Biomechanical Properties and Corrosion Resistance of Plasma-Sprayed Fish Scale Hydroxyapatite (FsHA) and FsHA-Doped Yttria-Stabilized Zirconia Coatings on Ti–6Al–4V Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. FsHA Powder Preparation
2.2. Plasma Coating
2.3. Surface Roughness Test
2.4. Microhardness Test
2.5. Electrochemical Corrosion Test
2.6. In Vitro Bioactivity Assessment
2.7. In Vitro Cytotoxicity Test of Plasma-Sprayed FsHA/YSZ Coating
- Extracts for the cell viability test were obtained from the coated surface of each sample after immersion in complete media for 24 h at 37 °C without agitation, with a weight-volume ratio of 200 mg/mL.
- The extraction vehicle (complete media) represented the negative control with no material.
- The pure extracts were then diluted with the complete media to make weight-vol-ume ratios of 100, 50 and 25 mg/mL. Subsequently, the pure extracts and the diluted extracts were added to a healthy monolayer of L929 cells (which were seeded with 3 × 105 cells/mL in 24-multiwell plates for 24 h) and incubated in a CO2 incubator (Bioevopeak, Shandong, China) at 37 °C/5% CO2 for 24 h.
- At the end of the 24 h incubation period, cell viability was tested using an Alamar Blue assay. The culture was stained with an Alamar Blue solution (1:10) and incubated for 4 h at 37 °C in a CO2 incubator.
