Magnetite Deposition Behavior on Alloy 600 and Alloy 690 Tubes in Simulated PWR Secondary Water
Abstract
:1. Introduction
2. Materials and Methods
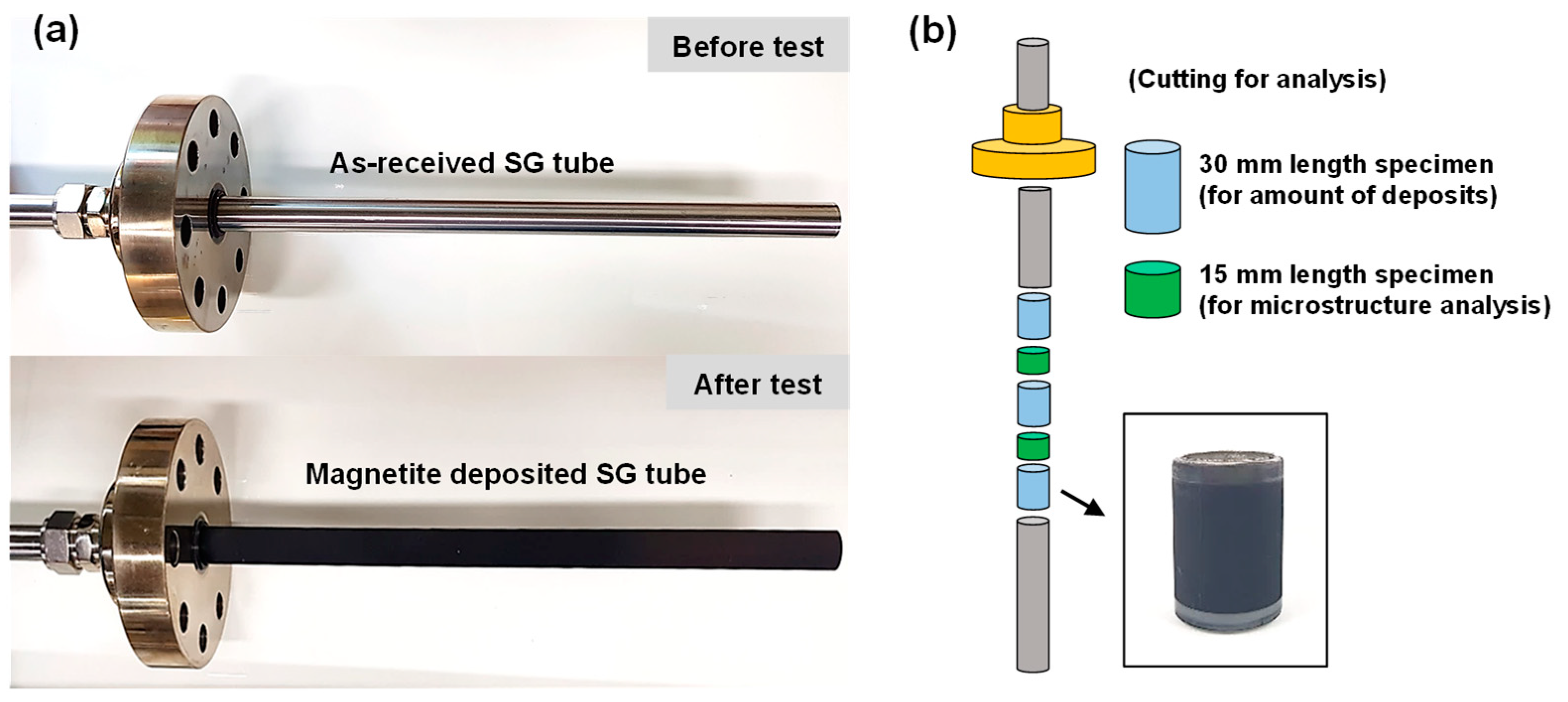
2.1. Tube Specimen Preparation
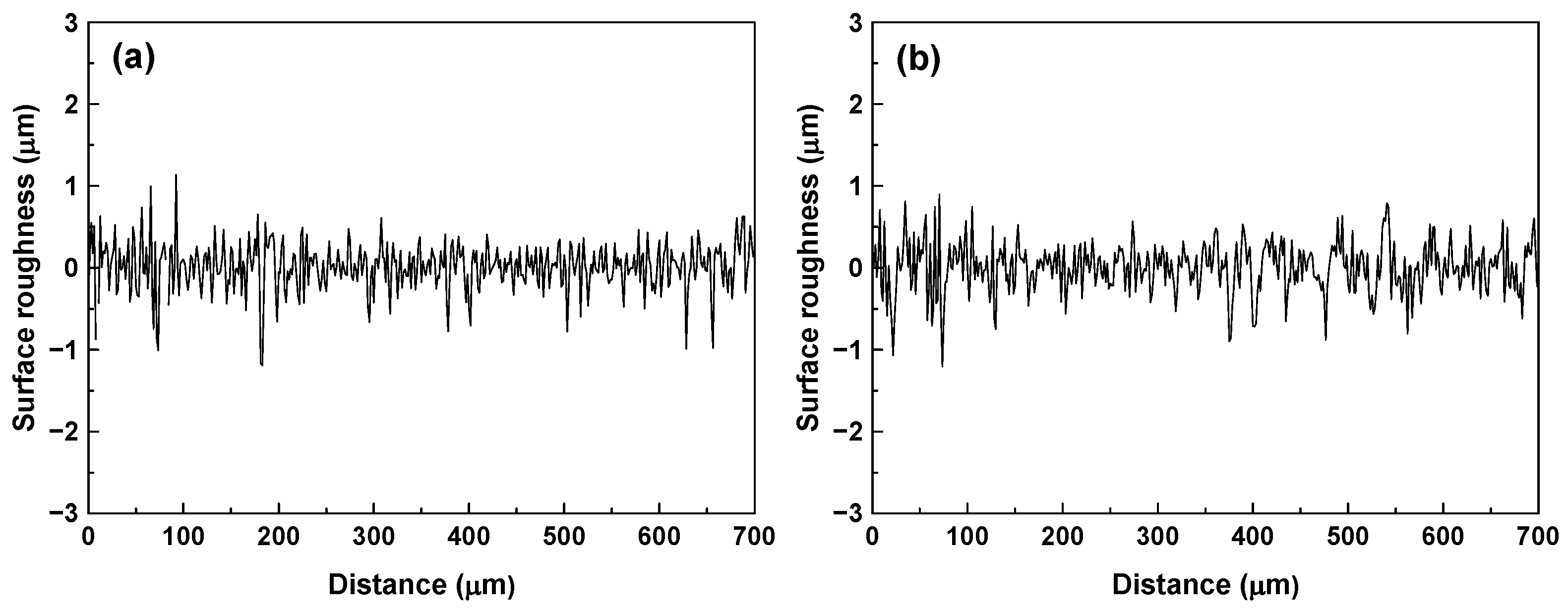
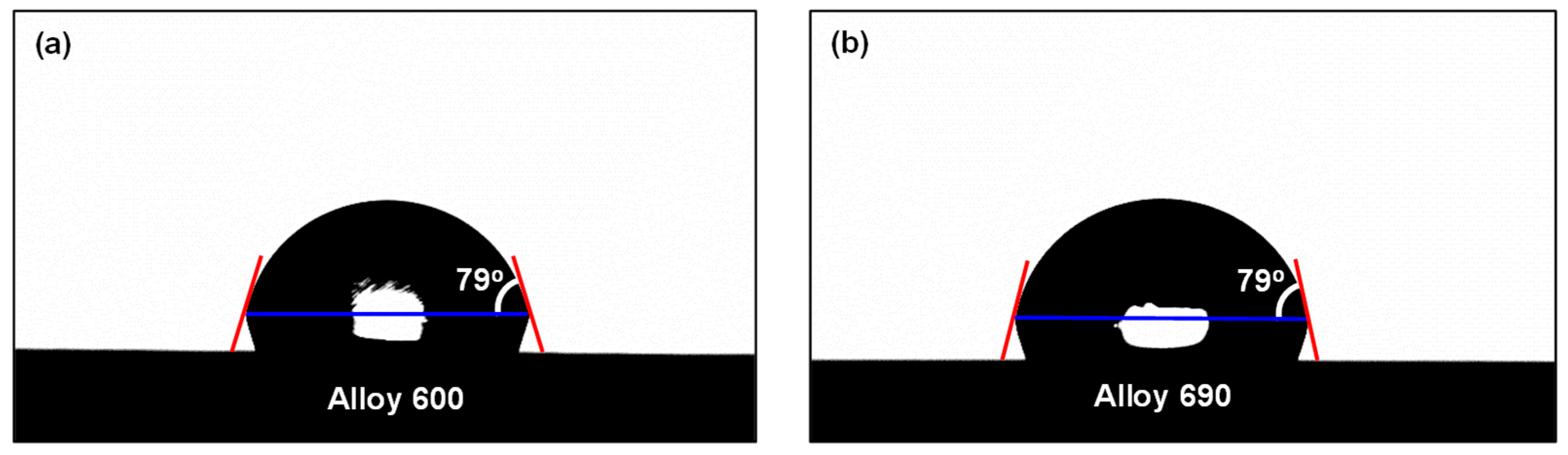
2.2. Surface Characterization of Specimens
2.3. Zeta Potential Measurements
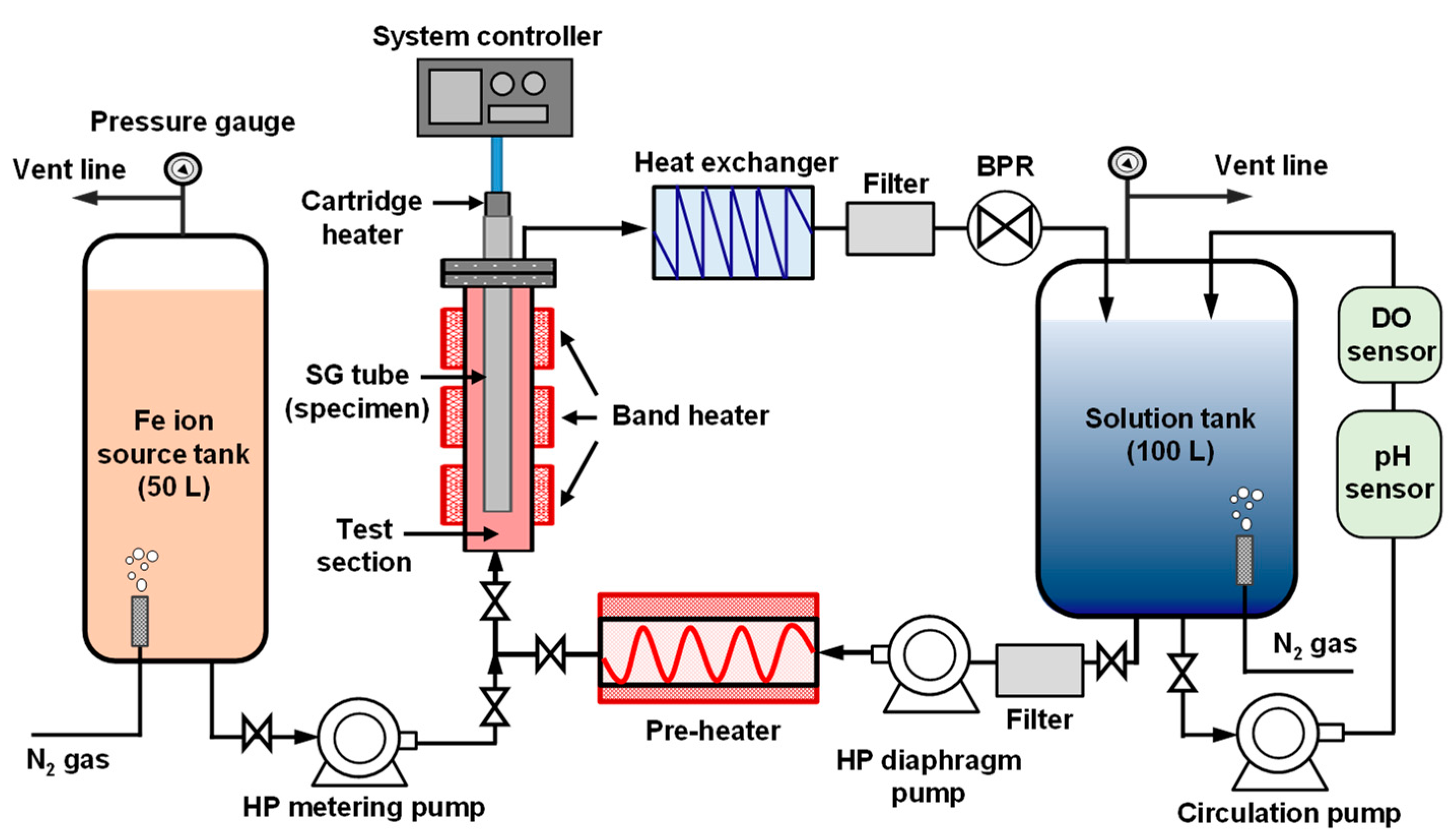
2.4. Magnetite Deposition Loop System
2.5. Microstructural Analysis and Amount of Magnetite Deposits
3. Results and Discussion
3.1. Surface Characteristics of SG Tubes before Deposition Tests
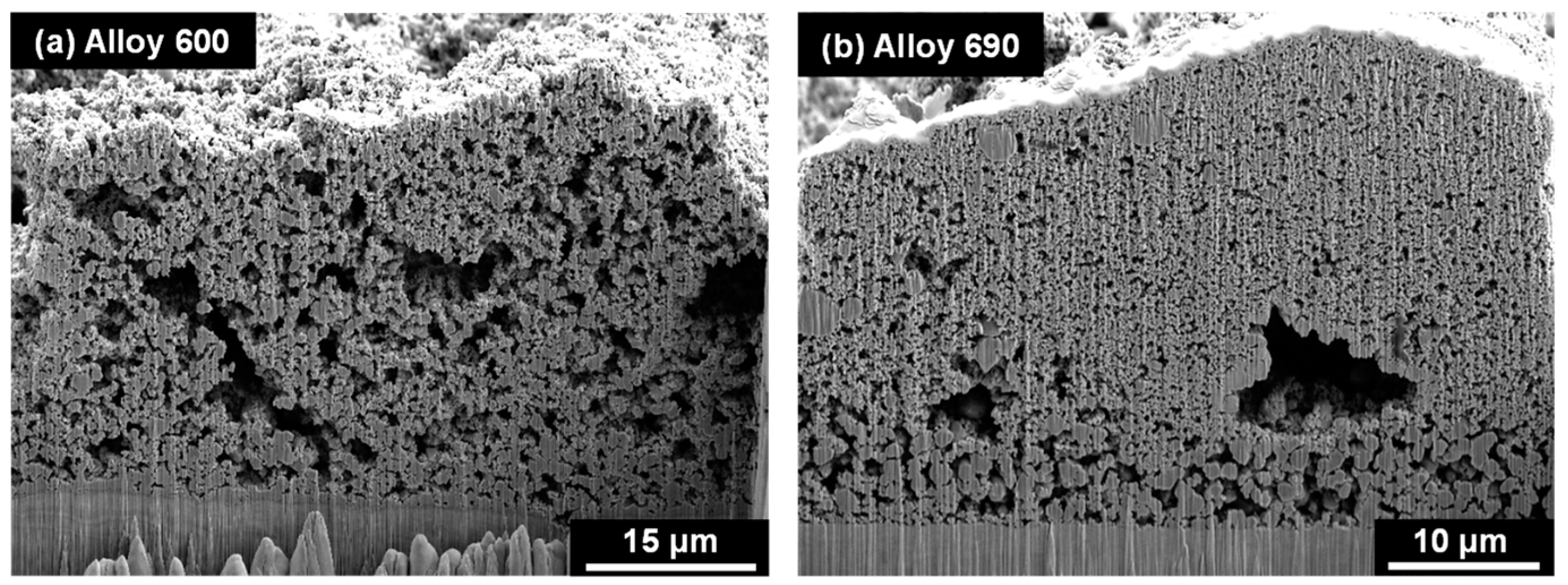
3.2. Characterization of Deposits on SG Tubes after Deposition Tests
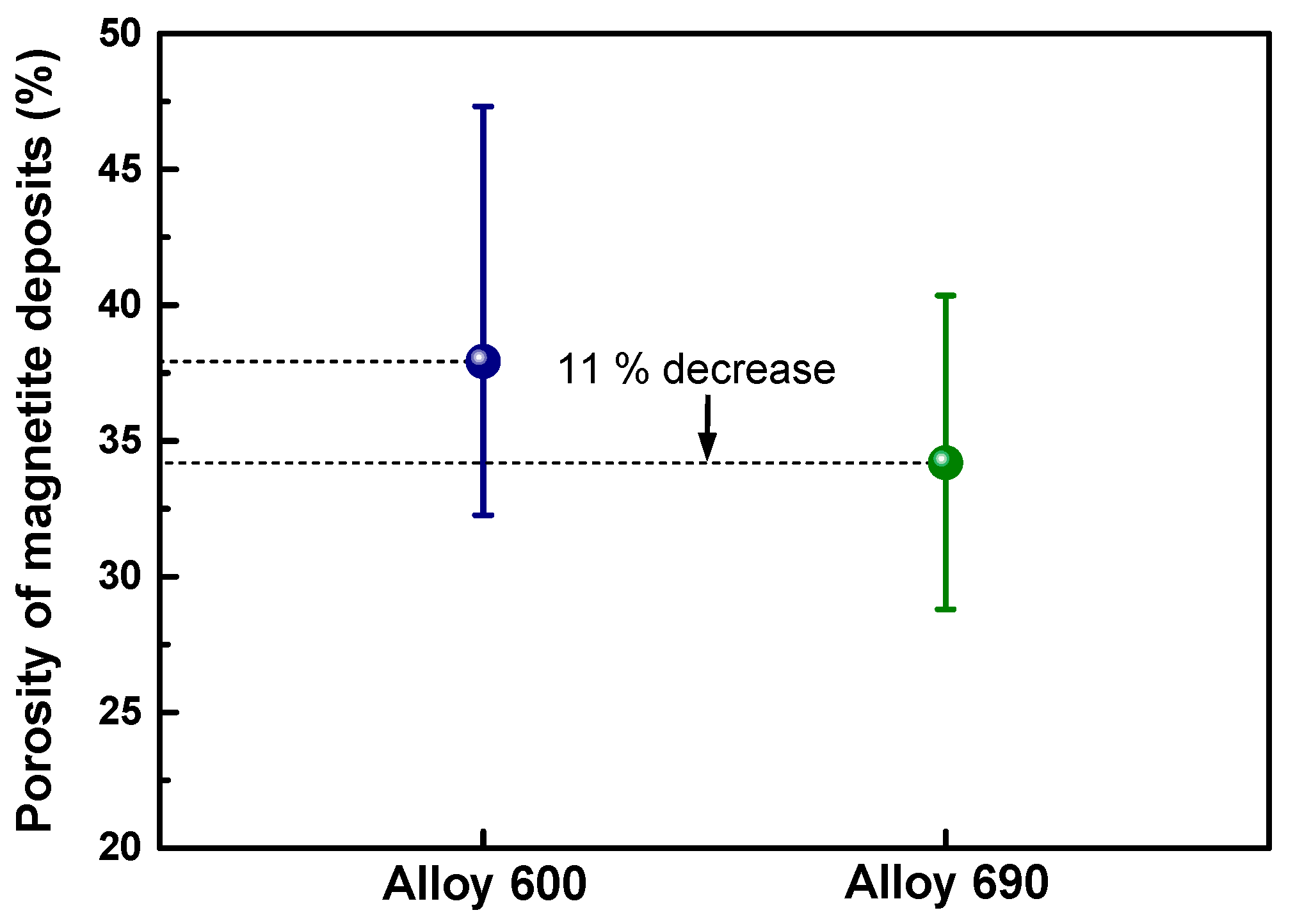
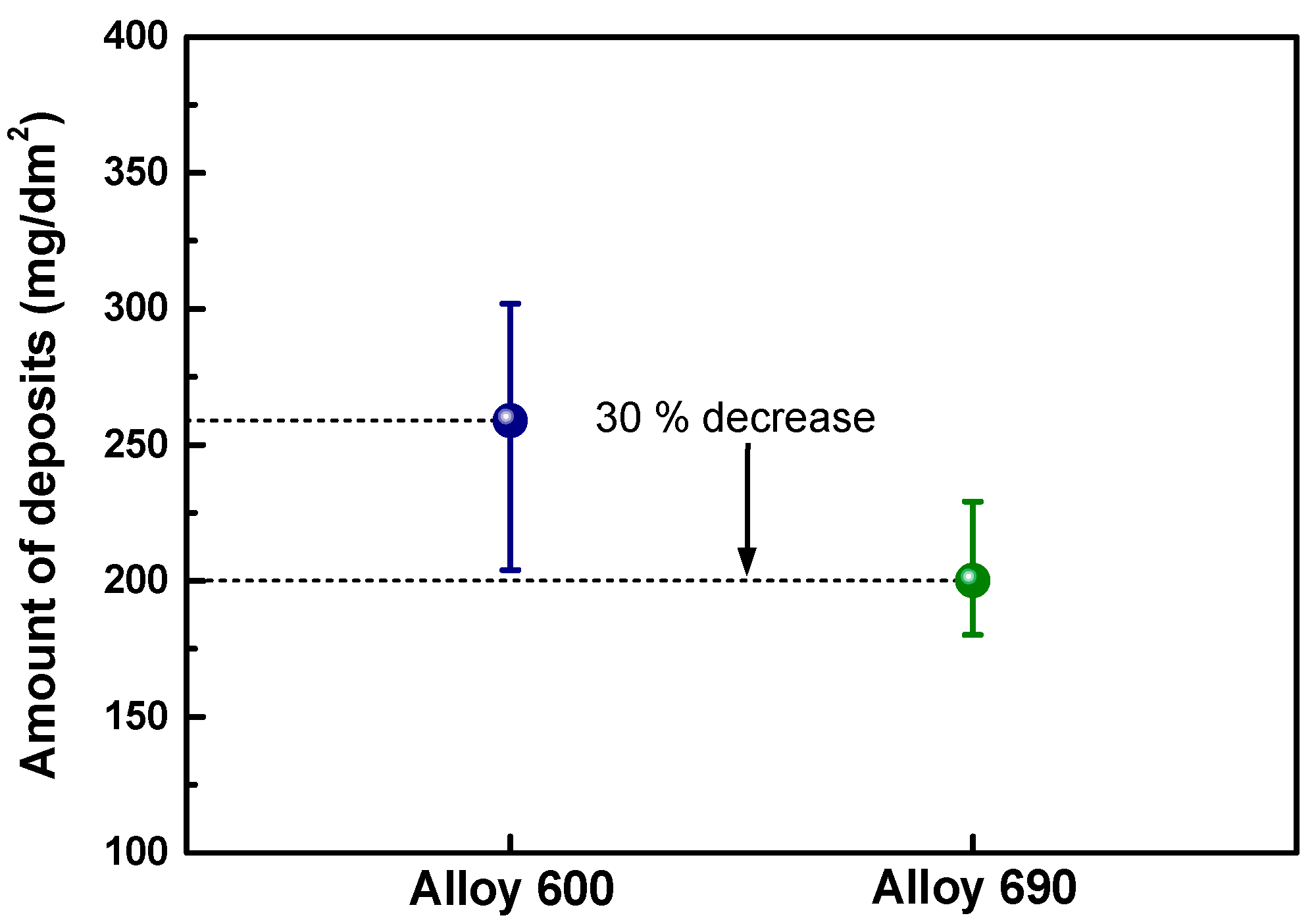
3.3. Amount of Magnetite Deposits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prince, A.A.M.; Velmurugan, S.; Narasimhan, S.V.; Ramesh, C.; Murugesan, N.; Raghavan, P.S.; Gopalan, R.J. Dissolution behaviour of magnetite film formed over carbon steel in dilute organic acid media. J. Nucl. Mater. 2001, 289, 281–290. [Google Scholar] [CrossRef]
- Ramesh, C.; Murugesan, N.; Prince, A.A.M.; Velmurugan, S.; Narasimhan, S.V.; Ganesan, V. Applied of polymer electrolyte based hydrogen sensor to study corrosion of carbon steel in acid medium. Corros. Sci. 2001, 43, 1865–1875. [Google Scholar] [CrossRef]
- Varrin, R.D., Jr. Deposit Accumulation in PWR Steam Generators. In Steam Generators for Nuclear Power Plants; Woodhead Publishing: Cambridge, UK, 2017; Chapter 11; pp. 323–363. [Google Scholar]
