dispersive X-ray (EDX) spectroscopy. The bonded interface was evaluated for adhesive–dentin penetration at the interface, using SEM. The study adhesive groups (EA, 5% TiO2 and 5% ZrO2) were analyzed for rheology and push-out bond strength of the fiber post to root dentin. Data were analyzed using analysis of variance and post hoc comparison. Both TiO2 and ZrO2 particles had irregular, non-uniform shapes. The EDX mapping showed the elemental presence of Ti (TiO2), Zr (ZrO2) and oxygen in formulated adhesives. The 5% TiO2 and 5% ZrO2 adhesives showed a decrease in viscosity, compared with the EA. Bond strength among the 5% TiO2 and 5% ZrO2 adhesives was statistically comparable (p > 0.05), but higher than the control group (10.57 ± 1.45 MPa) (p < 0.05). Reinforcement of the experimental dentin adhesive with 5% TiO2 or 5% ZrO2 increased the push-out bond strength of the fiber post to root dentin, in comparison with the EA. Particle-incorporated adhesives (5% TiO2 and 5% ZrO2) displayed decreased viscosity, compared with the EA (without particles).
1. Introduction
Dental posts are used in endodontically treated teeth to provide adequate tooth form for placement of a successful indirect restoration [1]. The success of a dental restoration depends on the effective bond of the dental post to the root dentin and the coronal restorative material. Critical success factors for post treatment include: post surface and bonding interface; treatment and preparation of root dentin; cementation material; and cementation procedure [2]. A reliable adhesive bond between the post and root dentin allows the root-post and core to form a monobloc construction, with transfer of occlusal forces, axially, to the periodontium [3]. By contrast, a compromised post–cement–dentin bond results in: microleakage; mechanical weakening of the post and root dentin; failure of the core; and restoration-, and tooth failure [4]. Treatment of the post and dentin bonding agents plays a critical role in developing a durable bond between root post, cement and dentin [5].
Dentin adhesives, or dentin bonding agents (DBAs), are complex mixtures of synthetic polymers acting as bridging agents, developed to optimize the attachment of polymeric composites to tooth dentin [6]. As dentin is a collagenous, hydrophilic, regenerating and dynamic tissue, its bond with polymeric adhesives is unstable and loses durability overtime [7,8]. Presence of moisture in dentin causes hydrolytic degradation of polymeric covalent bonds, resulting in leakage and loss of bond strength [9]. Therefore, DBAs are often modified to optimize their function, through the addition of fillers, with potential to improve their properties [10]. These fillers offer potential enhancements in anti-microbial, mechanical and biological properties [11,12]. The fillers with the maximum biocompatibility with oral tissue, giving them potential to be added to dentin adhesives, are zirconium oxide (ZrO2) and titanium dioxide (TiO2) [13].
ZrO2 is a tooth-colored material with excellent mechanical properties and chemical stability [14]. Stabilized Zr polycrystals are effectively used in tooth-and-implant supported oral rehabilitations, for replacing lost tooth structure [15,16]. Its use as a composite filler has shown improved mechanical and adhesive properties [17]. In a recent study by Lohbauer et al., incorporation of different percentage compositions of Zr particles revealed improved bond strength with dentin [18]. In addition, larger grain sizes of Zr particles tend to destabilize Zr products at low temperature aging [19]. Therefore, incorporation of micro-sized Zr particles may improve the durability of DBA in adhesive bonding. Another commonly used biomaterial in dentistry is Titanium oxide (TiO2). TiO2 and related alloys are thermally stable, chemically durable, highly biocompatible and mechanically strong [20,21,22]. Use of TiO2 has been shown to effect improvements in: mechanical fracture resistance of teeth; improved osseointegration of dental implants; antibacterial efficacy; and improved fit of dental restorations [23,24]. TiO2 particles have been used in dental adhesives, showing comparable outcomes to control adhesives, in solubility, biocompatibility and water sorption [25,26]. In addition, dental cements containing TiO2 have shown better degrees of conversion of modified DBAs [27].
Both ZrO2 and TiO2 particles have shown efficacy, and further potential, in improving adhesive ability of DBA to tooth dentin [18,26]. In a study by Al-Saleh et al., incorporation of ZrO2 and TiO2 particles into DBA showed an improvement in adhesive bonding of the resin composite to crown dentin [13]. The micro-structural differences of crown and root dentin compromise the root dentin’s ability to bond to root posts through resin adhesives [28]. However, root dentin adhesion was not assessed in the study by Al-Saleh et al. [13]. In addition, there is no evidence on the adhesive bond integrity of TiO2-, and ZrO2-incorporated dentin adhesive in bonding fiber posts to root dentin. It is hypothesized that the addition of ZrO2 and TiO2 filler particles to a polymer-based DBA would improve its rheological properties and enhance the adhesive bonding of fiber-based dental post to root dentin. Therefore, the aim of the present study was to prepare an experimental adhesive (EA) and assess the influence of 5% TiO2 and 5% ZrO2 particle reinforcement on its rheology and push-out bond strength of fiber post to root dentin.
2. Materials and Methods
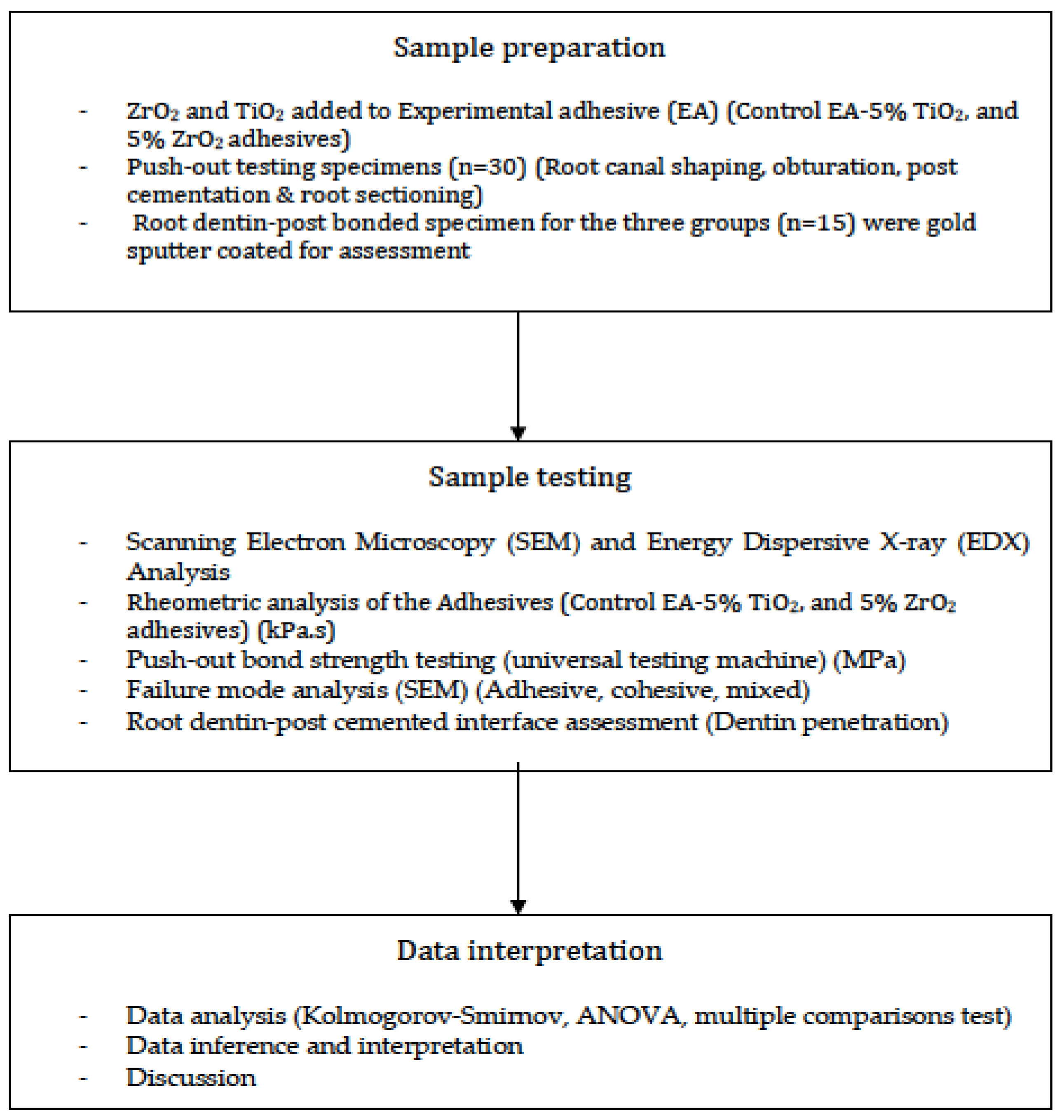
The present study protocol was approval from the institutional review board and ethics committee at King Saud University with approval No. E-20-5545. The study was designed in accordance with the principles stated in the Helsinki Declaration of 2013. Mandibular single-rooted premolars used in the experiments were acquired after gaining patient consent, following specialist dental care at a private practice. The steps in study methodology are presented in Figure 1. Sample size calculation for the required teeth, to be tested for bond strength, was performed using Graphpad Instat (Prism, San Diego, CA, USA), utilizing the means and SD from a previous (similar) study [13], at 90% power. The required samples were eight per group, however 10 samples in each bond-strength testing group were included.