- After the 4 h incubation, the stained culture was detected with absorbance using a Universal Microplate Reader at 570 nm.
3. Results and Discussion
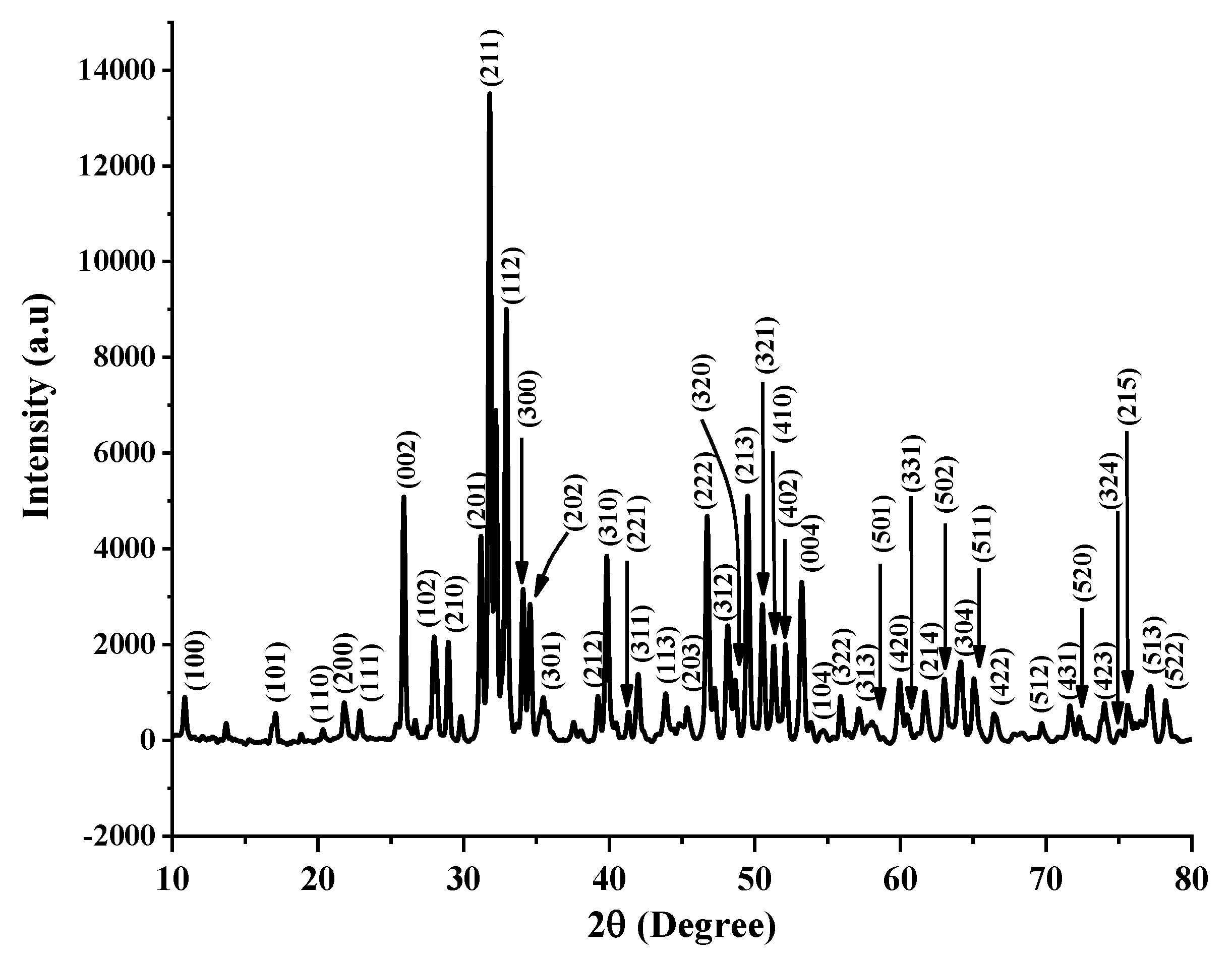
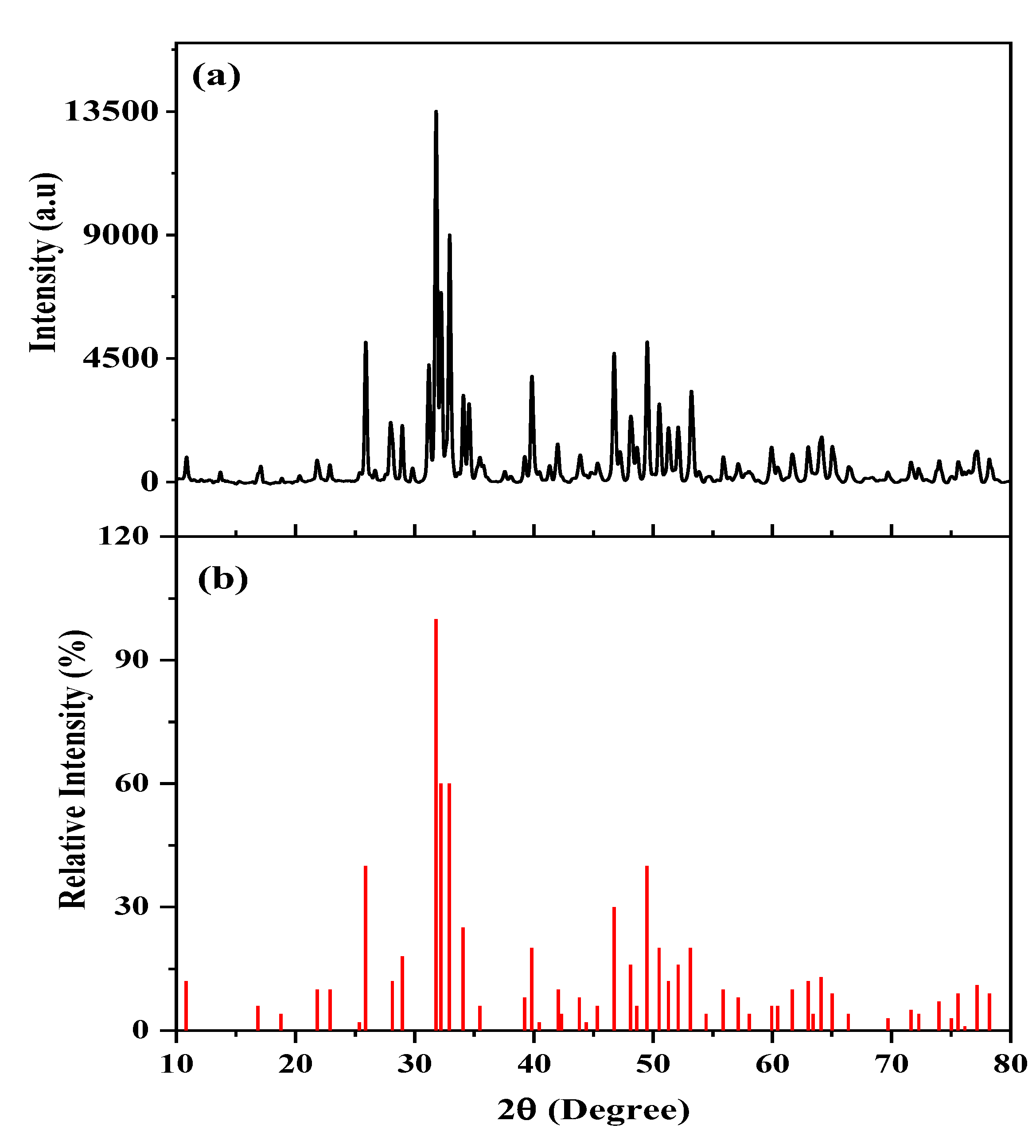
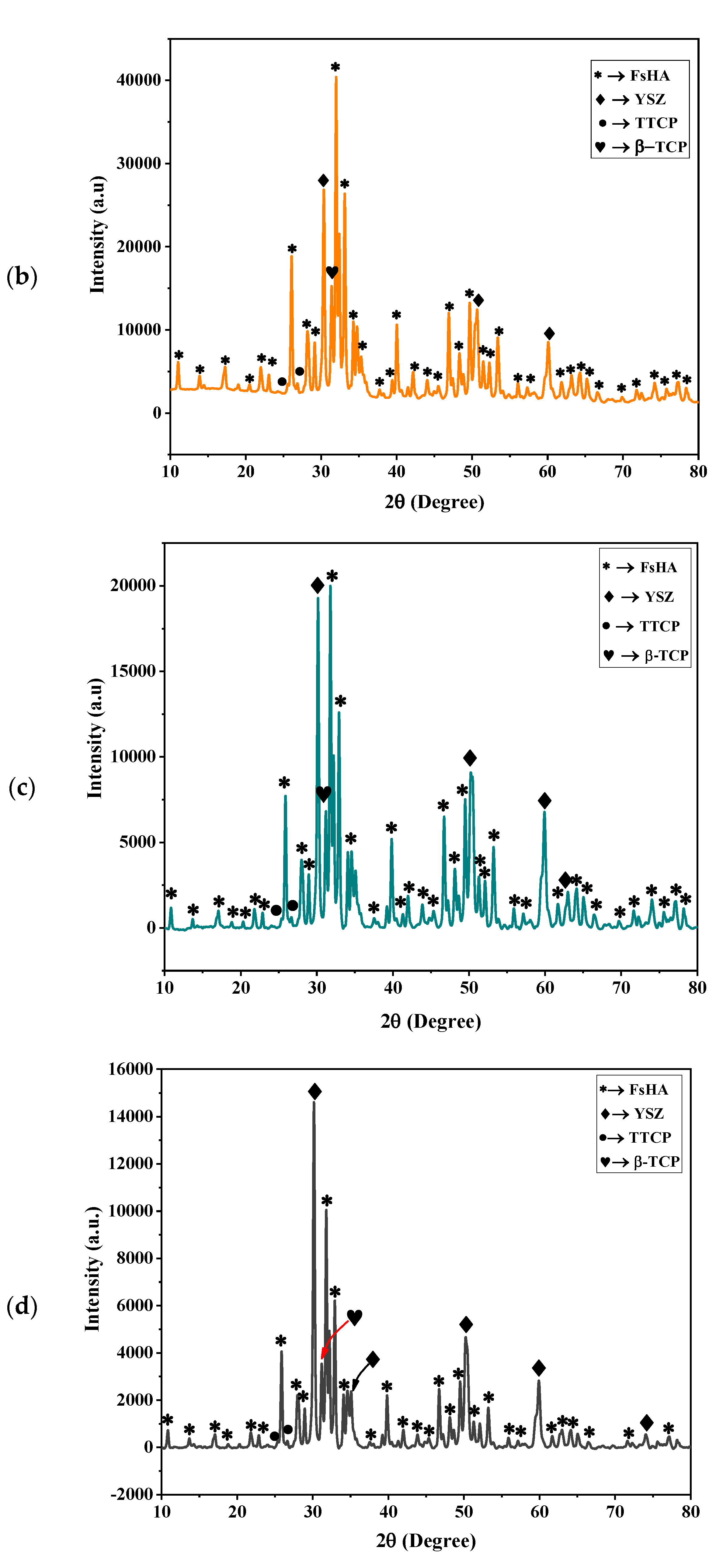
3.1. XRD Characterization of the FsHA/YSZ Powders
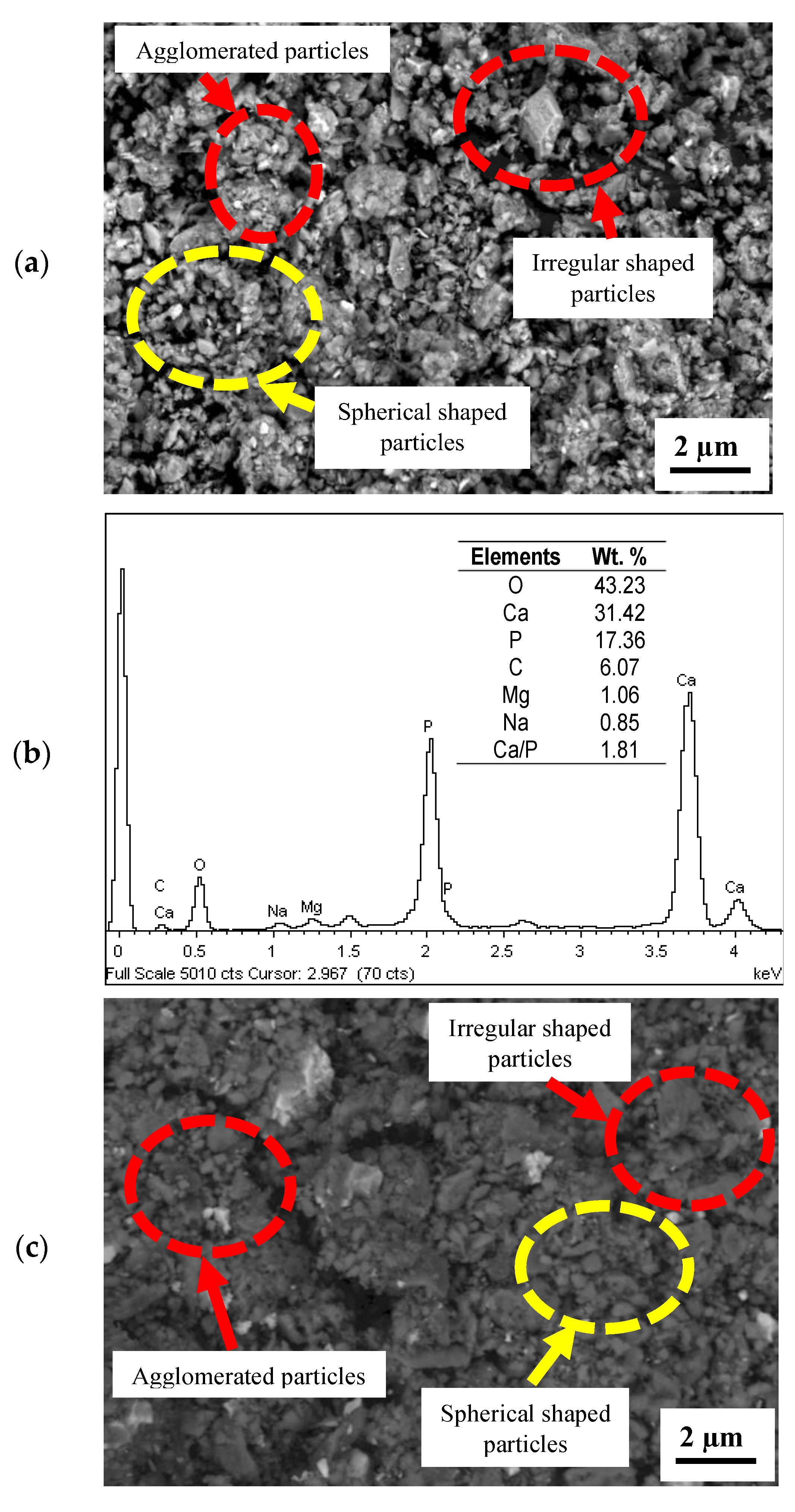
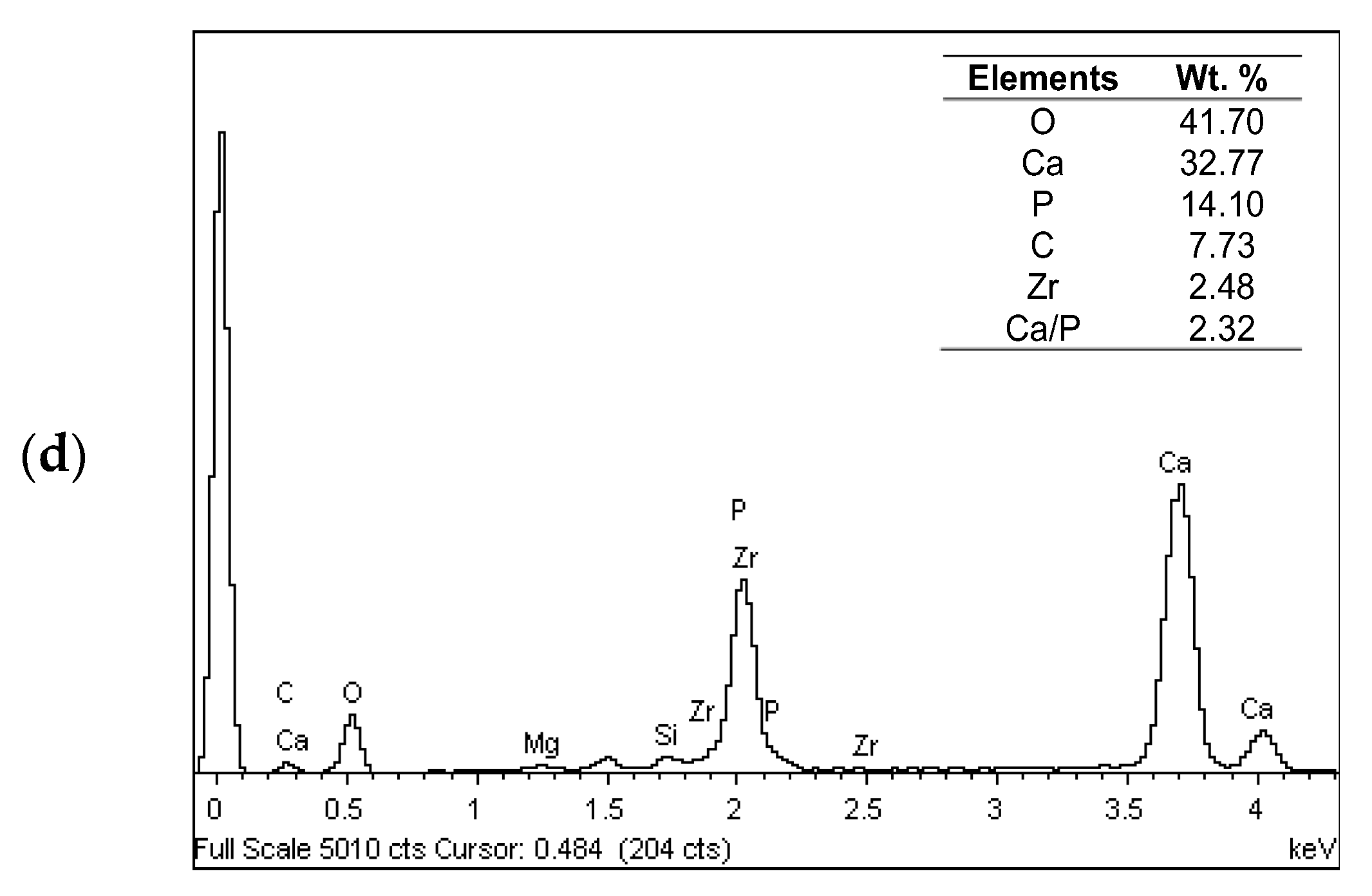
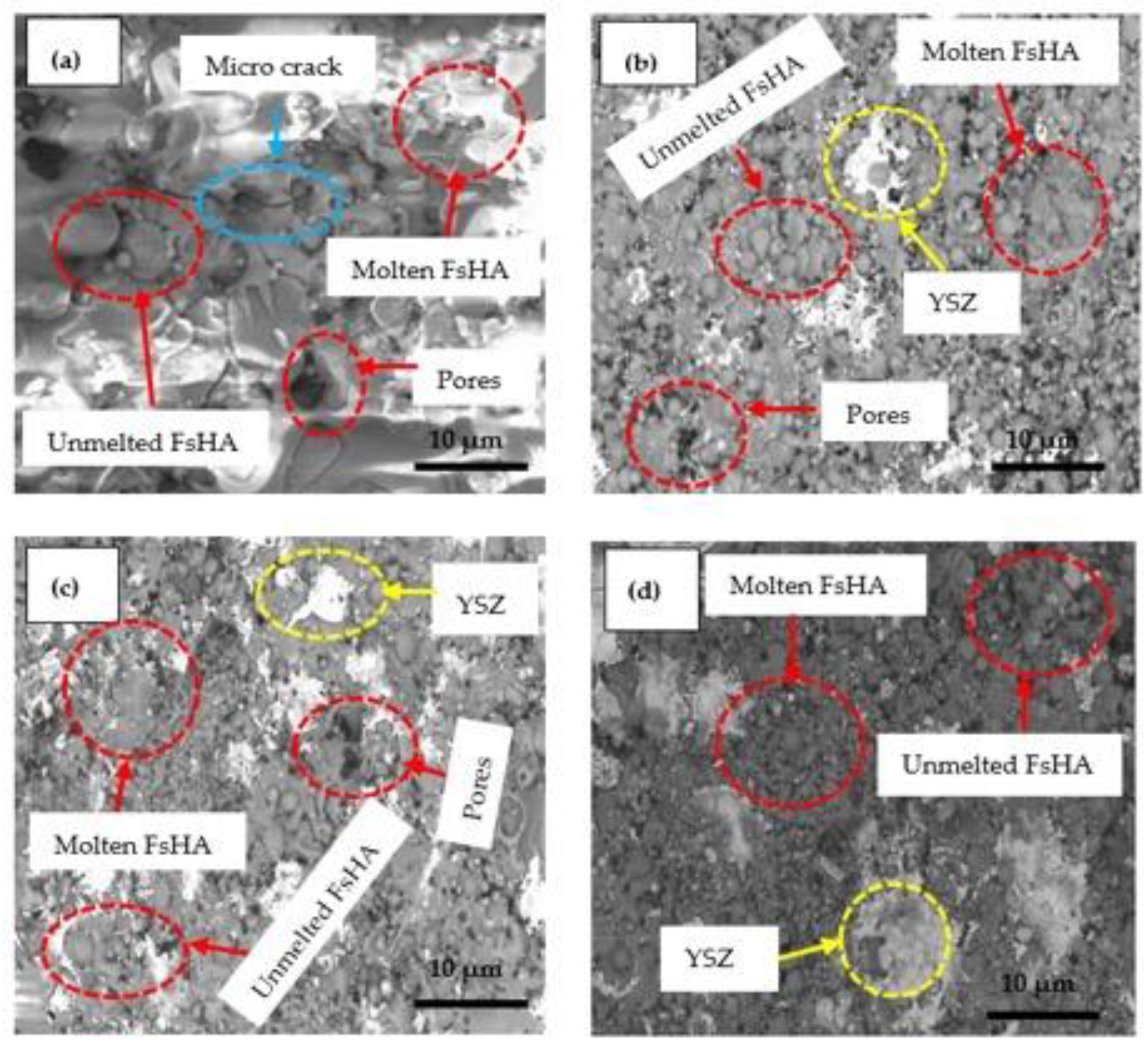
3.2. Microstructure Analysis
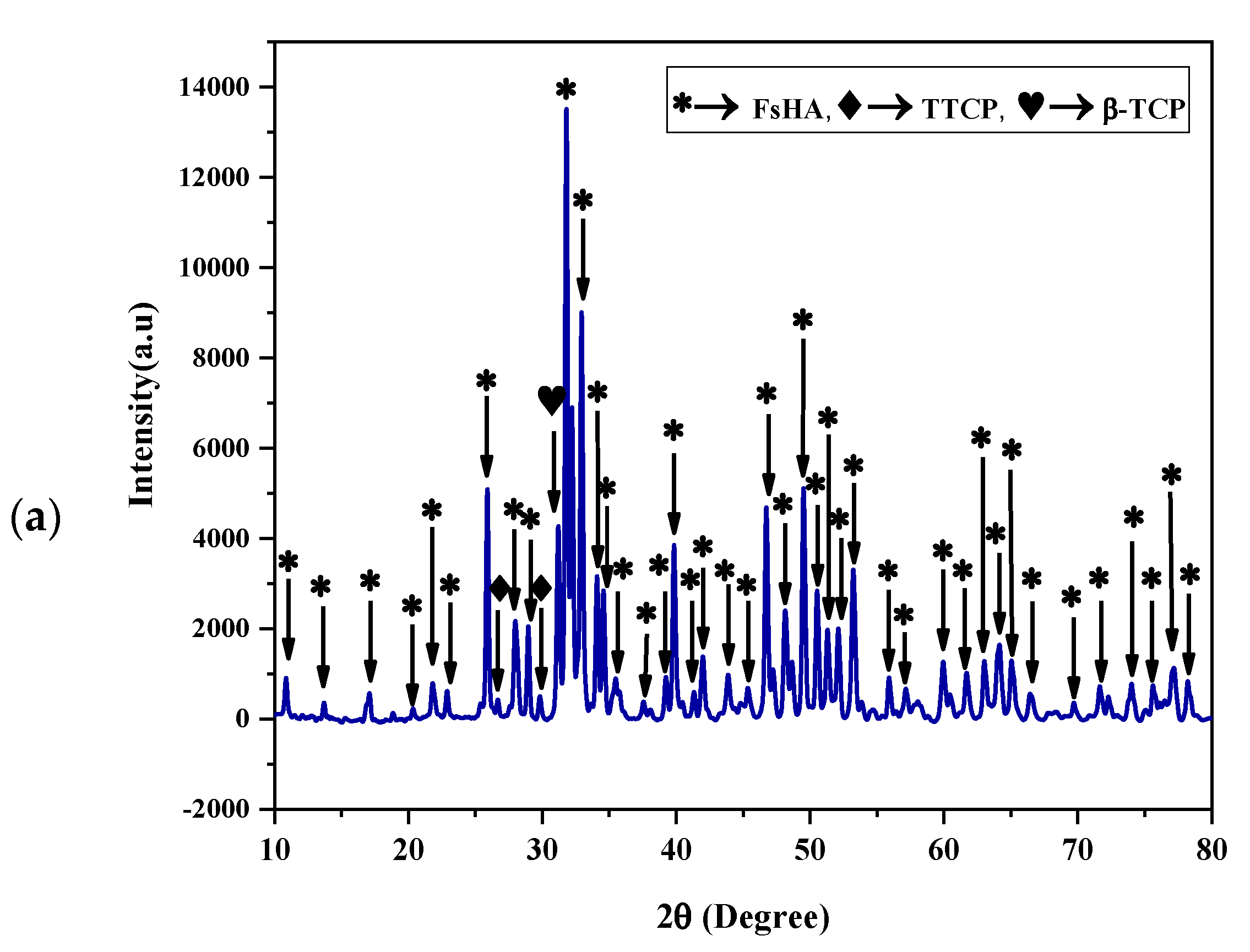
3.3. XRD Characterization of FsHA/YSZ Coatings
3.4. Surface Roughness
3.5. Microhardness
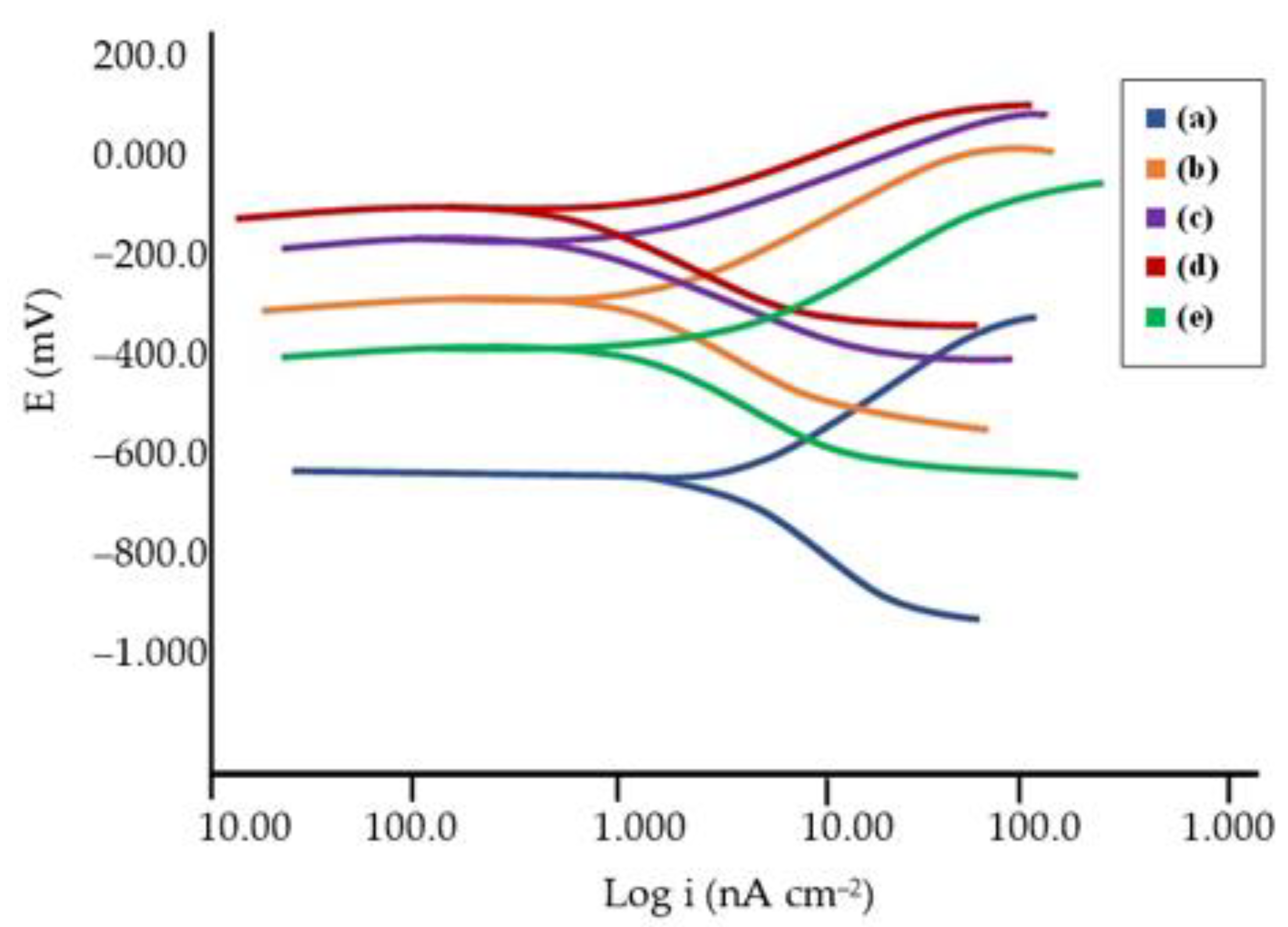
3.6. Corrosion Behavior
3.7. Evaluation of In Vitro Bioactivity of Plasma-Sprayed FsHA/YSZ Coatings
3.8. Evaluation of the In Vitro Cytotoxicity of FsHA/YSZ Coating
4. Conclusions
- The XRD patterns of the powders showed the highest peak intensity at 31.8°, representing the (211) crystal plane, which is the crystalline HA peak according to the JCPDS. The Crystallinity of the powders was above 96%, and the least Crystallinity of the plasma-sprayed coatings was 65.7%. Additionally, the XRD pattern of the undoped FsHA coating consisted of a sharp peak of HA, with lesser peak intensities for the CaO, TTCP, and β-TCP phases, while that of the FsHA/YSZ coatings had an extra sharp peak intensity of YSZ.
