Thermal Tunable Tribological Behavior of Shape Memory Biphenyl Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
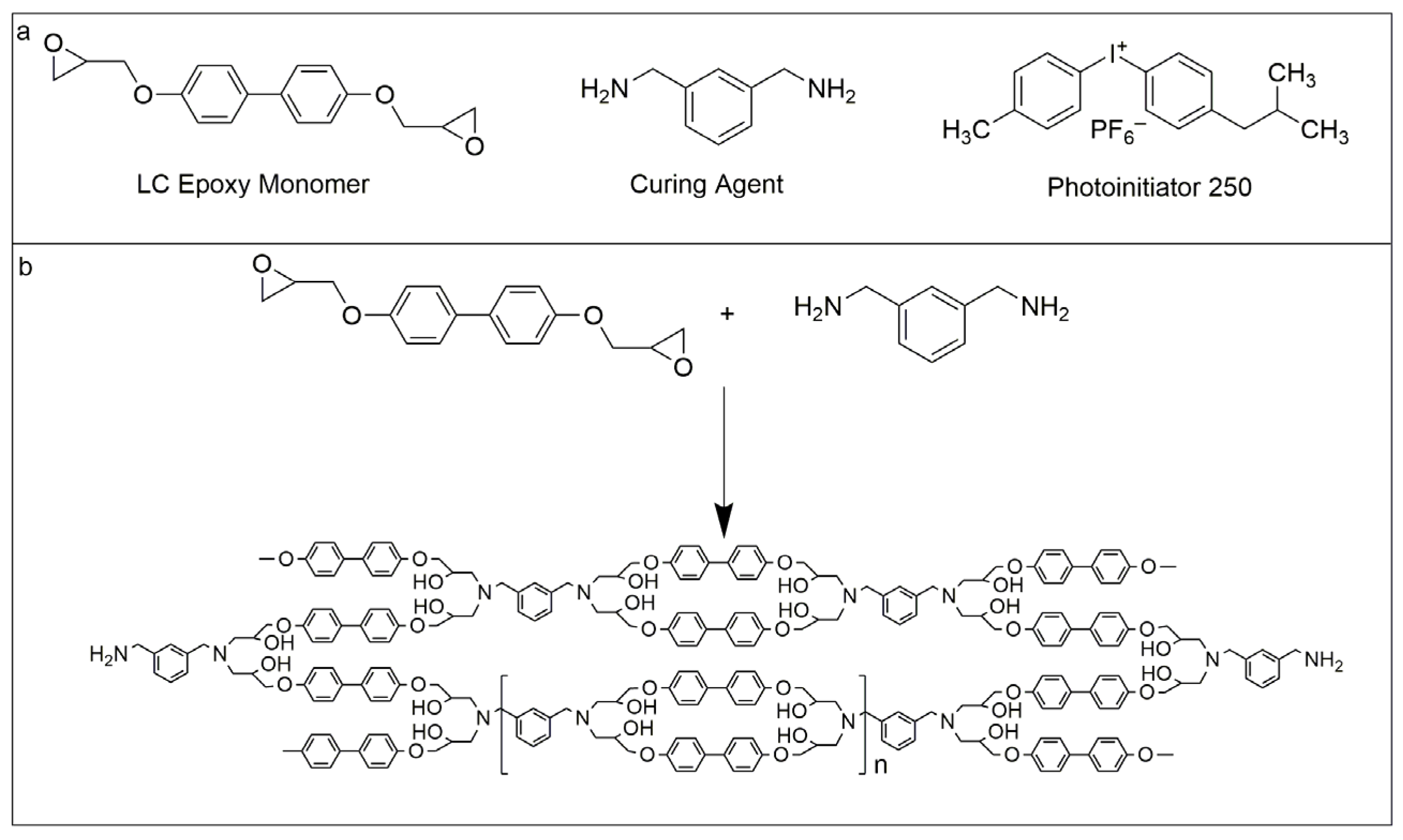
2.1. Materials
2.2. Preparation of Biphenyl Epoxy Resin Films (BPEPs)
2.3. Characterizations
3. Results
3.1. Physical and Chemical Properties of BPEPs
3.2. The Effect of the Molecular Structure on Friction
3.3. The Effect of the Crosslinking Density on the Friction
3.4. Thermal Tunable Tribological Behavior
4. Conclusions
- Due to the special molecular structure with sufficient biphenyl units, BPEPs presented an excellent self-lubricating property in a wide temperature range.