- Turner, C.W.; Khumsa-Ang, K. Corrosion Product Transport and Fouling in Nuclear Steam Generators. In Steam Generators for Nuclear Power Plants; Woodhead Publishing: Cambridge, UK, 2017; Chapter 9; pp. 215–271. [Google Scholar]
- Jeon, S.-H.; Hong, S.; Kwon, H.-C.; Hur, D.H. Characteristics of steam generator tube deposits in an operating pressurized water reactor. J. Nucl. Mater. 2018, 507, 371–380. [Google Scholar] [CrossRef]
- Varrin, R.D., Jr. Characterization of PWR Steam Generator Deposits; EPRI Report TR-106048; EPRI: Palo Alto, CA, USA, 1996. [Google Scholar]
- Klimas, S.J.; Miller, D.G.; Semmler, J.; Turner, C.W. The effect of the removal of steam generator tube ID deposits on heat transfer. In Proceedings of the 3th International Heat Exchanger and Steam Generator Conference, AECL, Toronto, ON, Canada, 21–24 June 1998; p. 11985. [Google Scholar]
- Turner, C.W.; Klimas, S.J.; Brideau, M.G. Thermal resistance of steam-generator tube deposits under single-phase forced convection and flow-boiling heat transfer. Can. J. Chem. Eng. 2000, 78, 53–60. [Google Scholar] [CrossRef]
- Gonzalez, F.; Spekkens, P. Corrosion of Inconel 600 under steam generator sludge piles. In Proceedings of the 4th International Symposium on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Jekyll Island, GA, USA, 6–10 August 1989; pp. 6–10. [Google Scholar]
- Paine, J.P.N.; Hobart, S.A.; Sawochka, S.G. Predicting steam generator crevice chemistry. In Proceedings of the 5th International Symposium on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Monterey, CA, USA, 25–29 August 1991; pp. 739–744. [Google Scholar]
