Erosive Wear Behavior of High-Chromium Cast Iron: Combined Effect of Erodent Powders and Destabilization Heat Treatments
Abstract
:1. Introduction
2. Materials and Methods
3. Results
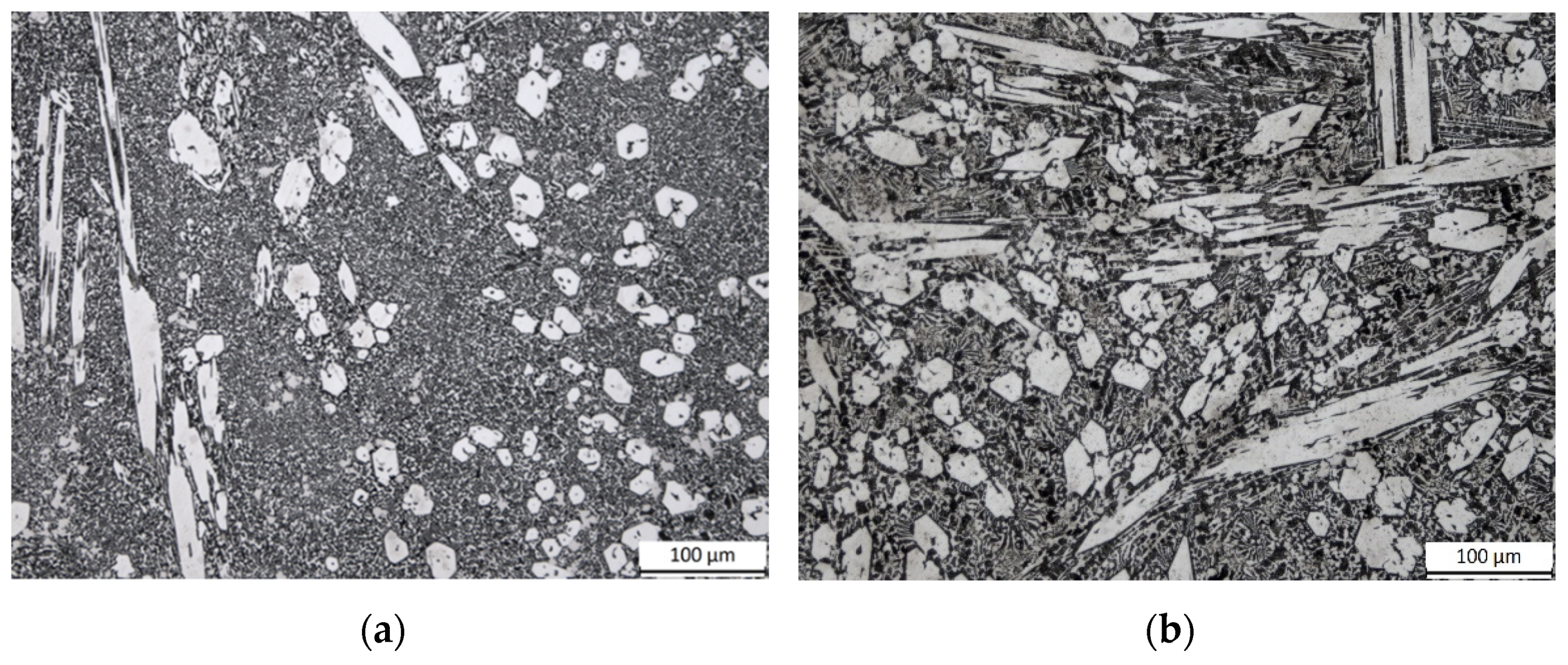
3.1. Microstructures of the HCCIs in the as-Received Condition
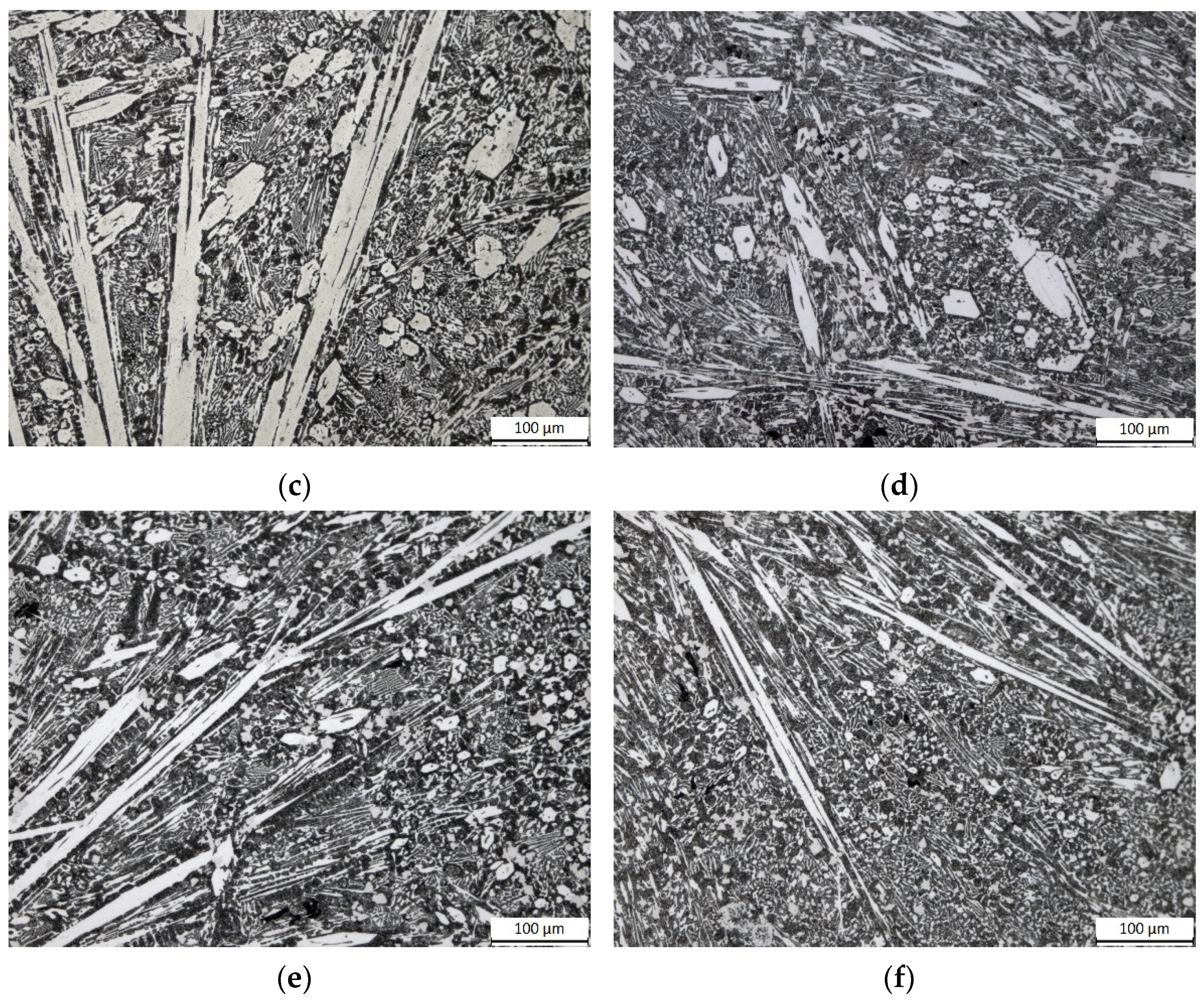
3.2. Microstructures of the HCCIs in the Heat-Treated Conditions
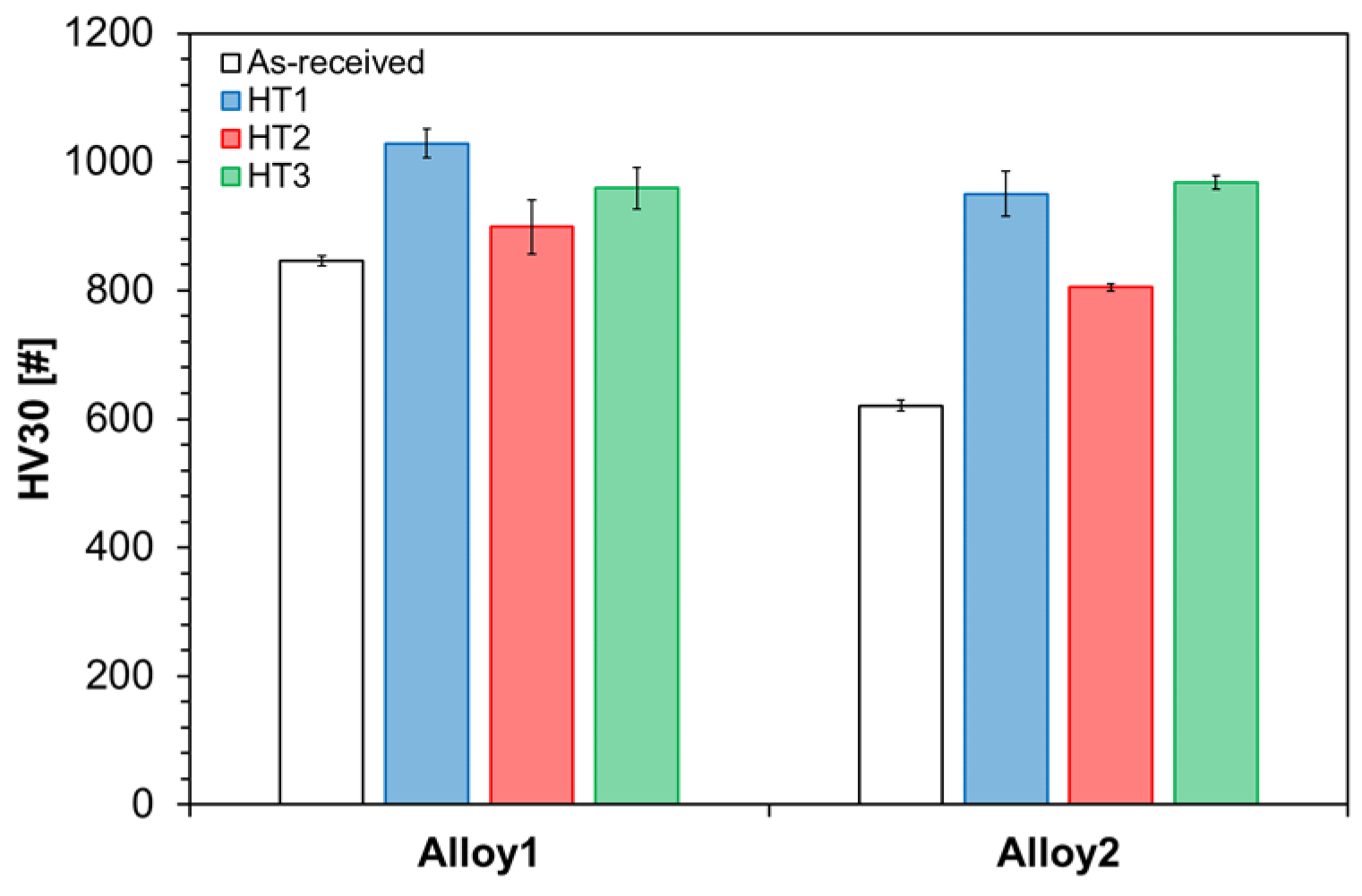
3.3. Bulk Hardness
3.4. XRD
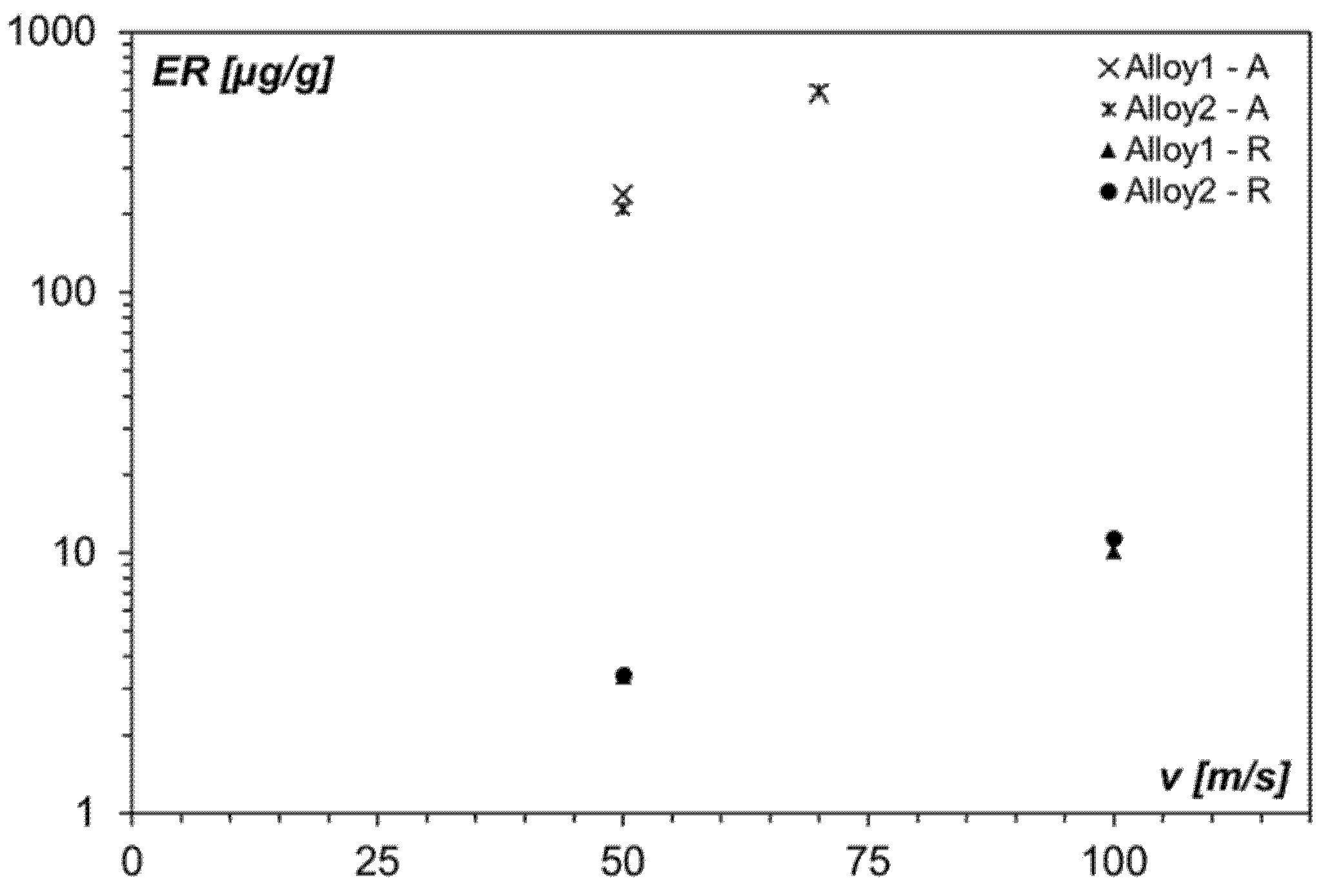
3.5. Erosion Behavior in the as-Received Condition
