Abstract
Naringin (NG), is a poorly water-soluble flavonoid that has reported to possess a variety of therapeutic efficacies. The present research work is designed to prepare and optimize Naringin hybrid nanoparticles (NG-HNs) using lipid (A), chitosan (B), and D-α-tocopheryl polyethylene glycol succinate (C). The formulations were optimized using a Box–Behnken Design (BBD), and the selection of optimized composition (NG-HNop) was carried out on the basis of low particle size (Y1) and high entrapment efficiency (Y2) using the point prediction method. The selected NG-HNop was further evaluated in order to study permeation, drug release, antimicrobial and antioxidant effect, and cell viability. The optimized nanoparticles (NG-HNop) showed a particle size and entrapment efficiency of 246 ± 8.3 nm and 83.5 ± 2.1%, with a polydispersibility index (PDI) of 0.23 and a Zeta potential of + 18.1 mV, indicating high stability. The optimized NG-HNop exhibited better drug release (89.62 ± 4.54%) and enhanced permeation (3.7 folds). A significant improvement in the antimicrobial activity was achieved against Escherichia coli with respect to Staphylococcus aureus with the hybrid nanoparticles. They also exhibited better activity in the tested cell line. On the basis of the study results, hybrid nanoparticles of Naringin are an alternative oral delivery method for treating cancer cells.
1. Introduction
The oral route is the most commonly used route for the treatment of different diseases. This route has the problem of poorly soluble drugs, with low solubility and dissolution leading to poor bioavailability [1]. Most bioactive compounds are poorly water-soluble, and belong to biopharmaceutical classification (BCS-II). Flavonoids are natural compounds that are used in the treatment of various diseases. They are found in various vegetables, fruits, and beverages [2,3]. Naringin (NG) belongs chemically to the flavanone -7-O-glycosides, which are mostly found in citrus fruits. Various pharmacological activities have been reported, such as antimicrobial [4], antioxidant [5], anti-arthritis [6], and anticancer activities [7], as well as antidiabetic activity [8]. The poor aqueous solubility is the major concern in the formulation of delivery systems. The bioavailability of Naringin is about 8%, due to its poor aqueous solubility [9]. Thus, improving its solubility through encapsulation in biodegradable polymers would be an alternative for enhancing therapeutic efficacy [10,11]
Different colloidal formulations have been reported to improve therapeutic efficacy. Formulations such as nanostructured lipid carriers [12], solid lipid nanoparticles [13], self-nano-micelles [14], liposomes [15], poly-(lactic-co-glycolic acid) nanoparticles [16], hybrid nanoparticles [17], and hybrid polymeric nanoparticles [18] have been reported to enhance in vitro/in vivo activities. Among them, hybrid nanoparticles (HNs) are a novel nano-carrier for the delivery of poorly soluble therapeutics with the aim of enhancing their therapeutic activity. They are prepared using drug, lipid, polymer, and surfactant. The composition contains a biocompatible and biodegradable lipid (soya lecithin) and polymer (chitosan, CS). Chitosan has also been reported for its non-toxic, bio-adhesive, and antimicrobial properties [19]. D-α-tocopheryl polyethylene glycol succinate (TPGS) is used as a surfactant in the formulation, because it increases solubility by conjugating with CS, increasing the stability of the HNs. This helps in the preparation of HNs due to the electrostatic attraction between the negative charge of lecithin and the positive charge of CS [20].
There are various research publications related to the use of HNs to improve the therapeutic efficacy of poorly soluble drugs. Perez-Ruiz et al. formulated epicatechin-loaded nanoparticles using lecithin, CS and TPGS that exhibited a four-fold lower IC50 value than pure epicatechin [21]. Fereig et al. formulated tacrolimus HNs for the management of psoriasis. The in vivo results revealed excellent anti-psoriatic efficacy. The results also revealed a sin deposition of 74.9%, in contast to that of the marketed product (13.4%) [22]. Recently insulin-loaded HPNs were prepared using D-α-Tocopheryl polyethylene glycol 1000 succinate, sodium deoxycholate and sulfobutyl ether-β-cyclodextrin [23]. The study results depicted desirable gastrointestinal stability and insulin release and a significant reduction in blood glucose levels. To date, no research has been reported describing Naringin (NG)-loaded HNs for the improvement of in vitro activity.
In the present study, hybrid nanoparticles of Naringin (NG) were prepared using lecithin, chitosan and TPGS. The formulations were optimized by means of a Box–Behnken design using lipid (A), chitosan (B), and TPGS (C) at low, medium, and high levels. The optimized formulation was further evaluated with the aim of studying physicochemical characterization, drug release, and permeation, as well as in vitro antioxidant and antimicrobial activity and cell viability.
2. Materials
Naringin (NG), Chitosan (CH) with low molecular weight (85% deacetylation), soya-lecithin (LC), and D-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) were obtained from Sigma Aldrich (St. Louis, MO, USA). The organic solvents ethanol, methanol and acetonitrile were procured from the SD Fine Chemicals, Mumbai, India. All other chemicals used for the studies were analytical grade.
3. Experimental Section
3.1. Box–Behnken Design
The optimization was performed using a three-factor three-level Box–Behnken Design (BBD) (Design-expert software, version 8.0.6, State-Ease Inc., Minneapolis, MO, USA). This design is based on the factorial points along with 5 center points and gives a total of 17 different compositions. For these factors, the independent levels were selected from the preliminary study. The variables lipid (A, 200–600 mg), chitosan (B, 0.1%–0.25%), and TPGS (C, 0.4%–1.4%) were used to prepare the HNs as shown in Table 1. The particle size (Y1) and entrapment efficiency (Y2) are the most important factors affecting the solubility, release and absorption of drugs. The effects of these variables on the particle size (Y1) and entrapment efficiency (Y2) were evaluated. The individual and combined effects of independent variables were evaluated, and the different models were used to select the optimum composition. The effect of the interaction on response can be represented by the following polynomial equation:
where β0 is the intercept, β 1 to β 33 are the regression coefficients obtained for the observed experimental values, and A, B and C are the coded values of the independent factors. Coefficients β 1, β 2 and β 3 refer to how individual parameters affect the response when two other parameters remain constant [24,25]. The interaction terms (such as β 12–β 23) indicates by what means the response is modified when the two factors are altered simultaneously. The fitness of the best model between the linear, two-factor (2F) interaction model and the quadratic model was determined on the basis of the analysis of variance p-value and the highest correlation R2, predicted R2, and adjusted R2 values. A p-value less than 0.05 was considered statistically significant.
Y (response) = β0 + β 1A + β 2B + β 3 C + β 12AB + β 13AC + β 23BC + β 11A2 + β 22B2 + β 33C2

Table 1.
Independent variables used to optimize Naringin hybrid nanoparticles (NG-HNs).
3.2. Preparation of Nanoparticles
Naringin hybrid polymeric nanoparticles (NG-HPNs) were developed as per the previously reported method with slight modification [26]. The formation of NG-HNs takes place after self-organization between NG-lecithin and CS. NG-HNs were prepared as per the composition shown in Table 2. Lecithin (LC) and NG solution were dissolved in the organic solvent. The sample was vortexed for complete solubilization. Separately, CS solution was prepared by being dissolved in glacial acetic acid (1% v/v). The weighed quantity of TPGS was also added to the CS solution. Then, LC-NG organic solution was added dropwise into the CS-TPGS solution (LC: CS solution, 1:12) with continuous stirring at 10,000 rpm for 1 h. The organic solvent was evaporated with stirring and pH was adjusted to 4.5. The prepared NG-HNs was separated from the suspension by ultracentrifugation. Finally, the NG-HNs was collected after washing with water and stored for further analysis.

Table 2.
Composition of Naringin hybrid nanoparticles (NG-HNs) with their experimental values.
3.3. Characterization
Particle Characterization
The prepared NG-HNs were evaluated with respect to their particle size (PS), polydispersibility index (PDI), and Zeta potential (ZP) using a particle size analyzer (Malvern ZS 900, Malvern, UK). The samples were diluted 100-fold with double-distilled water and transferred into a cuvette for the measurement of the PS and PDI. The analysis was performed at room temperature and at a scattering angle of 90°. The Zeta potential (ZP) of the same sample was analyzed to measure the surface charge using a special electrode cuvette. The particles showing values of PDI and ZP below 0.5 and ± 30 mV were considered to possess the optimum values [27,28].
