Novel Thermal Barrier Coatings with Phase Composite Structures for Extreme Environment Applications: Concept, Process, Evaluation and Performance
Abstract
:1. Introduction
2. Experimental Work
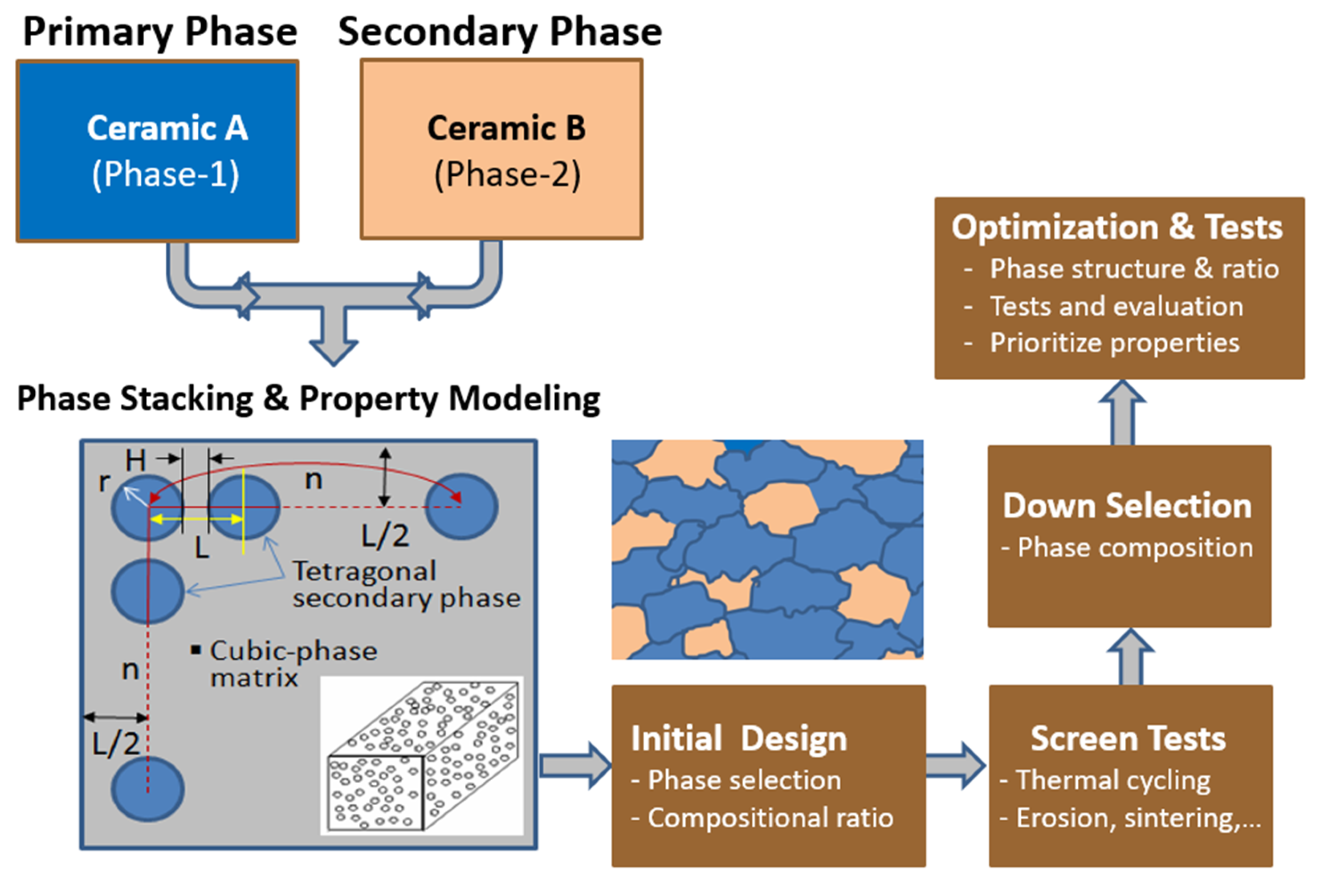
2.1. The Concept of Phase Composite Ceramics
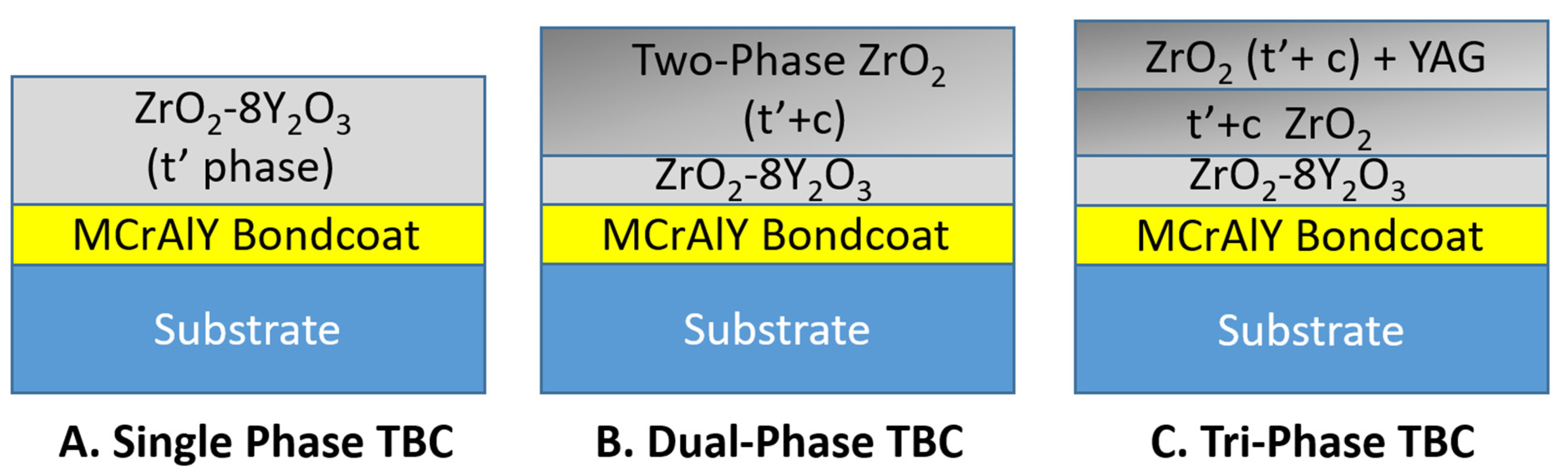
2.2. Process for Producing Dual-Phase TBC Coating
2.3. Process for Producing Tri-Phase TBC Coating
2.4. Thermal Stability Study
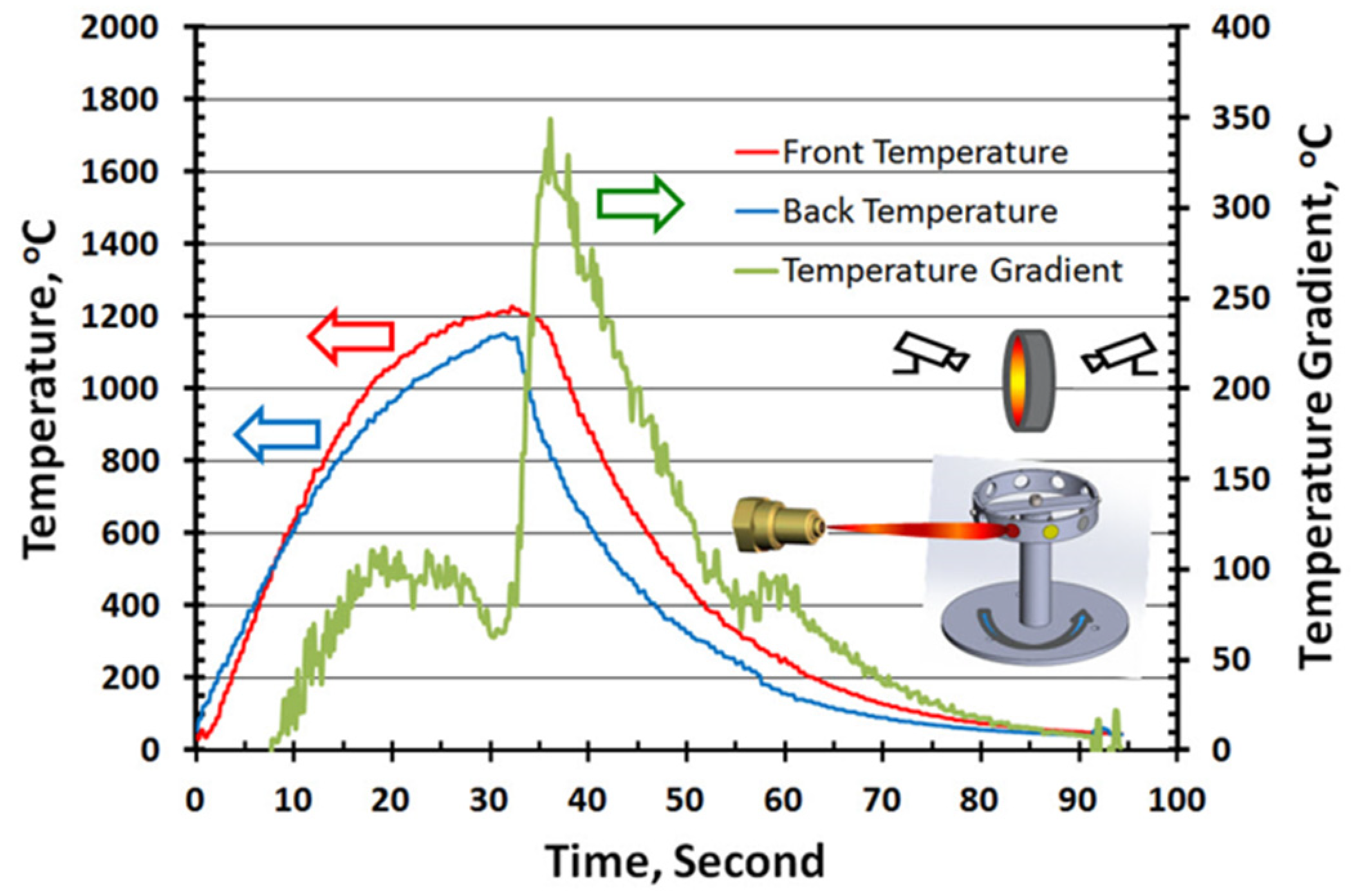
2.5. Thermal Shock Test
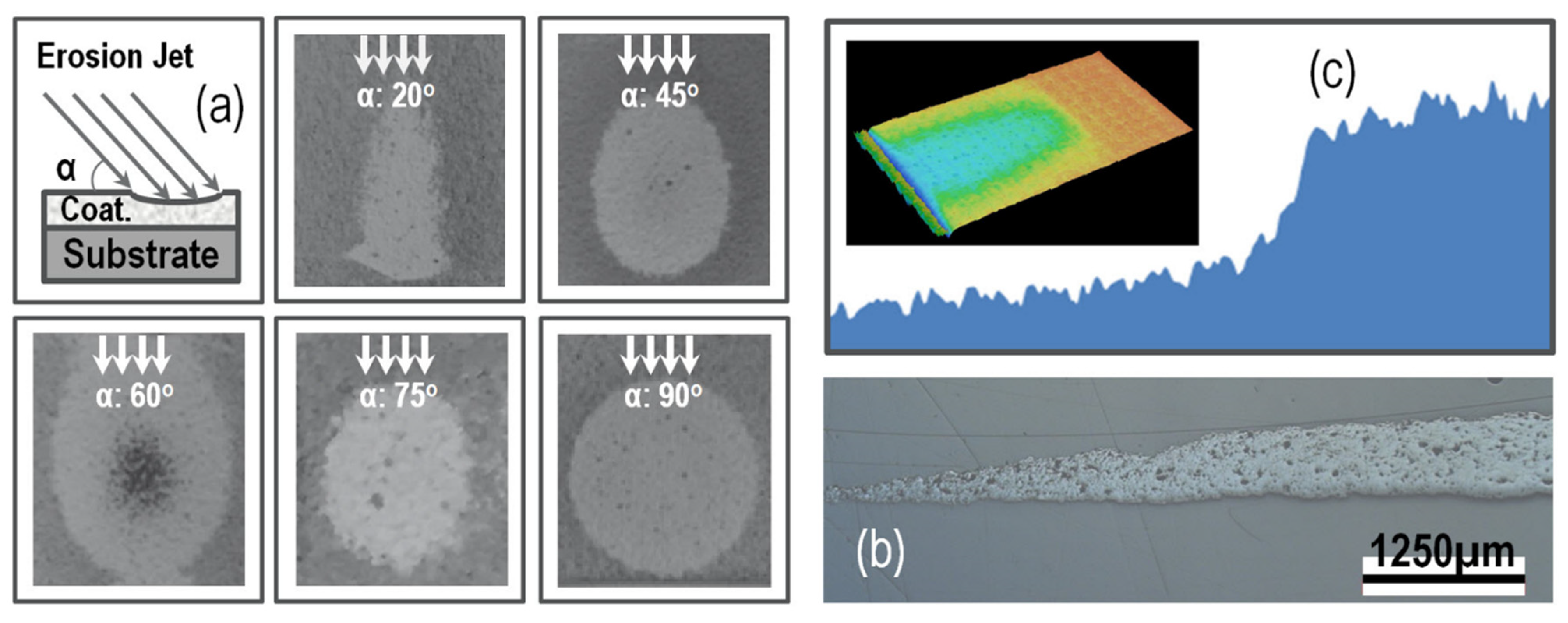
2.6. Solid Particle Erosion Test
2.7. CMAS Corrosion Test
3. Results and Discussion
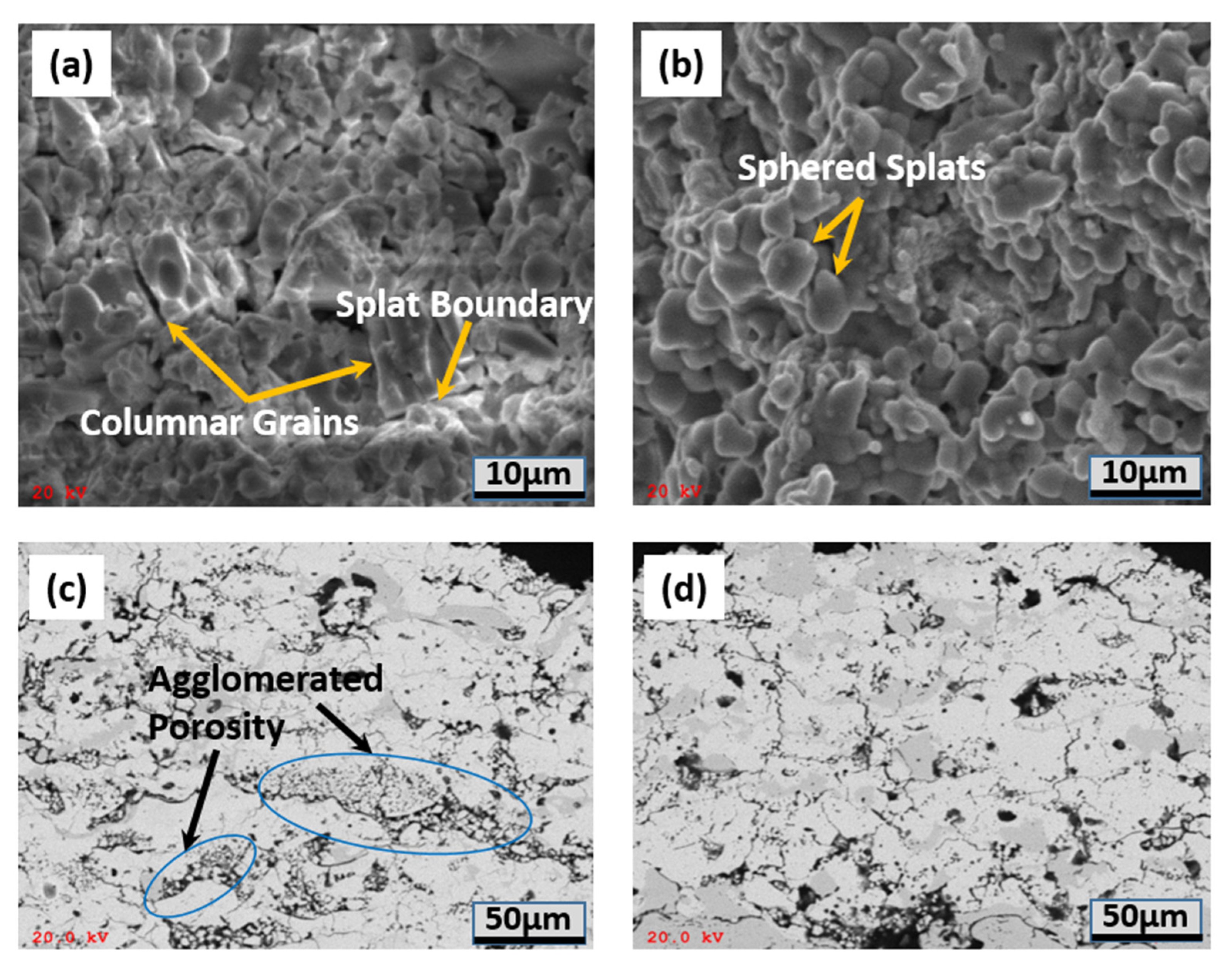
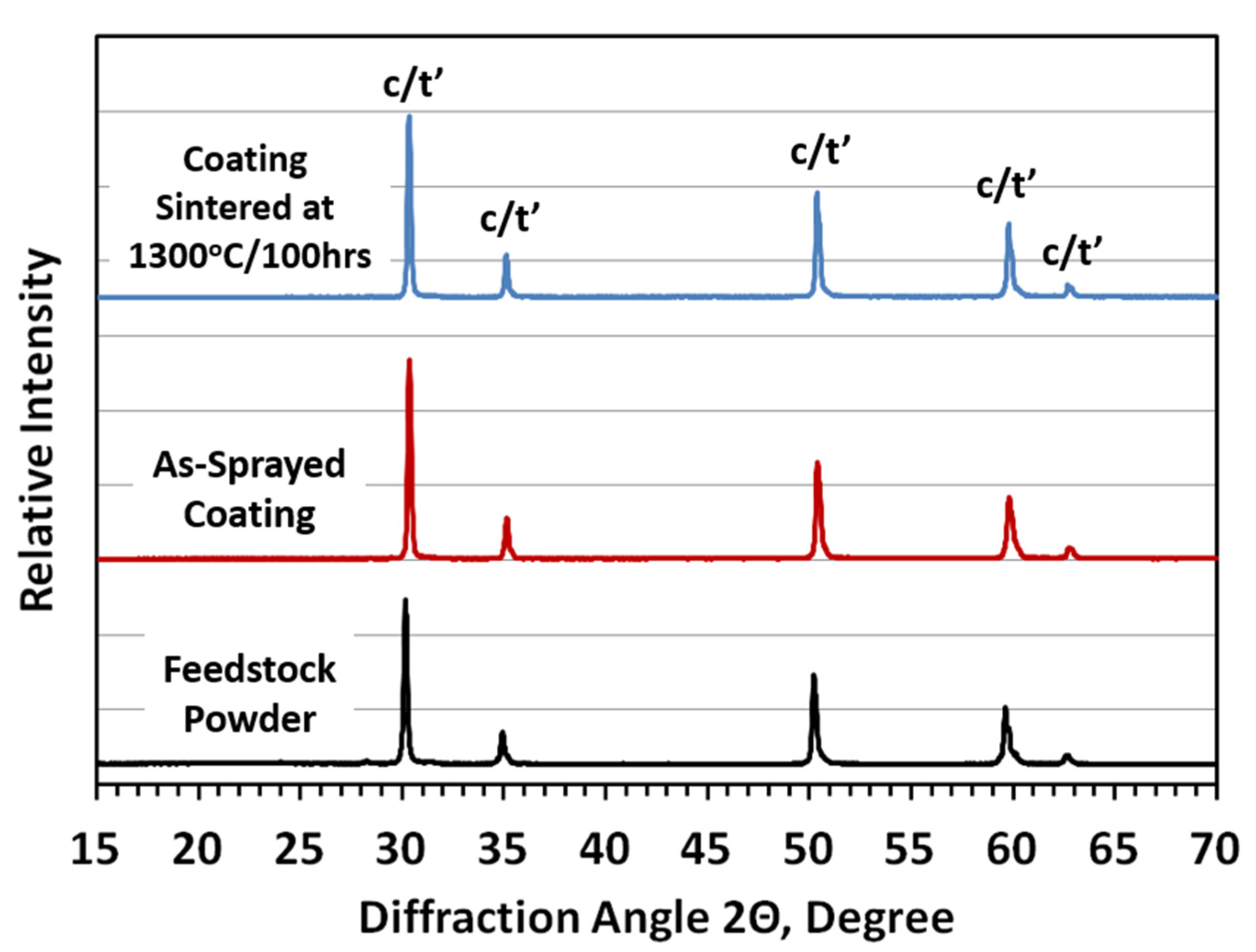
