Tailoring the Structural, Optical and Electrical Properties of Zinc Oxide Nanostructures by Zirconium Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Un-Doped and Zr-Doped ZnO (ZZO) Nanopartciles
2.2. Deposition of Un-Doped and Zr-Doped ZnO (ZZO) Films
2.3. Characterization Tools
3. Results and Discussion
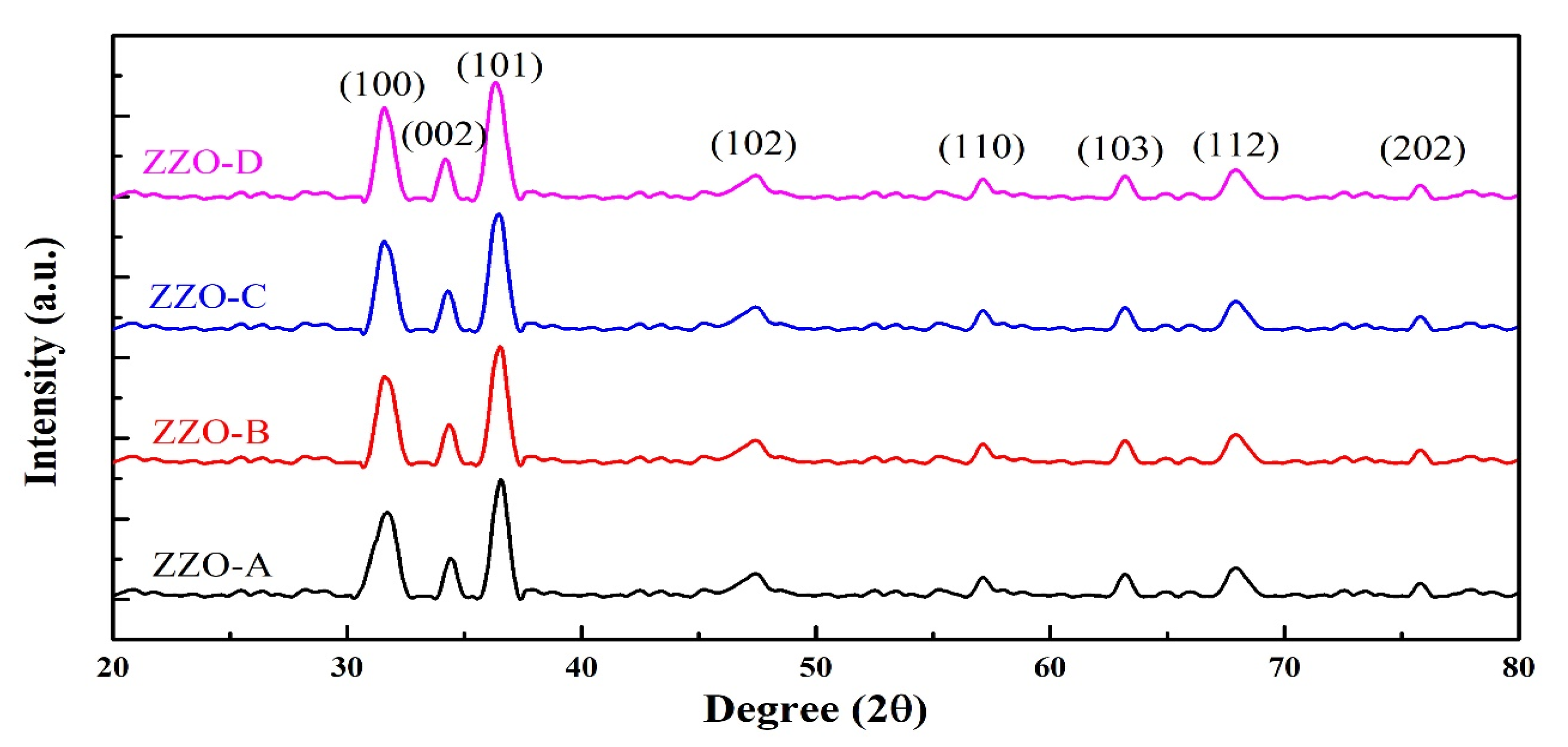
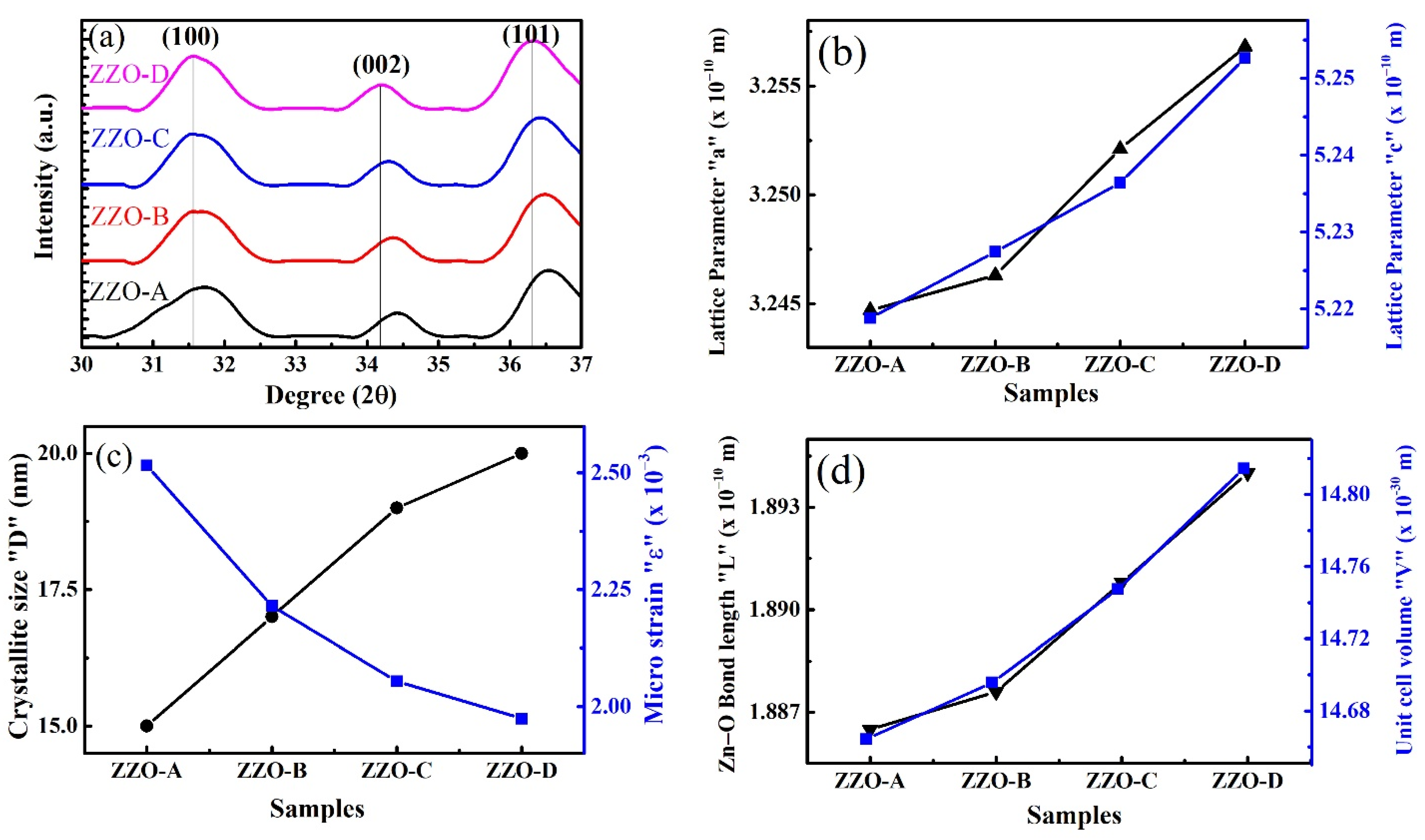