3.4. Entrapment Efficiency (EE)
The amount of NG entrapped in the prepared HNs was evaluated. An indirect estimation was performed using centrifugation and supernatant separation. Briefly, the samples were placed in a centrifugation tube and rotated at 6000 rpm for 45 min using a cooling centrifuge. The supernatant was collected, and the presence of unentrapped NG was measured using a UV spectrophotometer (Shimadzu 1800, Kyoto, Japan). The absorbance was noted in order to calculate the NG concentration for each formulation. The following equation was used to calculate the NG encapsulation:
3.5. In Vitro Release Study
Release studies were performed for the pure NG and NG-HNop using phosphate buffer (pH 6.8) as the release medium. The release medium was placed in a beaker, and the temperature was maintained at 37 ± 0.5 °C for the duration of the study. pure NG suspension and NG-HNop (equivalent to 5 mg NG) were placed in a pretreated dialysis bag (12 kDa) and immersed in the release medium. The release medium was stirred continuously at 75 rpm and the temperature was fixed at 37 °C for the duration of the study. The released content (2 mL) was collected at a specific time and replaced with fresh release medium to maintain uniform study conditions. The released samples at each time point were measured using a UV spectrophotometer at an absorbance of 285 nm. The precentage released was calculated, and a graph plotting drug release (%) as a function of time (h) was produced.
3.6. Ex Vivo Permeation Study
Permeation studies of the prepared NG-HNop and pure NG dispersion were performed using a goat intestinal membrane. The goat intestine was collected from a local slaughterhouse and washed with Ringer’s solution to remove any food residue. The intestinal membrane was filled with samples containing 5 mg of NG and tied at both ends. The samples were dipped into permeation media (25 mL, phosphate buffer pH 6.8), and the temperature was maintained at 37 ± 0.5 °C. The content of NG-HNop and pure NG dispersion (1 mL) released was collected at a specific time, and the same volume was added in order to maintain a constant volume of the permeated media. The amount of NG permeation was evaluated using the previously developed HPLC method [29]. The study was performed using acetonitrile and phosphate buffer (pH 3.5) at a ratio of 3:7 v/v and a flow rate of 0.75 mL/min. Detection was performed using a UV-detector at 285 nm.
3.7. Antioxidant Study
The antioxidant activities of NG-HNop and pure NG were determined as per the reported DPPH method with slight modification [30]. Stock solutions of pure NG and NG-HNop were prepared separately at concentrations of 1 mg/mL. Then, they were diluted to prepare different concentrations in the range of 10–500 µg/mL. DPPH solution (0.02%) in methanol was prepared. The different concentrations of each sample (500 µL) were added to the DPPH solution (125 µL). The mixture was vortexed and incubated for 1 h in darkness to complete the reaction mixture. After that, the color of the samples changed to become colorless, with the maximum change in color from violet to colorless indicating greater antioxidant potential. The samples were analyzed at 517 nm using a UV spectrophotometer with ascorbic acid serving as a reference standard. The percent scavenging activity was calculated using the following formula [31]:
3.8. Antibacterial Activity
The antibacterial activities of the NG-HNop and pure NG were evaluated using the cup plate method using Gram-negative and Gram-positive organisms in a nutrient agar medium. The agar medium was prepared and sterilized at 121 °C. The bacterial strain was grown in the sterile broth. The bacterial culture of Staphylococcus aureus and Escherichia coli (100 µL, 106 CFU/mL) was added to the agar medium, transferred to Petri dishes under aseptic conditions and allowed to stand in order to achieve solidification. The wells were made using a sterile borer (6 mm), and the NG-HNop and pure NG samples were added. Sterile water and ciprofloxacin were used as normal control and standard. The plates were kept aside for 1 h at room temperature and then incubated in an incubator for 24 h. The zone of inhibition of each tested sample was measured to compare the results.
3.9. Cell Viability Study
A cell viability study of the pure NG and NG-HNop was performed by means of MTT assay. The breast cancer cell line (MCF 7) was seeded in 96-well plates at a density of 5 × 104 cells per well in 100 µL solution. The cells were incubated for 24 h for complete growth. The cells were treated with pure NG and NG-HNop in different concentrations (5–500 µg/mL). The blank HN (control) was also tested with the same composition as that used for NG-HNop. DMSO was used as a solvent to solubilize the NG, and the concentration of DMSO was kept below 0.2%. After the treatment, the samples were kept for an incubation period of 48 h to achieve a high degree of killing. The blank cells received no treatment. MTT solution was added to each well, and incubated for a further 4 h. MTT formazan crystals were dissolved in DMSO (100 µL), and the plates were shaken. The absorbance of each well was measured using a microplate reader at 492 nm. The cell viability (%) was calculated using the following equation:
Cell Viability (%) = [OD]treatment − [OD]blank/[OD] control − [OD] blank) × 100
4. Statistical Analysis
All experiments were performed in triplicate, and the results are shown as mean ± SD. Statistical analysis was performed using one-way ANOVA following Tukey analysis between different samples.
5. Results and Discussion
5.1. Optimization
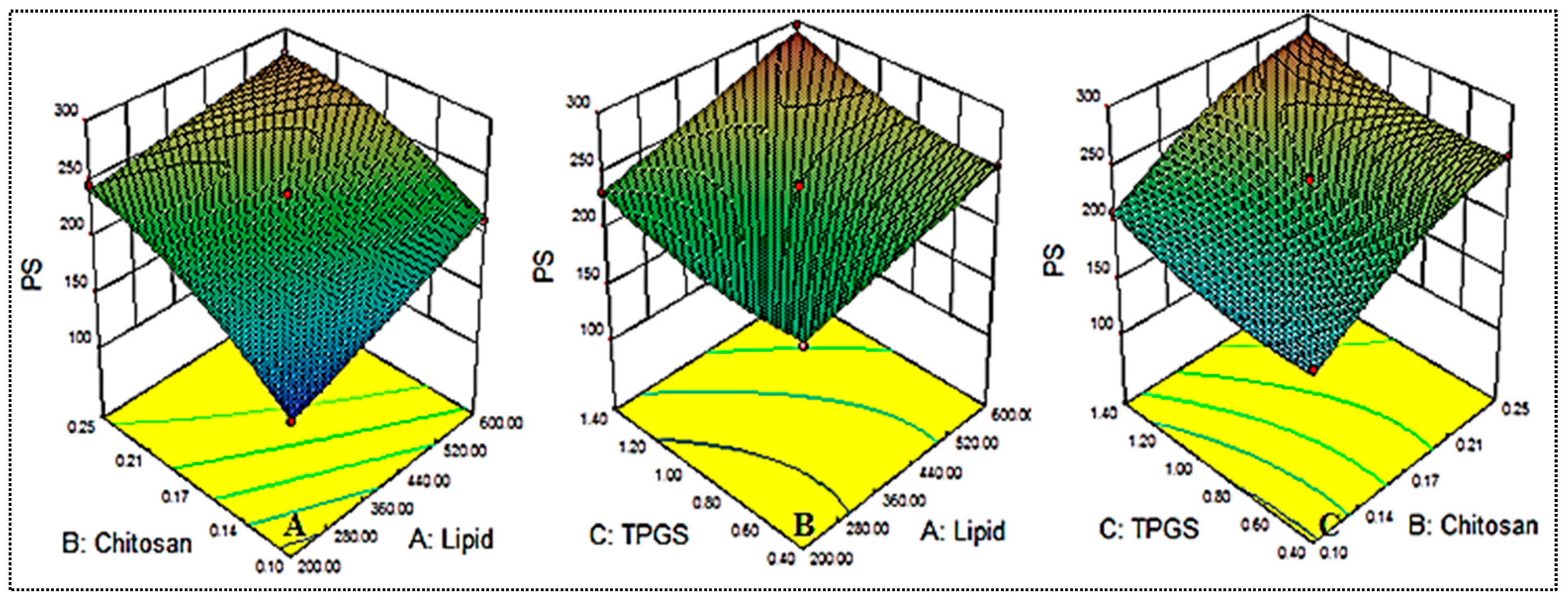
The approach to formulation design presented 17 formulation runs with five common compositions as shown in Table 2. The effect of the independent variables on particle size (Y1) and entrapment efficiency (Y2) was observed. Statistical analysis was performed in order to select the optimum model. The best fit model was found to be quadratic due to the non-significant lack of fit and low value of precision among the tested models (Table 3). Polynomial equations with positive and negative signs showed the synergistic and antagonistic effects of the independent variables on the response [32]. The effects of independent factors like lipid (A), CH (B) and TGPS (C) were also used to interpret the effects on particle size (Y1) and entrapment efficiency (Y2), as shown in the 3D model graphs.