Figure 1.
Study methodology.
2.1. Preparation of the ZrO2 and TiO2 Added Experimental Adhesive (EA)
An experimental adhesive was prepared, using a mixture of 50%-Bis-GMA, 25%-TEGDMA, and 25%-HEMA (60%) (weight), with ethanol (25%-m/m) as a solvent. Solutions of 0.5% (n/n) ethyl 4-dimethylamino benzoate and 0.5% camphorquinone were incorporated as a photoinitiator, in accordance with the monomer molarity. In addition, 1.0% (n/n) diphenyliodonium hexafluorophosphate (DPIHP) was integrated, as an electron initiator, into the adhesive mix. A three-necked flask (using a magnetic stirrer) and a condenser (SA300; Sansyo, Tokyo, Japan) were used to synthesize the experimental adhesive (EA). This new EA was stored in a dark plastic container to avoid photo-polymerization. Filler particles, including TiO2 and ZrO2 were acquired (TiO2: particle mixture-634662-Merck SA, Darmstadt, Germany; and ZrO2: 643028-Merck SA, Darmstadt, Germany) and particles of 5 wt.% of TiO2 and 5 wt.% of ZrO2 were added to the EA separately, to form three adhesive groups (EA, 5% TiO2, and 5% ZrO2). For homogenized dispersion of the EA with the particles, they were sonicated in a high-speed mixer. These newly prepared adhesives (EA, 5% TiO2, and 5% ZrO2) were stored at 4 °C and used for further experimentation.
2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX) Analysis
Using SEM and EDX analysis, TiO2 and ZrO2 filler particles were characterized. The material particles were mounted on the SEM aluminum stub and gold coated, before being observed via an SEM (Jeol JSM 6360, Scanning Electron Microscope, Peabody, MA, USA), operating at accelerating voltage of 30 kV. These samples were evaluated at different magnifications. The elemental distribution of the filler particles was ascertained using EDX spectroscopy.
2.3. Rheometric Analysis of the Adhesives
The experimental synthesized adhesives were assessed for changes in rheology using an MCR-72 rheometer (Anton Paar, Graz, Austria). Implementing a rotation mode and frequency sweep pre-set state of 8 mm (parallel plate) and 0.25 mm (opening), the adhesive rheology was investigated. Using a wide range of angular frequencies, ranging from 0.1 to 100 rad/s at 25 °C, viscosity of the samples (EA, 5% TiO2, and 5% ZrO2) was assessed. Ten samples were measured and assessed for each group.
2.4. Specimen Preparation for Push-Out Bond Strength Assessment
Thirty single-rooted human mandibular premolars, with developed apices and free of root canal treatments, were collected. Attached debris and plaque was removed with an ultrasonic scaler (Woodpecker, Unicorn DenMart Limited, Delhi, India) and disinfection with Chloramine T trihydrate (A.B. Enterprises, Mumbai, India) was performed for 48 h at 4 °C. The crowns were decoronated below the cementoenamel junction with a slow-speed, water-cooled diamond disc (BVM International, New Delhi, India). The root apex was removed to a length of 15 mm and was embedded perpendicularly in heat-cure acrylic resin (Meadway Dentals Pvt. Ltd., New Delhi, India). For standardization of apical size, a master apical file of #20 K-file was employed. A Protaper universal NiTi system (Dentsply, Philldelphia, PA, USA) was used at a rotation speed of 300 rpm, with a moderate in-and-out motion, in a crown-down fashion, for root canal preparation, flaring and finishing. SX and S1 shaping files were used for flaring of coronal, middle and apical thirds of the canal. An S2 file shaped the apical canalportion, followed by the ProTaper finishing files F1, F2, and F3, with repeated irrigation. Distilled-water irrigation was performed after the cleaning and shaping of each specimen. A 17% EDTA (Forbes Pharmaceuticals, Mumbai, India) irrigation was undertaken for 1 min, with a 30-gauge needle tip, producing apical constriction. The canals were then washed with normal saline and dried with wabsorbent paper points (Diadent Paper point Pro T, Hundal Dental Traders, Amritsar, India).
Root canal obturation was performed with gutta percha (GP) points and AH Plus sealer (MAARC, Resin-based Sealer, India), to the working length, via an F3 master apical cone. Using lateral condensation technique, accessory cones were inserted, excess was removed, and push-out bond-strength analysis was performed after 2 weeks. Post-space preparation was initiated with a Peeso reamer No. 3 drill, to a depth of 12 mm, keeping 3 mm at the apex. This was followed by the use of a size 1 post drill (3M Rely X Fiber Post Drill, 3M ESPE, Minneapolis, MN, USA) to finalize the preparation. NaOCl was used for irrigation, and preparation was verified radiographically. The canal space was etched for 20 s using 37% phosphoric acid gel (Ho Dental, Las Vegas, NV, USA). The post space was rinsed with saline and dried with paper points. A bonding agent (ExciTE F DSC) (Ivoclar; Vivadent, Schaan, Liechtenstein) was applied to the fiber post surface (RelyX™ Fiber Post 3M, ESPE) and allowed to dry. The root-canal-space dentin (ten teeth for each group) was treated with the specific adhesives prepared in the study (EA, 5% TiO2, and 5% ZrO2) (n = 10) and polymerized for 20 s (Bluephase C8, Ivoclar Vivadent, Schaan, Liechtenstein). A dual-cure polymerizing resin material, Multicore (Ivoclar; Vivadent, Schaan, Liechtenstein), was used as cement. Posts were cemented under finger pressure and excess was removed prior to photo-polymerization for 40 s.
2.5. Push-Out Bond Strength Assessments
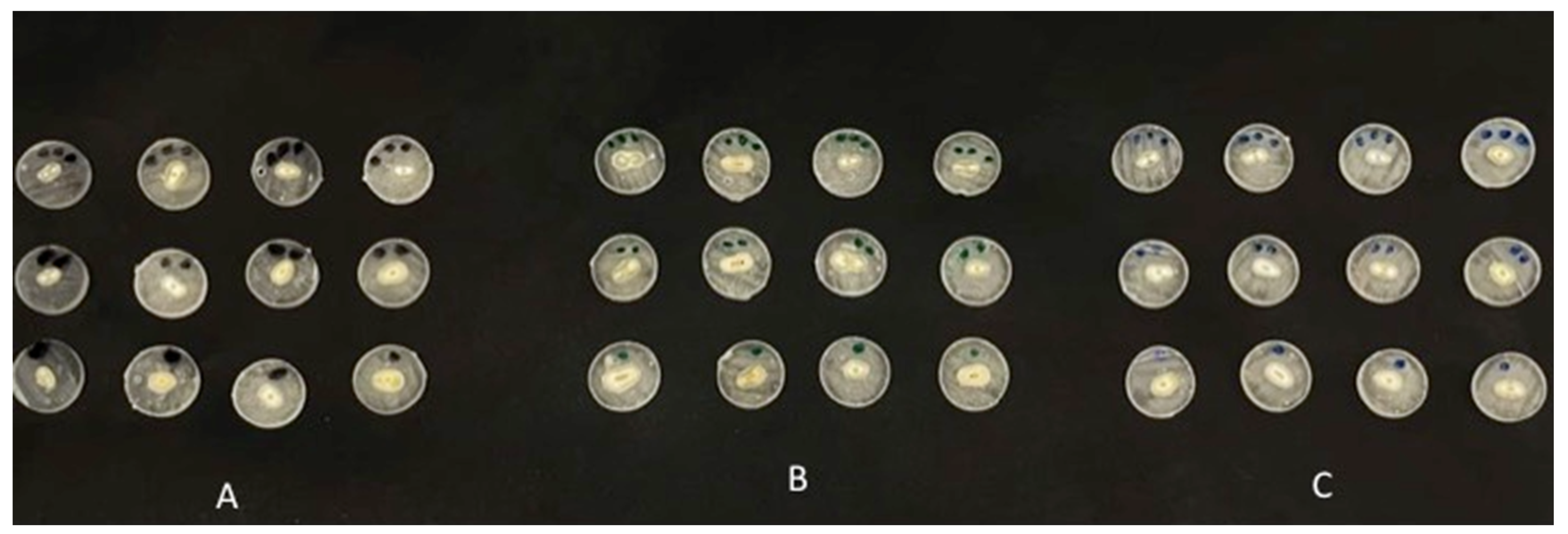
All root sections were embedded in clear acrylic resin, circumferentially. The roots with cemented fiber posts, in the three groups (EA, 5% TiO2, and 5% ZrO2), were sectioned perpendicularly to the long axis. Three perpendicular slices of 2 mm thickness (three slices representing the coronal, middle and apical regions of the post space) were obtained from each specimen, using a low-speed diamond saw (Micracut; Metkon, Bursa, Turkey) (Figure 2). In a universal testing machine (Instron, Norwood, MA, USA) the root sections obtained, were attached individually to the machine and at 0.5 mm/min, (the crosshead speed), the apical side of the disks underwent loading, facing a cylindrical plunger with a metal rod (0.8 and 1.2 mm in diameter for the apical and coronal slices, respectively) until failure. A total of 30 root sections in each group underwent the push-out bond strength test.