- Millet, P.J.; Fenton, J.M. A detailed model of localized concentration processes in porous deposits of SGs. In Proceedings of the 5th International Symposium on Environmental Degradation of Materials in Nuclear Power Systems-Water Reactors, Monterey, CA, USA, 25–29 August 1991; pp. 745–751. [Google Scholar]
- Jeon, S.-H.; Song, G.D.; Hur, D.H. Micro-galvanic corrosion of steam generator materials within pores of magnetite flakes in alkaline solutions. Metals 2018, 8, 899. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Pointeau, E.; Tevissen, E.; Chagnes, A. A review on clogging of recirculating steam generators in pressurized-water reactors. Prog. Nucl. Energy 2017, 97, 182–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-M.; Jeon, S.-H.; Kim, K.-S.; Han, J.O.; Hur, D.H. Effects of pH control agents on magnetite deposition on steam generator tubes. Ann. Nucl. Energy 2020, 143, 729. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Lee, J.-M.; Hur, D.H.; Lee, J.-H.; Jeon, S.-H. Effects of advanced amines on magnetite deposition of steam generator tubes in secondary system. Coatings 2021, 11, 514. [Google Scholar] [CrossRef]
- Lee, J.-M.; Jeon, S.-H.; Han, J.O.; Hur, D.H. Effects of NaCl on magnetite deposition on the outer surfaces of steam generator tubes. Ann. Nucl. Energy 2021, 151, 107886. [Google Scholar] [CrossRef]
- Wolfe, R. Steam Generator Management Program: Steam Generator Deposit Characterization Sourcebook; EPRI Report TR-3002002794; EPRI: Palo Alto, CA, USA, 2014. [Google Scholar]
- ISO 4287; Geometrical Product Specifications (GPS)-Surface Texture: Profile Method-Terms, Definitions and Surface Texture Parameters. International Organization for Standardization (ISO): Geneva, Switzerland, 1997.