Table 3.
Statistical model fit summary report of hybrid nanoparticles.
5.2. Influence of Independent Factors on Particle size (Y1)
The independent variables used exerted an effect on the particle size, and the results are shown in Table 2 and Figure 1. The mean particle size was found to be between 151.65 ± 7.8 (NG-HN1) and 295.37 ± 13.2 (NG-HN8). The formulation NG-HN1, prepared with lipid 200 mg, chitosan 0.1 % and TPGS 0.9%, exhibited the lowest particle size, while the maximum particle size was observed for the composition lipid 600 mg, chitosan 0.17% and TPGS 1.4%. The different compositions exhibited significant (p < 0.05) variation in the size due to the changes in the concentrations of lipid, chitosan and TPGS. Increasing lipid (A) and chitosan (B) concentrations directly affected the particle size. When the concentration of lipid was increased from 200 mg to 400 mg, the size increased. A possible reason for this effect is the increased viscosity of the medium, leading to reduced emulsification [18,33]. On the other hand, increasing TGPS concentration resulted in increased particle size (NG-HN6, NG-HN8, NG-HN9, NG-HN11) due to the re-coalescence of the particles during preparation at excess lipid concentrations. Another reason might be the interaction between the lipid and chitosan, and the fact that it does not completely emulsify. Similar findings have been observed in previously reported literature [34,35]. On the basis of the polynomial equation (Equation (1)), all three factors, with their positive coefficient values, were observed to have an effect on particle size.
Particle size (Y1) = +236.12 + 23.99 A + 39.02 B+16.39 C − 6.96 AB + 4.47 AC − 0.48 BC + 0.063 A2 − 15.59 B2 + 10.37 C2
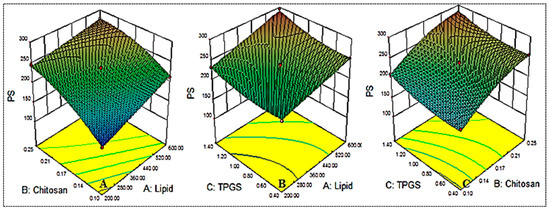
Figure 1.
Effect of the constraints used ((lipid (A), chitosan (B), TPGS (C) = on particle size (Y1).
Further, it was observed by means of ANOVA that factors like A, B, C, AB, B2, and C2 exerted a significant effect (p < 0.05) on particle size (Y1), while other factors, such as BC, and A2, presented a non-significant effect (p > 0.05). Among the different models tested, the best fitting model was found to be the quadratic model, as shown in Table 3.
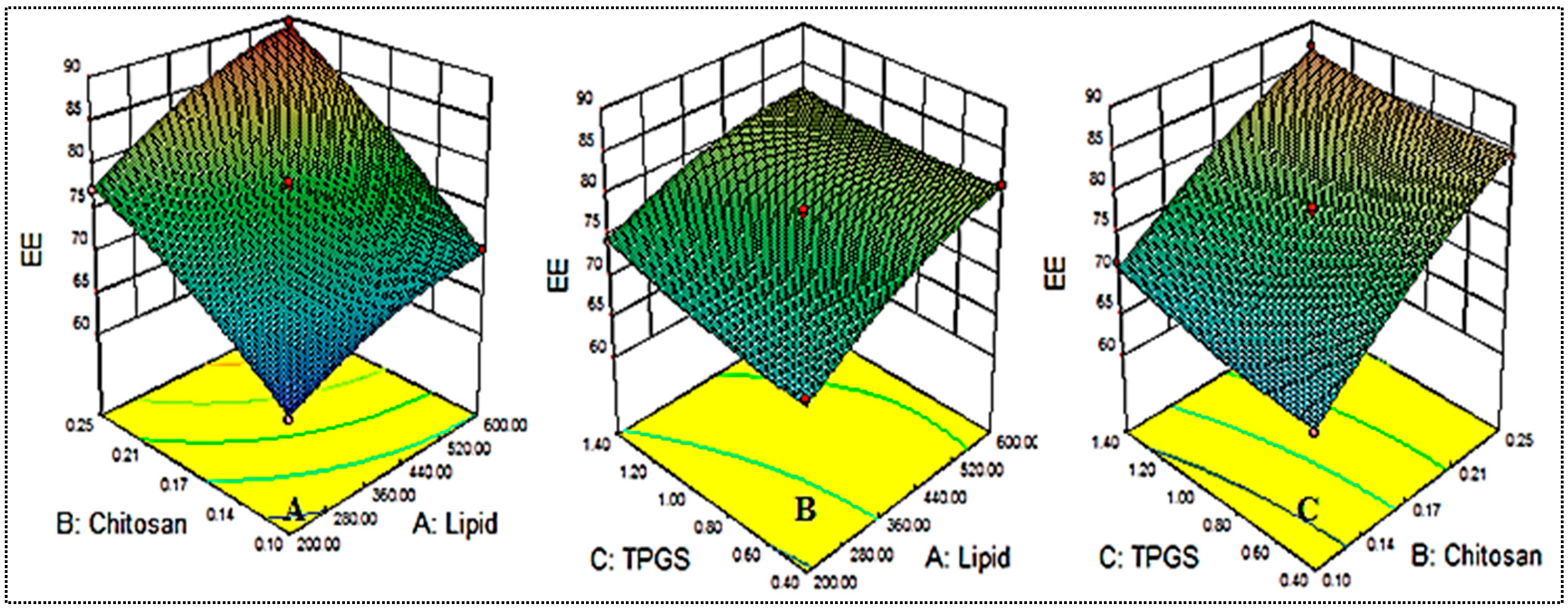
5.3. Influence of Independent Constraints on Entrapment Efficiency (Y2)
The independent variables used exerted an effect on the entrapment efficiency, and the results are shown in Table 2 and Figure 2. The mean entrapment efficiency was found to be between 62.56 ± 2.3 (NG-HN1) and 89.45 ± 3.4 (NG-HN4). The formulation NG-HN1, prepared with lipid 200 mg, chitosan 0.1%, and TPGS 0.9%, presented the lowest entrapment efficiency, while the maximum was observed for the composition with lipid 600 mg, chitosan 0.25% and TPGS 0.9%. The different compositions showed significant (p < 0.05) variations in entrapment efficiency due to changes in the concentrations of lipid, chitosan and TPGS. All three factors lipid, CH and TGPS were observed to exert a positive effect on entrapment efficiency. With increasing lipid concentration, the entrapment efficiency increased (NG-HN1, NG-HN2, NG-HN3, NG-HN4). This might be due to the availability of more space to accommodate a larger amount of drug, which may lead to increased entrapment efficiency [34,35]. With increasing concentration of chitosan, entrapment efficiency was also found to increase. TGPS also increased the entrapment efficiency by increasing the solubility and reducing the interfacial tension. The obtained results were found to be in good agreement with those presented in previous reports [18,33]. ANOVA provided a polynomial equation (Equation (3)) to evaluate the effect of formulation variables on entrapment efficiency:
Entrapment efficiency = +77.62 + 4.65 A + 8.42 B + 1.59 C + 1.21 AB − 0.96 AC − 0.56 BC − 1.64 A2 − 1.19 B2 + 0.76 C2
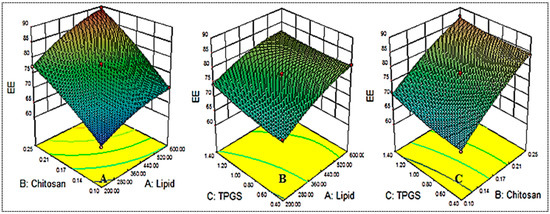
Figure 2.
Effect of the constraints used (lipid (A), chitosan (B), TPGS (C) on entrapment efficiency (Y2).
By means of the equation presented above, all three factors with positive coefficient values were found to exhibit a favorable (positive) effect. Further, it was observed using ANOVA that the factors A, B, C, AB, A2, and B2 had a significant effect (p < 0.05) on the entrapment efficiency, while other factors, such as AB, BC, and C2, had a nonsignificant effect (p > 0.05). The quadratic model was considered the best-fit model on the basis of its maximum regression value (Table 3).
5.4. Selection of Optimized Composition
On the basis of the results presented in Table 2, the polynomial equations, and the 3D model graphs (Figure 1 and Figure 2), it was concluded that all three formulation variables (lipid (A), CH (B), TGPS (C)) exerted a significant effect on the responses, i.e., particle size (Y1) and entrapment efficiency (Y2). Among the 17 formulations, the formulation composition NG-HN13 was selected for further optimization on the basis of the results of the point prediction method (Table 4).