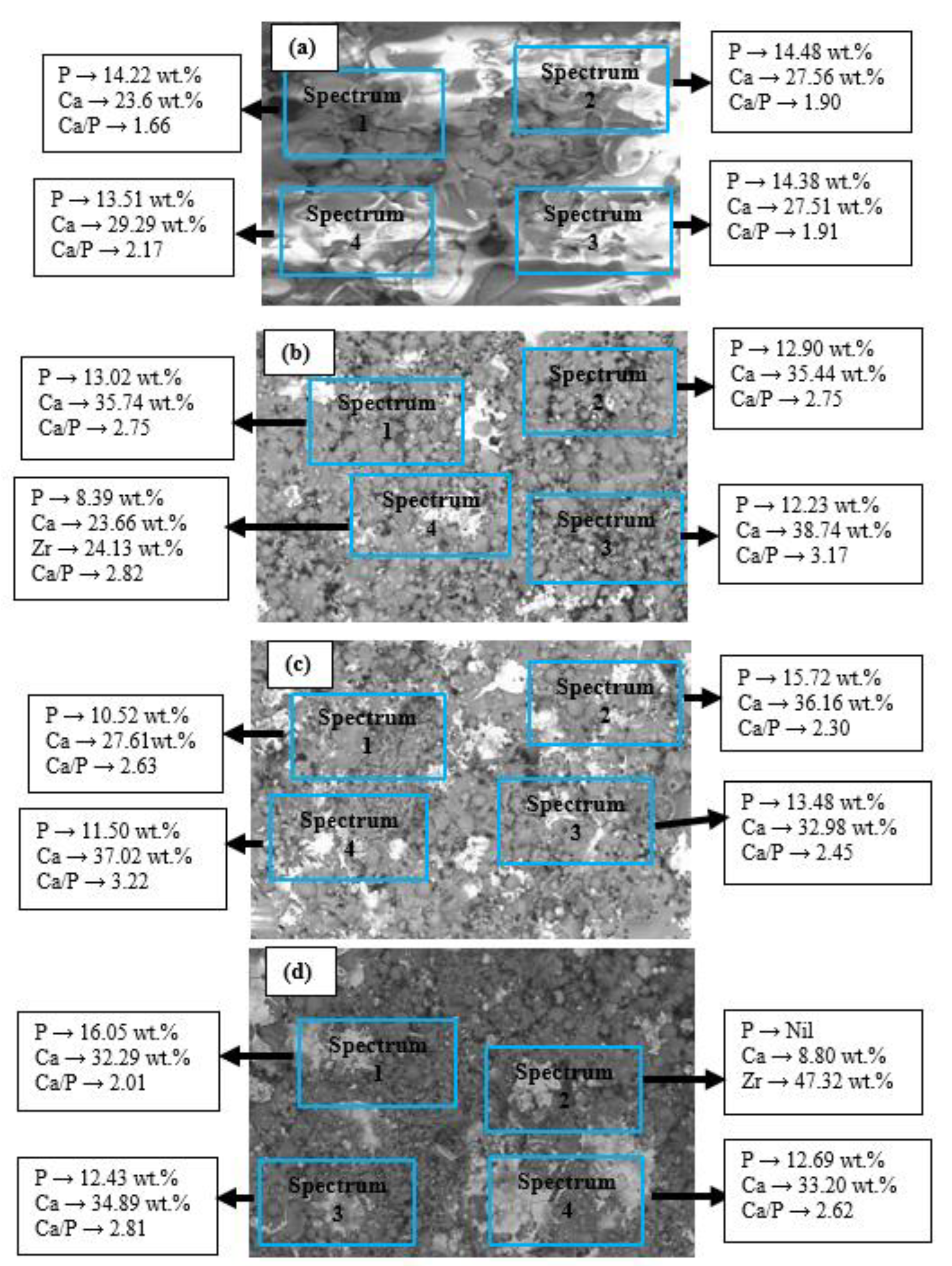
- The microstructures of the coatings showed significant amounts of Ca and P. The micrograph of the FsHA + 0 wt.% YSZ coating revealed micropores, microcracks, and molten and unmelted spheroidized FsHA particles, whereas the FsHA/YSZ coatings showed an increased amount of melted FsHA particles, lesser pores, fine microcracks, and ZrO2 particles.
- The highest hardness of 558.5 Hv was obtained with the FsHA + 20 wt.% YSZ coating as a result of the solid solution strengthening of YSZ in FsHA.
- The FsHA + 10 wt.% YSZ-coated sample had the least surface roughness (4.205 µm) compared with the other coated samples: FsHA + 0 wt.% YSZ (4.316 µm), FsHA + 15 wt.% YSZ (4.252 µm), and FsHA + 20 wt.% YSZ (4.218 µm). This showed that the YSZ addition slightly reduced the roughness of the doped coatings.