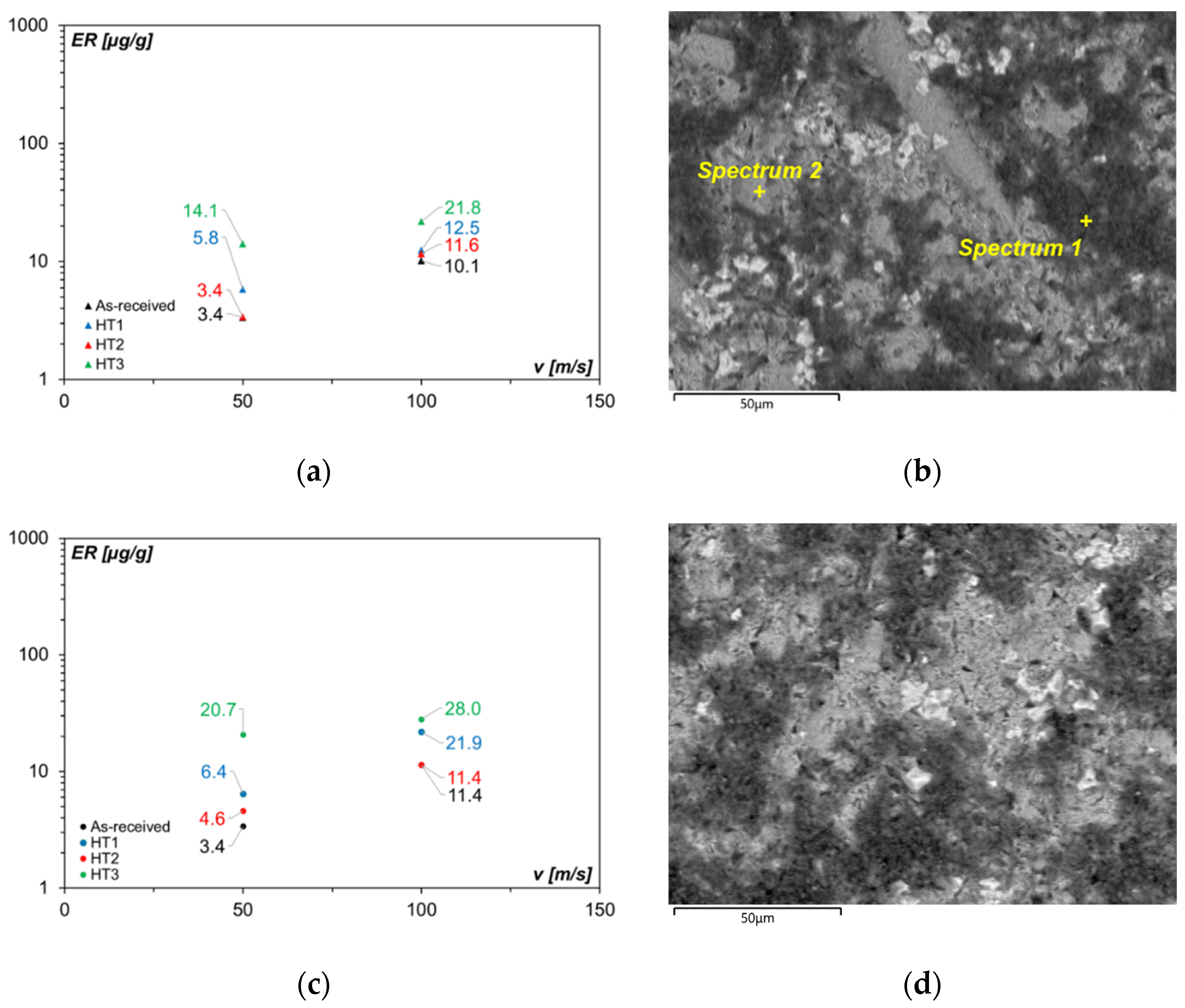
3.6. Erosion Behavior in the Heat-Treated Conditions
3.7. Erosion Behavior vs. Hardness Ratio
4. Discussion
5. Conclusions
- For all the investigated heat treatments, the precipitation of secondary carbides and, for Alloy 2, the overall transformation of the austenite into martensite were detected. Size and distribution of secondary carbides were affected by the temperature and time parameters: after HT1 treatment (the highest temperature and soaking time), they always appear as fine granular distributed particles.
- The obtained bulk hardness values were influenced not only by the retained austenite and martensite contents but also depended on soaking temperature and time that affects carbides’ dimension and distribution.
- For the same erodent powder, the ER of both Alloy 1 and Alloy 2 is comparable, and it increased as the impact velocity increased. Alloy 1 and Alloy 2 offered good erosion resistance when the erodent particles were softer than the target material: the ER with Al2O3 powder is between one and two orders of magnitude higher with respect to raw meal powder.
- The ER of Alloy 1 and Alloy 2 tested with both raw meal powder and Al2O3 powder worsened in the heat-treated conditions. Besides, irrespective of impact velocity and alloy composition, the samples treated with HT3 route, exhibit in all the tested conditions the highest ER. With raw meal powder, the lowest values of ER were for the as-received condition, but the highest values of ER were about three times greater. Hence, the overall hardness of the alloy is not a comprehensive index of erosive wear resistance.