Table 4.
Point prediction optimization using the Box–Behnken design.
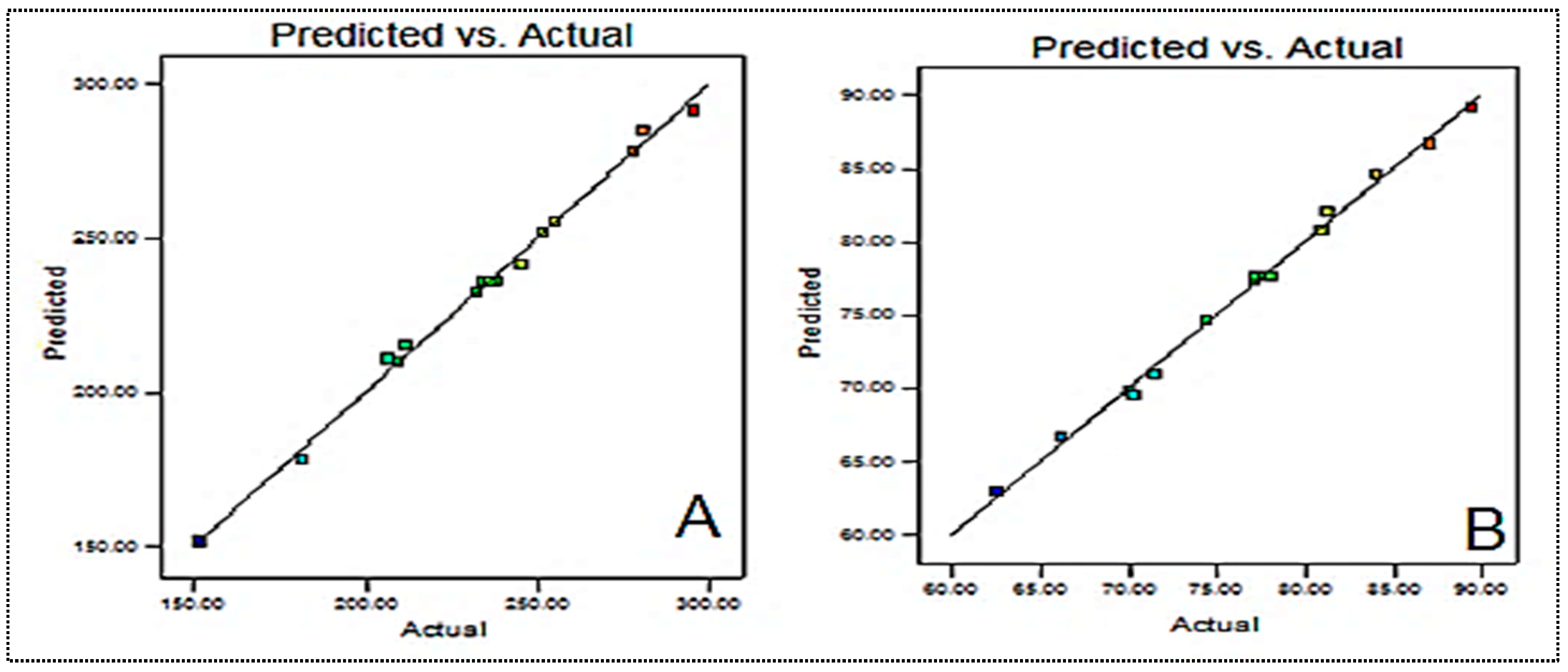
The composition was slightly changed in order to obtain the minimum particle size and high entrapment efficiency. The particle size and entrapment efficiency of the optimized formulation were found to be 246.4 ± 8.3 nm and 83.5 ± 4.1%, respectively. These values were found to be close to the predicted values of 249.5 nm for particle size and 82.75% for entrapment efficiency. Figure 3 presents the actual and predicted graphs for each variable. There is a close agreement between the actual and predicted values, as can be observed from the graph. The desirability value was found to be closer to 1 (0.987).
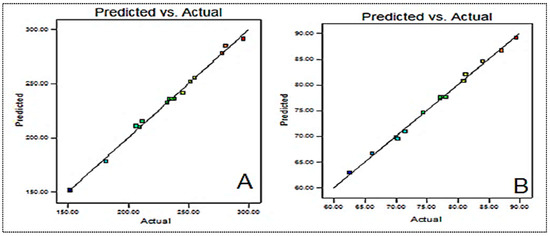
Figure 3.
Predicted and actual results of (A) particle size and (B) entrapment efficiency.
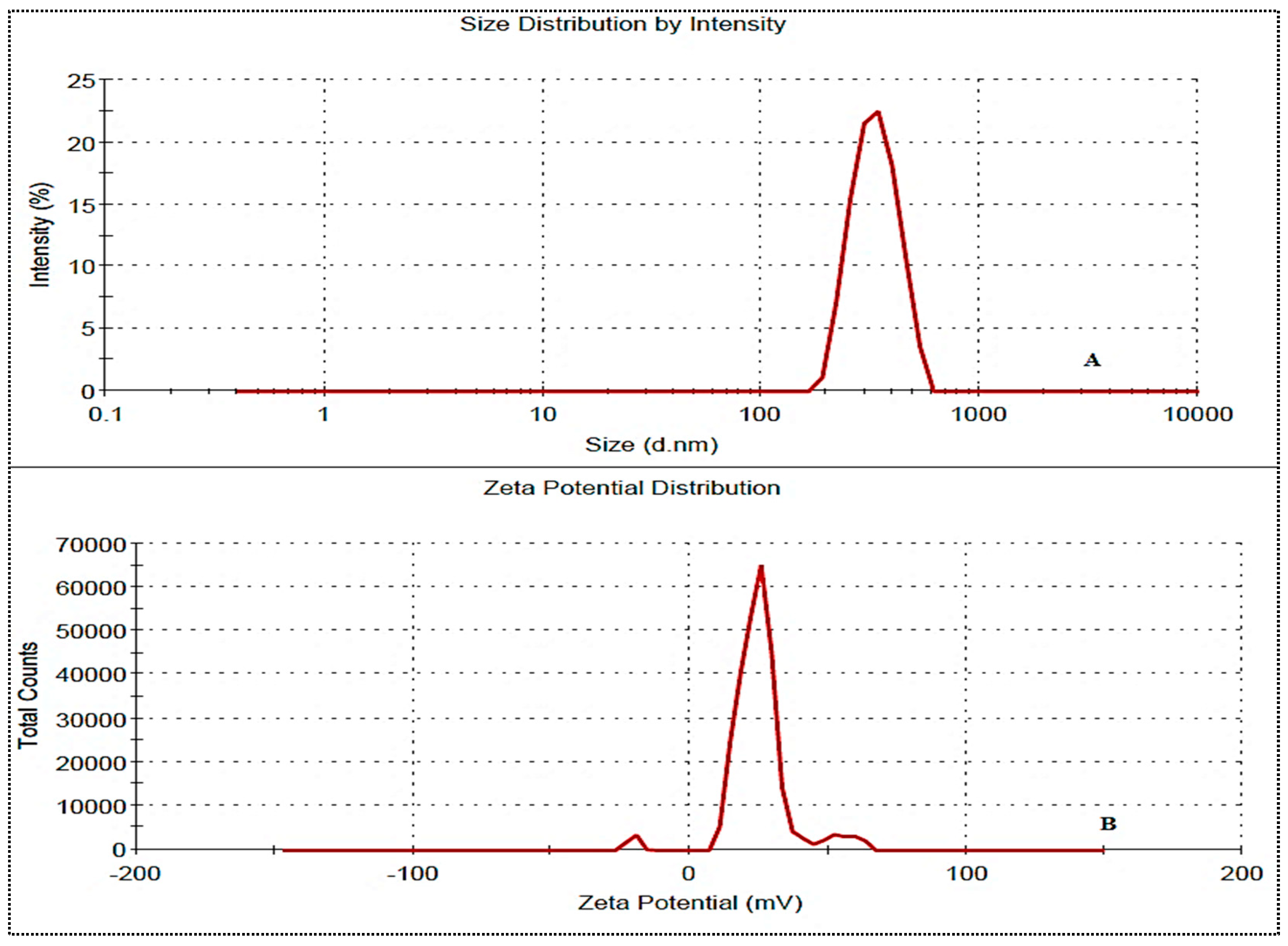
The prepared NG-HN1 to NG-HN17 exhibited a positive Zeta potential for all formulations. The values were found to be between 10.2 mV and 23.1 mV, and were therefore below the standard limit of ±30 mV. The selected optimized formulation NG-HNop presented values of particle size, PDI, and Zeta potential of 246.4 ± 8.3 nm (Figure 4A), >0.5 (0.23) and +18.1 mV (Figure 4B), respectively, indicating the high stability of the NG-HNs.