3.1. Thermal Stability and Sintering Behavior
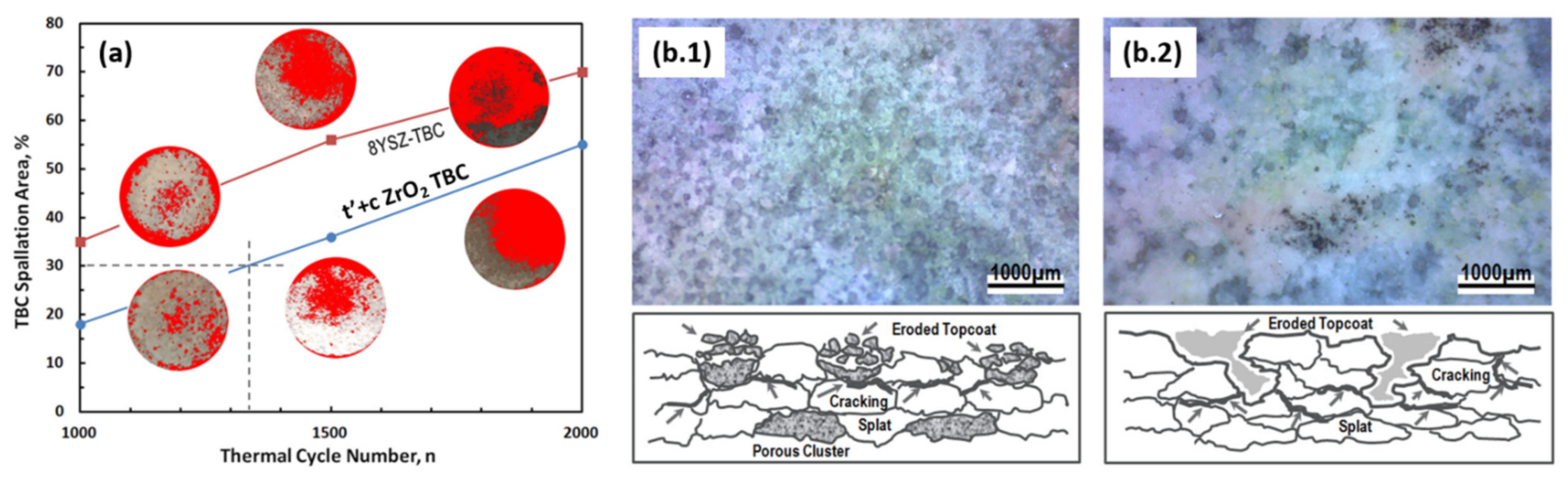
3.2. Thermal Shock Test
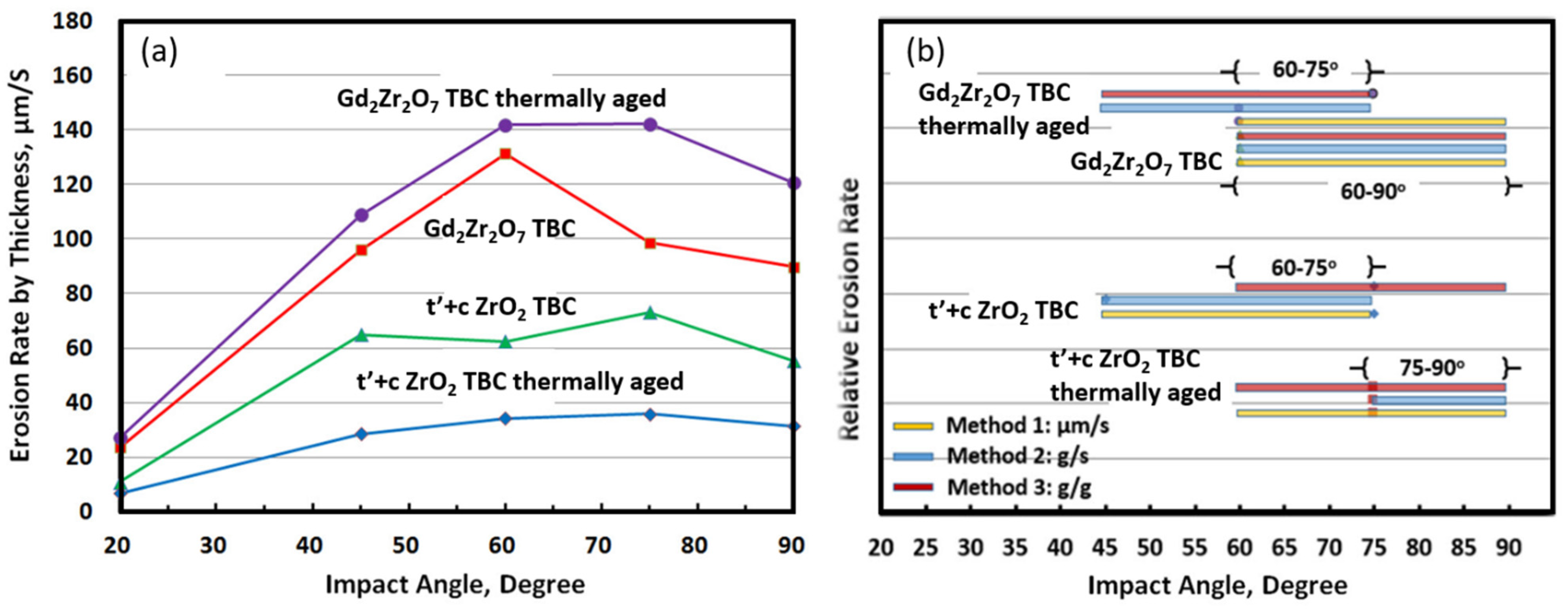
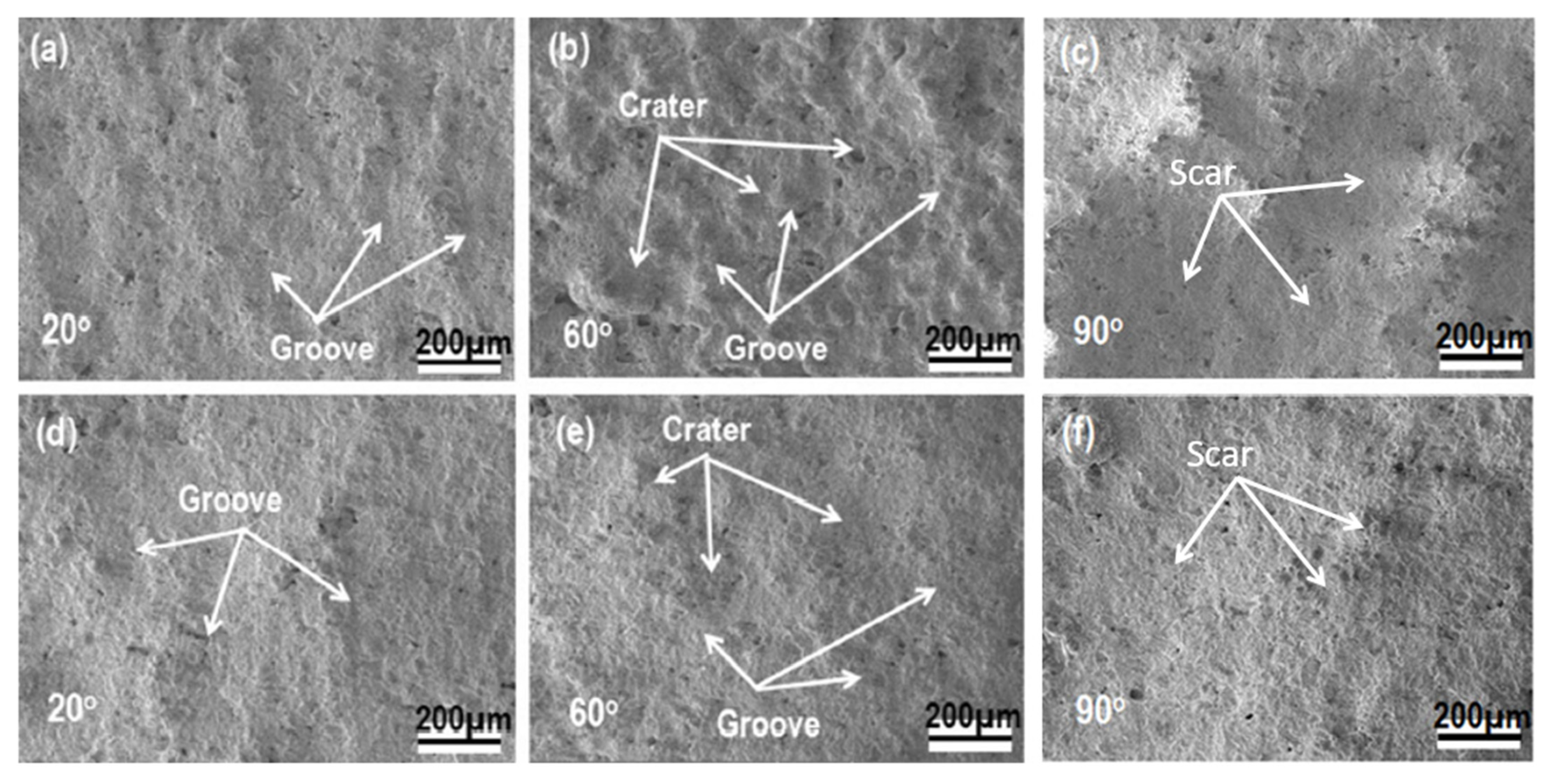
3.3. Solid Particle Erosion Test
- Among all the specimens, the group of phase composite TBCs showed much lower SPE erosion rates than the group of zirconate TBCs.
- The sintering treatment at 1300 °C was more influential for the t’+c phase TBCs in reducing the erosion rate relative to the Gd2Zr2O7 TBCs. However, the thermal aging of the Gd2Zr2O7 TBCs had little effect on the erosion rate.
- It has been clearly demonstrated that the impinging angle of the TBCs has a substantial impact on their erosion