- Friction induced the self-assembly of the biphenyl unit aligned along the direction of sliding and enhanced the thermal conductivity and hardness that counteract the decreased modulus with temperature increasing; therefore, the COF from 0.175 to 0.214 maintained a relatively low value at a temperature above Tg.
- The COF and wear rate of BPEP1 friction at Tg + 20 °C increase gradually with the decrease in crosslinking density, which is because the decrease in modulus and the increase in hydrogen bond increase the sliding resistance and adhesion caused by material deformation in the friction.
- Thermal tunable tribological behavior of BPEPs is based on the mobility varying of the polymer chain and uses the thermal of the friction interface to achieve shape recovery, which is beneficial for reducing friction coefficient and reducing wear.
- In this study, we first proposed a wear self-compensation mechanism based on shape memory polymer in high-temperature friction experiments, which is conducive to promoting the development of shape memory polymers in the field of tribology. In addition, shape memory polymers can be combined with fillers capable of photothermal conversion, allowing them to achieve intelligent tribological properties by remote control of temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Part B-Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Liu, L.; Fang, G. Microstructure and thermal properties of cetyl alcohol/high density polyethylene composite phase change materials with carbon fiber as shape-stabilized thermal storage materials. Appl. Energy 2017, 200, 19–27. [Google Scholar] [CrossRef]
- Khun, N.W.; Sun, D.W.; Huang, M.X.; Yang, J.L.; Yue, C.Y. Wear resistant epoxy composites with diisocyanate-based self-healing functionality. Wear 2014, 313, 19–28. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Q.; Deng, X.; Cui, J. Earthworm-Inspired Rough Polymer Coatings with Self-Replenishing Lubrication for Adaptive Friction-Reduction and Antifouling Surfaces. Adv. Mater. 2018, 30, 1802141. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Xie, G.; Xu, W.; Wu, S.; Zhang, L.; Luo, J. Self-Cleaning of Interfacial Oil Between Polymer Composites with Porous Zeolite Microparticles and Their Self-Lubrication Properties. Adv. Mater. Interfaces 2019, 6, 1801889. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Zabinski, J.S. Smart Nanocomposite Coatings with Chameleon Surface Adaptation in Tribological Applications. In NATO Science Series II: Mathematics, Physics and Chemistry; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Ren, Y.; Liu, G.; Yang, H.; Tong, T.; Xu, S.; Zhang, L.; Luo, J.; Zhang, C.; Xie, G. Dynamic wear sensor array based on single-electrode triboelectric nanogenerators. Nano Energy 2020, 68, 104303. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, Y.; Song, F.; Wang, Q.; Wang, C.; Wang, T. Efficacy of hierarchical pore structure in enhancing the tribological and recyclable smart lubrication performance of porous polyimide. Friction 2022. [Google Scholar] [CrossRef]
- Ruan, H.; Shao, M.; Zhang, Y.; Wang, Q.; Wang, C.; Wang, T. Supramolecular Oleogel-Impregnated Macroporous Polyimide for High Capacity of Oil Storage and Recyclable Smart Lubrication. ACS Appl. Mater. Interfaces 2022, 14, 10936–10946. [Google Scholar] [CrossRef]