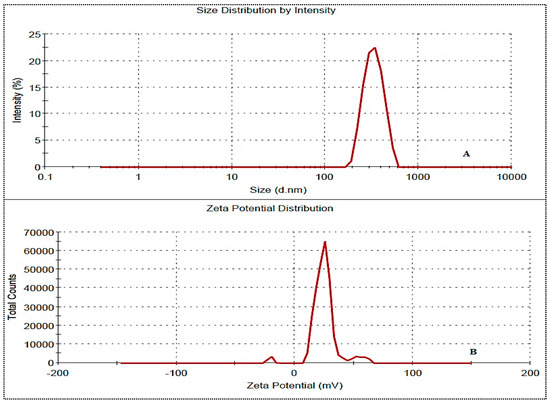
Figure 4.
Particle size (A) and Zeta potential (B) of NG-HNop.
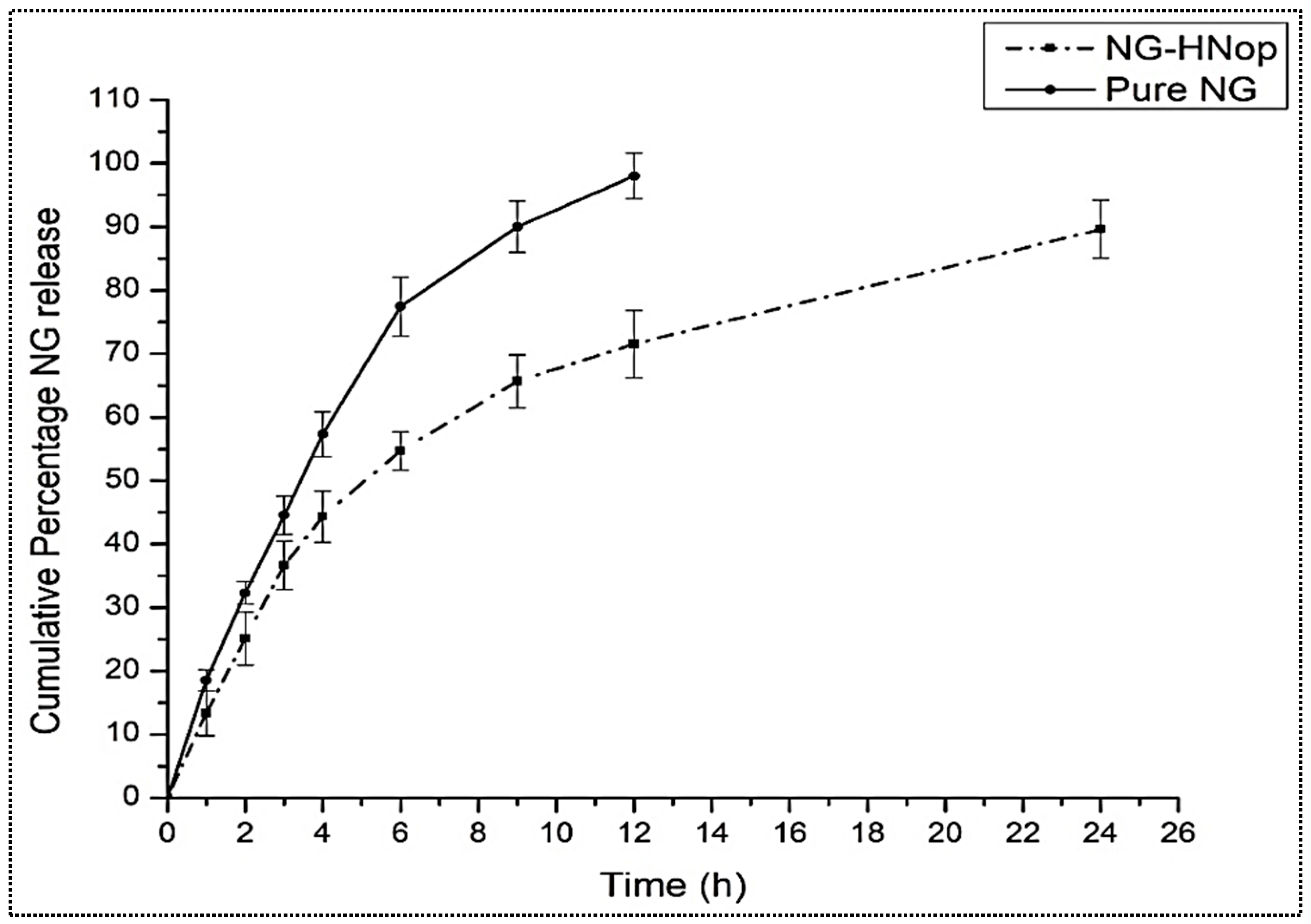
5.5. In Vitro Release Study
The comparative in vitro release profiles of pure NG and NG-HNop are expressed in Figure 5. The pure NG exhibited a maximum NG release of 98.34 ± 4.1% in 12 h of study. A significantly (p < 0.05) prolonged release was achieved by the prepared NG-HNop. The prepared NG-HNop exhibited 71.54 ± 3.8% release after 12 h, and 89.6 ± 4.5% release after 24 h. There was a significant (p < 0.05) difference in the release observed after 12 h. The release was found to be biphasic for NG-HNop, with an initial rapid release of 25.11 ± 4.3% in 2 h. This rapid release was due to the mechanism of diffusion from the outer layer of the HNs, as well as the adherence of the drug molecule to the surface [36]. Subsequently, between 2 and 24 h, the total NG release was found to be slower. The slower release of NG from the HNs between 2 h and 24 h was ascribed to the release from the lipophilic inner shells of the hybrid solid matrix, which is composed of lipid and CS [37]. The presence of chitosan in the nanoparticles led to a retarded release, resulting in prolonged release.
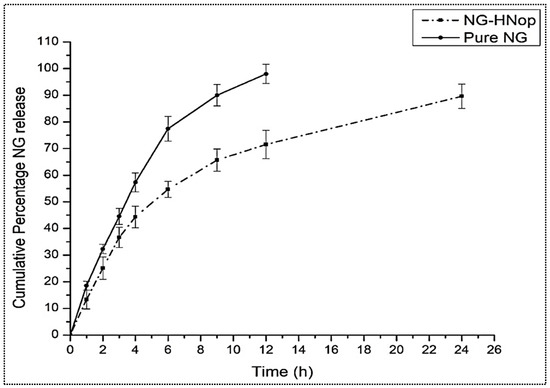
Figure 5.
Comparative in vitro release of pure Naringin (NG) and Naringin hybrid nanoparticles (NG-HNop). The study was performed in triplicate and the data are shown as mean ± SD.
5.6. In Vitro Permeation Study
The comparative permeation profiles of pure NG and NG-HNop were determined. The intestinal permeation flux results revealed a value of 55.28 µg/h.cm2 for NG-HNop and 15.94 µg/h.cm2 for pure NG. The permeation flux was found to be 3.7-fold higher than that of pure NG. Further, a significant (p < 0.05) increase in the apparent permeability coefficient (APC) was also observed for NG-HNop. NG-HNop exhibited a 3.46-fold higher value of APC (9.21 × 10−3 cm/min) compared to the pure NG dispersion (2.65 × 10−3 cm/min). The significant (p < 0.05) improvement in the intestinal permeability of NG-HNop was ascribed to the small particle size, which provides a significantly greater surface area for intestinal absorption. The other factor involved in the higher absorption was the cationic surface charge of the particle. This interacts with the intestinal mucosa and opens the tight junctions, thereby significantly improving drug permeation [38].
