Calcium Sulfate in Implantology (Biphasic Calcium Sul-Fate/Hydroxyapatite, BCS/HA, Bond Apatite®): Review of the Literature and Case Reports
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Selection of Studies
3.2. Study Design
3.3. Characteristics of the Participants
3.4. Characteristics and Results of the Studies
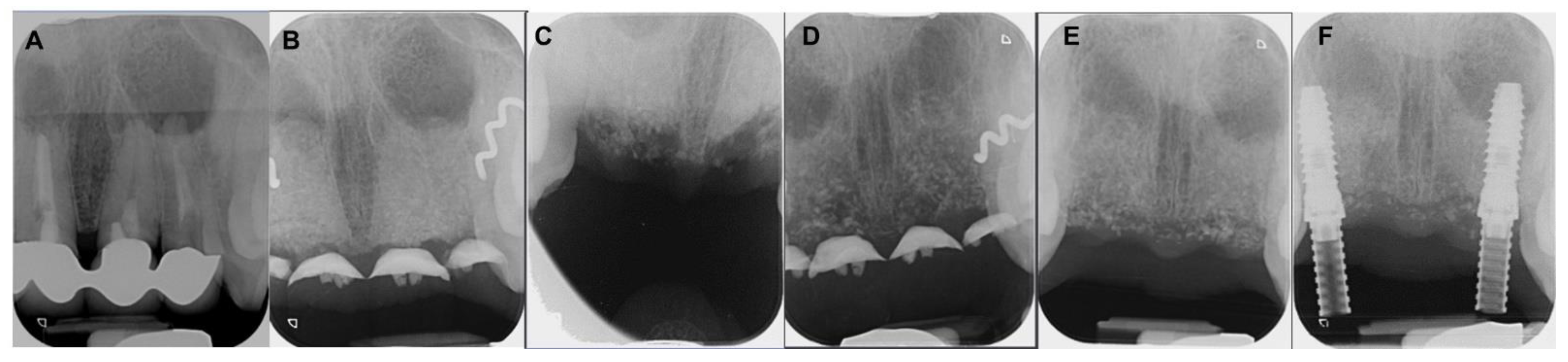
4. Clinical Cases
4.1. Patient No. 1
4.2. Patient No. 2
4.3. Patient No. 3
4.4. Patient No.4
4.5. Patient No.5
4.6. Patient No.6
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrokovski, J.; Massler, M. Alveolar ridge resorption following tooth extraction. J. Prosthet. Dent. 1967, 17, 21–27. [Google Scholar] [CrossRef]
- Youngson, C. Summary of: The influence of specialty training, experience, discussion and reflection on decision making in modern restorative treatment planning. Br. Dent. J. 2011, 210, 164–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irinakis, T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J. Can. Dent. Assoc. 2006, 72, 917–922. [Google Scholar] [PubMed]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: A clinical and histologic study in humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Turri, A.; Dahlin, C. Comparative maxillary bone-defect healing by calcium-sulphate or deproteinized bovine bone particles and extra cellular matrix membranes in a guided bone regeneration setting: An experimental study in rabbits. Clin. Oral Implants Res. 2015, 26, 501–506. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef]
- Chau, A.M.T.; Mobbs, R.J. Bone graft substitutes in anterior cervical discectomy and fusion. Eur. Spine J. 2009, 18, 449–464. [Google Scholar] [CrossRef]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Raghoebar, G.M.; Louwerse, C.; Kalk, W.W.I.; Vissink, A. Morbidity of chin bone harvesting. Clin. Oral Implants Res. 2001, 12, 503–507. [Google Scholar] [CrossRef]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Baranes, D.; Kurtzman, G.M. Biphasic Calcium Sulfate as an Alternative Grafting Material in Various Dental Applications. J. Oral Implant. 2019, 45, 247–255. [Google Scholar] [CrossRef]
- Peltier, L.F.; Bickel, E.Y.; Lillo, R.; Thein, M.S. The Use of Plaster of Paris to Fill Defects in Bone. Ann. Surg. 1957, 146, 61–69. [Google Scholar] [CrossRef]
- Thomas, M.V.; Puleo, D.A. Calcium sulfate: Properties and clinical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 597–610. [Google Scholar] [CrossRef]
- Walsh, W.R.; Morberg, P.; Yu, Y.; Yang, J.L.; Haggard, W.; Sheath, P.C.; Svehla, M.; Bruce, W.J. Response of a calcium sulfate bone graft substitute in a confined cancellous defect. Clin. Orthop. Relat. Res. 2003, 406, 228–236. [Google Scholar] [CrossRef]
- Crespi, R.; Capparé, P.; Gherlone, E. Magnesium-Enriched Hydroxyapatite Compared to Calcium Sulfate in the Healing of Human Extraction Sockets: Radiographic and Histomorphometric Evaluation at 3 Months. J. Periodontol. 2009, 80, 210–218. [Google Scholar] [CrossRef]
- Gitelis, S.; Piasecki, P.; Turner, T.; Haggard, W.; Charters, J.; Urban, R. Use of a calcium sulfate-based bone graft substitute for benign bone lesions. Orthopedics 2001, 24, 162–166. [Google Scholar] [CrossRef]