- ISO 11562; Geometrical Product Specifications (GPS)-Surface Texture: Profile Method-Metrological Characteristics of Phase Correct Filters. International Organization for Standardization (ISO): Geneva, Switzerland, 1996.
- Kaszuba, M.; Corbett, J.; Watson, F.M.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [Green Version]
- Levitin, E.Y.; Kokodiy, N.G.; Timanjuk, V.A.; Vedernikova, I.O.; Chan, T.M. Measurement of the size and refractive index of Fe3O4 nanoparticles. Inorg. Meter. 2014, 50, 817–820. [Google Scholar] [CrossRef]
- Tang, L.; Casas, J.; Venkataramasubramani, M. Magnetic nanoparticle mediated enhancement of localized surface plasmon resonance for ultrasensitive bioanalytical assay in human blood plasma. Anal. Chem. 2013, 85, 1431–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite nanoparticles: Synthesis and application in optics and nanoparticles. Materials 2022, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-S.; Park, M.-S.; Baek, S.H.; Hur, D.H. Effect of aluminum oxide coated on fuel cladding surface on crud deposition in simulated PWR primary water. Ann. Nucl. Energy 2018, 121, 607–614. [Google Scholar] [CrossRef]
- Fruzzetti, K. Pressurized Water Reactor Secondary Water Chemistry Guidelines-Revision 8; EPRI Report 3002010645; EPRI: Palo Alto, CA, USA, 2017. [Google Scholar]
- Wolfe, R. Steam Generator Management Program: Steam Generator Deposit Removal Strategies Sourcebook; EPRI TR-30020050902015; EPRI: Palo Alto, CA, USA, 2015. [Google Scholar]
- Helyer, M.L.; Glaves, C.L. Chemical Cleaning of PWR Steam Generator Sludge Piles; NP-4708; EPRI: Palo Alto, CA, USA, 1986. [Google Scholar]
- Forch, H.; Oliver, T.; Hertle, W. Chemical cleaning of PWR steam generators with a low temperature process. Nucl. Eng. Des. 1993, 147, 115–118. [Google Scholar] [CrossRef]