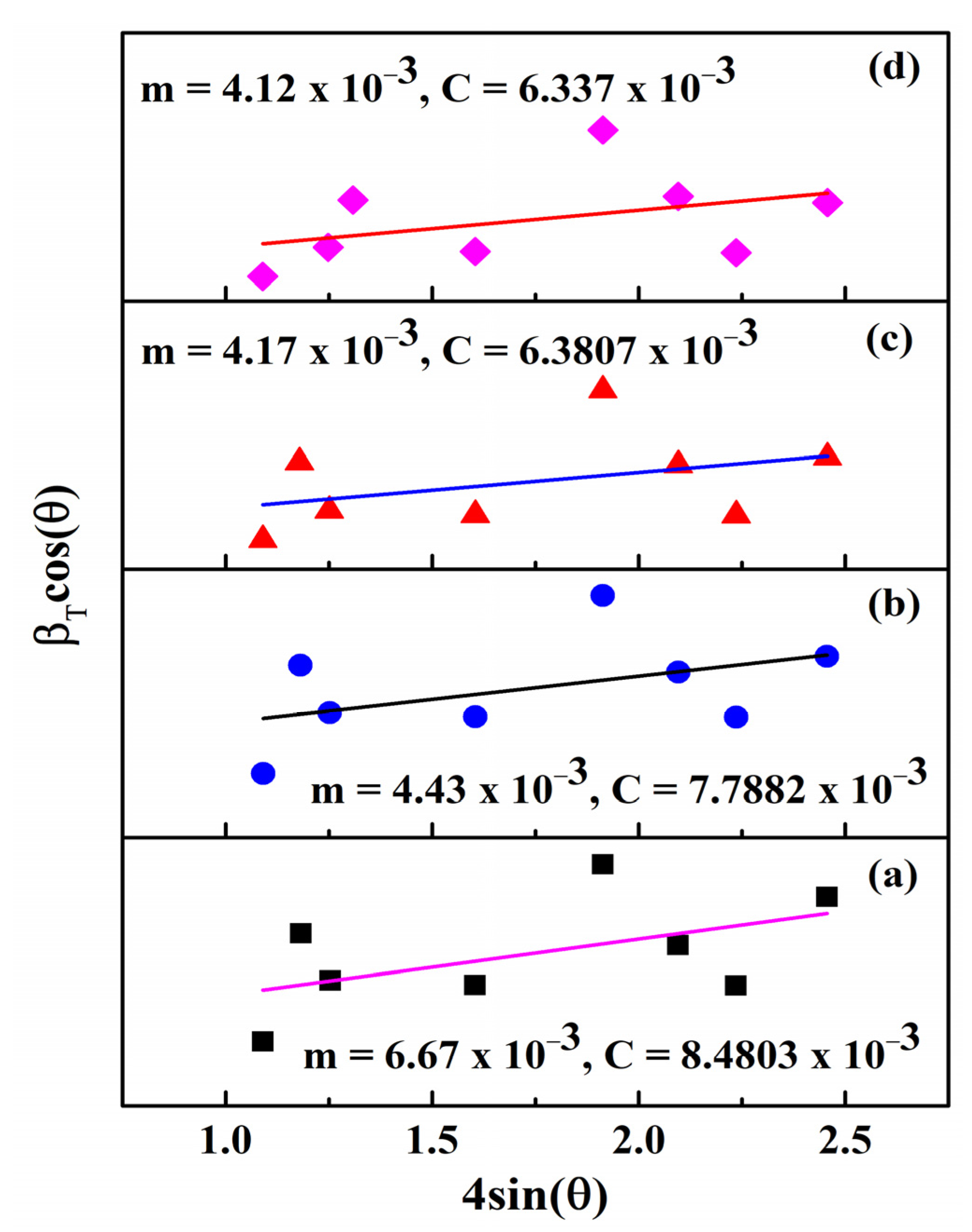
3.1. XRD Analysis
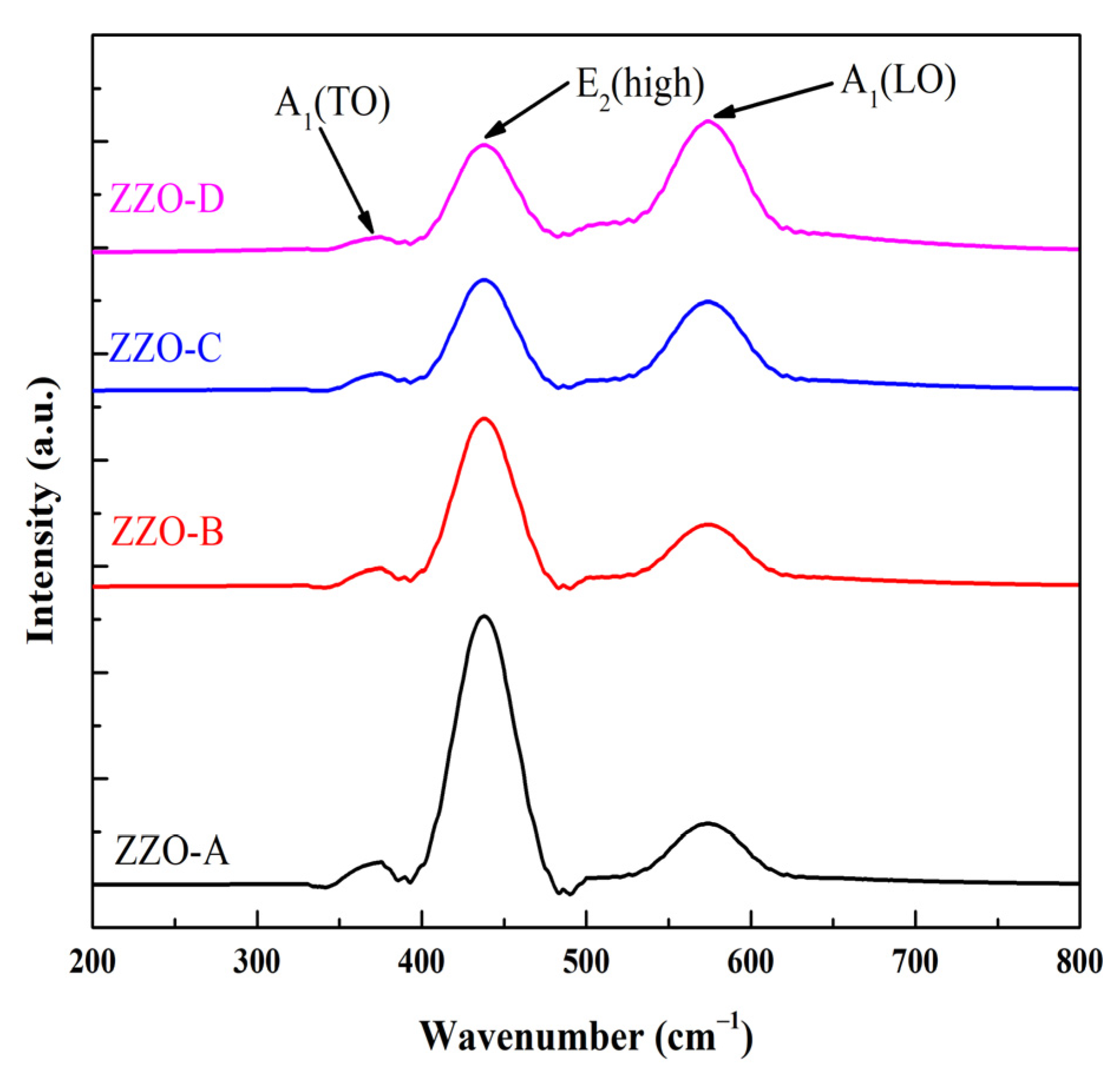