- The He/Ht ratio affects the ER: whether it is less than 1, the ER showed a stronger dependence on the ratio of the hardness of the erodent to that of the target material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Studnicki, A.; Kilarski, J.; Przybył, M.; Suchoń, J.; Bartocha, D. Wear resistance of chromium cast iron—research and application. Manuf. Eng. 2006, 16, 63–73. [Google Scholar]
- Tabrett, C.P.; Sare, I.R.; Ghomashchi, M.R. Microstructure-property relationships in high chromium white iron alloys. Int. Mater. Rev. 1996, 41, 59–82. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Xing, X.; Wang, J.; Ren, X.; Yang, Q. Growth characteristics of primary M7C3 carbide in hypereutectic Fe-Cr-C alloy. Sci. Rep. 2016, 6, 32941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiengmoon, A. Carbides in high chromium cast irons. Naresuan Univ. Eng. J. 2011, 6. [Google Scholar]
- Ma, S.; Xing, J.; He, Y.; Li, Y.; Huang, Z.; Liu, G.; Geng, Q. Microstructure and crystallography of M7C3 carbide in chromium cast iron. Mater. Chem. Phys. 2015. [Google Scholar] [CrossRef]
- Chotěborský, R.; Hrabě, P.; Müller, M.; Válek, R.; Savková, J.; Jirka, M. Effect of carbide size in hardfacing on abrasive wear. Res. Agric. Eng. 2009, 55, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Buchely, M.F.; Gutierrez, J.C.; León, L.M.; Toro, A. The effect of microstructure on abrasive wear of hardfacing alloys. Wear 2005, 259, 52–61. [Google Scholar] [CrossRef]
- Correa, E.O.; Alcântara, N.G.; Valeriano, L.C.; Barbedo, N.D.; Chaves, R.R. The effect of microstructure on abrasive wear of a Fe–Cr–C–Nb hardfacing alloy deposited by the open arc welding process. Surf. Coatings Technol. 2015, 276, 479–484. [Google Scholar] [CrossRef]
- Chotěborský, R.; Hrabě, P.; Müller, M.; Savková, J.; Jirka, M. Abrasive wear of high chromium Fe-Cr-C hardfacing alloys. Res. Agric. Eng. 2008, 54, 192–198. [Google Scholar] [CrossRef]
- Cruz-Crespo, A.; Fernández-Fuentes, R.; Ferraressi, A.V.; Gonçalves, R.A.; Scotti, A. Microstructure and Abrasion Resistance of Fe-Cr-C and Fe-Cr-C-Nb Hardfacing Alloys Deposited by S-FCAW and Cold Solid Wires. Soldag. Inspeção 2016, 21, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Jilleh, A.; Kishore Babu, N.; Thota, V.; Anis, A.L.; Harun, M.K.; Talari, M.K. Microstructural and wear investigation of high chromium white cast iron hardfacing alloys deposited on carbon steel. J. Alloys Compd. 2021, 857, 157472. [Google Scholar] [CrossRef]
- Jindal, C.; Singh Sidhu, B.; Kumar, P.; Singh Sidhu, H. Performance of hardfaced/heat treated materials under solid particle erosion: A systematic literature review. Mater. Today Proc. 2022, 50, 629–639. [Google Scholar] [CrossRef]
- Tarodiya, R.; Levy, A. Surface erosion due to particle-surface interactions—A review. Powder Technol. 2021, 387, 527–559. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, T.K. Solid particle erosion behaviour of hardfacing deposits on cast iron—Influence of deposit microstructure and erodent particles. Wear 2006, 261, 1069–1079. [Google Scholar] [CrossRef]
- Purba, R.H.; Shimizu, K.; Kusumoto, K.; Todaka, T.; Shirai, M.; Hara, H.; Ito, J. Erosive wear characteristics of high-chromium based multi-component white cast irons. Tribol. Int. 2021, 159, 106982. [Google Scholar] [CrossRef]
- Adler, T.A.; Doğan, Ö.N. Erosive wear and impact damage of high-chromium white cast irons. Wear 1999, 225–229, 174–180. [Google Scholar] [CrossRef]
- Sapate, S.G.; Rama Rao, A.V. Effect of material hardness on erosive wear behavior of some weld-deposited alloys. Mater. Manuf. Process. 2002, 17, 187–198. [Google Scholar] [CrossRef]
- Sapate, S.G.; Rama Rao, A.V. Effect of carbide volume fraction on erosive wear behaviour of hardfacing cast irons. Wear 2004, 256, 774–786. [Google Scholar] [CrossRef]