behaviors. Samples exposed to angles greater than 60° exhibited significantly increased rates of erosion. It appears that a mode combining both brittle and rough material properties is best suited for describing the erosion behavior of both groups.
- The comparison of erosion rates obtained from three separate methods revealed the presence of commonly overlapping regions of high erosion rates, as a function of the impact angle. For instance, in the case of an as-sprayed composite TBC, an overlapping region of high erosion rates was observed to be between 60°~75°, while in the aged composite TBC, this shifted to 75°~90°. This shift may suggest that the sintering treatment has caused a variation in the primary cause of erosion losses. Consequently, these common regions for each type of TBC can serve to indicate the dominant erosive behavior with respect to impact angle. The maximum erosion rate observed can be attributed to two distinct mechanisms, namely, a ductile erosion mechanism for alloy materials at lower impact angles and a brittle erosion mechanism for ceramic materials at higher impact angles. The topcoat coating is composed of a ZrO2-based ceramic material, which is known for its high toughness as well as its porosity. This composite material exhibits both ductile and brittle properties when subject to an intermediate impact angle.
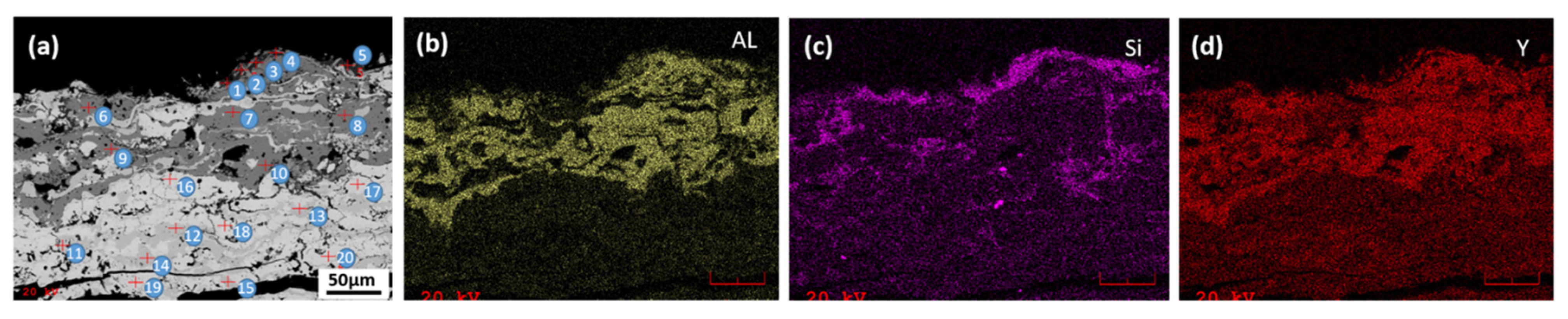
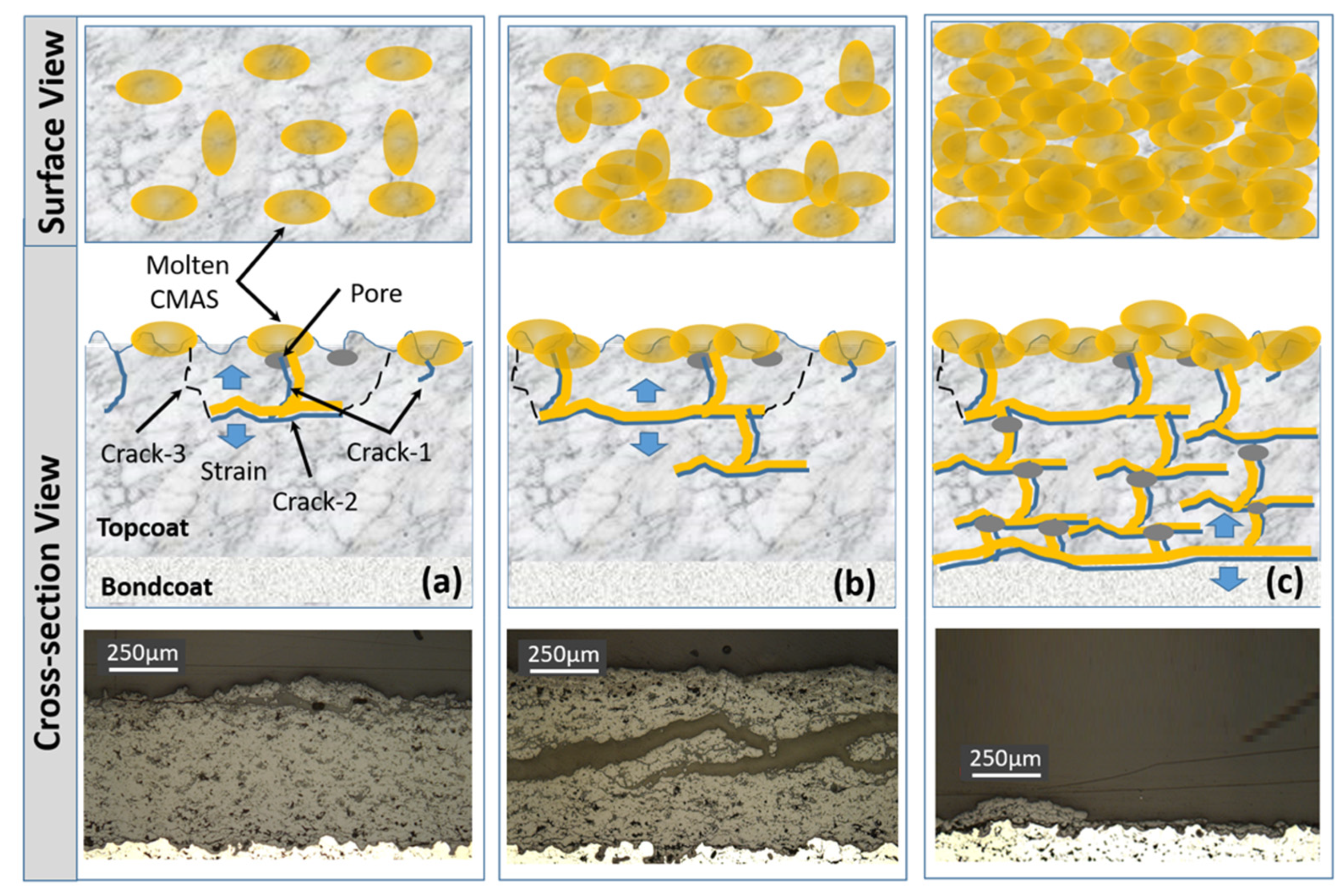