Figure 2.
Specimen sections in the study groups. (A) EA (control). (B) 5% TiO2. (C) 5% ZrO2.
The Formula for calculating the push-out bond strength is as follows:
where, F is maximum failure load [N] and A is bonding area [mm2] of post segments. Using a digital caliper (IP54-6-Inch Digital Caliper, Baileigh industrial, Chicago, IL, USA) the thickness of the sections and the diameter of coronal-, and apical post segments were assessed. As each group consisted of 10 root samples, which were further divided into three sections each, a total of 30 push-out tests were performed for each group.
τ = F/A,
Failure modes among the fractured root sections were assessed using SEM, which were classified as adhesive, cohesive and mixed failures.
2.6. SEM Observation of Adhesive-Dentin Interface
Five bonded sections from each adhesive group (EA, 5% TiO2, and 5% ZrO2), not used in push-out bond strength assessment, were assessed for adhesive penetration and resin-tag formation, using scanning electron microscopy (SEM). The specimens were wet-polished (Beuhler Polisher, Lake Bluff, IL, USA) followed by placement in an ultrasonic bath (distilled water for 5 min) (Bandelin Digital-Sigma-Aldrich Darmstadt, Germany). The specimens were then conditioned with 36% phosphoric acid (DeTrey conditioner, Dentsply, York, PA, USA), followed by washing and immersion in 5.25% NaOCl solution for 15 min. Washed specimens were dehydrated by placement in ethanol solution (80%, 90% and 100%). Specimens were mounted and gold coated as explained earlier, in Section 2.2. The specimens for EA, 5% TiO2, and 5% ZrO2 were observed at different magnifications, using SEM (Joel JSM 6360, Scanning Electron Microscope, Salem, OR, USA) operating at 30 kV voltage.
2.7. Statistical Analysis
The results of the push-out bond strength tests, in the study groups, were tabulated and assessed using statistical program for social sciences (SPSS-20.0, IBM, Chicago, IL, USA). The data normality was identified using the Kolmogorov–Smirnov test. The means and standard deviations were compared using ANOVA and post hoc Tukey–Kramer multiple comparison test.
3. Results
3.1. Outcomes of the Characterization of the Filler Particles
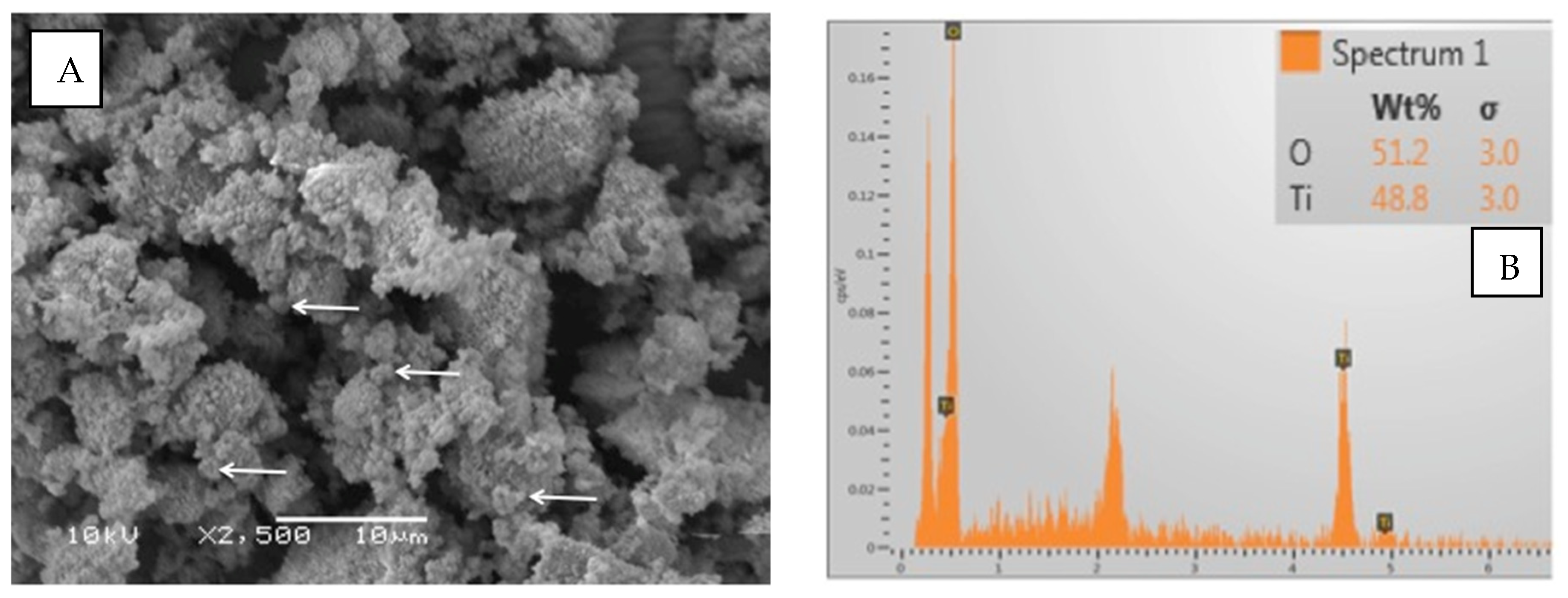
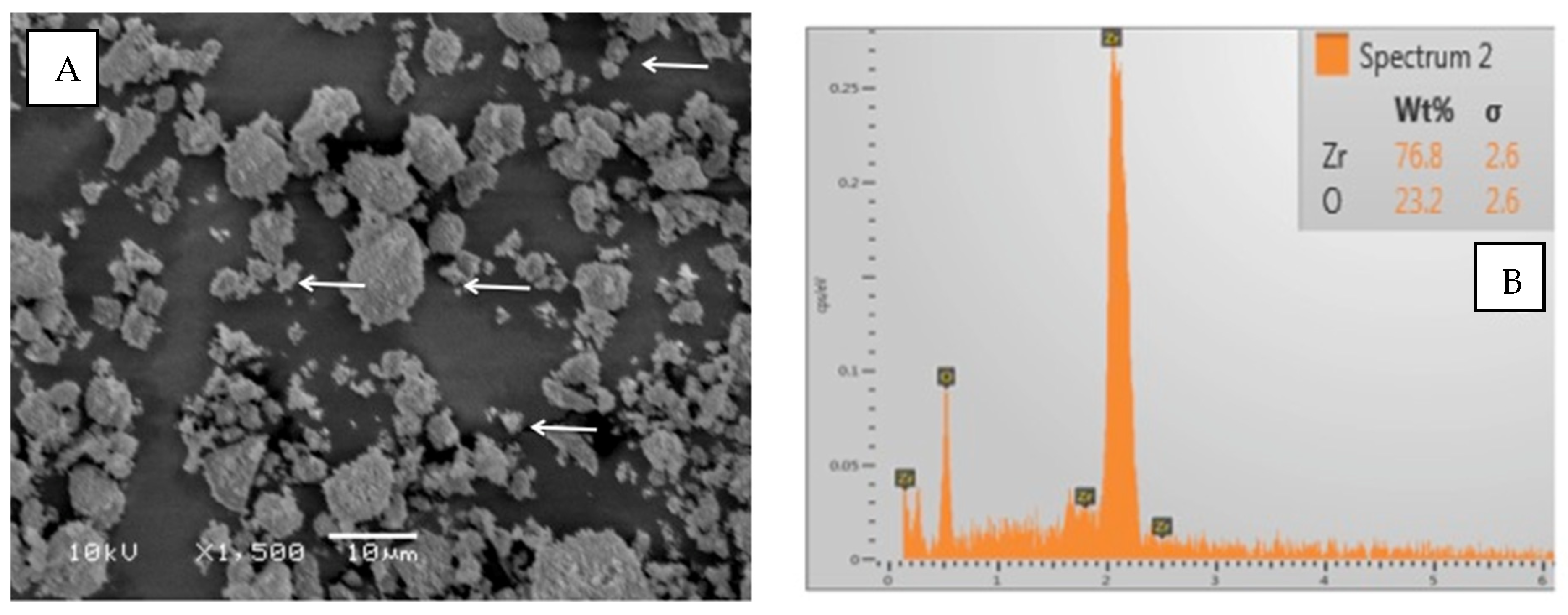
The particles of TiO2 demonstrated irregular shapes on the SEM micrograph (Figure 3A). The filler particles’ size ranged between 500 and 1000 nm. The EDX analysis showed almost equal quantities of titanium (48.8%) and oxygen (51.2%) in the TiO2 particles (Figure 3B). Regarding ZrO2 particles, the shape was irregularly spherical (Figure 4A). The ZrO2 particles were comparatively smaller than TiO2 particles, ranging from 500 to 700 nm. The EDX elemental analysis of ZrO2 particles revealed 76.2% Zr and 23.8% O (oxygen) as constituent elements of the powder (Figure 4B).