- Sapate, S.G.; RamaRao, A.V. Erosive wear behaviour of weld hardfacing high chromium cast irons: Effect of erodent particles. Tribol. Int. 2006, 39, 206–212. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, Q.B.; Zhang, Y.W.; Lim, C.Y.H.; Khoo, B.C. Effect of particle size on erosion characteristics. Wear 2016, 348–349, 126–137. [Google Scholar] [CrossRef]
- Stevenson, A.N.; Hutchings, I. Wear of hardfacing white cast irons by solid particle erosion. Wear 1995, 186–187, 150–158. [Google Scholar] [CrossRef]
- Fortini, A.; Suman, A.; Vulpio, A.; Merlin, M.; Pinelli, M. Microstructural and erosive wear characteristics of a high chromium cast iron. Coatings 2021, 11, 490. [Google Scholar] [CrossRef]
- Filipovic, M.; Kamberovic, Z.; Korac, M.; Gavrilovski, M. Microstructure and mechanical properties of Fe-Cr-C-Nb white cast irons. Mater. Des. 2013, 47, 41–48. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, C.-M.; Chen, J.-H.; Wu, W. The effects of additive elements on the microstructure characteristics and mechanical properties of Cr–Fe–C hard-facing alloys. J. Alloys Compd. 2010, 498, 30–36. [Google Scholar] [CrossRef]
- Tang, X.H.; Chung, R.; Li, D.Y.; Hinckley, B.; Dolman, K. Variations in microstructure of high chromium cast irons and resultant changes in resistance to wear, corrosion and corrosive wear. Wear 2009, 267, 116–121. [Google Scholar] [CrossRef]
- Li, P.; Yang, Y.; Shen, D.; Gong, M.; Tian, C.; Tong, W. Mechanical behavior and microstructure of hypereutectic high chromium cast iron: The combined effects of tungsten, manganese and molybdenum additions. J. Mater. Res. Technol. 2020, 9, 5735–5748. [Google Scholar] [CrossRef]
- Zhi, X.; Xing, J.; Fu, H.; Xiao, B. Effect of niobium on the as-cast microstructure of hypereutectic high chromium cast iron. Mater. Lett. 2008, 62, 857–860. [Google Scholar] [CrossRef]
- Bedolla-Jacuinde, A.; Correa, R.; Quezada, J.G.; Maldonado, C. Effect of titanium on the as-cast microstructure of a 16%chromium white iron. Mater. Sci. Eng. A 2005, 398, 297–308. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Z.; Xing, X.; Ren, X.; Zhou, Y.; Yang, Q. Effect of Nb additive on wear resistance and tensile properties of the hypereutectic Fe-Cr-C hardfacing alloy. Mater. Today Commun. 2020. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Lekatou, A.; Diavati, E. Effect of Destabilization Heat Treatments on the Microstructure of High-Chromium Cast Iron: A Microscopy Examination Approach. J. Mater. Eng. Perform. 2009, 18, 1078–1085. [Google Scholar] [CrossRef]
- Tabrett, C.P.; Sare, I.R. Effect of high temperature and sub-ambient treatments on the matrix structure and abrasion resistance of a high-chromium white iron. Scr. Mater. 1998. [Google Scholar] [CrossRef]
- Doğan, Ö.N.; Hawk, J.A.; Laird, G. Solidification structure and abrasion resistance of high chromium white irons. Metall. Mater. Trans. A 1997, 28, 1315–1328. [Google Scholar] [CrossRef]
- Doǧan, Ö.N.; Hawk, J.A. Effect of carbide orientation on abrasion of high Cr white cast iron. Wear 1995, 189, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Maratray, F.; Poulalion, A. Austenite Retention in High-Chromium White Irons. Trans. Am. Foundrymen’s Soc. 1982, 90. [Google Scholar]
- Karantzalis, A.E.; Lekatou, A.; Kapoglou, A.; Mavros, H.; Dracopoulos, V. Phase Transformations and Microstructural Observations During Subcritical Heat Treatments of a High-Chromium Cast Iron. J. Mater. Eng. Perform. 2012, 21, 1030–1039. [Google Scholar] [CrossRef]
- Gasan, H.; Erturk, F. Effects of a destabilization heat treatment on the microstructure and abrasive wear behavior of high-chromium white cast iron investigated using different characterization techniques. Metall. Mater. Trans. A 2013, 44, 4993–5005. [Google Scholar] [CrossRef]
- Guitar, M.A.; Suárez, S.; Prat, O.; Duarte Guigou, M.; Gari, V.; Pereira, G.; Mücklich, F. High chromium cast irons: Destabilized-subcritical secondary carbide precipitation and its effect on hardness and wear properties. J. Mater. Eng. Perform. 2018, 27, 3877–3885. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Lekatou, A.; Mavros, H. Microstructural Modifications of As-Cast High-Chromium White Iron by Heat Treatment. J. Mater. Eng. Perform. 2009. [Google Scholar] [CrossRef]
- Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Optimization of thermal processes applied to hypoeutectic white cast iron containing 25% Cr aimed at increasing erosive wear resistance. Metals 2020, 10, 359. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Influence of thermal parameters related to destabilization treatments on erosive wear resistance and microstructural variation of white cast iron containing 18% Cr. application of design of experiments and rietveld structural analysis. Materials 2019, 12, 3252. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pociño, A.; Alvarez-Antolin, F.; Asensio-Lozano, J. Erosive wear resistance regarding different destabilization heat treatments of austenite in high chromium white cast iron, alloyed with Mo. Metals 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.K.; Lee, S.; Jung, J.-Y. Effects of heat treatment on wear resistance and fracture toughness of duo-cast materials composed of high-chromium white cast iron and low-chromium steel. Metall. Mater. Trans. A 2006, 37, 633–643. [Google Scholar] [CrossRef] [Green Version]
- He-Xing, C.; Zhe-Chuan, C.; Jin-Cai, L.; Huai-Tao, L. Effect of niobium on wear resistance of 15%Cr white cast iron. Wear 1993. [Google Scholar] [CrossRef]
- Aldi, N.; Casari, N.; Pinelli, M.; Suman, A.; Vulpio, A.; Saccenti, P.; Beretta, R.; Fortini, A.; Merlin, M. Erosion behavior on a large-sized centrifugal fan. In Proceedings of the 13th European Turbomachinery Conference on Turbomachinery Fluid Dynamics and Thermodynamics, Lausanne, Switzerland, 8–12 April 2019. [Google Scholar]
- Bedolla-Jacuinde, A.; Guerra, F.; Mejia, I.; Vera, U. Niobium Additions to a 15%Cr–3%C White Iron and Its Effects on the Microstructure and on Abrasive Wear Behavior. Metals 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Guitar, M.A.; Nayak, U.P.; Britz, D.; Mücklich, F. The Effect of Thermal Processing and Chemical Composition on Secondary Carbide Precipitation and Hardness in High-Chromium Cast Irons. Int. J. Met. 2020, 14, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Suman, A.; Vulpio, A.; Fortini, A.; Fabbri, E.; Casari, N.; Merlin, M.; Pinelli, M. Experimental analysis of micro-sized particles time-wise adhesion: The influence of impact velocity and surface roughness. Int. J. Heat Mass Transf. 2021, 165, 120632. [Google Scholar] [CrossRef]
- Aldi, N.; Casari, N.; Pinelli, M.; Suman, A.; Vulpio, A.; Saccenti, P. Performance Modification of an Erosion-Damaged Large-Sized Centrifugal Fan. In Proceedings of the 13th ASME Turbo Expo 2021: Turbomachinery Technical Conference and Exposition, Virtual, 7–11 June 2021. [Google Scholar]
- Bedolla-Jacuide, A.; Arias, L.; Hernández, B. Kinetics of Secondary Carbides Precipitation in a High-Chromium White Iron. J. Mater. Eng. Perform. 2003, 12, 371–382. [Google Scholar] [CrossRef]
- Kim, C. X-ray method of measuring retained austenite in heat treated white cast irons. J. Heat Treat. 1979. [Google Scholar] [CrossRef]
- Sapate, S.G.; Haque, N. Effect of microstructure on slurry erosion behaviour of weld hardfacing alloys. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2011, 225, 49–59. [Google Scholar] [CrossRef]
- Finnie, I. Erosion of surfaces by solid particles. Wear 1960, 3, 87–103. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, C.-M.; Chen, J.-H.; Hsieh, C.-C.; Wu, W. Microstructural evolution of hypoeutectic, near-eutectic, and hypereutectic high-carbon Cr-based hard-facing alloys. Metall. Mater. Trans. A 2009, 40, 1031–1038. [Google Scholar] [CrossRef]
- Pearce, J.T.H. Examination of M7C3 carbides in high chromium cast irons using thin foil transmission electron microscopy. J. Mater. Sci. Lett. 1983, 2, 428–432. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Liu, H.; Yang, H.; Shen, B.; Gao, S.; Huang, S. The precipitation and transformation of secondary carbides in a high chromium cast iron. Mater. Charact. 2006, 56, 73–78. [Google Scholar] [CrossRef]