- The undoped FsHA coating showed a significantly improved corrosion resistance, with a 43% reduction in the corrosion rate compared with the uncoated substrate, and the FsHA/YSZ coatings showed further improvements in corrosion resistance, with the FsHA + 20 wt.% YSZ coating having the least corrosion rate of 9.467 mmpy.
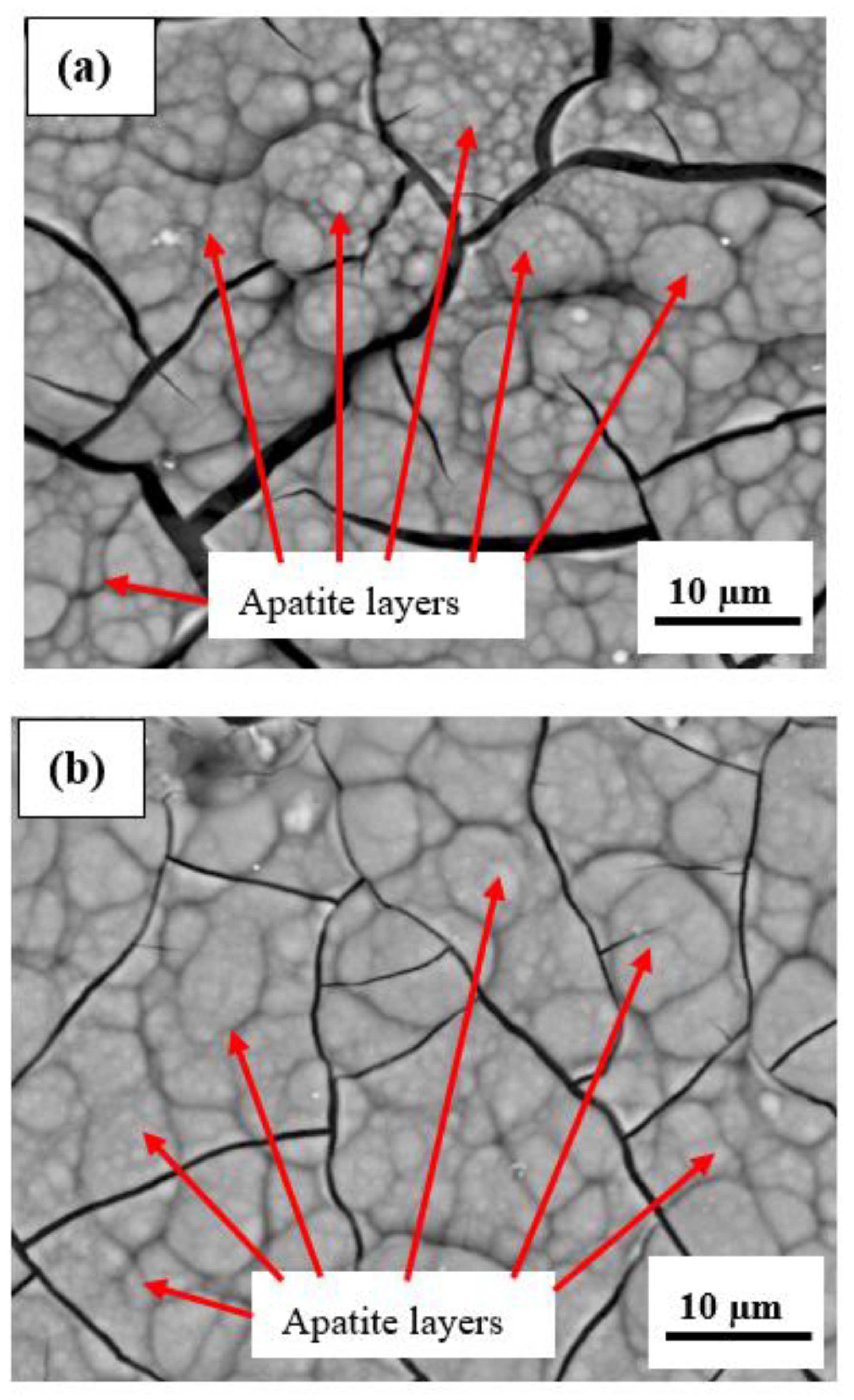
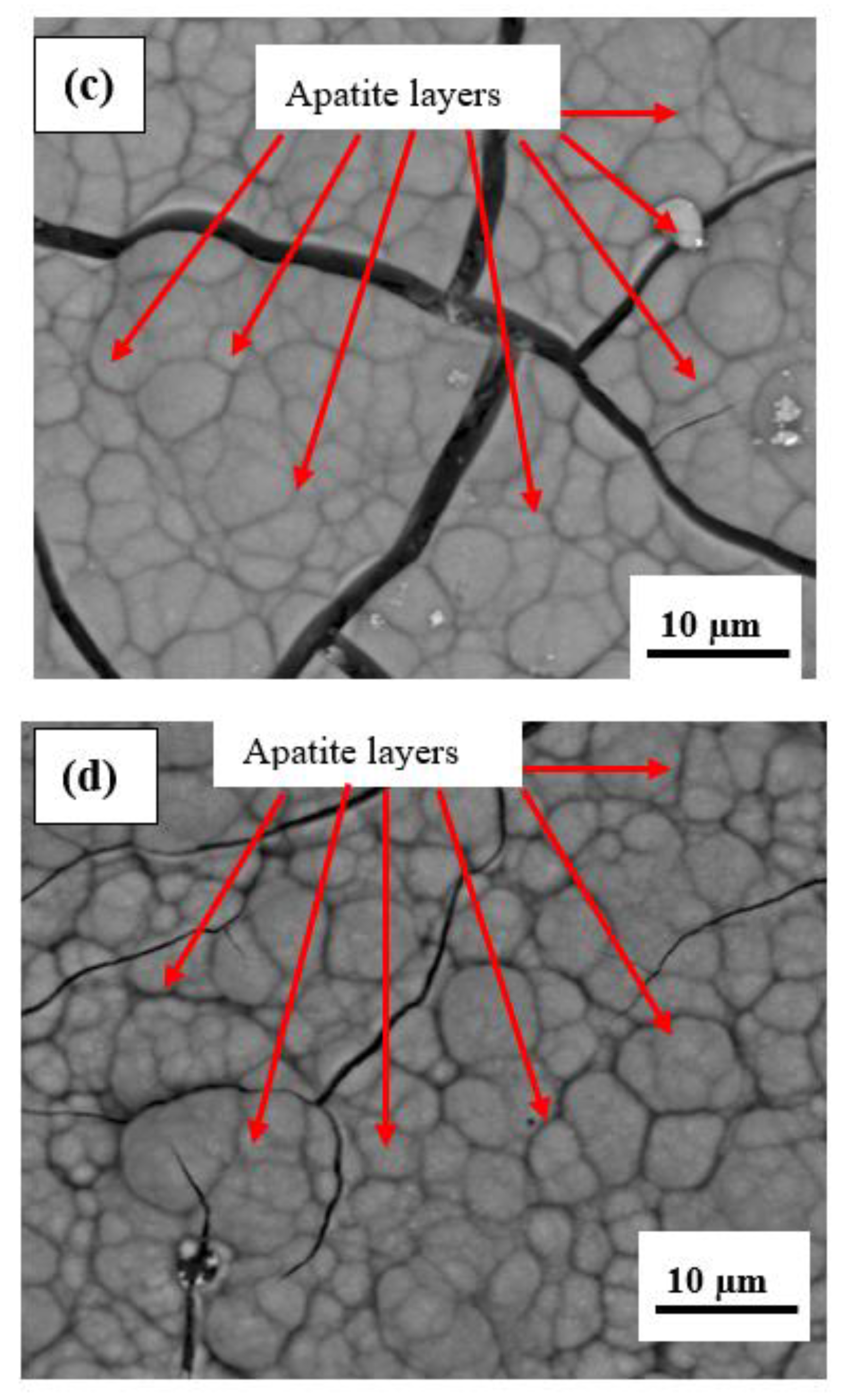
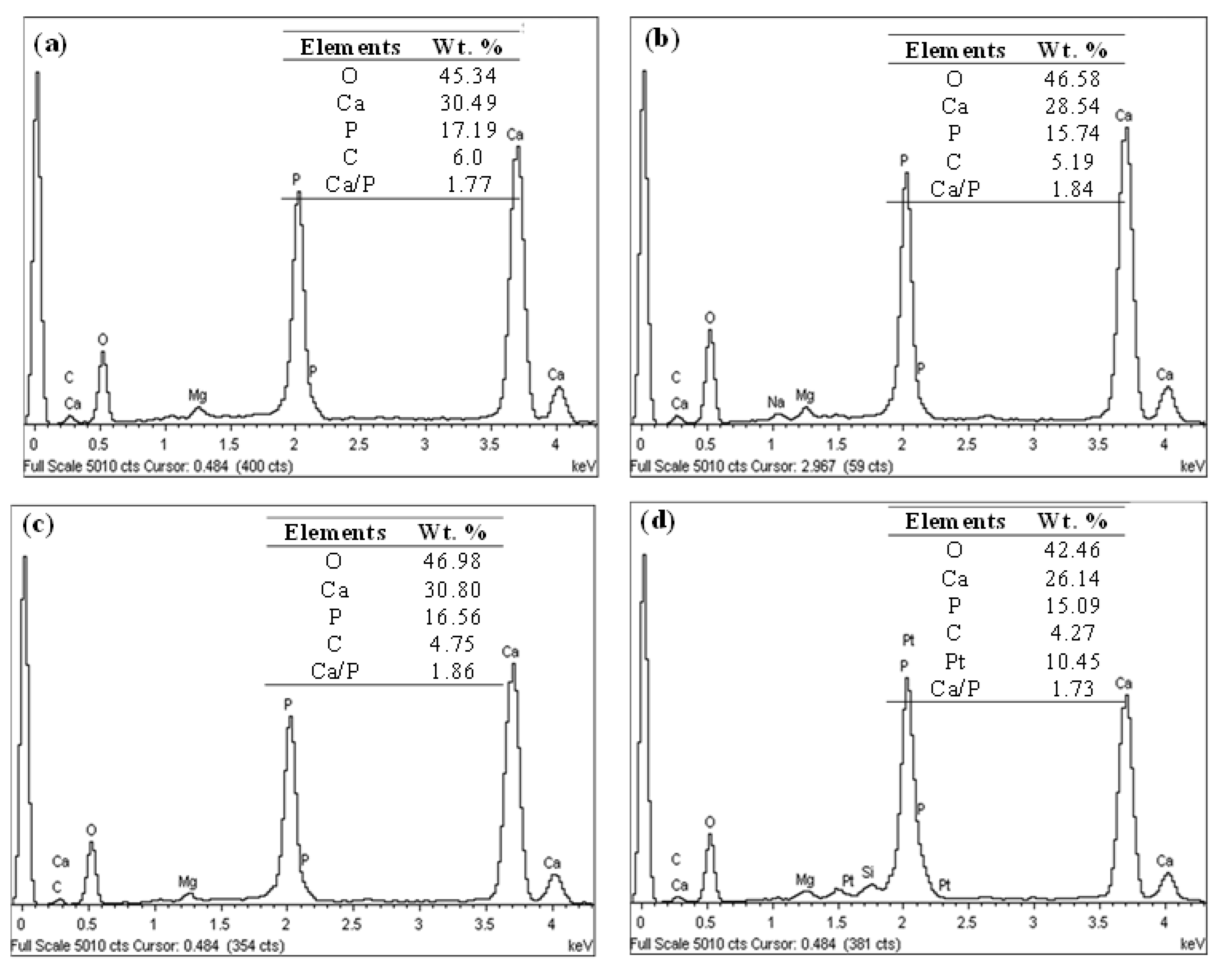
- The microstructure of the plasma-sprayed FsHA/YSZ coatings after 14 days of immersion in SBF revealed enlarged cracks and delaminated segments with well-grown apatite spherulite layers on the whole surface of the coatings. The EDX analysis of the coatings after 14 days of immersion in SBF confirmed the strong presence of Ca and P, with Ca/P ratios in the range of 1.73–1.86, which were close to the stoichiometric ratio of 1.67. This indicated the occurrence of multiple reactions between the coatings and the SBF solution that led to the formation of bone-like apatite (Ca10−x(PO4)6−x(CO3)x(OH)2).