- Shao, M.; Li, S.; Duan, C.; Yang, Z.; Qu, C.; Zhang, Y.; Zhang, D.; Wang, C.; Wang, T.; Wang, Q. Cobweb-like Structural Stimuli-Responsive Composite with Oil Warehouse and Transportation System for Oil Storage and Recyclable Smart-Lubrication. ACS Appl. Mater. Interfaces 2018, 10, 41699–41706. [Google Scholar] [CrossRef]
- Adibnia, V.; Olszewski, M.; De Crescenzo, G.; Matyjaszewski, K.; Banquy, X. Superlubricity of Zwitterionic Bottlebrush Polymers in the Presence of Multivalent Ions. J. Am. Chem. Soc. 2020, 142, 14843–14847. [Google Scholar] [CrossRef]
- Oak, S.; Pashazanusi, L.; Sengel, S.B.; Omarova, M.; Hemstock, J.L.; He, W.; He, J.; John, V.; Sahiner, N.; Pesika, N.S. Tunable Friction Through Stimuli Responsive Hybrid Carbon Microspheres. Langmuir 2019, 35, 15849–15854. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Feng, Y.; Zhao, N.; Chen, Z.; Shi, J.; Zhou, F. Polymer-based lubricating materials for functional hydration lubrication. Chem. Eng. J. 2022, 429, 132324. [Google Scholar] [CrossRef]
- Tang, S.; Li, S.; Zhu, S.; Ma, L.; Tian, Y. Light-controlled friction realized by a photorheological fluid. Tribol. Int. 2022, 176, 107914. [Google Scholar] [CrossRef]
- Hua, J.; Björling, M.; Larsson, R.; Shi, Y. Controlling friction in Ionic Liquid/Glycerol Aqueous Solution lubricated contacts by adjusting CO2 and water content. Tribol. Int. 2021, 161, 107070. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, S.; Yang, W.; Yu, B.; Pei, X.; Zhou, F.; Liu, W. Sundew-Inspired Simultaneous Actuation and Adhesion/Friction Control for Reversibly Capturing Objects Underwater. Adv. Mater. Technol. 2019, 4, 1800467. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Z.; Zhang, X.; Tao, L.; Wei, Y.; Ji, Y. Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy. Chem. Sci. 2014, 5, 3486–3492. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Jiang, Z.C.; Tong, X.; Zhao, Y. Biomimetic Locomotion of Electrically Powered “Janus” Soft Robots Using a Liquid Crystal Polymer. Adv. Mater. 2019, 31, 1903452. [Google Scholar] [CrossRef]
- Zheng, N.; Fang, G.Q.; Cao, Z.L.; Zhao, Q.; Xie, T. High strain epoxy shape memory polymer. Polym. Chem. 2015, 6, 3046–3053. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-Memory Polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Zheng, F.; Bai, Y.; Wang, Q.; Wang, T. Switchable friction properties induced by shape memory effect. J. Mater. Sci. 2014, 49, 8394–8401. [Google Scholar] [CrossRef]
- Liu, D.; Broer, D.J. Self-assembled Dynamic 3D Fingerprints in Liquid-Crystal Coatings Towards Controllable Friction and Adhesion. Angew. Chem. Int. Ed. 2014, 53, 4542–4546. [Google Scholar] [CrossRef]
- Xia, F.; Feng, L.; Wang, S.; Sun, T.; Song, W.; Jiang, W.; Jiang, L. Dual-Responsive Surfaces That Switch between Superhydrophilicity and Superhydrophobicity. Adv. Mater. 2006, 18, 432–436. [Google Scholar] [CrossRef]
- Liu, D.; Broer, D.J. Light controlled friction at a liquid crystal polymer coating with switchable patterning. Soft Matter 2014, 10, 7952–7958. [Google Scholar] [CrossRef] [PubMed]
- Myshkin, N.K.; Kovalev, A. Polymer mechanics and tribology. Ind. Lubr. Tribol. 2018, 70, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Tao, L.M.; Cao, P.R.; Yang, Z.H.; Zhang, X.R.; Wang, Q.H.; Wang, T.M.; Luo, H.M.; Zhang, Y.M. Biphenyl Containing Shape Memory Epoxy Resin with Post-heating Adjustable Properties. Macromol. Mater. Eng. 2021, 306, 2100185. [Google Scholar] [CrossRef]