- Puzzuoli, F.V.; Leinonen, P.J.; Lowe, G.A.; Murchie, B. Steam generator cleaning campaigns at Bruce A: 1993–1996. In Proceedings of the 4th International Conference on CANDU Maintenance, Toronto, ON, Canada, 16–18 November 1997. [Google Scholar]
- Lee, H.C.; Sung, K.B. Analysis of Chemical cleaning for the Top of Tubesheet of NPP’s steam generator. J. Korean Acad.—Ind. Coop. Soc. 2013, 14, 2043–2048. [Google Scholar]
- Han, J.; Kim, S.J.; Lee, Y.K.; Hur, D.H. Chemical cleaning of magnetite deposits on the flow mini-channels of printed circuit heat exchanger in an EDTA-based solution. Materials 2022, 15, 1471. [Google Scholar] [CrossRef]
- Jone, B.J.; McHale, J.P.; Garimella, S.V. The influence of surface roughness on nucleate pool boiling heat transfer. J. Heat Transf. 2009, 131, 121009. [Google Scholar] [CrossRef] [Green Version]
- McHale, J.P.; Garimella, S.V. Bubble nucleation characteristics in pool boiling of a wetting liquid on smooth and rough surfaces. Int. J. Multiph. Flow 2010, 36, 249–260. [Google Scholar] [CrossRef] [Green Version]
- Alam, T.; Lee, P.S.; Yap, C.R. Effects of surface roughness on flow boiling in silicon microgap heat sinks. Int. J. Heat Mass Transf. 2013, 64, 28–41. [Google Scholar] [CrossRef]
- Cheedarala, R.K.; Park, E.; Kong, K.; Park, Y.B.; Park, H.W. Experimental study on critical heat flux of highly efficient soft hydrophilic CuO-chitosan nanofluid templates. Int. J. Heat Mass Transf. 2016, 100, 396–406. [Google Scholar] [CrossRef]
- Harada, T.; Nagakura, H.; Okawa, T. Dependence of bubble behavior in subcooled boiling on surface wettability. Nucl. Eng. Des. 2010, 240, 3949–3955. [Google Scholar] [CrossRef]