3.4. CMAS Corrosion Test
4. Conclusions
- The thermal exposure test at 1300 °C was performed to evaluate the phase thermal stability and sintering behavior of two-phase composite TBCs. The XRD analysis results indicate that high phase stability was achieved without phase transformation. In terms of the single-phase 8YSZ-TBC, the phase composite TBCs show better thermal sintering resistance.
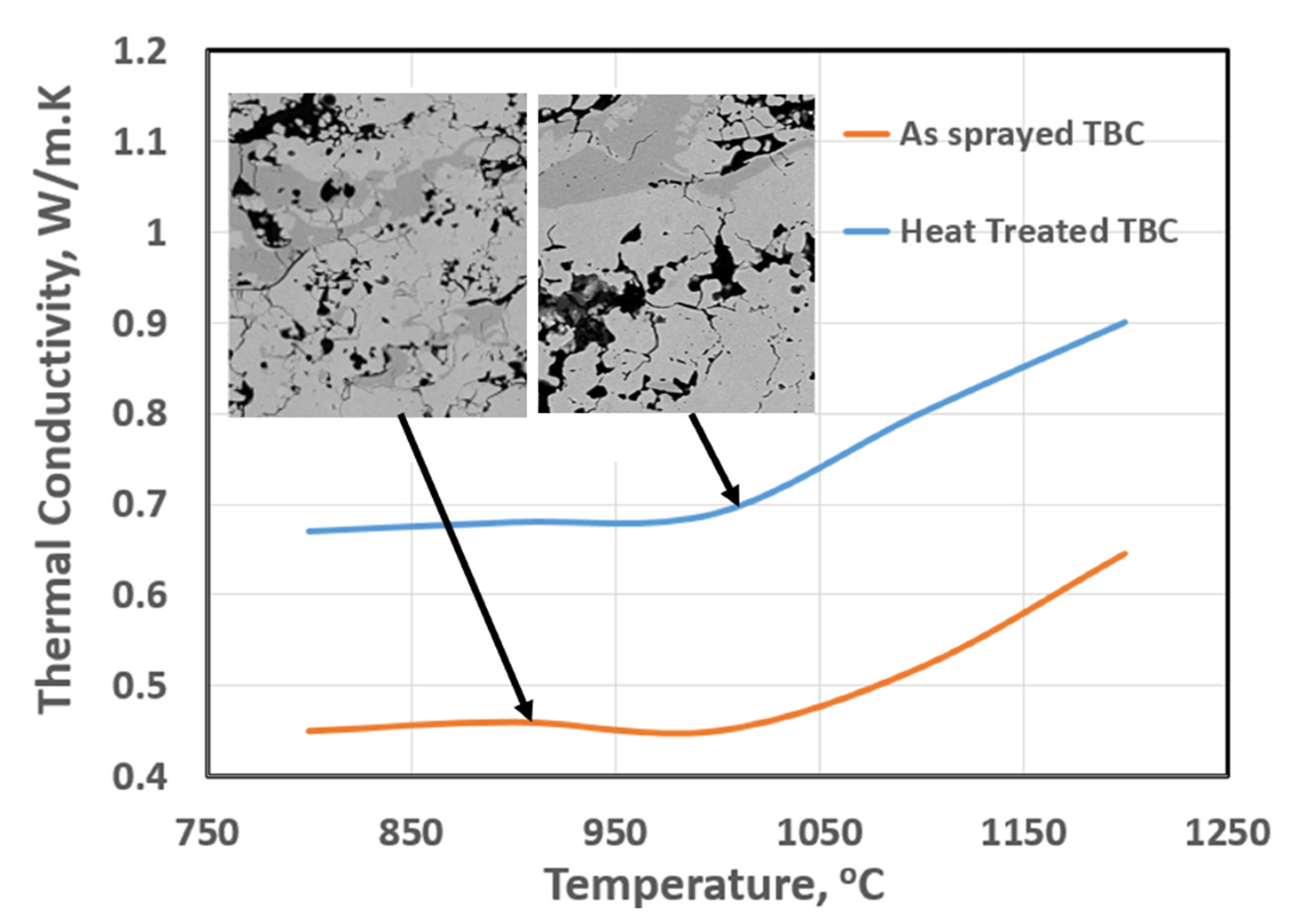
- Thermal conductivity K, for the as-sprayed two-phase TBCs was measured as 0.45~0.65 W/m K at the temperature range of 1000~1200 °C. This demonstrates the merit of low-thermal conductivity TBC. Thermal aging at 1300 °C led to an increase in K values.
- The burner-rig test was proven to significantly accelerate the TBCs’ deterioration and failure. The thermal cycling lifetime of the t’+c phase composite TBC is acceptable and is comparable to that of the baseline of the single t’-phase, 8YSZ-TBC.
- The results of the solid-particle erosion show that the erosion rates of the TBCs tested strongly depend on the impingement angle, while the highest erosion rates existed in the medium- and high angle ranges from 60° to 90°. The t’+c zirconia TBC has demonstrated low erosion rates relative to the single-phase zirconate TBC. The combined mode of ductile or tough and brittle modes had been utilized to elaborate the TBCs’ erosion behaviors and erosion mechanisms.