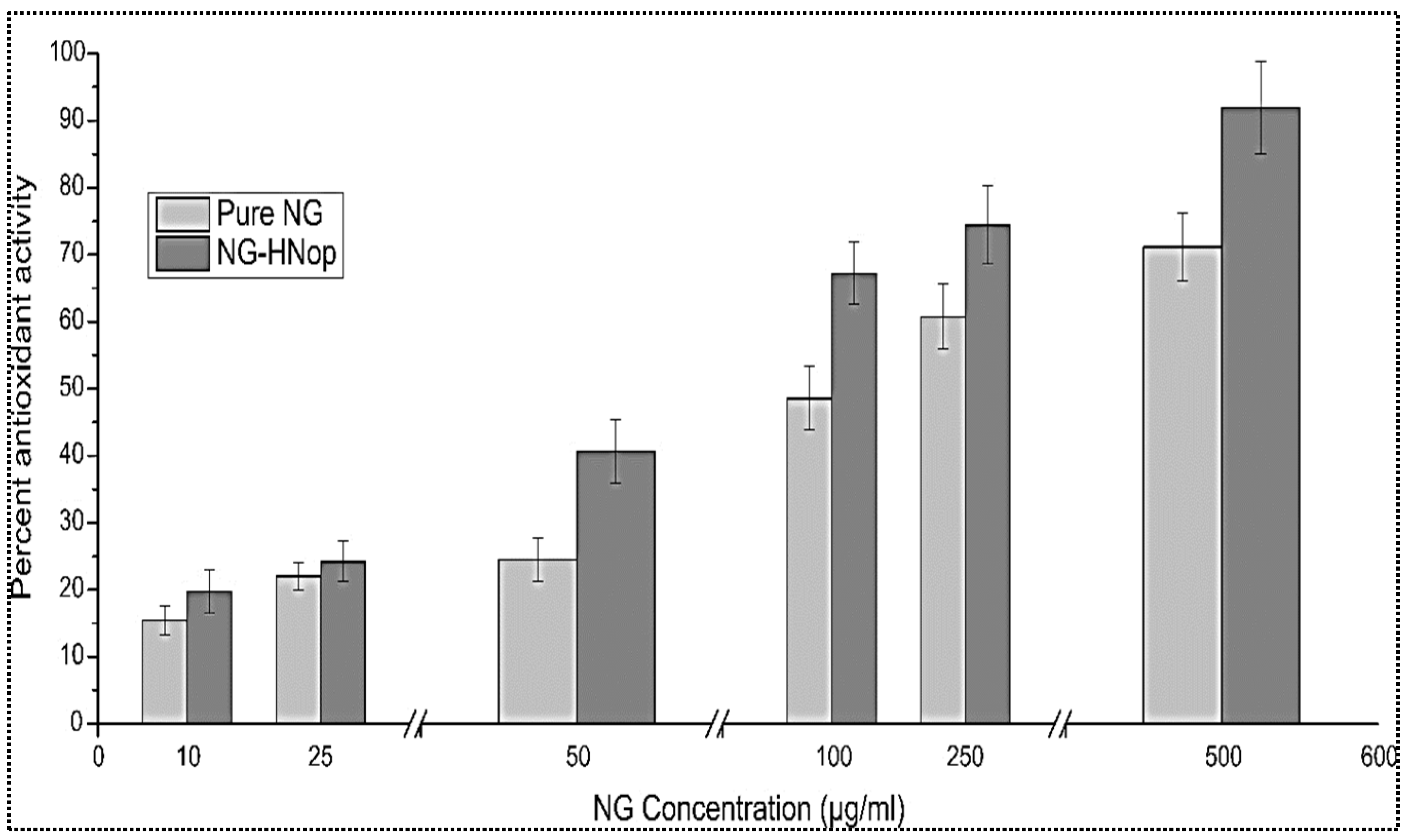
5.7. Antioxidant Activity
This study works on the principle of the conversion of the free radical form into a stable form, with the DPPH solution changing from a violet color to a yellow color following reduction in the presence of antioxidants. The antioxidant activities of NG-HNop and pure NG were assessed at a variety of concentrations (10 to 500 µg/mL), and the data are presented in Figure 6. The effect was found to be concentration dependent. The pure NG exhibited antioxidant properties between 15.45 ± 2.11 and 71.11 ± 5.48, respectively. However, NG-HNop presented significantly (p < 0.05) enhanced antioxidant properties (20.1 ± 3.32 to 91.91 ± 6.87) at all tested concentrations. A non-significant enhancement of antioxidant properties was achieved at lower concentrations. At higher concentrations, the difference was found to be significant. The increase in activity was due to the increased solubility of NG following encapsulation in NPs [39].
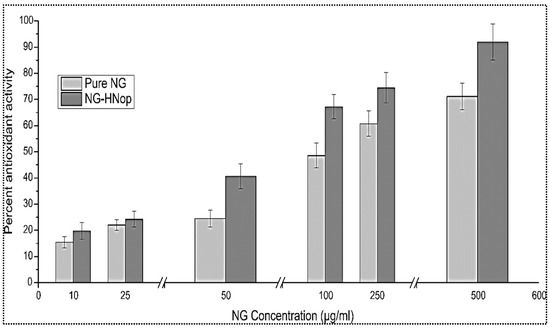
Figure 6.
In vitro antioxidant activity of pure Naringin (NG) and Naringin hybrid nanoparticles (NG-HNop). The study was performed in triplicate and the data are shown as mean ± SD.
5.8. Antibacterial Activity
The antibacterial activity/zone of inhibition (ZOI) and the minimum inhibitory concentration (MIC) value of NG-HNop and pure NG dispersion were determined with respect to S. aureus and E. coli in order to assess any differences in their activity (Table 5). The MIC values of the pure NG and NG-HNop were evaluated against E. coli and S. aureus. The pure NG showed an MIC value of 50 ± 3.1 µg/mL, and a significantly lower value of MIC was found for NG-HNop (20 ± 1.6 µg/mL) against E. coli. This result is close to that reported in the literature. The MIC value was also determined against S. aureus, and the pure NG obtained a value of 70 ± 2.4 µg/mL. A significant (p < 0.05) reduction in MIC value was found for NG-HNop 30 ± 2.8 µg/mL. The MIC values were 2.5-fold smaller for E. coli and 2.3-fold for S. aureus. This significant reduction in the MIC value was due to the size of the nanoparticles, their prolonged dissolution, and their enhanced permeation across the cell wall. The pure NG exhibited ZOI values of 14.2 ± 1.3 mm and 16.8 ± 1.5 mm, respectively, against S. aureus and E. coli. The prepared NG-HNop showed a significantly (p < 0.05) enhanced activity against both of the tested organisms. It exhibited ZOI values of 23.4 ± 1.9 mm and 24.1 ± 2.2 mm, respectively, against the same organisms. NG showed greater activity against E. coli (Gram-negative) than S. aureus (Gram-positive). On the basis of these results, it can be observed that the antibacterial activity of NG was significantly enhanced after encapsulation within the nanoparticles. NG-HNop resulted in greater permeation of NG to the bacterial cell, presenting enhanced antibacterial properties. This leads to destabilization effects in the outer bacterial membrane, thus facilitating entry of the drug into the cell [40]. The solubility of NG also improved after encapsulation into the prepared NPs, providing higher therapeutic efficacy. The nano-size of NG-HNop and its enhanced entrapment efficiency also help lend it greater therapeutic effect. The smaller size of the particles provides a greater effective surface area for absorption, resulting in greater antibacterial properties [11]. The presence of chitosan in the nanoparticles also promotes antibacterial activity. It acts by binding with the negatively charged bacterial cell wall, altering membrane permeability, inhibiting DNA replication, and leading to cell death [41]. The combination of chitosan with NG exhibited synergistic action against the tested organisms, making the vesicles ideal delivery systems. Antibacterial activity was also tested for the standard compound ciprofloxacin, which showed highly significantly (p < 0.001) improved antibacterial activity against S. aureus (ZOI of 25.8 ± 1.4 mm) and E. coli (23.7 ± 1.8 mm) compared to pure NG. A non-significant difference in the ZOI was found between ciprofloxacin and pure NG.

Table 5.
MIC and zone of inhibition results for pure Naringin (NG) and Naringin hybrid nanoparticles (NG-HNop). The study was performed in triplicate and data are shown as mean ± SD. **—Highly significant with respect to pure Naringin.