- Deliberador, T.M.; Nagata, M.J.; Furlaneto, F.A.; Melo, L.G.; Okamoto, T.; Sundefeld, M.L.; Fucini, S.E. Autogenous Bone Graft with or Without a Calcium Sulfate Barrier in the Treatment of Class II Furcation Defects: A Histologic and Histometric Study in Dogs. J. Periodontol. 2006, 77, 780–789. [Google Scholar] [CrossRef]
- Mayer, Y.; Zigdon-Giladi, H.; Machtei, E.E. Ridge Preservation Using Composite Alloplastic Materials: A Randomized Control Clinical and Histological Study in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 1163–1170. [Google Scholar] [CrossRef]
- Machtei, E.E.; Rozitsky, D.; Zigdon-Giladi, H.; Levin, L. Bone preservation in dehiscence-type defects using composite biphasic calcium sulfate plus biphasic hydroxyapatite/β-tricalcium phosphate graft: A histomorphometric case series in canine mandible. Implant Dent. 2013, 22, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.E.; Zou, Y.; Dziubla, T.D.; Puleo, D.A. Effects of composition and setting environment on mechanical properties of a composite bone filler. J. Biomed. Mater. Res. A 2013, 101, 973–980. [Google Scholar] [CrossRef]
- Wang, L.; Barbieri, D.; Zhou, H.; de Bruijn, J.D.; Bao, C.; Yuan, H. Effect of particle size on osteoinductive potential of microstructured biphasic calcium phosphate ceramic. J. Biomed. Mater. Res. Part A 2014, 103, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Yahav, A.; Kurtzman, G.M.; Katzap, M.; Dudek, D.; Baranes, D. Bone Regeneration: Properties and Clinical Applications of Biphasic Calcium Sulfate. Dent. Clin. N. Am. 2020, 64, 453–472. [Google Scholar] [CrossRef]
- Pecora, G.; Andreana, S.; Margarone, J.E., 3rd; Covani, U.; Sottosanti, J.S. Bone regeneration with a calcium sulfate barrier. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 424–429. [Google Scholar] [CrossRef]
- Strocchi, R.; Orsini, G.; Iezzi, G.; Scarano, A.; Rubini, C.; Pecora, G.; Piattelli, A. Bone regeneration with calcium sulfate: Evidence for increased angiogenesis in rabbits. J. Oral Implantol. 2002, 28, 273–278. [Google Scholar] [CrossRef]
- Ebell, M.H.; Siwek, J.; Weiss, B.D.; Woolf, S.H.; Susman, J.; Ewigman, B.; Bowman, M. Strength of recommendation taxonomy (SORT): A patient-centered approach to grading evidence in the medical literature. Am. Fam. Physician 2004, 69, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.; Sharma, A.; Jain, A.; Bali, D.; Malik, R.; Gugnani, S. The use of nanocrystalline and two other forms of calcium sulfate in the treatment of infrabony defects: A clinical and radiographic study. J. Indian Soc. Periodontol. 2015, 19, 545–553. [Google Scholar]
- Mandlik, V.; Roy, S.; Jha, A. Comparative evaluation of bioglass with calcium sulphate β-hemihydrate for the treatment of intraosseous defects—A clinico-radiological study. Med. J. Armed Forces India 2012, 68, 42–47. [Google Scholar] [CrossRef]
- Machtei, E.E.; Mayer, Y.; Horwitz, J.; Zigdon-Giladi, H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: Clinical and histological findings. Clin. Implant Dent. Relat. Res. 2018, 21, 14–20. [Google Scholar] [CrossRef]
- Lindhe, J.; Araújo, M.G.; Bufler, M.; Liljenberg, B. Biphasic alloplastic graft used to preserve the dimension of the edentulous ridge: An experimental study in the dog. Clin Oral Implants Res. 2013, 24, 1158–1163. [Google Scholar] [CrossRef]