- Tri-phase TBCs were obtained by adding a third YAG phase to the t’+c zirconia topcoat, then they were investigated for CMAS corrosion tests at 1250 °C for the maximum exposure time of 120 h. In all the tested TBC samples, the CMAS attack was found to be very aggressive and caused coating damage. Compared with single-phase 8YSZ TBC, the degradation of the t’+c TBC and its YAG-modified TBC is inhibited, to some degree. In particular, the YAG additive is more effective in slowing down the process of CMAS infiltration, due to the formation of crystalline compounds with low mobility in the chemical reactions of the YAG phase with molten CMAS.
- The overall performance of the phase composite ceramics in the experimental tests proves their potential as promising TBC topcoats for next-generation advanced gas turbine engines that are operating in extreme environments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondal, K.; NuñezIII, L.; Downey, C.; Rooyen, I. Recent advances in the thermal barrier coatings for extreme environments. Mater. Sci. Energy Technol. 2021, 4, 208–210. [Google Scholar] [CrossRef]
- Lee, K.; Zhu, D.; Lima, R. Perspectives on environmental barrier coatings (EBCs) manufactured via air plasma spray (APS) on ceramic matrix composites (CMCs): A tutorial paper. J. Therm. Spray Technol. 2021, 30, 40–58. [Google Scholar] [CrossRef]
- Clarke, D.; Levi, C. Materials design for the next generation thermal barrier coatings. Ann. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.; Steinke, T.; Mack, D.; Stover, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Perepezko, J. The hotter the engine, the better. Science 2009, 326, 1068–1069. [Google Scholar] [CrossRef]
- Hossain, M.; Rube, M.H.K.; Akbar, M.A.; Ahmed, M.H.; Haque, N.; Rahman, M.F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Intl. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Miller, R. Thermal barrier coatings for aircraft engines: History and directions. J. Therm. Spray Technol. 1997, 6, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Stecura, S. Effects of compositional changes on the performance of a thermal barrier coating system. In Proceedings of the Third Annual Conference on Composite and Advanced Materials, Merritt Island, FL, USA, 21–24 January 1979. NASA TM-78976 Report. [Google Scholar]
- Stabican, V. Phase equilibria and metastabilities in the systems ZrO2-MgO, ZrO2-CaO and ZrO2-Y2O3. Adv. Ceram. 1984, 24A, 71–85. [Google Scholar]
- Hasselman, D. Thermal diffusivity & conductivity of dense polycrystalline ZrO2 ceramics: A survey. Am. Ceram. Soc. Bull. 1987, 66, 799–806. [Google Scholar]
- Schlichting, W.; Padture, N.; Klemens, P. Thermal conductivity of dense and porous yttria-stabilized zirconia. J. Mater. Sci. 2001, 36, 3003–3010. [Google Scholar] [CrossRef]
- Hutchings, I. Transitions, threshold effects and erosion maps. Key Eng. Mater. 1992, 71, 75–92. [Google Scholar]
- Ramachandran, C.; Balasubramanian, V.; Ananthapadmanabhan, P. Erosion of atmospheric plasma sprayed rare earth oxide coatings under air suspended corundum particles. Ceram. Int. 2013, 39, 649–672. [Google Scholar] [CrossRef]
- Mahade, S.; Curry, N.; Bjorklund, S.; Markocsan, N.; Nylen, P.; Vaßen, R. Erosion performance of gadolinium zirconate-based thermal barrier coatings processed by suspension plasma spray. J. Therm. Spray Technol. 2017, 26, 108–115. [Google Scholar] [CrossRef]
- Mahade, S.; Zhou, D.; Curry, N.; Markocsan, N.; Nylen, P.; Vaßen, R. Tailored microstructures of gadolinium zirconate/YSZ multi-layered thermal barrier coatings produced by suspension plasma spray: Durability and erosion testing. J. Mater. Proc. Technol. 2019, 264, 283–294. [Google Scholar] [CrossRef]
- Mahade, S.; Ruelle, C.; Curry, N.; Holmberg, J.; Bjorklund, S.; Markocsan, N.; Nylen, P. Understanding the effect of material composition and microstructural design on the erosion behavior of plasma sprayed thermal barrier coatings. Appl. Surf. Sci. 2019, 488, 170–184. [Google Scholar] [CrossRef]
- Kramer, S.; Yang, J.; Levi, C.; Johnson, C. Thermochemical interaction of thermal barrier coatings with molten CaO-MgO-Al2O3-SiO2 (CMAS) deposits. J. Am. Ceram. Soc. 2006, 9, 3167–3175. [Google Scholar] [CrossRef]
- Levi, C.; Hutchinson, J.; Vidal-Setif, M.; Johnson, C. Environmental degradation of thermal barrier coatings by molten deposits. MRS Bull. 2012, 37, 932–941. [Google Scholar]
- Kim, J.; Dunn, M.; Baran, A.; Wade, D.; Tremba, E. Deposition of volcanic materials in the hot sections of two gas turbine engines. J. Eng. Gas Turbines Power 1993, 115, 641–651. [Google Scholar] [CrossRef]
- Clarke, D.; Oechsner, M.; Padture, N. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef] [Green Version]
- DeWet, D.; Taylor, R.; Stott, F. Corrosion mechanisms of ZrO2-Y2O3 thermal barrier coatings in the presence of molten middle-east sand. J. Phys. IV 1993, 3, 655–663. [Google Scholar]
- Craig, M.; Ndamka, N.; Wellman, R.; Nicholls, J. CMAS degradation of EB-PVD TBCs: The effect of basicity. Surf. Coat. Technol. 2015, 270, 145–153. [Google Scholar] [CrossRef]
- Aygun, A.; Vasiliev, A.; Padture, N.; Ma, X. Novel thermal barrier coatings that are resistant to high-temperature attack by glassy deposits. Acta Mater. 2007, 55, 6734–6745. [Google Scholar] [CrossRef]
- Kramer, S.; Yang, J.; Levi, C. Infiltration-inhibiting reaction of gadolinium zirconate thermal barrier coatings with CMAS melts. Am. Ceram. Soc. 2008, 91, 576–583. [Google Scholar] [CrossRef]
- Kumar, R.; Jordan, E.; Gell, M.; Roth, J.; Jiang, C.; Wang, J.; Rommel, S. CMAS behavior of yttrium aluminum garnet (YAG) and yttria-stabilized zirconia (YSZ) thermal barrier coatings. Surf. Coat. Technol. 2015, 327, 126–138. [Google Scholar] [CrossRef]
- Ma, X.; Rivellini, K.; Ruggiero, P.; Wildridge, G. Toward durable thermal barrier coating with composite phases and low thermal conductivity. J. Therm. Spray Technol. 2020, 29, 423–432. [Google Scholar] [CrossRef]