Figure 3.
(A) SEM image of TiO2. The particles had irregular and spherical shapes. The particle size was approximately 500–1000 nm (arrows denote small particles). (B) The representative EDX graph shows almost equal quantities of Ti and O in the tested powder.

Figure 4.
(A) SEM image of Zirconia oxide particles (ZrO2). The particles had an irregular shape. The particle size was 500 to 700 nm (arrows denote small particles). (B) The EDX analysis showed dominant presence of Zr with a small quantity of O in the tested powder.
3.2. Outcomes of the Assessment of the Rheological Properties
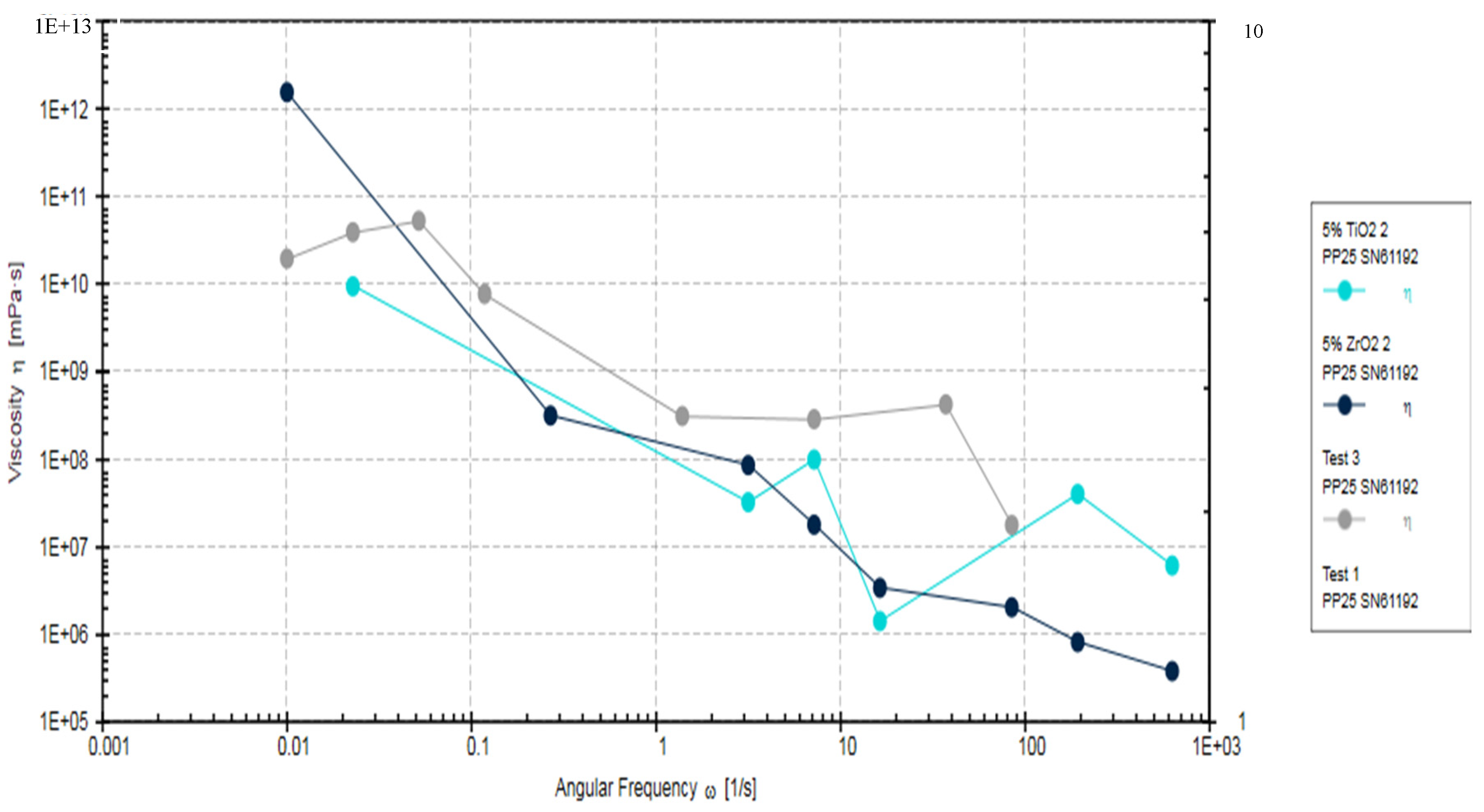
The Rheometric analysis of the adhesives fabricated in the study (EA, 5% TiO2, and 5% ZrO2) showed a decrease in the viscosity of 5% TiO2, and 5% ZrO2 in comparison to EA (Control adhesive), as the angular frequency increased (Figure 5). The viscosity in the control group specimens was significantly higher (p < 0.05), at 5184.6 ± 65.71 kPa·s, compared to 5% ZrO2 (4784.3 ± 83 kPa·s) and 5% TiO2 (4627.2 ± 72 kPa·s). Viscosity for 5% ZrO2 (4784.3 ± 83 kPa·s) and 5% TiO2 (4627.2 ± 72 kPa·s) samples was statistically comparable (p > 0.05).
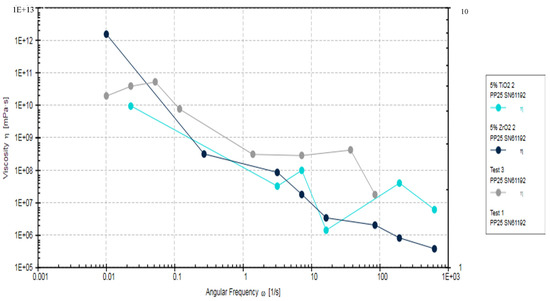
Figure 5.
Rheometric properties of EA, 5% TiO2 and 5% ZrO2 adhesives, showing complex viscosity of angular frequencies of 0.001 to 1000 rads/s.
This decrease in viscosity was gradual, and consistent with increasing angular frequency, for 5% ZrO2 adhesive. However, 5% TiO2 adhesive also showed lower viscosity compared to the control EA. The viscosities for 5% TiO2 and 5% ZrO2 were overlapping (Figure 5). The EA adhesive showed less decrease in viscosity compared with 5% TiO2 and 5% ZrO2 adhesives. All investigated study adhesives demonstrated non-Newtonian behavior (shear-thinning or pseudo-plasticity). The reduction in viscosity for filler-containing adhesive groups (TiO2 and ZrO2) suggests that the incorporation of particles caused improved fluidity in the resin and filler mixture. It was also observed that even at lower angular frequencies, there was no presence of Newtonian plateau. Although the EA showed higher viscosity compared to particles containing adhesives, this was not a consistent observation and some overlap was seen among the viscosities of the three adhesives at higher frequencies (Figure 5).