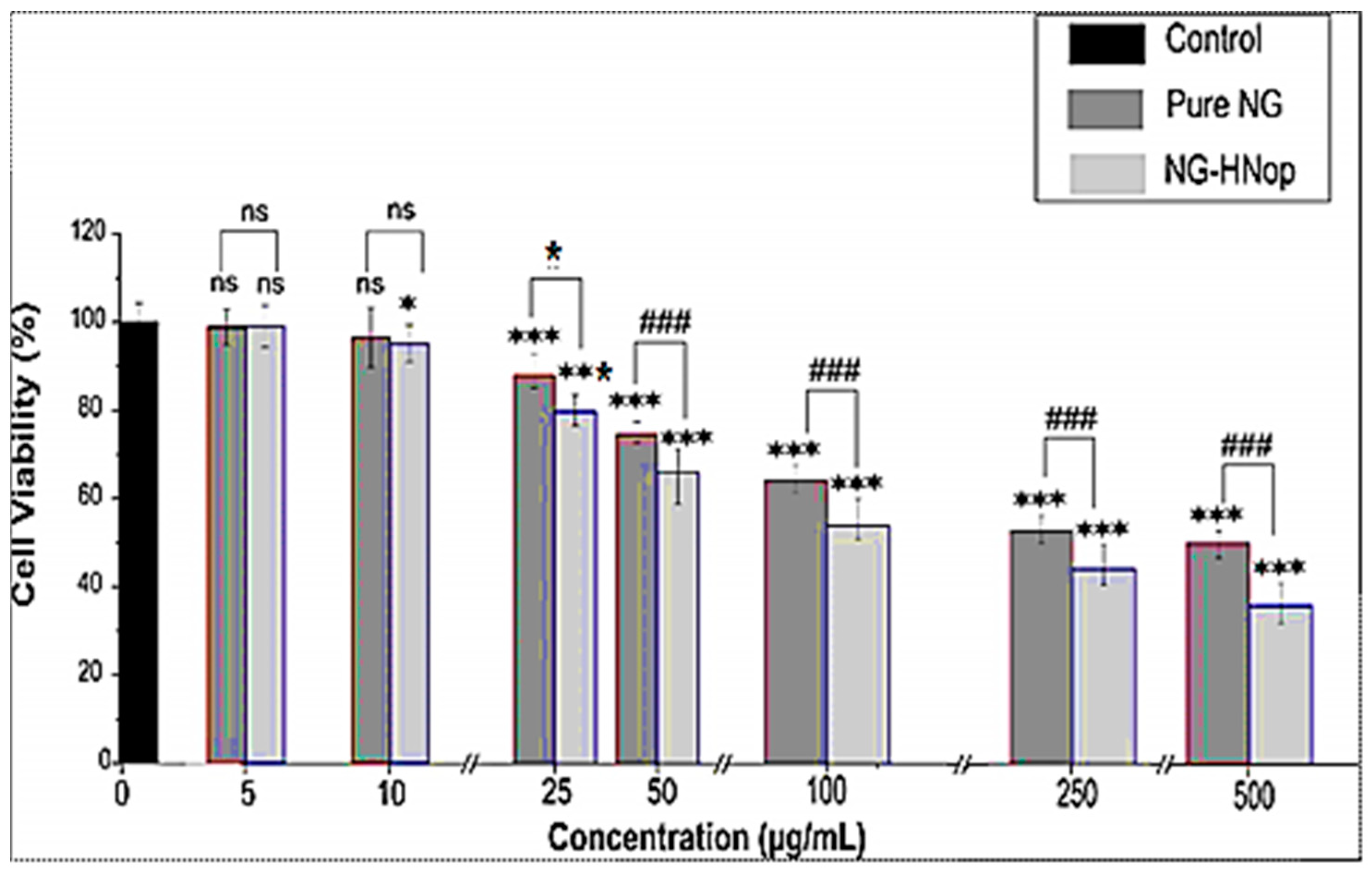
5.9. Cell Viability Study
The cell viability of the pure NG and NG-HNop was evaluated against MCF-7 cell lines at 48 h, as shown in Figure 7. The blank HNs showed negligible cell cytotoxicity against the tested cell lines. A comparative study of the results obtained for pure NG and NG-HNop revealed concentration- and time-dependent cell viability against the cell line. A non-significant effect was observed at lower concentrations (5 µg/mL and 10 µg/mL at 48 h). Significantly greater (p < 0.05) effects were observed for NG-HNop, which showed reduced cell viabilities at concentrations of 25 µg/mL, 50 µg/mL, 100 µg/mL, 250 µg/mL and 500 µg/mL from NG-HNop, compared to pure NG. The pure NG showed reduced cell viability at concentrations of 100 µg/mL (69.8%), 250 µg/mL (57.1%) and 500 µg/mL (51.9%). NG-HNop-treated cell lines showed a significant (p < 0.05) reduction in cell viability from a concentration of 25 µg/mL (80.23%) onwards, with cell viabilities of 63.76% (50 µg/mL), 52.9% (100 µg/mL), 44.2% (250 µg/mL) and 35.8% (500 µg/mL) being observed. The cell viability gradually decreased with increasing concentration of NG. The pure NG and NG-HNop showed highly significant differences in cell viability, observed at each concentration, when compared to control (p < 0.001). The maximum activity was observed at 500 µg/mL for both samples at 48 h. The activity was further evaluated at 48 h, and a higher activity than that at 24 h was observed. The pure NG presented cell viability results of 65.7% (100 µg/mL), 57.2% (250 µg/mL) and 44.2% (500 µg/mL). The prepared NG-HNop presented cell viabilities of 49.8 % (at 50 µg/mL), 41.1% (100 µg/mL), 28.8% (250 µg/mL) and 23.5% (500 µg/mL). These findings confirm that the prepared NG-HNop shows great effectiveness at killing at both time points when compared to the free NG. The enhanced activity can be ascribed to the small particle size as well as the controlled release [42]. The IC50 was calculated at both time points, with the pure NG and NG-HNop having values of 420.4 ± 14.9 µg/mL and 110.5 ± 18.2 µg/mL, respectively, at 24 h. After 48 h incubation time, IC50 values were found to be 245.1 ± 9.6 µg/mL and 75.3 ± 12.7 µg/mL, respectively. The results of this study indicate that NG-HNop is more lethal against MCF-7 cell lines than pure NG. This higher activity is due to the enhanced solubility of NG after encapsulation into the NPs, its nano-metric size range, which provides a much larger surface area for exposure to cancer cells, and the slow drug release profile [43]. Another reason for the greater activity is the presence of chitosan in the NPs. This promotes anticancer activity by promoting drug permeation through an enhanced mucoadhesion effect, helping to open the tight junction of the cell wall [44]. This accelerates cell death by promoting apoptosis [45]. The presence of cationic charge in the amino group provides a higher degree of attraction towards the cancer cells than the anionic charge in the cancer cells [46].
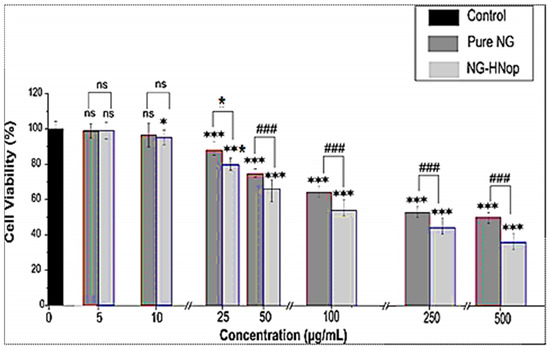
Figure 7.
Cell viability activity of pure Naringin (NG) and Naringin hybrid nanoparticles (NG-HNop). The study was performed in triplicate and data are shown as mean ± SD. Statistical analysis was performed by Tukey–Kramer test. ***—Highly significant with respect to control; ###—highly significant with respect to pure NG; *—significant with respect to pure NG; ns—non-significant.
6. Conclusions
The present research work aimed to prepare Naringin-loaded hybrid nanoparticles using chitosan and lecithin as a carrier. The formulations were optimized using a Box–Behnken design, and their effects were evaluated on particle size and entrapment efficiency. The selected optimized NG-HNop exhibited values of particle size, PDI, Zeta potential, and entrapment efficiency of 246 ± 8.39 nm, 0.23, 28 mV and 83.5 ± 2.1%, respectively. NG-HNop exhibited prolonged drug release and enhanced permeation (55.28 µg/h.cm2). The permeation flux was found to be 3.7-fold higher than the pure NG. Enhanced antioxidant properties (20.1 ± 3.32 to 91.91 ± 6.87) were also observed at all tested concentrations. The antibacterial study results revealed that NG possessed greater activity against E. coli (Gram-negative) than S. aureus (Gram-positive). The cell viability activity results showed enhanced cell viability potential against the tested cancer cell lines. NG-HNop was found to be more lethal against MCF-7 cell lines than pure NG.