- Ma, X.; Rivellini, K.; Ruggiero, P.; Wildridge, G. Evaluation and characterization of a durable composite phase thermal barrier coating in solid particle erosion and burner rig tests. J. Therm. Spray Technol. 2021, 30, 69–80. [Google Scholar] [CrossRef]
- Ma, X.; Ruggiero, P.; Wildridge, G. Evaluation of CMAS resistance and failure behavior for phase composite thermal barrier coatings. J. Therm. Spray Technol. 2023, 32. (under published). [Google Scholar]
- Gu, J.; Wei, B.; Berendt, A.; Ghoshal, A.; Walock, M.; Reidy, R.; Berman, D.; Aouadi, S. A comparative study of calcium–magnesium–aluminum–silicon oxide mitigation in selected self-healing thermal barrier coating ceramics. J. Mater. Res. 2020, 220, 2311–2320. [Google Scholar] [CrossRef]
- Keyvani, A.; Bahamirian, M.; Kobayashi, A. Effect of sintering rate on the porous microstructural, mechanical and thermomechanical properties of YSZ and CSZ TBC coatings undergoing thermal cycling. J. Alloys Compd. 2017, 727, 1057–1066. [Google Scholar] [CrossRef]
- Lv, B.; Mücke, R.; Fan, X.; Wang, T.J.; Guillon, O.; Vaßen, R. Sintering resistance of advanced plasma-sprayed thermal barrier coatings with strain-tolerant microstructures. J. Eur. Ceram. Soc. 2018, 38, 5092–5100. [Google Scholar] [CrossRef]
- Clarke, D. Materials selection guidelines for low thermal conductivity TBCs. Surf. Coat. Technol. 2003, 164, 67–74. [Google Scholar] [CrossRef]
- Zhu, D. Low conductivity thermal barrier coating. In Proceedings of the School of Materials Engineering Seminar, West Lafayette, IN, USA, 28 February 2005; NASA technical report, TM-2005-21385. pp. 1–29. [Google Scholar]
- Scheibel, J.; Fick, K.; Elbel, S.; Smith, J. Gas turbine low conductivity thermal barrier coating validation and demonstration. In The Future of Gas Turbine Technology, Proceedings of the 8th International Gas Turbine Conference, Brussels, Belgium, 12–13 October 2016; 48-IGTC16; European Turbine Network: Brussels, Belgium, 2016; pp. 1–10. [Google Scholar]
- Li, C.; Yang, G.; Ohmori, A. Relationship between particle erosion and lamellar microstructure for plasma-sprayed alumina coating. Wear 2006, 260, 1166–1172. [Google Scholar] [CrossRef]
- Goretta, K.; Pena, J.; Orera, V.; Chen, N.; Singh, D.; Routbort, J. Solid-particle erosion of directionally solidified Al2O3-ZrO2 (Y2O3) eutectics. Wear 2010, 268, 571–578. [Google Scholar] [CrossRef]
- Kitamura, J.; Tang, Z.; Mizuno, H.; Sato, K.; Burgess, A. Structural, mechanical and erosion properties of yttrium oxide coatings by axial suspension plasma spraying for electronics applications. J. Therm. Spray Technol. 2011, 20, 170–185. [Google Scholar] [CrossRef]
- Mishra, S.; Prakash, S.; Chandra, K. Studies on erosion behaviour of plasma sprayed coatings on a Ni-based superalloy. Wear 2006, 260, 422–432. [Google Scholar] [CrossRef]
- Eaton, H.; Novak, R. Particulate erosion of plasma-sprayed porous ceramics. Surf. Coat. Technol. 1987, 30, 41–50. [Google Scholar] [CrossRef]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Kahn, R.; Levy, R.; Verduzco, C.; Villeneuve, P.J. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Environ. Health Perspect. 2010, 118, 847–855. [Google Scholar] [CrossRef] [Green Version]
- Thorsteinsson, T.; Jóhannsson, T.; Stohl, A.; Kristiansen, N.I. High levels of particulate matter in Iceland due to direct ash emissions by the Eyjafjallajokull eruption and resuspension of deposited ash. J. Geophys. Res. Solid Earth 2012, 117, B00C05. [Google Scholar] [CrossRef]
- Kueppers, U.; Cimarelli, C.; Hess, K.-U.; Taddeucci, J.; Wadsworth, F.B.; Dingwell, D.B. The thermal stability of Eyjafjallajokull ash versus turbine ingestion sands. J. Appl. Volcanol. 2014, 3, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Turcer, L.; Krause, A.; Garces, H.; Zhang, L.; Padture, N. Environmental-barrier coating ceramics for resistance against attack by molten calcia-magnesia-alumino silicate (CMAS) glass: Part II, β-Yb2Si2O7 and β-Sc2Si2O7. J. Eur. Ceram. Soc. 2018, 38, 3914–3924. [Google Scholar] [CrossRef]
- Dimitrov, V.; Sakka, S. Electronic oxide polarizability and optical basicity of simple oxides. J. Appl. Phys. 1996, 79, 1736–1740. [Google Scholar] [CrossRef]
- Ndamka, N.; Wellman, R.; Nicholls, J. The degradation of thermal barrier coatings by molten deposits: Introducing the concept of basicity. Mater. High Temp. 2016, 33, 44–50. [Google Scholar] [CrossRef]



| TBC Samples | 1. Thermal Stability Test | 2. Thermal Shock Test | 3. Erosion Test | 4. CMAS Test |
|---|---|---|---|---|
| ZrO2-8Y2O3 (t’) | √ | √ | √ | |
| ZrO2-20Y2O3 (c) | √ | |||
| Gd2Zr2O7 | √ | |||
| ZrO2 (t’+c) | √ | √ | √ | √ |
| ZrO2 (t’+c) + YAG | √ |
| Point# | t’+c+YAG TBC: SEM/EDXS Results, wt. % Average | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Detection Layer/Zone | Main Elements | ||||||||
| Zr | Al | Ca | Y | Si | Mg | Na | RE | ||
| P1-5 | Dark surface layer | 0 | 28 | 20.4 | 7.8 | 42.3 | 0.3 | 1.1 | |
| P6-10 | Dark gray affected zone | 0 | 26.6 | 0.9 | 70.9 | 1 | 0.6 | ||
| P11-15 | Light gray affected zone | 88 | 0.5 | 0 | 11.3 | ||||
| P16-20 | Main coating zone | 69.7 | 0.1 | 0.2 | 6.4 | 0.5 | 0 | 23 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Rivellini, K.; Ruggiero, P.; Wildridge, G. Novel Thermal Barrier Coatings with Phase Composite Structures for Extreme Environment Applications: Concept, Process, Evaluation and Performance. Coatings 2023, 13, 210. https://doi.org/10.3390/coatings13010210
Ma X, Rivellini K, Ruggiero P, Wildridge G. Novel Thermal Barrier Coatings with Phase Composite Structures for Extreme Environment Applications: Concept, Process, Evaluation and Performance. Coatings. 2023; 13(1):210. https://doi.org/10.3390/coatings13010210
Chicago/Turabian StyleMa, Xinqing, Kristina Rivellini, Peter Ruggiero, and George Wildridge. 2023. "Novel Thermal Barrier Coatings with Phase Composite Structures for Extreme Environment Applications: Concept, Process, Evaluation and Performance" Coatings 13, no. 1: 210. https://doi.org/10.3390/coatings13010210