3.2. Raman Analysis
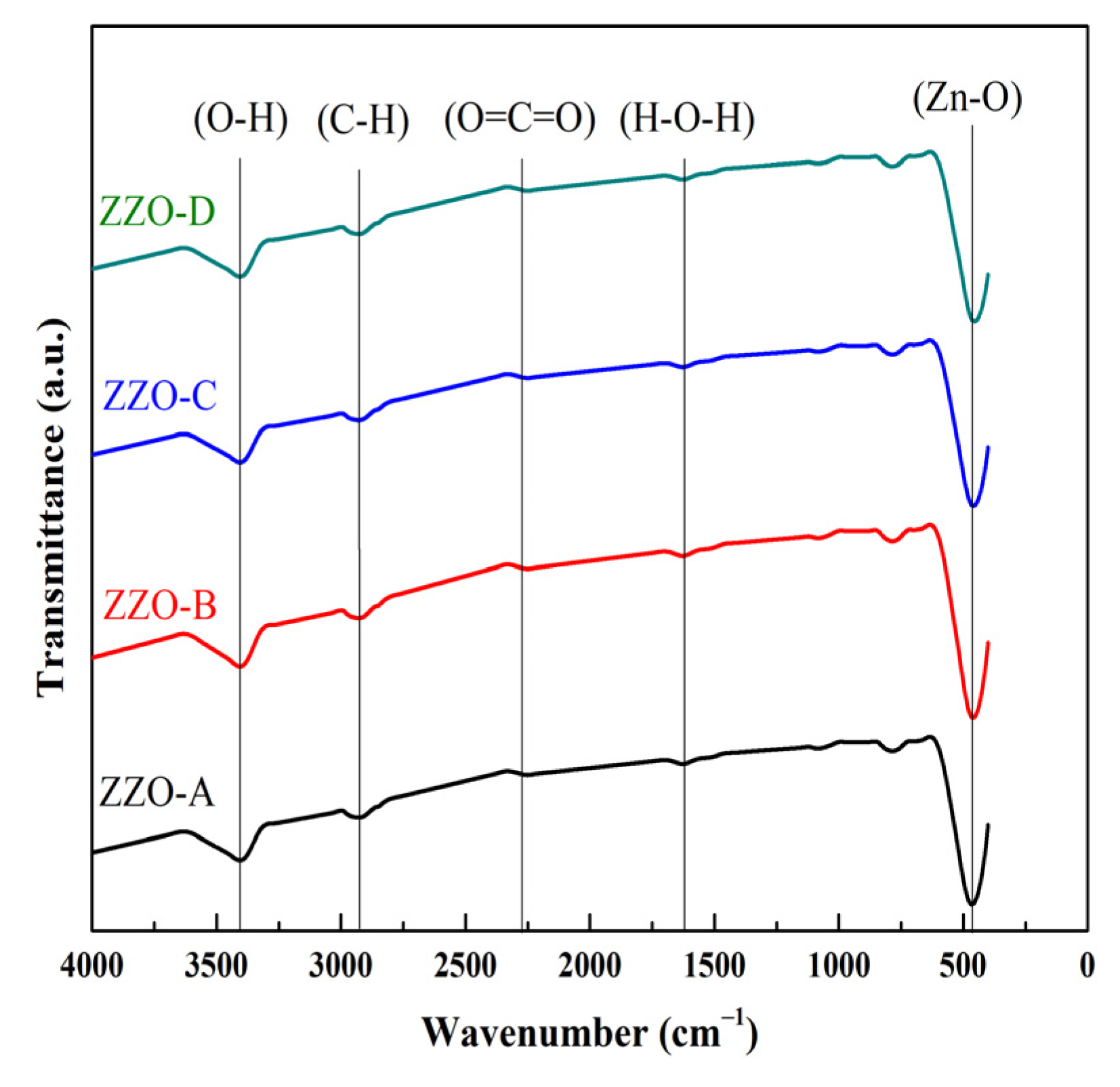
3.3. FTIR Analysis