3.3. Outcomes of Push-Out Bond Strength and Failure Mode Analysis
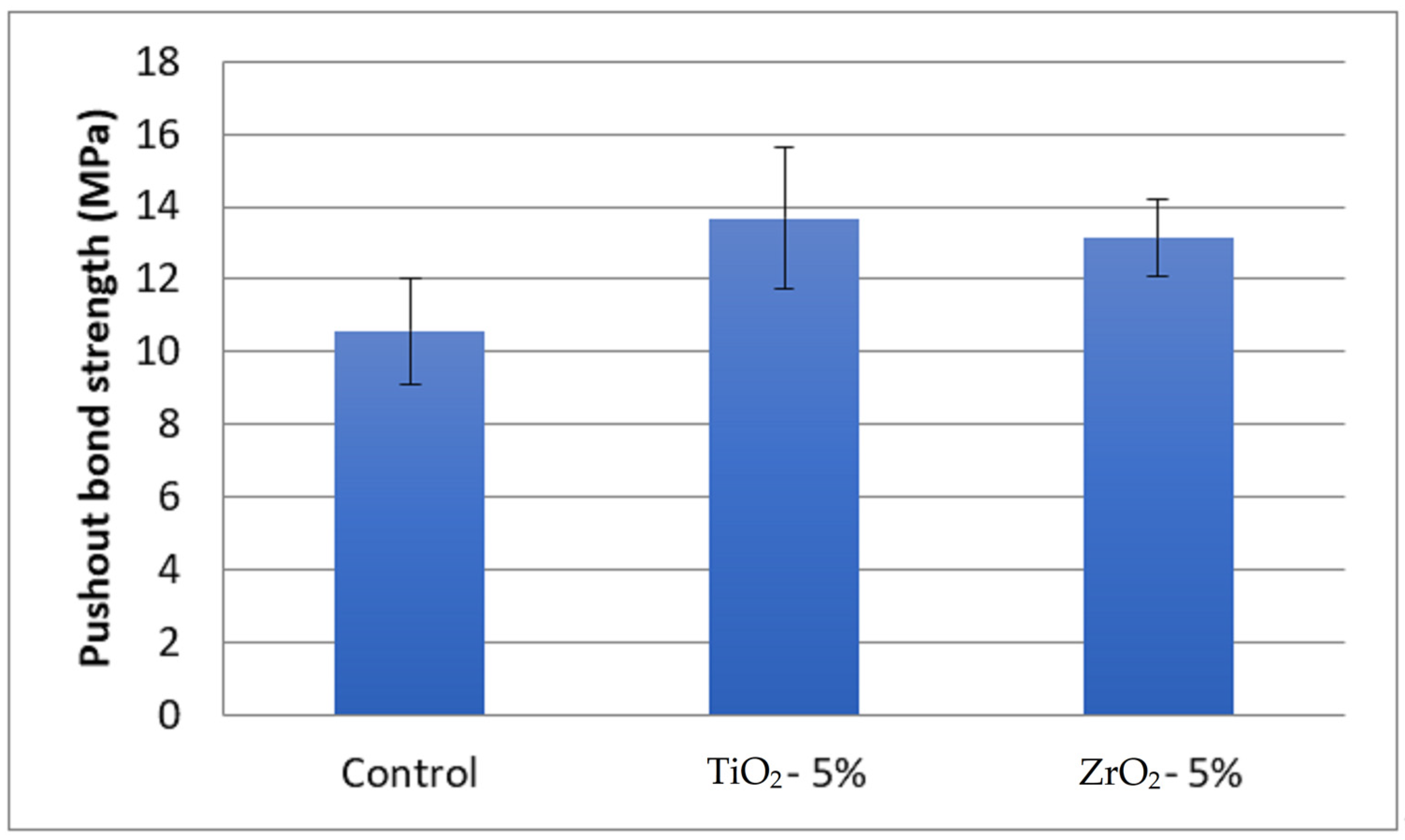
The highest push-out bond strength was observed for 5% TiO2 (13.67 ± 1.95 MPa) adhesive and the lowest was in the EA control adhesive (10.57 ± 1.45 MPa) (Table 1). The push-out bond strength in 5% ZrO2 adhesive samples was 13.14 ± 1.08 MPa. The bond strength among the groups was statistically different (p < 0.05). The bond strength in the 5% TiO2 and 5% ZrO2 adhesives was statistically comparable (p > 0.05) (Figure 6). The specimens in the control group (10.57 ± 1.45 MPa) showed significantly lower bond strength values compared to 5% TiO2 (13.67 ± 1.95 MPa) and 5% ZrO2 (13.14 ± 1.08 MPa) adhesives, respectively.

Table 1.
Mean and standard deviation of push-out bond strengths of experimental adhesives (MPa).
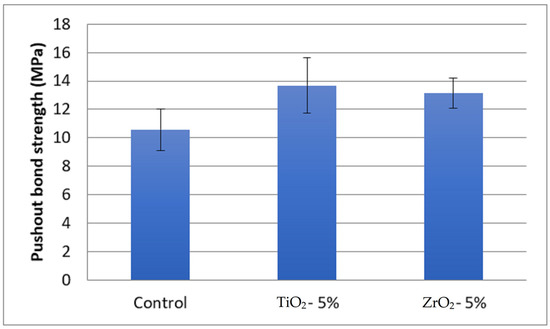
Figure 6.
Comparison of means and standard deviations of push-out bond strength (MPa) among study groups.
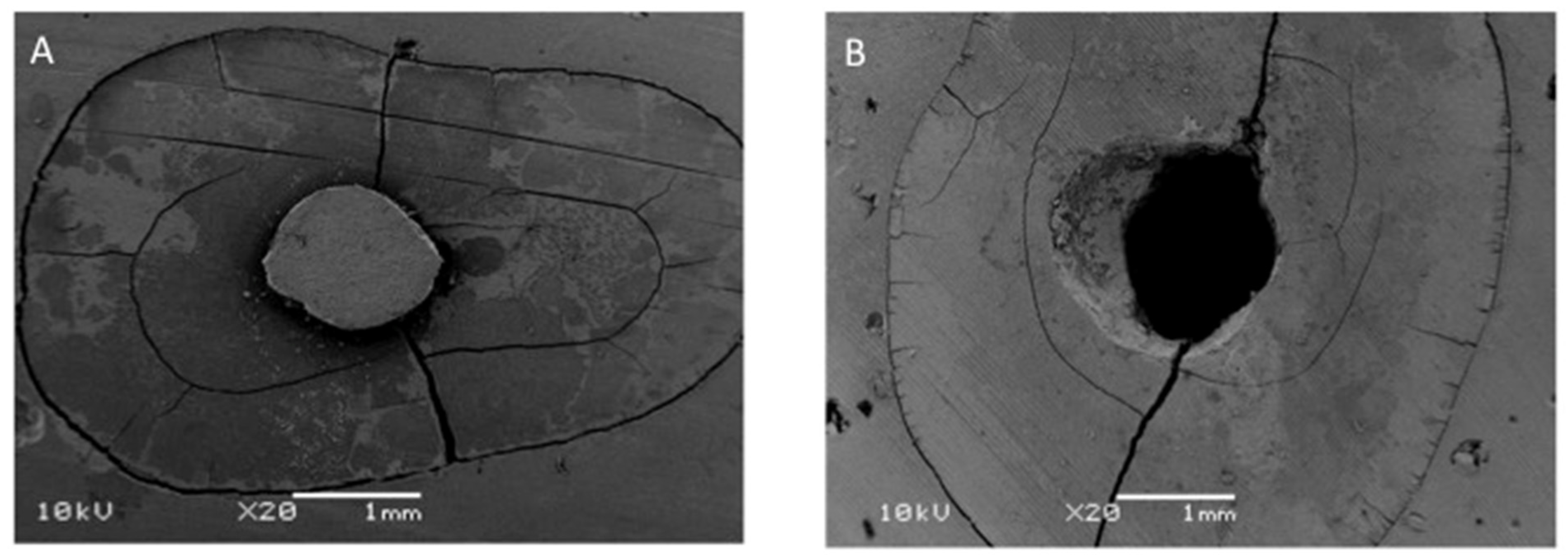
The most common failure mode, following the push-out bond strength tests, among the study groups, was in adhesives (range 80%–100%) (Table 1). The EA group samples showed 100% of adhesive failures. The second most common failure mode was a mixed type, with 20% in 5% TiO2 and 10% in 5% ZrO2 adhesives (Figure 7).

Figure 7.
SEM analysis for Failure mode analysis. (A) Adhesive failure. (B) Mixed failure.