- The in vitro cytotoxicity results showed that the L929 cells demonstrated a good cell viability of 95% at the highest concentration (200 mg/mL) of the coated specimen in contrast to the positive control cultures with a cell viability of 5%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rey, C.; Combes, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichert, D.; Drouet, C.; Sfihi, H.; Rey, C.; Combes, C. Nanocrystalline apatite-based biomaterials: Synthesis processing and characterization. In Biomaterials Research Advances; Kendall, J.B., Ed.; Nova Science Publishers: New York, NY, USA, 2008. [Google Scholar]
- Xue, W.; Moore, J.L.; Hosick, H.L.; Bose, S.; Bandyopadhyay, A.; Lu, W.W.; Cheung, K.M.C.; Luk, K.D.K. Osteoprecursor cell response to strontium-containing hydroxyapatite ceramics. J. Biomed. Mater. Res. A 2006, 79, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Suchanek, W.; Yoshimura, M. Processing, Properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- de Bruijn, J.D.; van Blitterswilk, C.A.; Davis, J.E. Initial bone matrix formation at the hydroxyapatite interface in vivo. J. Biomed. Mater. Res. 1995, 29, 89–99. [Google Scholar] [CrossRef]
- Grootde, K.; Wolke, J.G.C.; Jansen, J.A. Calcium phosphate coatings or medical Implants. Proc. Inst. Mech. Eng. H 1998, 212, 137–147. [Google Scholar] [CrossRef]
- Hing, K.A.; Revell, P.A.; Smith, N.; Buckland, T. Effect of silicon level on rate, quality and progression of bone healing within silicate substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [Google Scholar] [CrossRef]
- Morks, M.F. Fabrication and characterization of plasma-sprayed HA/SiO2 coatings for biomedical application. J. Mech. Behav. Biomed. Mater. 2008, 1, 105–111. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Pan, D.; Cheang, P. Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti–6Al–4V composite coatings in simulated body fluid. Biomaterials 2004, 25, 3177–3185. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Chang, J.; Woods, J.; Chen, Y.; Zreiqat, H. The effect of Zn contents on phase composition, chemical stability and cellular bioactivity in Zn-Ca-Si system ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 346–353. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.; Zheng, X.; Ding, C.; Chu, P.K. Improved stability of plasma-sprayed dicalcium silicate/zirconia composite coating. Thin Solid Film. 2006, 515, 1214–1218. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.W.; Hübsch, C. Low-temperature degradation of different zirconia ceramics for dental applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Chou, B.Y.; Chang, E.; Yao, S.Y.; Chen, J.M. Phase transformation during plasma spraying of hydroxyapatite–10-wt.%-zirconia composite coating. J. Am. Ceram. Soc. 2002, 85, 661–669. [Google Scholar] [CrossRef]
- Chou, B.Y.; Chang, E. Plasma-sprayed hydroxyapatite coating on titanium alloy with ZrO2 second phase and ZrO2 intermediate layer. Surf. Coat. Technol. 2002, 153, 84–92. [Google Scholar] [CrossRef]
- Fu, L.; Khor, K.A.; Lim, J.P. Effects of yttria stabilized zirconia on plasma sprayed hydroxyapatite/yttria stabilized zirconia composite coatings. J. Am. Ceram. Soc. 2002, 85, 800–806. [Google Scholar] [CrossRef]
- Kasugu, T.; Yoshida, M.; Ikushima, A.J.; Tuchiya, M.; Kusakari, H. Stability of zirconia-toughened bioactive glass-ceramics—In vivo study using dogs. J. Mater. Sci. Mater. Med. 1993, 4, 36–49. [Google Scholar] [CrossRef]
- Duong, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Post-Spinning Treatments to Carbon Nanotube Fibers; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Tebyanian, H.; Norahan, M.H.; Eyni, H.; Movahedin, M.; Mortazavi, S.M.J.; Karami, A.; Nourani, M.R.; Baheiraei, N. Effects of collagen/β-tricalcium phosphate bone graft to regenerate bone in critically sized rabbit calvarial defects. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018820490. [Google Scholar] [CrossRef] [Green Version]
- Deram, V.; Minichiello, C.; Vannier, R.N.; le Maguer, A.; Pawlowski, L.; Murano, D. Microstructural characterizations of plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2003, 166, 153–159. [Google Scholar] [CrossRef]
- Lawrence, S.K.; Gertrude, S.M. Studies on the relationship of the chemical constituents of blood and cerebrospinal fluid. J. Exp. Med. 1925, 42, 565–591. [Google Scholar]
- Cachinho, S.C.P.; Correia, R.N. Titanium scaffolds for osteointegration: Mechanical, in vitro and corrosion behavior. J. Mater. Sci. Mater. Med. 2008, 19, 451–457. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process: An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Gaona, M.; Guilemany, J.M. Tribological study of plasma hydroxyapatite coatings. Key Eng. Mater. 2004, 16, 383–386. [Google Scholar] [CrossRef]
- Habibovic, P.; Barrère, F.; de Groot, K. Learning from Nature How to Design New Implantable Biomaterials: From Biomineralization Fundamentals to Biomimetic Materials and Processing Routes. NATO Sci. Ser. 2005, 171, 105–121. [Google Scholar]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Milovac, D.; Gamboa-martínez, T.C.; Ivankovic, M.; Gallego, G.; Ivankovic, H. PCL coated hydroxyapatite scaffold derived from cuttle fish bone: In vitro cell culture studies. Mater. Sci. Eng. C 2014, 42, 264–272. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, E01588. [Google Scholar] [CrossRef] [Green Version]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Elsevier: Toronto, ON, Canada, 2004. [Google Scholar]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.H.; Braissant, O.A. A comparative physicochemical study of bioactive glass and bone-derived hydroxyapatite. Ceram. Int. 2011, 37, 1601–1607. [Google Scholar] [CrossRef]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Andronescu, E.; Grumezescu, A.M.; Madalina-Ionela, G.; Holban, A.; Ilie, F.; Irimia, A.; Nicoara, I.; Tone, M. Nano-hydroxyapatite: Novel approaches in biomedical applications. Nano Biomater. Hard Tissue Eng. 2016, 4, 189–213. [Google Scholar]
- Wang, H.X.; Guan, S.K.; Wang, X.; Ren, C.X.; Wang, L.G. In vitro degradation and mechanical integrity of Mg-Zn-Ca alloy coated with Ca-deficient hydroxyapatite by the pulse electrodeposition process. Acta Biomater. 2010, 6, 1743–1748. [Google Scholar] [CrossRef]
- Zainol, I.; Alwi, N.M.; Abidin, M.Z.; Haniza, H.M.Z.; Ahmad, M.S.; Ramli, A. Physicochemical properties of hydroxyapatite extracted from fish scales. J. Adv. Mater. Res. 2012, 545, 239–253. [Google Scholar]
- Burnat, B.; Walkowiak-Przybyło, M.; Błaszczyk, T.; Klimek, L. Corrosion behaviour of polished and sandblasted titanium alloys in PBS solution. Acta Bioeng. Biomech. 2013, 15, 87–95. [Google Scholar] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ASTM F1926/F1926M; Standard Test Method for Dissolution Testing of Calcium Phosphate Granules, Fabricated Forms, and Coatings. ASTM International: West Conshohocken, PA, USA, 2021.
- Wolf, M.F.; Coleman, K.P.; Lewerenz, G.M. In Vitro Assessment of Cell and Tissue Compatibility. In Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 593–608. [Google Scholar]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Vernier Geneva, Switzerland, 2009.
- ISO 13779-2008; Implants for Surgery-Hydroxyapatite Part 3: Chemical Analysis and Characterization of Crystallinity and Phase Purity. International Organization for Standardization: Geneva, Switzerland, 2008.