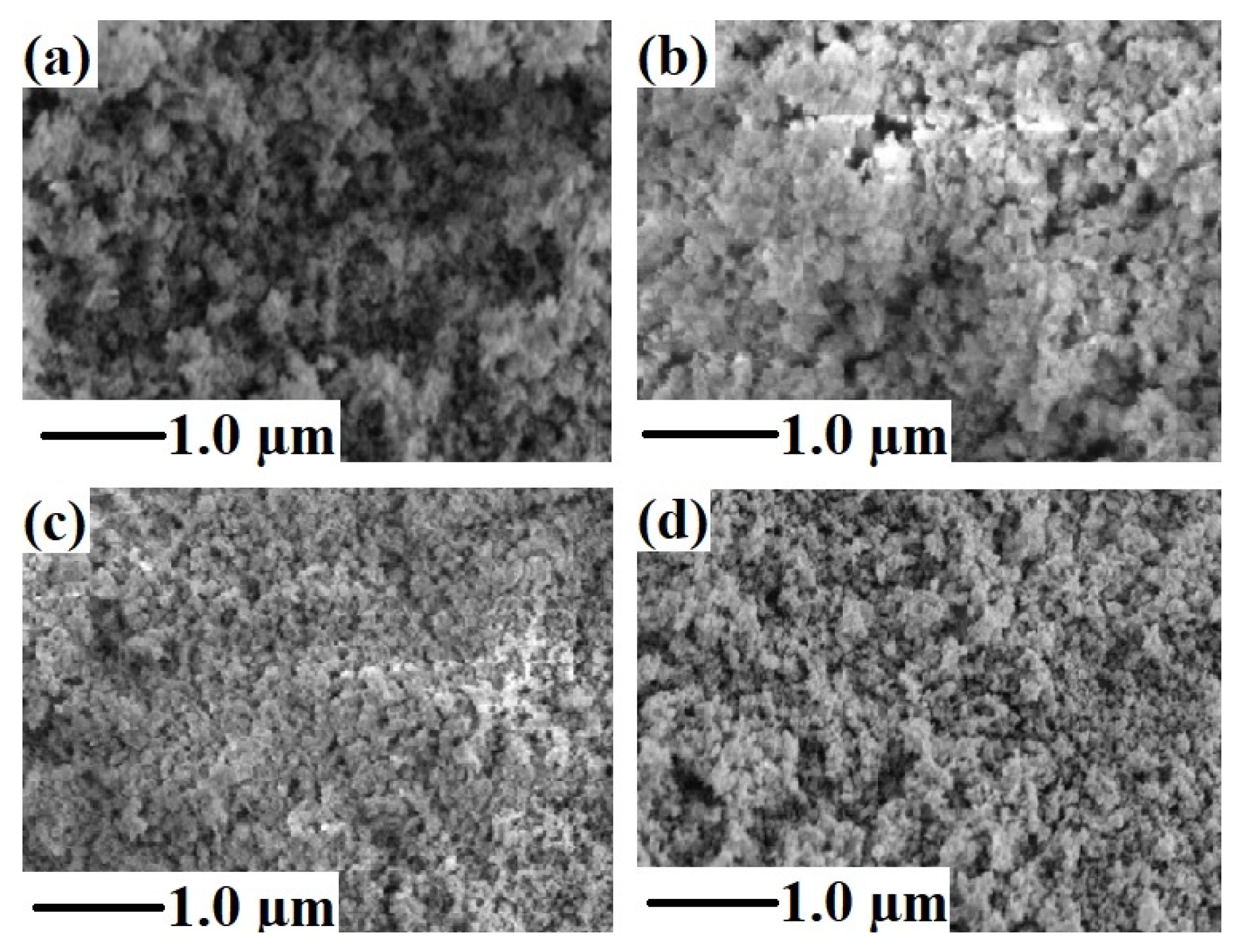
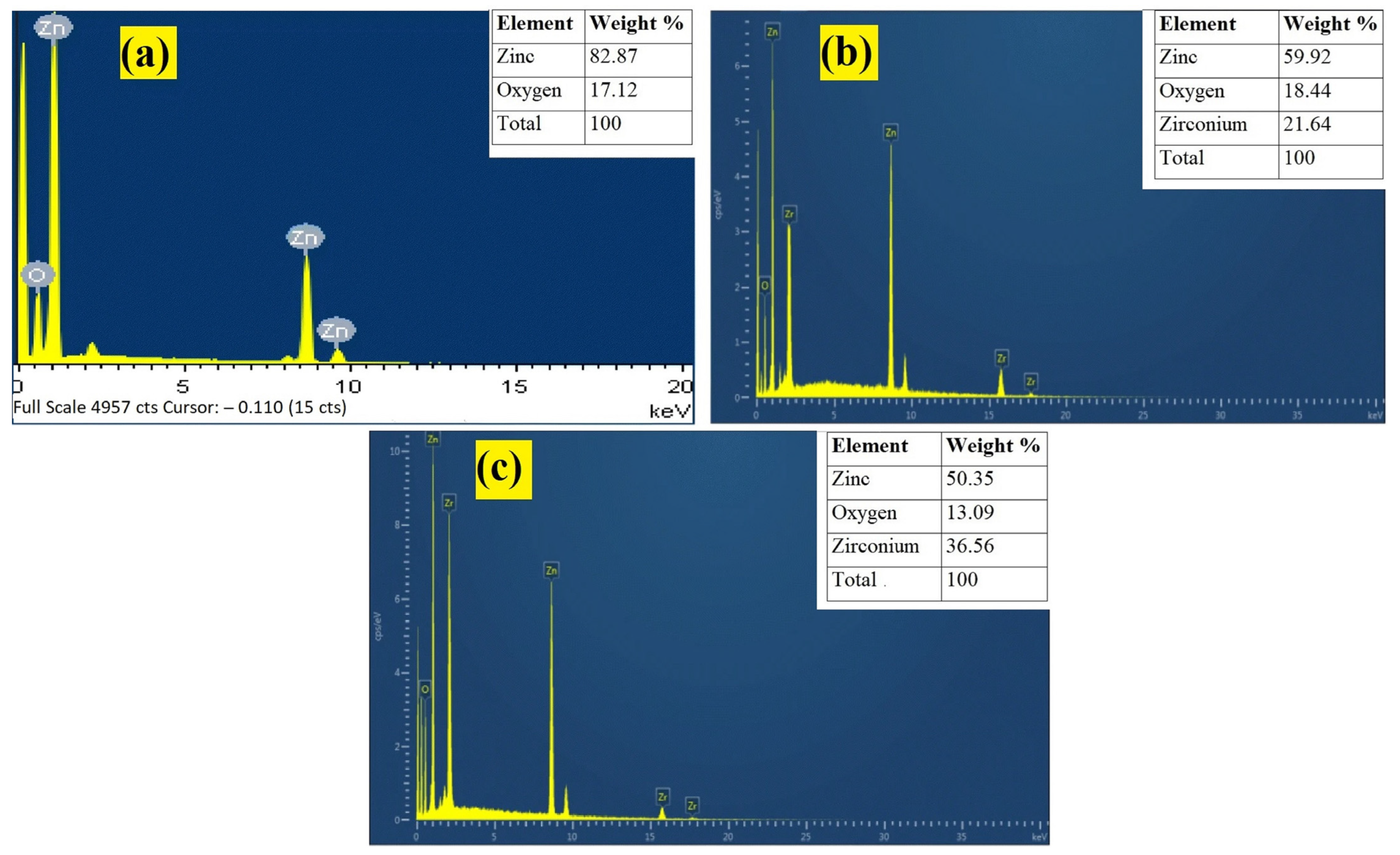
3.4. SEM and EDX Analysis