- Yang, L.X.; Chao, Y.M.; Jia, L.; Li, C.B. Wettability and boiling heat transfer study of black silicon surface produced using the plasma immersion ion implantation method. Appl. Therm. Eng. 2016, 99, 253–261. [Google Scholar] [CrossRef]
- Park, M.-S.; Shim, H.-S.; Baek, S.H.; Kim, J.G.; Hur, D.H. Effects of oxidation states of fuel cladding surface on crud deposition in simulated primary water of PWRs. Ann. Nucl. Energy 2017, 103, 275–281. [Google Scholar] [CrossRef]
- Baek, S.H.; Shim, H.-S.; Kim, J.G.; Hur, D.H. Effect of chemical etching of fuel cladding surface on crud deposition behavior in simulated primary water of PWRs at 328 °C. Ann. Nucl. Energy 2018, 116, 69–77. [Google Scholar] [CrossRef]
- Baek, S.H.; Shim, H.-S.; Kim, J.G.; Hur, D.H. Effects of heat flux on fuel crud deposition and sub-cooled nucleate boiling in simulated PWR primary water at 13 MPa. Ann. Nucl. Energy 2019, 133, 178–185. [Google Scholar] [CrossRef]
- Shields, K.J. State-of-Knowledge on Deposition Part 1: Parameters Influencing Deposition in Fossil Units; EPRI Report 1004194; EPRI: Palo Alto, CA, USA, 2002. [Google Scholar]
- Smith, D.S.; Alzina, A.; Bourret, J.; Nait-Ali, B.; Pennec, F.; Tessier-Doyen, N.; Otsu, K.; Matsubara, H.; Elser, P.; Gonzenbach, U.T. Thermal conductivity of porous materials. J. Mater. Res. 2013, 28, 2260–2272. [Google Scholar] [CrossRef] [Green Version]
- Song, G.D.; Jeon, S.H.; Kim, J.G.; Hur, D.H. Synergistic effect of chloride ions and magnetite on the corrosion of Alloy 690 in alkaline solutions. Corros. Sci. 2017, 131, 71–80. [Google Scholar] [CrossRef]