Author Contributions
Conceptualization, S.S.I. and M.M.A.; Methodology, software and writing—original draft preparation, A.Z.; validation, S.S.I. and M.M.A., formal analysis, R.A.; investigation and resources, S.A. and M.N.b.J.; data curation, S.S.I. and R.A.; writing—review and editing and funding acquisition, S.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research project is funded from the research project supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research project is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary Flavonoids in the Prevention of T2D: An Overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Aihaiti, Y.; Song Cai, Y.; Tuerhong, X.; Ni Yang, Y.; Ma, Y.; Zheng, H.S.; Xu, K.; Xu, P. Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation. Front. Pharmacol. 2021, 12, 672054. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Abu Hashim, I.I.; Yusif, R.M.; Shaaban, A.A.A.; El-Sheakh, A.R.; Hamed, M.F.; Badria, F.A.E. Polymeric micelles for potentiated antiulcer and anticancer activities of naringin. Int. J. Nanomed. 2018, 13, 1009–1027. [Google Scholar] [CrossRef]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Hsiu, S.L.; Huang, T.Y.; Hou, Y.C.; Chin, D.H.; Chao, P.D. Comparison of metabolic pharmacokinetics of naringin and naringenin in rabbits. Life Sci. 2002, 70, 1481–1489. [Google Scholar] [CrossRef]
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Ahmed, S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 2017, 174, 243–252. [Google Scholar] [CrossRef]
- Hussain, K.; Ali, I.; Ullah, S.; Imran, M.; Parveen, S.; Kanwal, T.; Shah, S.A.; Saifullah, S.; Shah, M.R. Enhanced Antibacterial Potential of Naringin Loaded β Cyclodextrin Nanoparticles. J. Clust. Sci. 2022, 33, 339–348. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. Surface modification of solid lipid nanoparticles for oral delivery of curcumin: Improvement of bioavailability through enhanced cellular uptake, and lymphatic uptake. Eur. J. Pharm. Biopharm. 2017, 117, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Hou, Y.; Geng, Y.; Wu, X. Novel self-nanomicellizing formulation based on Rebaudioside A: A potential nanoplatform for oral delivery of naringenin. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 112, 110926. [Google Scholar] [CrossRef]
- Duong, T.T.; Yen, T.T.H.; Nguyen, L.T.; Nguyen, T.D.; Nguyen, T.Q.; Nghiem, T.H.; Pham, H.T.; Raal, A.; Heinämäki, J.; Pham, T.M. Berberine-loaded liposomes for oral delivery: Preparation, physicochemical characterization and in vivo evaluation in an endogenous hyperlipidemic animal model. Int. J. Pharm. 2022, 616, 121525. [Google Scholar] [CrossRef]
- Feng, S.S.; Mei, L.; Anitha, P.; Gan, C.W.; Zhou, W. Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 2009, 30, 3297–3306. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Y.; Liu, J.; Li, Z.; Fan, Q.; Jiang, Z.; Yan, F.; Wang, Z.; Huang, P.; Feng, N. Chitosan-functionalized lipid-polymer hybrid nanoparticles for oral delivery of silymarin and enhanced lipid-lowering effect in NAFLD. J. Nanobiotechnol. 2018, 16, 64. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Ahmed, M.M.; Yasir, M.; Warsi, M.H.; Alquraini, A.; Ghoneim, M.M.; Alshehri, S. Development and Optimization of Hybrid Polymeric Nanoparticles of Apigenin: Physicochemical Characterization, Antioxidant Activity and Cytotoxicity Evaluation. Sensors 2022, 22, 1364. [Google Scholar] [CrossRef]
- Ozcan, I.; Azizoglu, E.; Senyigit, T.; Ozyazıcı, M.; Ozer, O. Enhanced dermal delivery of diflucortolone valerate using lecithin/chitosan nanoparticles: In-vitro and in-vivo evaluations. Int. J. Nanomed. 2013, 8, 461–475. [Google Scholar] [CrossRef] [Green Version]
- Senyigit, T.; Sonvico, F.; Barbieri, S.; Ozer, O.; Santi, P.; Colombo, P. Lecithin/chitosan NPs of clobetasol-17-propionate capable of accumulation in pigskin. J. Control. Release 2010, 142, 368–373. [Google Scholar] [CrossRef]
- Perez-Ruiz, A.G.; Ganemb, A.; Olivares-Corichi, I.M.; García-Sánchez, J.R. Lecithin–chitosan–TPGS nanoparticles as nanocarriers of (−)-epicatechin enhanced its anticancer activity in breast cancer cells. RSC Adv. 2018, 8, 34773–34782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.G.; Abdel-Mottaleb, M.M.A. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Agardan, N.B.; Han, S. In vitro and in vivo evaluations on nanoparticle and phospholipid hybrid nanoparticles with absorption enhancers for oral insulin delivery. Pharm. Dev. Technol. 2021, 26, 157–166. [Google Scholar] [CrossRef]
- Baig, A.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box-Behnken design for preparation of levofloxacin-loaded stearic acid solid lipid nanoparticles for ocular delivery: Optimization, in vitro release, ocular tolerance, and antibacterial activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef]
- Fasolo, D.; Pippi, B.; Meirelles, G.; Zorzi, G.; Fuentefria, A.M.; von Poser, G.; Teixeira, H.F. Topical delivery of antifungal Brazilian red propolis benzophenones-rich extract by means of cationic lipid nanoemulsions optimized by means of Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 56, 101573. [Google Scholar] [CrossRef]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; Di Bari, M.T.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh, D.F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar]
- Musmade, K.P.; Trilok, M.; Dengale, S.J.; Bhat, K.; Reddy, M.S.; Musmade, P.B.; Udupa, N. Development and Validation of Liquid Chromatographic Method for Estimation of Naringin in Nanoformulation. J. Pharm. 2014, 2014, 864901. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Gabriele, M.; Fernàndez-Busquets, X.; Valenti, D.; Fadda, A.M.; Pucci, L.; Manconi, M. Antioxidant activity of quercetin in Eudragit-coated liposomes for intestinal delivery. Int. J. Pharm. 2019, 565, 64–69. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [Green Version]
- Imam, S.S.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: In vitro characterization and in vivo pharmacokinetic study. Drug Deliv. 2015, 22, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.; Lovric, J.; Pepic, J.; Filipovic-Grcic, I. Lecithin/chitosan nanoparticles for transdermal delivery of melatonin. J. Microencapsul. 2011, 28, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Feng, S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release 2003, 86, 33–48. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Ozgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbouSamra, M.M.; Salama, A.H.; Awad, G.E.A.; Mansy, S.S. Formulation and evaluation of novel hybridized nanovesicles for enhancing buccal delivery of Ciclopirox olamine. AAPS PharmSciTech. 2020, 21, 283. [Google Scholar] [CrossRef]
- Thakur, K.; Sharma, G.; Singh, B.; Chhibber, S.; Patil, A.B.; Katare, O.P. Chitosan-tailored lipidic nano constructs of Fusidic acid as a promising vehicle for wound infections: An explorative study. Int. J. Biol. Macromol. 2018, 115, 1012–1025. [Google Scholar] [CrossRef]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P.; Pillay, V. Improved antioxidant, antimicrobial and anticancer activity of naringenin on conjugation with pectin. 3 Biotech 2019, 9, 312. [Google Scholar] [CrossRef]
- Athanassiou, G.; Michaleas, S.; Lada-Chitiroglou, E.; Tsitsa, T.; Antoniadou-Vyza, E. Antimicrobial activity of β-lactam antibiotics against clinical pathogens after molecular inclusion in several cyclodextrins. A novel approach to bacterial resistance. J. Pharm. Pharmacol. 2003, 55, 291–300. [Google Scholar] [CrossRef]
- Nagy, A.; Harrison, A.; Sabbani, S.; Munson, R.S.; Dutta, P.K., Jr.; Waldman, W.J. Silver nanoparticles embedded in zeolite membranes: Release of silver ions and mechanism of antibacterial action. Int. J. Nanomed. 2011, 6, 1833. [Google Scholar]
- Tahir, N.; Perwez, A.; Mir, S.R.; Rizvi, M.M.; Amin, S. Exemestane encapsulated polymer-lipid hybrid nanoparticles for improved efficacy against breast cancer: Optimization, in vitro characterization and cell culture studies. Nanotechnology 2021, 32, 415101. [Google Scholar]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Mäkilä, E.; Salonen, J.; Santos, H.A. Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimardhani, Y.S.; Suniarti, D.F.; Freisleben, H.J.; Wanandi, S.I.; Siregar, N.C.; Ikeda, M.A. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Xia, P.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).