- Powell, G.L.F.; Laird, G. Structure, nucleation, growth and morphology of secondary carbides in high chromium and Cr-Ni white cast irons. J. Mater. Sci. 1992, 27, 29–35. [Google Scholar] [CrossRef]
- Kishore, K.; Kumar, U.; Dinesh, N.; Adhikary, M. Effect of Soaking Temperature on Carbide Precipitation, Hardness, and Wear Resistance of High-Chromium Cast Iron. J. Fail. Anal. Prev. 2020. [Google Scholar] [CrossRef]
- Gahr, K.-H.Z. Wear by hard particles. Tribol. Int. 1998, 31, 587–596. [Google Scholar] [CrossRef]
- Bitter, J.G.A. A study of erosion phenomena part I. Wear 1963, 6, 5–21. [Google Scholar] [CrossRef]
- Goodwin, J.E.; Sage, W.; Tilly, G.P. Study of Erosion by Solid Particles. Proc. Inst. Mech. Eng. 1969, 184, 279–292. [Google Scholar] [CrossRef]
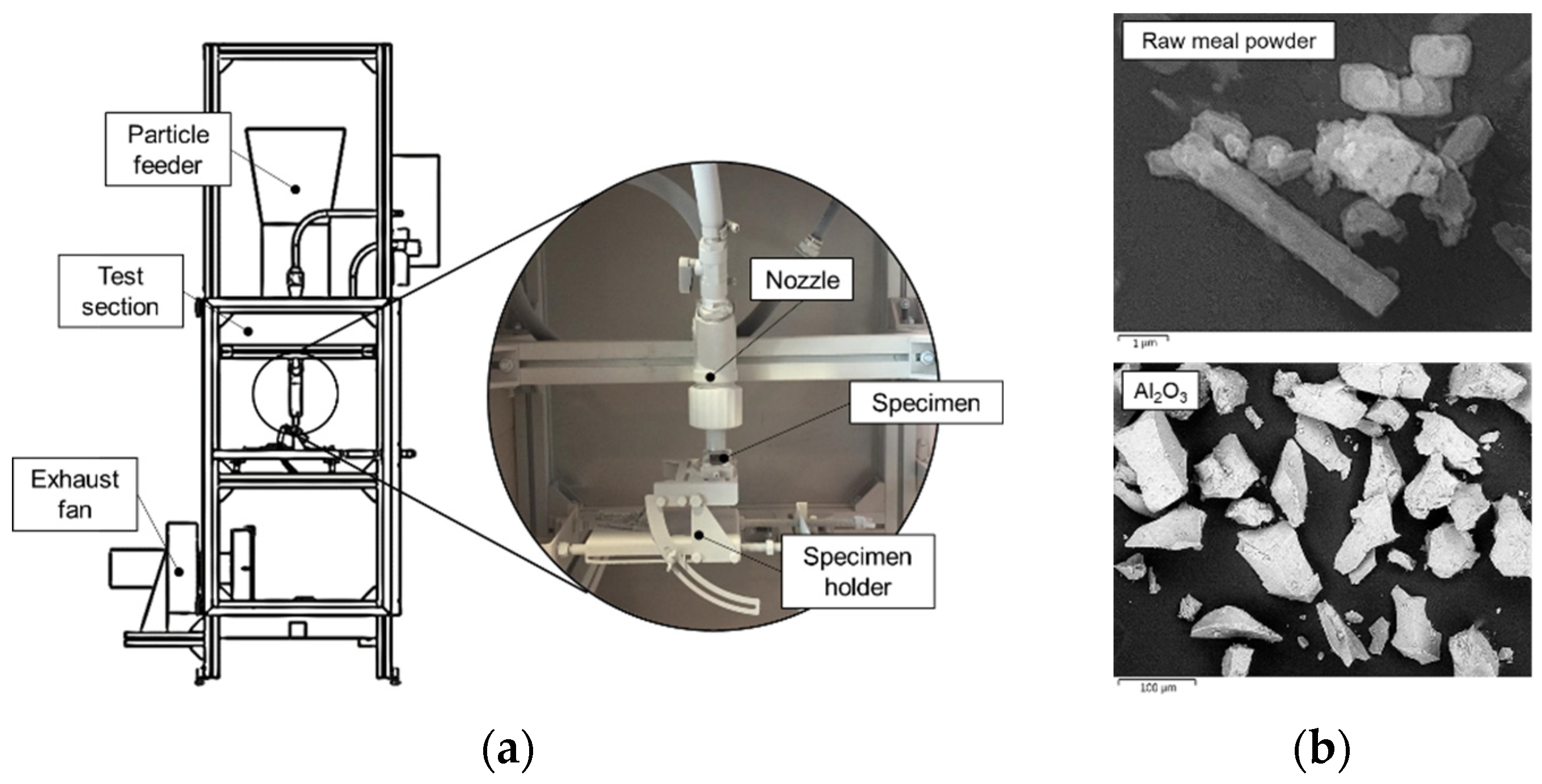





| Alloy | Composition (wt.%)—Fe Balance | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | Cr | Mo | Nb | W | V | |
| Alloy 1 | 3.83 | - | 0.88 | 20.22 | - | 3.75 | - | - |
| Alloy 2 | 4.15 | 0.56 | 1.08 | 21.04 | 2.78 | 4.09 | 0.86 | 0.69 |
| Name | Dwelling T [°C] | Dwelling t [min] | Cooling Medium | Microstructural Effects | Reference |
|---|---|---|---|---|---|
| HT1 | 1000 | 480 | Oil | By using a long dwelling time at the destabilization temperature of austenite promote the increment of Ms temperature and, in turn, favors a decrease in retained austenite in the final microstructure. | [41] |
| HT2 | 900 | 30 | Air | Secondary carbides precipitation occurred within the matrix, which was partially transformed into martensite. This increases the overall hardness and abrasive wear resistance. | [45] |
| HT3 | 980 | 90 | Air | Carbide precipitation and austenite/martensite transformation caused the increment of the material hardness compared to the as-cast material. | [46] |
| Al | Ca | Si | Cu | Na | HV0.1 |
|---|---|---|---|---|---|
| 69.09 ± 9.56 | 24.52 ± 5.35 | 1.53 ± 0.78 | 2.98 ± 0.36 | 1.88 ± 0.52 | 412 ± 95 |
| Alloy | Condition | Phases | Wt.% | Alloy | Condition | Phases | Wt.% |
|---|---|---|---|---|---|---|---|
| Alloy 1 | As-received | α | 30.1 (4) | Alloy 2 | As-received | α | 22.3 (5) |
| γ | 6.8 (3) | γ | 33.3 (5) | ||||
| M7C3 | 47.9 (6) | M7C3 | 38.4 (6) | ||||
| NbC | 5.6 (1) | NbC | 6.0 (1) | ||||
| M23C6 | 9.7 (6) | ||||||
| HT1 | α | 39.4 (5) | HT1 | α | 45 (2) | ||
| γ | 0.4 (1) | γ | 20 (4) | ||||
| M7C3 | 43.6 (5) | M7C3 | 26 (2) | ||||
| NbC | 9.1 (2) | NbC | 9.5 (5) | ||||
| M23C6 | 7.5 (6) | ||||||
| HT2 | A | 43.6 (6) | HT2 | α | 39 (2) | ||
| Γ | 0.7 (1) | γ | 30.9 (2) | ||||
| M7C3 | 38.1 (6) | M7C3 | 22 (4) | ||||
| NbC | 6.4 (1) | NbC | 8.6 (5) | ||||
| M23C6 | 11.2 (7) | ||||||
| HT3 | α | 44.5 (6) | HT3 | α | 56.9 (4) | ||
| γ | 0.7 (3) | γ | 1.1 (2) | ||||
| M7C3 | 42.8 (6) | M7C3 | 35.5 (4) | ||||
| NbC | 7.2 (2) | NbC | 6.5 (2) | ||||
| M23C6 | 4.8 (6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortini, A.; Suman, A.; Zanini, N.; Cruciani, G. Erosive Wear Behavior of High-Chromium Cast Iron: Combined Effect of Erodent Powders and Destabilization Heat Treatments. Coatings 2022, 12, 1218. https://doi.org/10.3390/coatings12081218
Fortini A, Suman A, Zanini N, Cruciani G. Erosive Wear Behavior of High-Chromium Cast Iron: Combined Effect of Erodent Powders and Destabilization Heat Treatments. Coatings. 2022; 12(8):1218. https://doi.org/10.3390/coatings12081218
Chicago/Turabian StyleFortini, Annalisa, Alessio Suman, Nicola Zanini, and Giuseppe Cruciani. 2022. "Erosive Wear Behavior of High-Chromium Cast Iron: Combined Effect of Erodent Powders and Destabilization Heat Treatments" Coatings 12, no. 8: 1218. https://doi.org/10.3390/coatings12081218
APA StyleFortini, A., Suman, A., Zanini, N., & Cruciani, G. (2022). Erosive Wear Behavior of High-Chromium Cast Iron: Combined Effect of Erodent Powders and Destabilization Heat Treatments. Coatings, 12(8), 1218. https://doi.org/10.3390/coatings12081218