- A Horowitz, R.; Rohrer, M.D.; Prasad, H.S.; Tovar, N.; Mazor, Z. Enhancing extraction socket therapy with a biphasic calcium sulfate. Compend. Contin. Educ. Dent. 2012, 33, 420–426, 428. [Google Scholar]
- Dudek, D.; Reichmann-Warmusz, E.; Kurtzman, G.M.; Mahesh, L. The use of grafting material biphasic calcium sulfate for the treatment of osseous defects resulting from radicular cysts. Clinical study and six-month follow up. J. Osseointegr. 2020, 12, 716–721. [Google Scholar] [CrossRef]
- Laino, L.; Troiano, G.; Giannatempo, G.; Graziani, U.; Ciavarella, D.; Dioguardi, M.; Muzio, L.L.; Lauritano, F.; Cicciù, M. Sinus Lift Augmentation by Using Calcium Sulphate. A Retrospective 12 Months Radiographic Evaluation Over 25 Treated Italian Patients. Open Dent. J. 2015, 9, 414–419. [Google Scholar] [CrossRef]
- Peltier, L.F.; Jones, R.H. Treatment of unicameral bone cysts by curettage and packing with plaster-of-Paris pellets. J. Bone Jt. Surg. Am. 1978, 60, 820–822. [Google Scholar] [CrossRef]
- Mahesh, L.; A Salama, M.; Kurtzman, G.M.; Joachim, F.P.C. Socket grafting with calcium phosphosilicate alloplast putty: A histomorphometric evaluation. Compend. Contin. Educ. Dent. 2012, 33, e109–e115. [Google Scholar]
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50. [Google Scholar] [CrossRef]
- Trombelli, L.; Heitz-Mayfield, L.J.; Needleman, I.; Moles, D.; Scabbia, A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J. Clin. Periodontol. 2002, 29 (Suppl. 3), 117–135. [Google Scholar] [CrossRef]
- Ricci, J.; Alexander, H.; Nadkarni, P.; Hawkins, M.; Turner, J.; Rosenblum, S.; Brezenoff, L.; Deleonardis, D.; Pecora, G. Biological mechanisms of calcium sulfate replacement by bone. In Bone Engineering; Em2 Inc.: Mississauga, ON, Canada, 2000; pp. 332–344. [Google Scholar]
- Kadhim, D.R.; Hamad, T.I.; Fatalla, A.A. Use of Eggshells as Bone Grafts around Commercially Pure Titanium Implant Screws Coated with Nano Calcium Sulfate. Int. J. Biomater. 2022, 2022, 8722283. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The Efficacy of Bone Replacement Grafts in the Treatment of Periodontal Osseous Defects. A Systematic Review. Ann. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef]
- Guarnieri, R.; Grassi, R.; Ripari, M.; Pecora, G. Maxillary sinus augmentation using granular calcium sulfate (surgiplaster sinus): Radiographic and histologic study at 2 years. Int. J. Periodontics Restor. Dent. 2006, 26, 79–85. [Google Scholar]
- Kher, U.; Ioannou, A.L.; Kumar, T.; Siormpas, K.; Mitsias, M.E.; Mazor, Z.; Kotsakis, G.A. A clinical and radiographic case series of implants placed with the simplified minimally invasive antral membrane elevation technique in the poste-rior maxilla. J. Craniomaxillofac. Surg. 2014, 42, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Khoshkam, V. Guided bone regeneration for the reconstruction of alveolar bone defects. Ann. Maxillofac. Surg. 2017, 7, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Beitlitum, I.; Artzi, Z.; Nemcovsky, C.E. Clinical evaluation of particulate allogeneic with and without autogenous bone grafts and resorbable collagen membranes for bone augmentation of atrophic alveolar ridges. Clin. Oral Implants Res. 2010, 21, 1242–1250. [Google Scholar] [CrossRef]
- Sakka, S.; Coulthard, P. Bone Quality: A Reality for the Process of Osseointegration. Implant Dent. 2009, 18, 480–485. [Google Scholar] [CrossRef]