- Xia, D.H.; Behnamian, Y.; Luo, J.L. Review—Factors influencing sulfur induced corrosion on the secondary side in pressurized water reactors (PWRs). J. Electrochem. Soc. 2019, 166, 49–64. [Google Scholar] [CrossRef]
- Fruzzetti, K. Multivariable Assessment of Flow Accelerated Corrosion and Steam Generator Fouling, Literature Review; EPRI Report 1003619; EPRI: Palo Alto, CA, USA, 2003. [Google Scholar]
- Iwahori, T. Role of surface chemistry in crud deposition on heat transfer surface. Corrosion 1979, 35, 345–350. [Google Scholar] [CrossRef]
- Chang, S.H.; Bang, I.C.; Baek, W.P. A photographic study on the near-wall bubble behavior in subcooled flow boiling. Int. J. Therm. Sci. 2002, 41, 609–618. [Google Scholar] [CrossRef]
- Gaertner, R.F. Photographic study of nucleate pool boiling on a horizontal surface. ASEM J. Heat Transf. 1965, 17, 17–29. [Google Scholar] [CrossRef]
- Sakurai, A.; Shiotsu, M.; Hata, K.; Fukuda, K. Photographic study on transitions from non-boiling and nucleate boiling regime to film boiling due to increasing heat inputs in liquid nitrogen and water. Nucl. Eng. Des. 2000, 200, 39–54. [Google Scholar] [CrossRef]
- Nishikawa, K.; Fujita, Y.; Uchida, S.; Ohta, H. Effect of surface configuration on nucleate boiling heat transfer. Int. J. Heat Mass Trans. 1984, 27, 1559–1571. [Google Scholar]
- Ryu, W.S.; Park, D.G.; Song, U.S.; Park, J.S.; Ahn, S.B. Effects of irradiation on thermal conductivity of Alloy 690 at low neutron fluence. Nucl. Eng. Technol. 2013, 45, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, S.S.; Kim, D.W. Production Method for Alloy 690 Ordered Alloy of Improved Thermal Conductivity, and Alloy 690 Ordered Alloy Produced Thereby. U.S. Patent 20160145730A1, 26 May 2016. [Google Scholar]