3.4. Qualitative Assessment of Resin-Dentin Interface
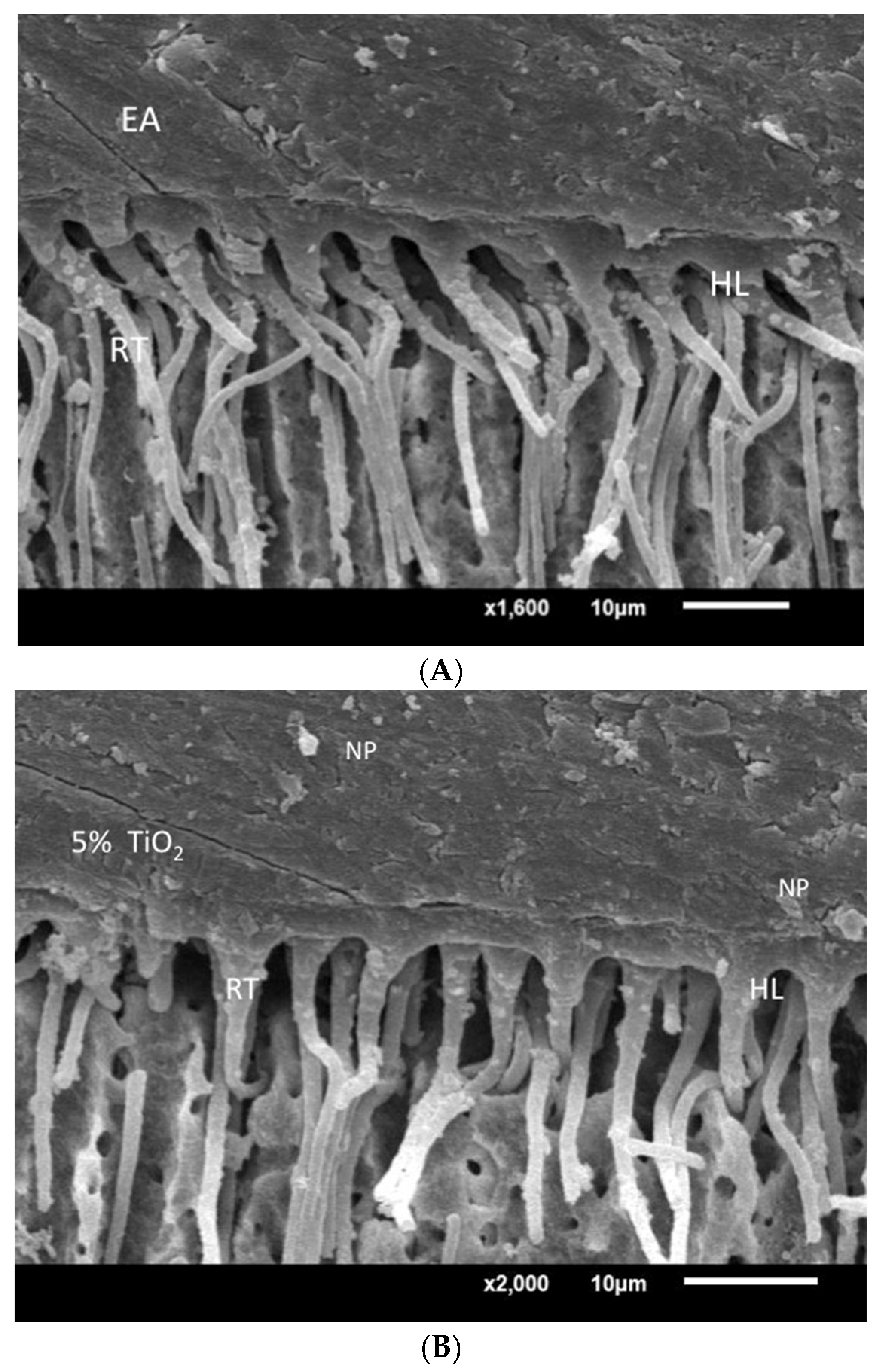
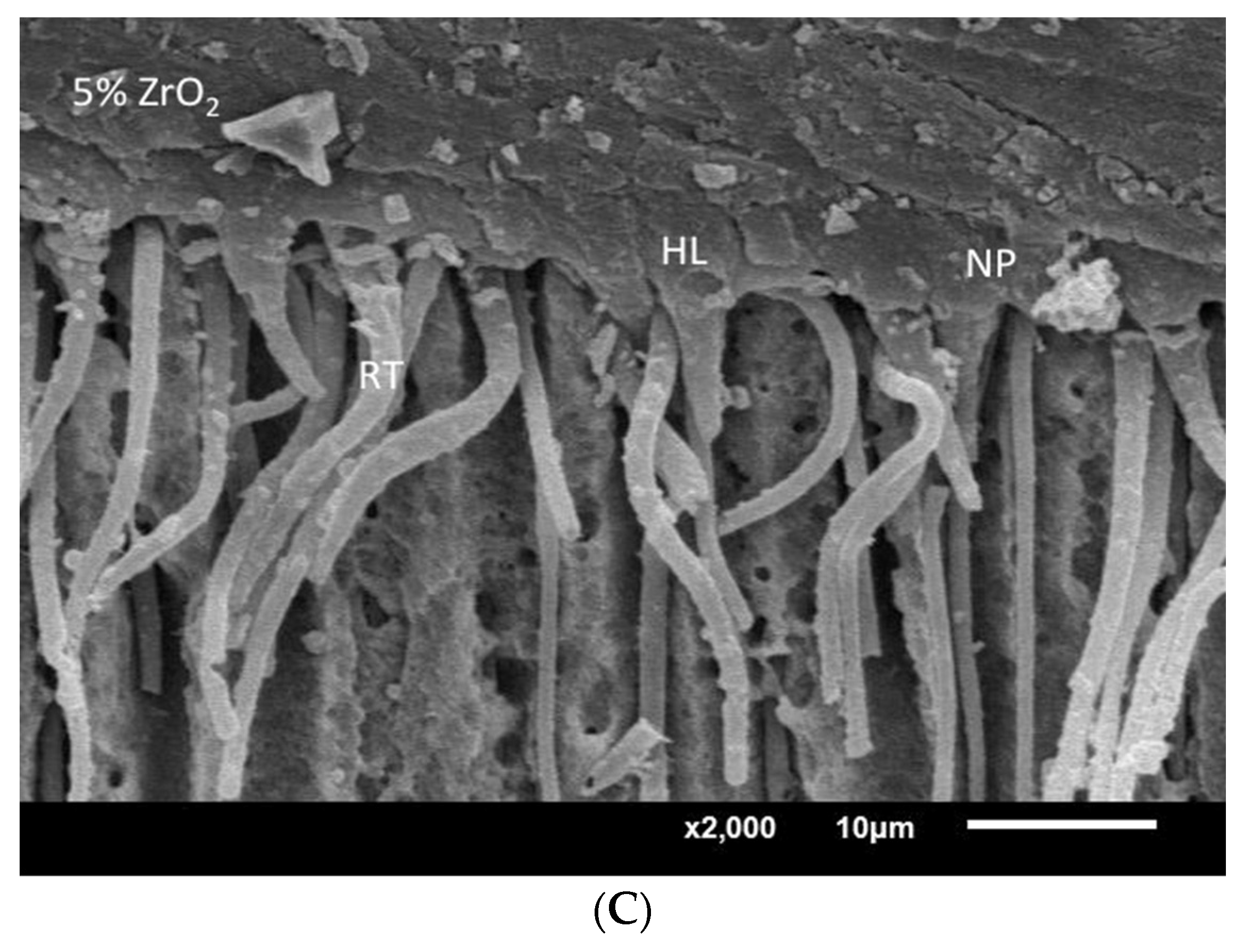
SEM assessment of resin-dentin interface specimens bonded with EA—5% TiO2 and 5% ZrO2 adhesives—revealed comparable density, and dimensions, of adhesive resin tags from filled dentinal tubules. A well-developed hybrid layer, comprising adhesive resin tags and dentinal tubules, was observed for 5% TiO2 and 5% ZrO2 (Figure 8). Some of the dispersed particles were also observed at the resin–dentin interface (Figure 8B,C). Presence of dispersed particles within the hybrid layer suggests interaction between adhesive and particles. The improvement of the adhesive bond of the TiO2 and 5% ZrO2 adhesives can be associated with the density and length of resin tags formed through the dentinal tubules at the resin–dentin interface. In addition, the thickness of the hybrid layer reduces the shear stresses at the interface, as both particle-infused adhesives showed thick hybrid layers with better bond strength outcomes.


Figure 8.
SEM assessment of resin–dentin interface specimens bonded with EA, 5% TiO2 and 5% ZrO2. (A) EA showing dentin penetration with resin tag (RT) and hybrid layer (HL) formation. (B) 5% TiO2 adhesive showing thick hybrid layer and resin tags similar to EA samples with dispersed particles (NP). (C) 5% ZrO2 adhesive showing developed HL and RT with isolated ZrO2 NP.
4. Discussion
The present study was based on the hypothesis that the addition of ZrO2 and TiO2 filler particles in a polymer based DBA would improve its rheological properties, and improve the adhesive bonding of a fiber-based dental post to root dentin. It was observed that 5% ZrO2 and 5% TiO2 adhesive showed lower viscosity and higher push-out bond strength, compared to the control adhesive (EA). As a result, the study hypothesis was accepted. Previous studies have confirmed the reinforcing effect of fillers in augmenting the mechanical properties of polymer-based adhesives [12,29,30]. This study presented novel evidence of ZrO2-, and TiO2-incorporated root dentin adhesive for fiber post bonding.