- Kinaia, B.M.; Shah, M.; Neely, A.L.; Goodis, H.E. Crestal Bone Level Changes Around Immediately Placed Implants: A Systematic Review and Meta-Analyses With at Least 12 Months’ Follow-Up After Functional Loading. J. Periodontol. 2014, 85, 1537–1548. [Google Scholar] [CrossRef]
























|
Author/Year Type of Study | Sample Size (n) Gender M/F Age | Type of Study | Results | Conclusions | |
|---|---|---|---|---|---|
| Pandit et al. [28] 2021 RCT | n: 16 20–64 years old Splitmouth Nanogen n: 15, Dentogen n: 15, BoneGen n: 15 | To evaluate the efficacy of calcium sulfate in the treatment of periodontal defects. Comparison of three materials Nanogen (NG), Dentogen (DG), and BoneGen (BG). | At 6 months Probing level reduction:NG 3.33 mm, DG 2.67 mm y BG 4 mm. Clinical insertion gain: NG 1.6 mm, DG 2.20 mm y BG 2.47 mm. Reduction of the periodontal defect: NG 2 mm, DG 2.07 mm, BG 2.07 mm. No statistically significant differences between groups. | Calcium sulfate is an effective material in the treatment of periodontal defects. | |
| Mandlik et al. [29] 2012 CT | n: 25 30–50 years old Splitmouth Group A n: 25, Group B n: 25 | To compare the efficacy of phosphosilicate (Group A) and calcium sulfate (Group B) in the treatment of periodontal defects. | At 9 months Probing level: Group A 7.52 ± 1.074 → 2.20 ± 0.040 mm Group B 7.20 ± 1.069 → 2.14 ± 0.351 mm Clinical insertion level: Group A 7.52 ± 1.0359 → 2.48 ± 0,614 mm Group B 7.20 ± 1.069 → 2.32 ± 0.471 Bone gain: Group A 58.93%/Group B 48.56% No statistically significant differences between groups. | No significant differences were observed between the two materials in terms of the efficacy of treating periodontal defects. | |
| Machtei et al. [30] 2018 RCT | n: 11 7M/4F 45–80 = 64 years old | To compare the dimensional changes and bone quality of calcium sulfate (BCS/HA) and bovine xenograft (BDX) in socket preservation cases. | At 4 months Bone height loss: BDX 0.25 mm, BCS/HA 0.65 mm, Control 1.7 mmBone width loss at −3 mm: BDX 1.56 ± 0.4 mm, BCS/HA 0.5 ± 0.4 mm Control 2.96 ± 0.3 mmNew bone formation: BDX 21,5%, BCS/HA 44.4% y Control 81,5%. Remaining graft material: BDX 44.18%, BCS/HA 16.51%. | Calcium sulfate can be used as the material of choice for socket preservation with similar and sometimes even better results than bovine xenograft. | |
| Mayer et al. [31] 2016 RCT | n: 36 13M/23F Splitmouth CS n: 14, Control n:15 | To evaluate the efficacy of calcium sulfate in cases of socket preservation. | At 4 months Bone height loss: CS 0.3 ± 2.01, Control 0.1 ± 2.03 Bone width loss at −3 mm: CS 0.03 ± 2.32 mm, Control 2.28 ± 2.36 mm Histopathological analysis: CS 47.7% bone, 36.3% connective tissue graft y 16% remaining graft material Control 52.6% bone y 46.7% connective tissue graft | Calcium sulfate is an effective material in socket preservation cases, providing better results than natural healing. | |
| Horowitz et al. [32] 2012 Case series | n: 40 | To evaluate the efficacy of calcium sulfate in cases of socket preservation. | At 4 months Bone volume and density were maintained. Calcium sulfate is completely reabsorbed, giving rise to new bone. | Calcium sulfate is an effective material in cases of socket preservation before implant placement. | |