- Islam, A.M.; Lim, H.; You, N.-H.; Ahn, S.; Goh, M.; Hahn, J.R.; Yeo, H.; Jang, S.G. Enhanced Thermal Conductivity of Liquid Crystalline Epoxy Resin using Controlled Linear Polymerization. ACS Macro Lett. 2018, 7, 1180–1185. [Google Scholar] [CrossRef]
- Yang, R.; Chen, L.; Ruan, C.; Zhong, H.-Y.; Wang, Y.-Z. Chain folding in main-chain liquid crystalline polyesters: From π–π stacking toward shape memory. J. Mater. Chem. C 2014, 2, 6155–6164. [Google Scholar] [CrossRef]
- Carfagna, C.; Amendola, E.; Giamberini, M.; Hakemi, H.; Pane, S. Liquid crystalline epoxy resins in polymer dispersed liquid crystal composites. Polym. Int. 1997, 44, 465–473. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, Y.; Li, S.; Yang, L.; Wang, C.; Wang, T.; Wang, Q. Effect of temperature on the friction and wear performance of porous oil-containing polyimide. Tribol. Int. 2021, 157, 106891. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, X.; Liu, X.; Liang, Z.; Ge, Z.; Luo, Y. Preparation and properties of waterborne polyurethane modified by aminoethylaminopropyl polydimethylsiloxane for fluorine-free water repellents. Prog. Org. Coat. 2020, 139, 105407. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation Of Surface Free Energy Of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Santiago, D.; Guzman, D.; Ferrando, F.; Serra, A.; De la Flor, S. Bio-Based Epoxy Shape-Memory Thermosets from Triglycidyl Phloroglucinol. Polymers 2020, 12, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmonte, A.; Lama, G.C.; Gentile, G.; Fernandez-Francos, X.; De la Flor, S.; Cerruti, P.; Ambrogi, V. Synthesis and Characterization of Liquid-Crystalline Networks: Toward Autonomous Shape-Memory Actuation. J. Phys. Chem. C 2017, 121, 22403–22414. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, F.; Tian, N.; Zhou, J. Effect of temperature on the tribological and dynamic mechanical properties of liquid crystalline polymer. Polym. Test. 2005, 24, 270–274. [Google Scholar] [CrossRef]
- You, Y.; Huang, X.; Pu, Z.; Jia, K.; Liu, X. Enhanced crystallinity, mechanical and dielectric properties of biphenyl polyarylene ether nitriles by unidirectional hot-stretching. J. Polym. Res. 2015, 22, 211. [Google Scholar] [CrossRef]
- Guo, H.L.; Li, Y.W.; Zheng, J.; Gan, J.Q.; Liang, L.Y.; Wu, K.; Lu, M.G. High thermo-responsive shape memory epoxies based on substituted biphenyl mesogenic with good water resistance. Rsc Adv. 2015, 5, 67247–67257. [Google Scholar] [CrossRef]
- Li, Y.Z.; Pruitt, C.; Rios, O.; Wei, L.Q.; Rock, M.; Keum, J.K.; McDonald, A.G.; Kessler, M.R. Controlled Shape Memory Behavior of a Smectic Main-Chain Liquid Crystalline Elastomer. Macromolecules 2015, 48, 2864–2874. [Google Scholar] [CrossRef]
- Kawamoto, S.; Fujiwara, H.; Nishimura, S. Hydrogen characteristics and ordered structure of mono-mesogen type liquid-crystalline epoxy polymer. Int. J. Hydrogen Energy 2016, 41, 7500–7510. [Google Scholar] [CrossRef]
- Ortiz, C.; Kim, R.; Rodighiero, E.; Ober, C.K.; Kramer, E.J. Deformation of a polydomain, liquid crystalline epoxy-based thermoset. Macromolecules 1998, 31, 4074–4088. [Google Scholar] [CrossRef]
- Choy, C.L. Thermal-Conductivity Of Polymers. Polymer 1977, 18, 984–1004. [Google Scholar] [CrossRef]