In the present study, a push-out bond strength test, following root filling and fiber post cementation, was performed. The push-out bond test typically determines the resistance to dislodgment in the root canal filler (fiber post) with root dentin. A compressive load is applied to the filling material in the root canal until failure, and push-out is considered a reliable and more representative testing method for root fillings than the conventional shear test [31,32]. Newer methods in push-out testing utilize precisely machined holes in root sections, however, the present study used the standard methodology to simulate clinical conditions and maintain standardization [33,34]. The push-out study was performed, followed by cleaning and shaping, then obturation, followed by post cementation with the experimental adhesives, in the present study. These steps can introduce inconsistencies in the root dentin, however bonding of polymeric agents to the unprepared root dentin does not provide clinical simulation and fails to completely eradicate microbial contamination and smear layer [35]. Interestingly, a push-out probe of a diameter smaller than 85% of the fiber post material [36] is recommended, however a larger diameter probe was used to transmit forces closer to the root dentin and adhesive under investigation.
TiO2 is a remarkably biocompatible material, with excellent mechanical properties [37]. It is commonly used in dental implants, however its incorporation in dental biomaterials enhances its physical and chemical properties, in addition to its influence on polymerization [27,38]. ZrO2 is a chemically inert material with high fracture-resistance and acceptable optical properties (18). SEM assessment of TiO2 particles showed irregular and spherical-shaped particles ranging between 500 and 1000 nm in size, which has been revealed in previous studies [39]. Similarly, ZrO2 particles also showed irregular and non-uniform shape when incorporated in the adhesive, which is in line with previous experiments [40,41]. EDX analysis of TiO2 and ZrO2 particles showed high percentages of Ti (48.8%), Zr (76.2%) and oxygen elements, which verifies the abundance of these elements in the experimental material.
Viscosity of the modified and experimental adhesives was assessed in the present study. The adhesives with incorporation of particles (TiO2 and ZrO2) showed a decrease in material viscosity as the angular frequency increased. The decrease in viscosity for the EA was less than the decrease in the particle-containing adhesives. Studies have suggested that adhesives containing particle reinforcement have displayed shear-thinning behavior, and a relation between increasing angular frequency and decreasing viscosity has been observed [42,43]. It is pertinent to mention that rheological assessments can show erratic outcomes, as they are easily influenced by material handling during experimentation [44].
The push-out bond strength of fiber posts using TiO2 and ZrO2 containing adhesives was significantly higher than the bond strength for the EA adhesive in the present study.The inorganic particles added to the adhesives increased the surface area of the interaction between the polymer and fillers. This enhanced the adhesive bonding of the DBA to root dentin [45]. In addition, the interfacial adhesion was also positively influenced by the fillers in the adhesive [46]. The improvement in adhesive bonding due to the incorporation of ZrO2 and TiO2 particles has been reported in previous studies [47]. Moreover, TiO2 mechanically reinforces biomaterials, including adhesives; therefore increased mechanical strength of TiO2-filled materials can improve the strength of the hybrid adhesive–dentin layer [48,49]. Furthermore, ZrO2 particles show high dispersion in adhesives, which potentially improves the mechanical bond of modified adhesive to root dentin [50]. Interestingly, the failure types in the present study were predominantly (>80%) adhesive, which provides validity to the push-out bond strength observed in the study. In addition, increased adhesive failures suggest that the weak link in the adhesive cementation of fiber post to the root dentin is the adhesive interface, which requires further investigation. Previous studies have shown a higher prevalence of adhesive failures in bond-strength assessment after incorporation of filler particles in the adhesives [51].
It was observed in a previous study that ZrO2-, and TiO2-incorporated adhesives showed improved bond strength to coronal tooth dentin [13]. The present study displayed the potential for adhesive reinforcement of EA with 5% ZrO2 and 5% TiO2 when cementing a fiber post to root dentin. Therefore, the practical use of these experimental adhesives, with incorporated particles, is recommended for root post cementation, contingent to further investigations. However, it is pertinent to mention that an experimental (and not commercially available) adhesive was used in the present study. In addition, the particles were not silanized, a process which is known to enhance the interaction between the inorganic particles and adhesive resin. The percentages of ZrO2 and TiO2 particles were standardized at 5%, a variation in this could result in variable outcomes. furthermore, the oral environment is dynamic, with changing temperatures, and fiber posts are exposed to fatigue loads, non-axial forces and acidic medium, however due to the in-vitro study model, these factors were not simulated in the study design. Therefore, further in-vivo studies are warranted, with different percentages of ZrO2 and TiO2 particle content in dentin adhesives, for adhesive bonding of fiber posts, with exposure to fatigue loading and saliva.
5. Conclusions
Reinforcement of the experimental dentin adhesive with 5% TiO2 or 5% ZrO2 increased the push-out bond strength of the fiber post to root dentin, in comparison to the EA. The 5% TiO2 and 5% ZrO2 adhesives showed root dentin interaction comparable to the EA. Particle-incorporated adhesives (5% TiO2 and 5% ZrO2) displayed decreased viscosity compared with the EA (without particles). These in-vitro findings are encouraging for the use of ZrO2 and TiO2 particle-incorporated root dentin adhesives, however further clinical studies, assessing their efficacy, are warranted.
Author Contributions
Conceptualization, S.A.-S., M.M.A., M.A., F.V., T.A., A.H.A. and A.A. (Abdullah Alateeq); methodology, A.A. (Abdullah Alateeq), A.H.A. and M.S.A.; validation, A.A. (Abdullah Alateeq), A.H.A., and M.S.A.; formal analysis, S.A.-S., M.M.A., M.A., F.V., T.A., A.A. (Abdullah Alshahrani) and A.A. (Abdullah Alateeq); investigation, S.A.-S., A.H.A., F.V., A.H.A. (Abdullah Alateeq), A.A. (Abdullah Alshahrani), A.H.A. and M.S.A.; data curation, S.A.-S., M.M.A., A.H.A., M.A., F.V., A.A. (Abdullah Alshahrani), T.A. and A.A. (Abdullah Alateeq); writing—original draft preparation, A.H.A., M.S.A. and A.H.A.; writing—review and editing, F.V., T.A. and A.A. (Abdullah Alateeq); supervision, F.V. and T.A. funding acquisition, T.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Researchers supporting project at King Saud University for funding through Researchers supporting project No. (RSP-2021-044).
Institutional Review Board Statement
The present study protocol was approved from the institutional review board and ethics committee at King Saud University with approval No. E-20-5545. The teeth extracted for orthodontic treatments, which were free from any apparent defects, were gathered after attaining the patients’ written informed consent and were utilized for the experiments in our study.
Informed Consent Statement
All the recommendations of the Helsinki Declaration and its later amendments were strictly followed. The teeth extracted for orthodontic treatments, which were free from any apparent defects, were gathered after attaining the patients’ written informed consent and were utilized for the experiments in our study.
Data Availability Statement
Data from the study are available by request from the corresponding author.