| Dudek et al. [33] 2020 CT | CS n: 30 14M/16F 28–68 = 55.6 years old | Xenograft n: 30 14M/16F 27–65 = 61.1 years old | To evaluate the efficacy of calcium sulfate in the regeneration of maxillary bone defects after surgical removal of radicular cysts compared to the use of xenografts. | Calcium sulfate achieves faster bone remodeling than bovine xenograft. Virtually complete reabsorption of calcium sulfate and replacement by new bone at 3 months. | The use of calcium sulfate proved to be a simple, inexpensive, and effective reconstructive treatment of bone defects after the enucleation of odontogenic cysts. |
| Laino et al. [34] 2015 CT | n: 27 49–75 = 59 years old | To evaluate the efficacy of calcium sulfate in lateral window sinus lifts. | At 6 months Mean bone height before surgery: 4.04 ± 1.48 Mean bone height in regenerated sites: 12.25 ± 3.20 mm Mean bone height gained: 8.21 ± 1.73 mm | The use of calcium sulfate in lateral window sinus lifts is an effective procedure. | |
| Patient Gender Age | Medical History of Interest [Toxic Habits] Type of Surgery | Closure by First Intention [Collagen Sponge] | I.M. | I. C. | Healing | Early Postoperative Complications | Late Postoperative Complications |
|---|---|---|---|---|---|---|---|
| 1 F 63 | NO [Tobacco: 2 cig/day] Horizontal Guided Bone Regeneration | Yes [No] | G | No | G | No | No |
| 2 M 52 | NO [-] Alveolar ridge preservation | No [Yes] | G | No | M | Graft loss and self-limited alveolitis | No |
| 3 M 61 | NO [-] Alveolar ridge preservation | Yes [No] | G | No | G | No | No osseointegration of the implant, replacement in 3 months, without problems and with good stability |
| 4 F 46 | NO [-] Alveolar ridge preservation | No [Yes] | G | No | M | Graft loss and self-limited alveolitis | No |
| 5 M 64 | NO [-] Sinus lift with lateral window | Yes [No] | G | No | G | No | No |
| 6 M 46 | NO [-] Sinus lift with lateral window | Yes [No] | G | No | G | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrejon-Moya, A.; Apalimova, A.; González-Navarro, B.; Zaera-Le Gal, R.; Marí-Roig, A.; López-López, J. Calcium Sulfate in Implantology (Biphasic Calcium Sul-Fate/Hydroxyapatite, BCS/HA, Bond Apatite®): Review of the Literature and Case Reports. Coatings 2022, 12, 1350. https://doi.org/10.3390/coatings12091350
Torrejon-Moya A, Apalimova A, González-Navarro B, Zaera-Le Gal R, Marí-Roig A, López-López J. Calcium Sulfate in Implantology (Biphasic Calcium Sul-Fate/Hydroxyapatite, BCS/HA, Bond Apatite®): Review of the Literature and Case Reports. Coatings. 2022; 12(9):1350. https://doi.org/10.3390/coatings12091350
Chicago/Turabian StyleTorrejon-Moya, Aina, Alina Apalimova, Beatriz González-Navarro, Ramiro Zaera-Le Gal, Antonio Marí-Roig, and José López-López. 2022. "Calcium Sulfate in Implantology (Biphasic Calcium Sul-Fate/Hydroxyapatite, BCS/HA, Bond Apatite®): Review of the Literature and Case Reports" Coatings 12, no. 9: 1350. https://doi.org/10.3390/coatings12091350
APA StyleTorrejon-Moya, A., Apalimova, A., González-Navarro, B., Zaera-Le Gal, R., Marí-Roig, A., & López-López, J. (2022). Calcium Sulfate in Implantology (Biphasic Calcium Sul-Fate/Hydroxyapatite, BCS/HA, Bond Apatite®): Review of the Literature and Case Reports. Coatings, 12(9), 1350. https://doi.org/10.3390/coatings12091350