- Essi, J.; Konsta, S.; Timo, S. Determining Zeta Potential of Magnetite Particles in PWR Secondary Side Water Treated with Ammonia or Ethanolamine by Using Streaming Potential Technique. In Proceedings of the 20th NPC International Conference, Brighton, UK, 2–7 October 2016; p. 151. [Google Scholar]
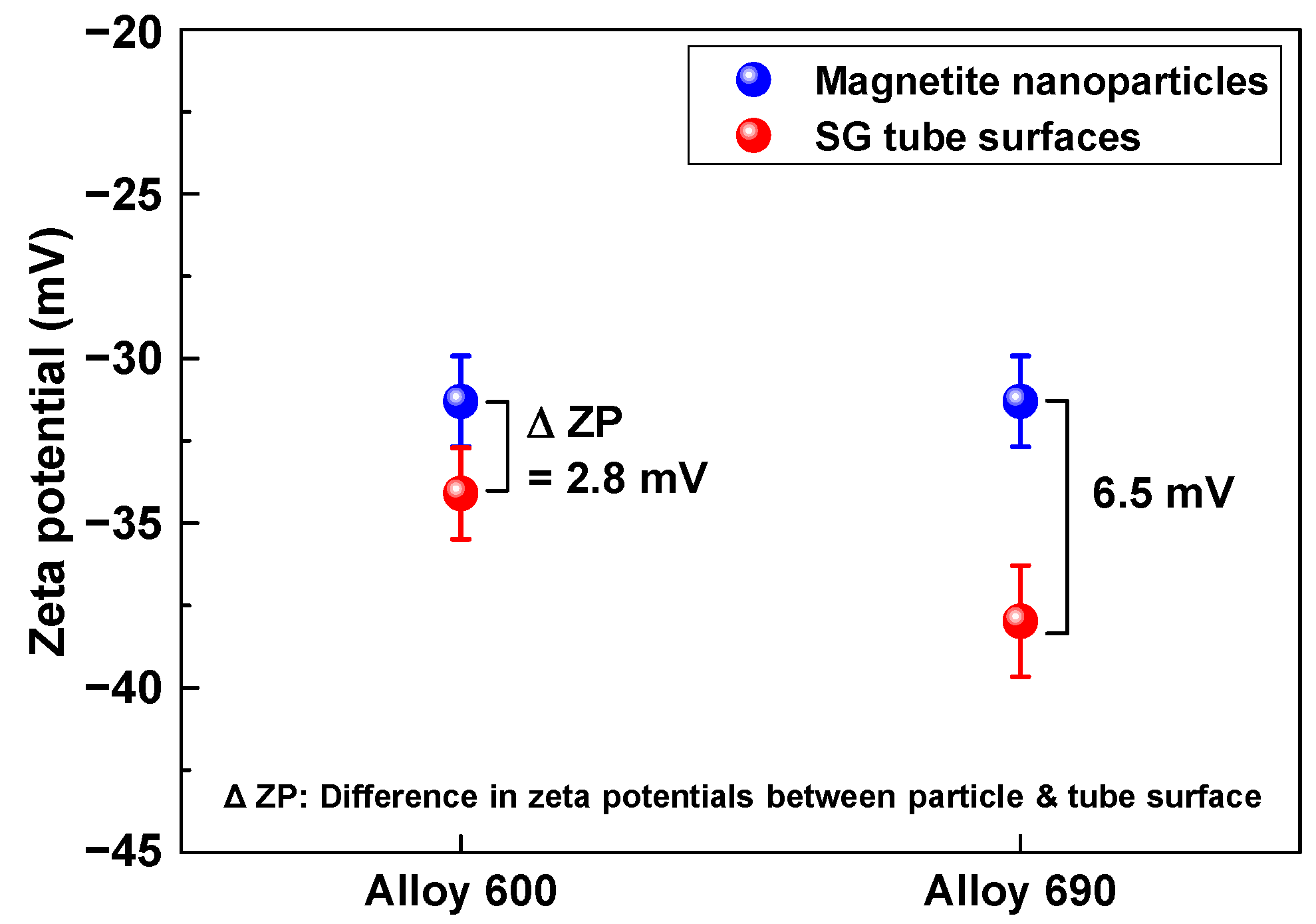

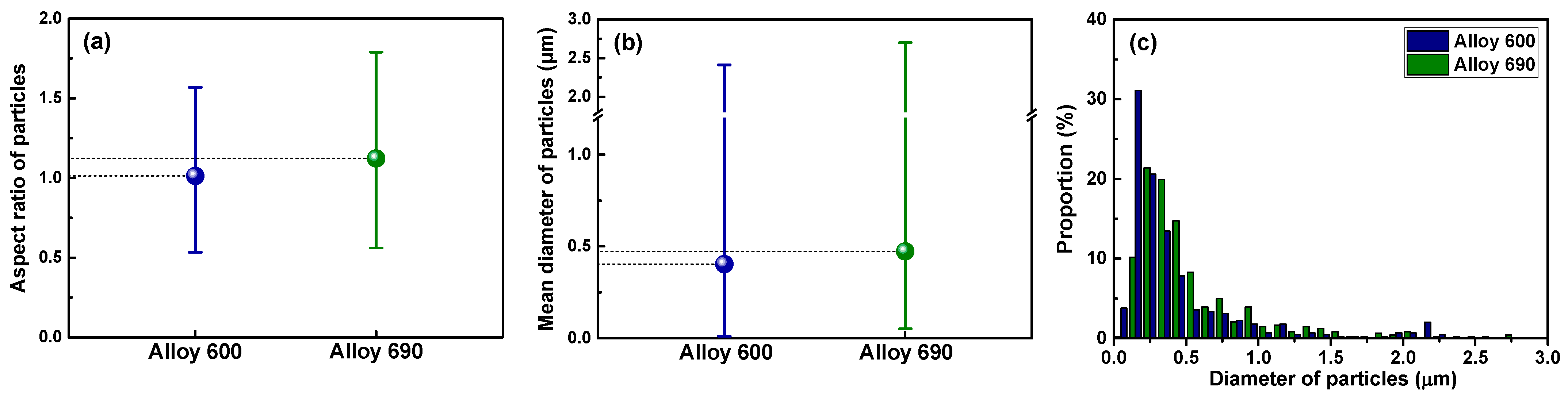
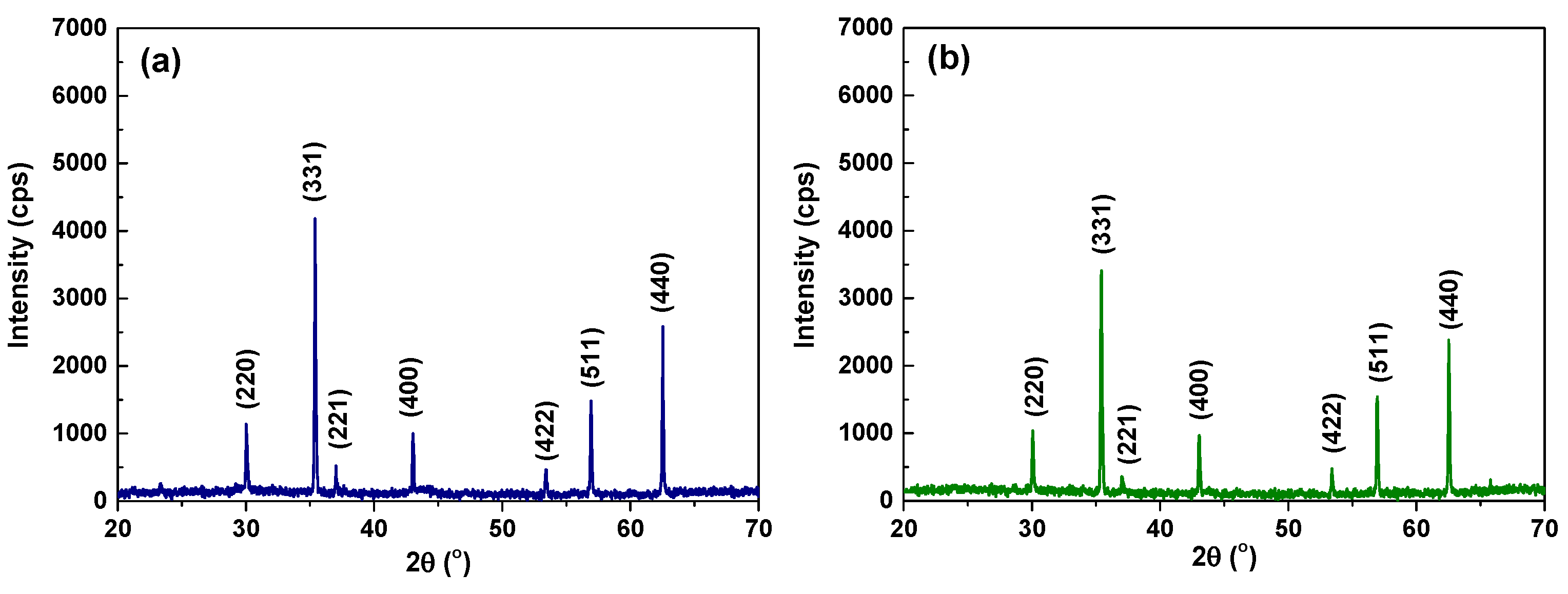
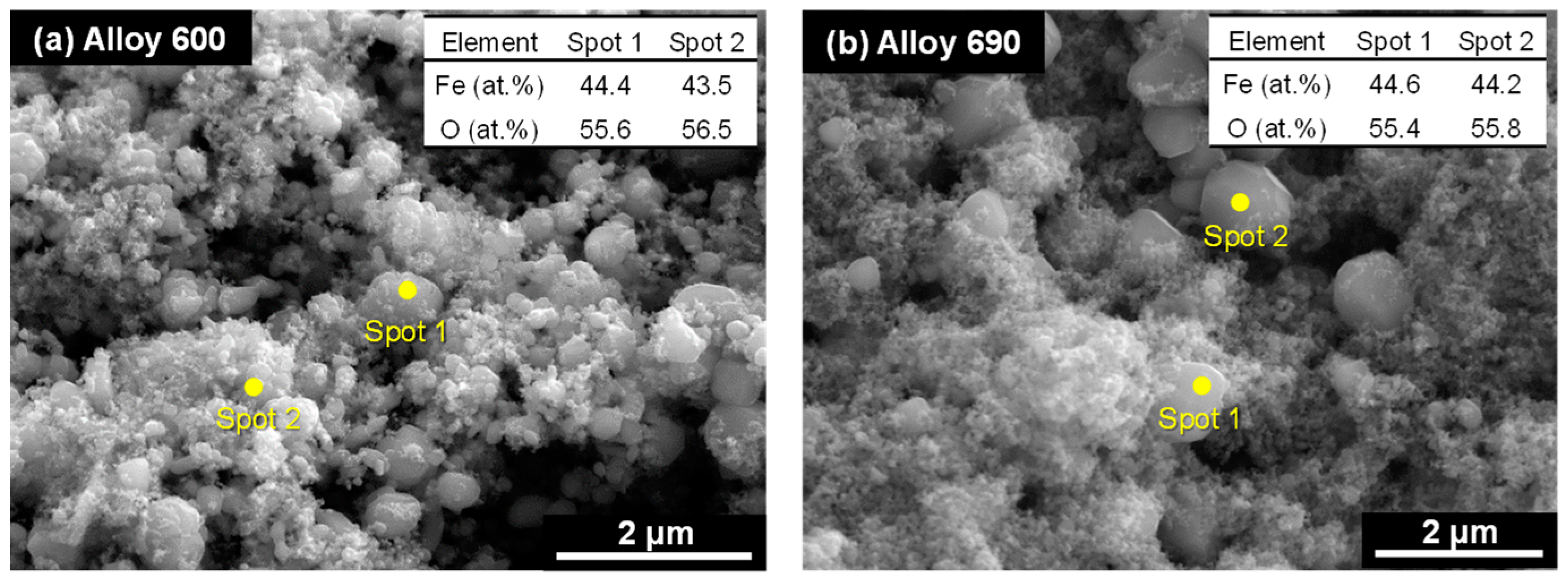
| Tubes | Ni | Cr | Fe | C | Si | Mn | Ti | Al | Cu | Co |
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy 600 | 76.01 | 15.20 | 7.82 | 0.02 | 0.26 | 0.21 | 0.25 | 0.17 | 0.034 | 0.02 |
| Alloy 690 | 59.54 | 29.31 | 10.09 | 0.02 | 0.30 | 0.29 | 0.25 | 0.17 | <0.01 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-H.; Lee, Y.-B.; Lee, K.-H.; Hur, D.-H. Magnetite Deposition Behavior on Alloy 600 and Alloy 690 Tubes in Simulated PWR Secondary Water. Coatings 2022, 12, 1231. https://doi.org/10.3390/coatings12091231
Jeon S-H, Lee Y-B, Lee K-H, Hur D-H. Magnetite Deposition Behavior on Alloy 600 and Alloy 690 Tubes in Simulated PWR Secondary Water. Coatings. 2022; 12(9):1231. https://doi.org/10.3390/coatings12091231
Chicago/Turabian StyleJeon, Soon-Hyeok, Yong-Beom Lee, Kyung-Hee Lee, and Do-Haeng Hur. 2022. "Magnetite Deposition Behavior on Alloy 600 and Alloy 690 Tubes in Simulated PWR Secondary Water" Coatings 12, no. 9: 1231. https://doi.org/10.3390/coatings12091231
APA StyleJeon, S.-H., Lee, Y.-B., Lee, K.-H., & Hur, D.-H. (2022). Magnetite Deposition Behavior on Alloy 600 and Alloy 690 Tubes in Simulated PWR Secondary Water. Coatings, 12(9), 1231. https://doi.org/10.3390/coatings12091231