- Rasul, G.; Kiziltas, A.; Hoque, S.B.; Banik, A.; Hopkins, P.E.; Tan, K.T.; Arfaei, B.; Shahbazian-Yassar, R. Improvement of the thermal conductivity and tribological properties of polyethylene by incorporating functionalized boron nitride nanosheets. Tribol. Int. 2022, 165, 107277. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Byrne, J.A.; McLaughlin, J.; Ahmed, W. Study of Human Serum Albumin Adsorption and Conformational Change on DLC and Silicon Doped DLC Using XPS and FTIR Spectroscopy. J. Biomater. Nanobiotechnol. 2013, 4, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Q.; Zeng, M.; Xu, Q.Y.; Hou, S.E.; Jin, H.Y.; Fan, L.R. Effects of glass-to-rubber transition of thermosetting resin matrix on the friction and wear properties of friction materials. Tribol. Int. 2012, 54, 51–57. [Google Scholar] [CrossRef]







| Liquid Sample | (mN/m) | (mN/m) | (mN/m) |
|---|---|---|---|
| Water | 72.8 | 29.1 | 43.7 |
| Diiodomethane | 50.8 | 49.5 | 1.3 |
| Samples | BPEP1 | BPEP2 | BPEP3 |
|---|---|---|---|
| Epoxy/amine | 1:0.5 | 1:0.68 | 1:0.86 |
| Tg 1 (°C) | 110 | 101 | 41 |
| Tg 2 (°C) | 83 | 71 | 48 |
| E′ (25 °C, GPa) | 2.8 | 2.6 | 1.6 |
| E′ (100 °C, MPa) | 25.7 | 19.2 | 11.4 |
| ve (103 mol·m−3) | 2.8 | 2.1 | 1.2 |
| εb (%) | 4.5 ± 1.4 | 29.8 ± 11.7 | 43.0 ± 8.3 |
| σb (MPa) | 29.9 ± 7.3 | 25.3 ± 6.6 | 20.9 ± 4.9 |
| E (MPa) | 2043.9 ± 382.1 | 1772.1 ± 166.5 | 1389.9 ± 284.3 |
| Temperature | Samples | Coefficient of Friction (COF) | Wear Rate (×10−5 mm3 N−1 m−1) |
|---|---|---|---|
| RT | BPEP1-RT | 0.125 ± 0.013 | 1.27 ± 0.54 |
| BPEP2-RT | 0.120 ± 0.014 | 1.38 ± 0.26 | |
| BPEP3-RT | 0.116 ± 0.007 | 1.70 ± 0.38 | |
| Tg + 20 °C | BPEP1-110 °C | 0.175 ± 0.010 | 6.94 ± 1.95 |
| BPEP2-90 °C | 0.205 ± 0.014 | 11.34 ± 2.65 | |
| BPEP3-70 °C | 0.214 ± 0.010 | 16.61 ± 1.15 |
| Sample | θ (Water)/° | θ (Diiodomethane)/° | (mN/m) |
|---|---|---|---|
| BPEP1 | 88.8 | 60.8 | 28.46 |
| BPEP2 | 91.6 | 66.4 | 25.29 |
| BPEP3 | 98.8 | 60.1 | 28.85 |
| Samples | COF | Wear Rate (×10−5 mm3 N−1 m−1) | E′ (MPa) |
|---|---|---|---|
| BPEP1-25 °C | 0.125 ± 0.013 | 1.27 ± 0.54 | 2842.4 |
| BPEP1-50 °C | 0.100 ± 0.015 | 1.41 ± 0.16 | 908.2 |
| BPEP1-70 °C | 0.131 ± 0.012 | 2.01 ± 0.38 | 183.1 |
| BPEP1-90 °C | 0.165 ± 0.015 | 3.85 ± 0.59 | 46.2 |
| BPEP1-110 °C | 0.175 ± 0.010 | 6.94 ± 1.95 | 19.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Cao, P.; Gao, K.; Ding, C.; Chen, S.; Zhang, X.; Wang, T.; Wang, Q.; Zhang, Y. Thermal Tunable Tribological Behavior of Shape Memory Biphenyl Epoxy Resin. Coatings 2023, 13, 166. https://doi.org/10.3390/coatings13010166
Yang J, Cao P, Gao K, Ding C, Chen S, Zhang X, Wang T, Wang Q, Zhang Y. Thermal Tunable Tribological Behavior of Shape Memory Biphenyl Epoxy Resin. Coatings. 2023; 13(1):166. https://doi.org/10.3390/coatings13010166
Chicago/Turabian StyleYang, Jing, Pengrui Cao, Ketian Gao, Chang Ding, Shoubing Chen, Xinrui Zhang, Tingmei Wang, Qihua Wang, and Yaoming Zhang. 2023. "Thermal Tunable Tribological Behavior of Shape Memory Biphenyl Epoxy Resin" Coatings 13, no. 1: 166. https://doi.org/10.3390/coatings13010166
APA StyleYang, J., Cao, P., Gao, K., Ding, C., Chen, S., Zhang, X., Wang, T., Wang, Q., & Zhang, Y. (2023). Thermal Tunable Tribological Behavior of Shape Memory Biphenyl Epoxy Resin. Coatings, 13(1), 166. https://doi.org/10.3390/coatings13010166