- Tanya, J.L. Optimisation of Plasma Sprayed Hydroxyapatite Coatings. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2008. [Google Scholar]
- Lee, T.M.; Tsai, R.S.; Chang, E.; Yang, C.Y.; Yang, M.R. The cell attachment and morphology of neonatal rat calvarial osteoblasts on the surface of Ti6Al4V and plasma sprayed HA coating: Effect of surface roughness and serum contents. J. Mater. Sci. Mater. Med. 2002, 13, 341–350. [Google Scholar] [CrossRef]
- Liu, J.; Long, X.; Zhu, H.; Zhu, W.; Chen, Z.; He, D.; Song, N.; Wang, X. Effects of La2O3 contents on microstructure and properties of laser-cladded 5 wt.% CaB6/HA bioceramic coating. Biomed. Mater. 2022, 17, 025007. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Sidhu, B.S. Corrosion behavior of plasma sprayed hydroxyapatite and hydroxyapatite-silicon oxide coatings on AISI 304 for biomedical application. Appl. Surf. Sci. 2013, 284, 811–818. [Google Scholar] [CrossRef]
- Cizek, J.; Aik Khor, K. Role of in-flight temperature and velocity of powder particles on plasma sprayed hydroxyapatite coating characteristics. Surf. Coat. Technol. 2012, 206, 2181–2191. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Anene, F.A.; Jaafar, C.N.A.; Zainol, I.; Hanim, M.A.A.; Suraya, M.T. Evaluation of Corrosion Inhibition of Plasma Sprayed FsHA/YSZ Coating on β-Titanium (Ti-13Nb-13Zr) Alloy Using Electrochemical Techniques. J. Biomim. Biomater. Biomed. Eng. 2022, 58, 45–57. [Google Scholar] [CrossRef]
- Tlotleng, M.; Akinlabi, E.; Shukla, M.; Pityana, S. Microstructures, hardness and bioactivity of hydroxyapatite coatings deposited by direct laser melting process. Mater. Sci. Eng. C 2014, 43, 189–198. [Google Scholar] [CrossRef]
- Hou, B.; Liua, Y.; Chena, H.; Yang, Y. In vitro Bioactivity, Bio-Corrosion Resistance and Antibacterial Property of Laser Cladded HA Coatings with Different Content of ZnO on Ti-6Al-4V Substrate. Mater. Res. 2019, 22 (Suppl. S2), e20180744. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Y.; Rehman, M.A.U. Improvement in the surface properties of stainless steel via zein/hydroxyapatite composite coatings for biomedical applications. J. Surf. 2020, 20, 589–624. [Google Scholar] [CrossRef]
- Solanke, S.; Gaval, V. Most commonly used metallic biomaterials for plasma sprayed hydroxyapatite coatings. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1168, 012013. [Google Scholar] [CrossRef]
- Ning, C.Q.; Zhou, Y. In vitro bioactivity of a biocomposite fabricated from HA and Ti powders by powder metallurgy method. Biomaterials 2002, 23, 2909–2915. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Murakami, H.; Kuroda, S. Effect of hollow spherical powder size distribution on porosity and segmentation cracks in thermal barrier coatings. J. Am. Ceram. Soc. 2006, 89, 3797–3804. [Google Scholar] [CrossRef]



| Ti–6Al–4V | ||||
|---|---|---|---|---|
| Element | Fi | Ni | Mi (g/mol) | NiFi/Mi |
| Ti | 0.895 | 4 | 47.90 | 0.0747 |
| Al | 0.060 | 3 | 26.98 | 0.00667 |
| V | 0.045 | 3 | 50.94 | 0.00265 |
| Ion | Ion Concentration (mM) | |
|---|---|---|
| Blood Plasma | SBF | |
| Na+ | 142.0 | 142.0 |
| K+ | 5.0 | 5.0 |
| Ca2+ | 2.5 | 2.5 |
| Mg2+ | 1.5 | 1.5 |
| Cl− | 103.0 | 147.8 |
| HCO3− | 27.0 | 4.2 |
| HPO42− | 1.0 | 1.0 |
| SO42− | 0.5 | 0.5 |
| pH | 7.2–7.4 | 7.4 |
| Parameters | Uncoated | FsHA + 0 wt.% YSZ | FsHA + 10 wt.% YSZ | FsHA + 15 wt.% YSZ | FsHA + 20 wt.% YSZ |
|---|---|---|---|---|---|
| Ra (µm) | 0.536 | 4.316 | 4.205 | 4.252 | 4.218 |
| Rz (µm) | 3.69 | 27.1 | 28.1 | 24.50 | 28.10 |
| Rmax (µm) | 5.24 | 30.1 | 31.0 | 33.80 | 35.60 |
| Hardness (Hv 0.3) | 360.3 | 459.1 | 497.4 | 531.6 | 558.5 |
| Parameters | Uncoated | FsHA+0 wt.% YSZ | FsHA + 10 wt.% YSZ | FsHA + 15 wt.% YSZ | FsHA + 20 wt.% YSZ |
|---|---|---|---|---|---|
| βa (mV/decade) | 359.1 | 160.7 | 64.8 | 82.93 | 183.2 |
| βc (mV/decade) | 202.9 | 192.6 | 58.6 | 75.76 | 70.11 |
| Ecorr (mV) | −609 | −258.9 | −143 | −87.60 | −366.3 |
| Icorr (nA cm−2) | 485 | 275.3 | 74.3 | 36.30 | 27.11 |
| CR (mmpy) | 169.37 | 96.137 | 25.95 | 12.676 | 9.467 |
| Rp (Ω cm2) | 0.1162 | 0.1384 | 0.1801 | 0.474 | 0.8132 |
| PE (%) | − | 16 | 35.5 | 75.5 | 85.7 |
| OCP (mV) | −613.5 | −233.7 | −129.3 | −55.25 | −286 |
| Negative Control | FsHA + 20 wt.% YSZ (mg/mL) | ||||
|---|---|---|---|---|---|
| 25 | 50 | 100 | 200 | ||
| OD (570 nm) | 0.685 | 0.667 | 0.699 | 0.663 | 0.718 |
| 0.689 | 0.753 | 0.697 | 0.714 | 0.671 | |
| 0.674 | 0.762 | 0.626 | 0.759 | 0.746 | |
| 0.855 | 0.718 | 0.829 | 0.694 | 0.629 | |
| Mean (OD) | 0.726 | 0.725 | 0.713 | 0.708 | 0.691 |
| Viability (%) | 100 | 99.9 | 98 | 98 | 95 |
| Negative Control | Phenol 1 v% (%) | ||||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | ||
| OD (570 nm) | 0.606 | 0.412 | 0.050 | 0.034 | 0.031 |
| 0.566 | 0.370 | 0.048 | 0.037 | 0.031 | |
| 0.576 | 0.365 | 0.048 | 0.031 | 0.031 | |
| 0.582 | 0.336 | 0.045 | 0.036 | 0.033 | |
| Mean (OD) | 0.583 | 0.371 | 0.048 | 0.035 | 0.032 |
| Viability (%) | 100 | 64 | 8 | 6 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anene, F.A.; Jaafar, C.N.A.; Mohamed Ariff, A.H.; Zainol, I.; Mohd Tahir, S.; Abdul Razak, B.; Salit, M.S.; Anene-Amaechi, J. Biomechanical Properties and Corrosion Resistance of Plasma-Sprayed Fish Scale Hydroxyapatite (FsHA) and FsHA-Doped Yttria-Stabilized Zirconia Coatings on Ti–6Al–4V Alloy for Biomedical Applications. Coatings 2023, 13, 199. https://doi.org/10.3390/coatings13010199
Anene FA, Jaafar CNA, Mohamed Ariff AH, Zainol I, Mohd Tahir S, Abdul Razak B, Salit MS, Anene-Amaechi J. Biomechanical Properties and Corrosion Resistance of Plasma-Sprayed Fish Scale Hydroxyapatite (FsHA) and FsHA-Doped Yttria-Stabilized Zirconia Coatings on Ti–6Al–4V Alloy for Biomedical Applications. Coatings. 2023; 13(1):199. https://doi.org/10.3390/coatings13010199
Chicago/Turabian StyleAnene, Franklin A., Che Nor Aiza Jaafar, Azmah Hanim Mohamed Ariff, Ismail Zainol, Suraya Mohd Tahir, Bushroa Abdul Razak, Mohd Sapuan Salit, and Joy Anene-Amaechi. 2023. "Biomechanical Properties and Corrosion Resistance of Plasma-Sprayed Fish Scale Hydroxyapatite (FsHA) and FsHA-Doped Yttria-Stabilized Zirconia Coatings on Ti–6Al–4V Alloy for Biomedical Applications" Coatings 13, no. 1: 199. https://doi.org/10.3390/coatings13010199
APA StyleAnene, F. A., Jaafar, C. N. A., Mohamed Ariff, A. H., Zainol, I., Mohd Tahir, S., Abdul Razak, B., Salit, M. S., & Anene-Amaechi, J. (2023). Biomechanical Properties and Corrosion Resistance of Plasma-Sprayed Fish Scale Hydroxyapatite (FsHA) and FsHA-Doped Yttria-Stabilized Zirconia Coatings on Ti–6Al–4V Alloy for Biomedical Applications. Coatings, 13(1), 199. https://doi.org/10.3390/coatings13010199