Acknowledgments
The authors are grateful to the Researchers supporting project at King Saud University for funding through Researchers supporting project No. (RSP-2021-044).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qualtrough, A.A.J.; Mannocci, F. Tooth-colored post systems: A review. Oper. Dent. 2003, 28, 86–91. [Google Scholar] [PubMed]
- Bonfante, E.A.; Pegoraro, L.F.; de Góes, M.F.; Carvalho, R.M. SEM observation of the bond integrity of fiber-reinforced composite posts cemented into root canals. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Butz, F.; Lennon, A.M.; Heydecke, G.; Strub, J.R. Survival rate and fracture strength of endodontically treated maxillary incisors with moderate defects restored with different post-and-core systems: An in vitro study. Int. J. Prosthodont. 2001, 14, 58–64. [Google Scholar]
- De Miranda Coelho, C.S.; Biffi, J.C.G.; da Silvagr, G.R.; Abrahão, A.; Campos, R.E.; Soares, C.J. Finite element analysis of weakened roots restored with composite resin and posts. Dent. Mater. J. 2009, 28, 671–678. [Google Scholar] [CrossRef] [PubMed]
- AlAali, K.A.; AlHelal, A.; Almahri, J.R.; Albakri, A.A.; Albani, R.M.; Alhaizan, Y.A.; Alhamdan, M.M.; Alaql, N.A.; Binhasan, M.; Alhamdan, E.M.; et al. Influence of conventional polymer, hybrid polymer and zinc phosphate luting agents on the bond strength of customized zirconia post in premolars—An in-vitro evaluation. Polymers 2022, 14, 758. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Zhao, Z.W.Q.; Zhao, J.; Zhao, B.; Ma, Z.; Zhang, C. Adhesion in teeth. Front. Mater. 2021, 7, 615225. [Google Scholar] [CrossRef]
- Ferracane, J.L. Models of caries formation around dental composite restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef]
- Iliev, G.; Hardan, L.; Kassis, C.; Bourgi, R.; Cuevas-Suarez, C.E.; Lukomska-Szymanska, M.; Mancino, D.; Haikel, Y.; Kharouf, N. Shelf life and storage conditions of universal adhesives: A literature review. Polymers 2021, 13, 2708. [Google Scholar] [CrossRef]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic effect of bioactive inorganic fillers in enhancing properties of dentin adhesives—A review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin bond integrity of filled and unfilled resin adhesive enhanced with silica particles—An SEM, EDX, micro-Raman, FTIR and micro-tensile bond strength study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, 84, 2082–2094. [Google Scholar] [PubMed]
- Al-Saleh, S.; Alateeq, A.; Alshaya, A.H.; Al-Qahtani, A.S.; Tulbah, H.I.; Binhasan, M.; Shabib, S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of TiO2 and ZrO2 particles on adhesive bond strength and viscosity of dentin polymer. A physical and chemical evaluation. Polymers 2021, 13, 3794. [Google Scholar] [CrossRef] [PubMed]
- Madfa, A.A.; Al-Sanabani, F.A.; Al-Quadami, N.H.; Al-Sanabani, J.S.; Amran, A.G. Use of zirconia in dentistry: An overview. Open Biomat. J. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Grech, J.A.E. Zirconia in dental prosthetics: A literature review. J. Mater. Res. Technol. 2019, 8, 4956–4964. [Google Scholar] [CrossRef]
- Apratim, A.; Eachempati, P.; Krishnappa Salian, K.K.; Singh, V.; Chhabra, S.; Shah, S. Zirconia in dental implantology: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 147–156. [Google Scholar] [CrossRef]
- Halder, S.; Ahemad, S.; Das, S.; Wang, J. Epoxy/glass fiber laminated composites integrated with amino functionalized ZrO2 for advanced structural applications. ACS Appl. Mater. Interfaces 2016, 8, 1695–1706. [Google Scholar] [CrossRef]
- Lohbauer, U.; Wagner, A.; Belli, R.; Stoetzel, C.; Hilpert, A.; Kurland, H.D.; Grabow, J.; Muller, F.A. Zirconia particles prepared by laser vaporization as fillers for dental adhesives. Acta Biomater. 2010, 6, 4539–4546. [Google Scholar] [CrossRef]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontol. 2000 2017, 73, 241–258. [Google Scholar] [CrossRef]
- Musial, J.; Krakowiak, R.; Mlynarczyk, D.T.; Goslinski, T.; Stanisz, B.J. Titanium dioxide particles in food and personal care products-what dowe know about their safety? Nanomaterials 2020, 10, 1110. [Google Scholar] [CrossRef]
- Sandu, A.V.; Baltatu, M.S.; Nabialek, M.; Savin, A.; Vizureanu, P. Characterization and mechanical proprieties of new TiMo alloys used for medical applications. Materials 2019, 12, 2973. [Google Scholar] [CrossRef] [PubMed]
- Spataru, M.C.; Butnaru, M.; Sandu, A.V.; Vulpe, V.; Vlad, M.D.; Baltatu, M.S.; Geanta, V.; Voiculescu, I.; Vizureanu, P.; Solcan, C. In-depth assessment of new Ti-based biocompatible materials. Mater. Chem. Phys. 2021, 258, 123959. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide particles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 2014, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The effect of silver, zinc oxide, and titanium dioxide particles used as final irrigation solutions on the fracture resistance of root-filled teeth. Clin. Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Esteban Florez, F.L.; Kraemer, H.; Hiers, R.D.; Sacramento, C.M.; Rondinone, A.J.; Silverio, K.G.; Khajotia, S.S. Sorption, solubility and cytotoxicity of novel antibacterial nanofilled dental adhesive resins. Sci. Rep. 2020, 10, 13503. [Google Scholar] [CrossRef]
- Ramos-Tonello, C.M.; Lisboa-Filho, P.N.; Arruda, L.B.; Tokuhara, C.K.; Oliveira, R.C.; Furuse, A.Y.; Rubo, J.H.; Borges, A.F.S. Titanium dioxide nanotubes addition to self-adhesive resin cement: Effect on physical and biological properties. Dent. Mater. 2017, 33, 866–875. [Google Scholar] [CrossRef]
- Chu, C.Y.; Kuo, T.C.; Chang, S.F.; Shyu, Y.C.; Lin, C.P. Comparison of the microstructure of crown and root dentin by a scanning electron microscopic study. J. Dent. Sci. 2010, 5, 14–20. [Google Scholar] [CrossRef]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin bond integrity of hydroxyapatite containing resin adhesive enhanced with graphene oxide nano-particles—An SEM, EDX, micro-Raman, and microtensile bond strength study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of hydroxyapatite nanospheres in dentin adhesive on the dentin bond integrity and degree of conversion: A scanning electron microscopy (SEM), Raman, fourier transform-infrared (FTIR), and microtensile study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Rodrigues, S.B.; Celeste, R.K.; Leitune, V.C.B.; Samuel, S.M.W. The influence of methodological variables on the push-out resistance to dislodgement of root filling materials: A meta-regression analysis. Int. Endod. J. 2016, 49, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Ureyen Kaya, B.; Kececi, A.D.; Orhan, H.; Belli, S. Micropush-out bond strengths of gutta-percha versus thermoplastic synthetic polymer-based systems—An ex vivo study. Int. Endod. J. 2008, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Brandao, M.C.; Fidel, R.A.; Fidel, S.R. The sealing ability of GuttaFlow in oval-shaped canals: An ex vivo study using a polymicrobial leakage model. Int. Endod. J. 2007, 40, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Akcay, M.; Arslan, H.; Durmus, N.; Mese, M.; Capar, I.D. Dentinal tubule penetration of AH plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: A confocal microscopic study. Lasers Surg. Med. 2016, 48, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.A.; Berzins, D.W.; Bahcall, J.K. An in vitro comparison of bond strength of various obturation materials to root canal dentin using a push-out test design. J. Endod. 2007, 33, 856–858. [Google Scholar] [CrossRef]
- Chen, W.P.; Chen, Y.Y.; Huang, S.H.; Lin, C.P. Limitations of push-out test in bond strength measurement. J. Endod. 2013, 39, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Lubojanski, A.; Dobrzynski, M.; Nowak, N.; Rewak-Soroczynska, J.; Sztyler, K.; Zakrzewski, W.; Dobrzynski, W.; Szymonowicz, M.; Rybak, Z.; Wiglusz, K.; et al. Application of selected nanomaterials and ozone in modern clinical dentistry. Nanomaterials 2021, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Sturmer, M.; Garcia, I.M.; Souza, V.S.; Visioli, F.; Scholten, J.D.; Samuel, S.M.W.; Leitune, V.C.B.; Collares, F.M. Titanium dioxide nanotubes with triazine-methacrylate monomer to improve physicochemical and biological properties of adhesives. Dent. Mater. 2021, 37, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Davoren, M.; Boertz, J.; Schins, R.P.; Hoffmann, E.; Dopp, E. Titanium dioxide particles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part. Fibre Toxicol. 2009, 6, 17. [Google Scholar] [CrossRef]
- Behbahani, A.R.S.; Esmaeilifar, A. Hydrothermal synthesis of zirconia particles from commercial zirconia. Proc. Eng. 2012, 42, 908–917. [Google Scholar] [CrossRef]
- Majedi, A.A.A.; Davar, F. Green synthesis of zirconia particles using the modified Pechini method and characterization of its optical and electrical properties. J. Sol.-Gel. Sci. Technol. 2016, 77, 542–552. [Google Scholar] [CrossRef]
- Balasubramanian, R.; Srinivasan, R.; Lee, J. Barrier, rheological, and antimicrobial properties of sustainable nanocomposites based on gellan gum/polyacrylamide/zinc oxide. Polymer Eng. Sci. 2021, 61, 2477–2486. [Google Scholar] [CrossRef]
- AlHamdan, E.M.; Al-Saleh, S.; AlRefeai, M.H.; Farooq, I.; Abrar, E.; Vohra, F.; Abduljabbar, T. Adhesive bond integrity of Y-TZP post with calcium fluoride infiltrated resin dentin adhesive: An SEM, EDX, FTIR and micro-Raman study. Surf. Int. Anal. 2021, 53, 956–962. [Google Scholar] [CrossRef]
- Lee, J.H.; Um, C.M.; Lee, I.B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Particles on the Interfacial/ Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Zare, Y.; Rhee, K.Y. Dependence of Z Parameter for Tensile Strength of Multi-Layered Interphase in Polymer Nanocomposites to Material and Interphase Properties. Nanoscale Res. Lett. 2017, 12, 42. [Google Scholar] [CrossRef]
- Felemban, N.H.; Ebrahim, M.I. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral Health 2017, 17, 43. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Sakagami, H.; Morales-Luckie, R.A.; Nakajima, H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J. Appl. Oral Sci. 2015, 23, 321–328. [Google Scholar] [CrossRef]
- Sun, L.G.R.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A review. Comp. Sci. Technol. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of different conditioning treatments on the bond integrity of root dentin to rGO infiltrated dentin adhesive. SEM, EDX, FTIR and microraman study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).