3.5. Optical Analysis
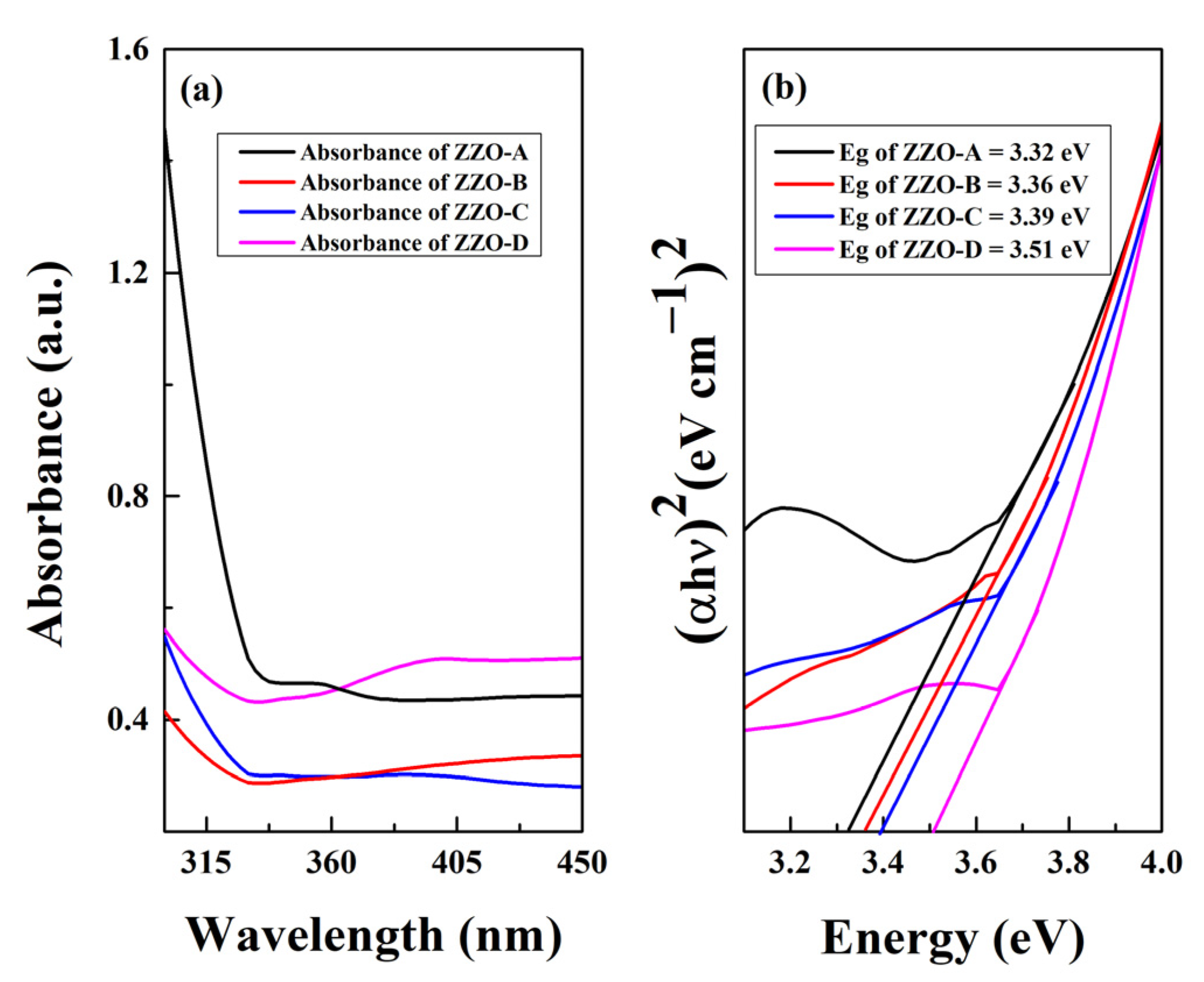
3.5.1. UV-Vis Spectroscopy
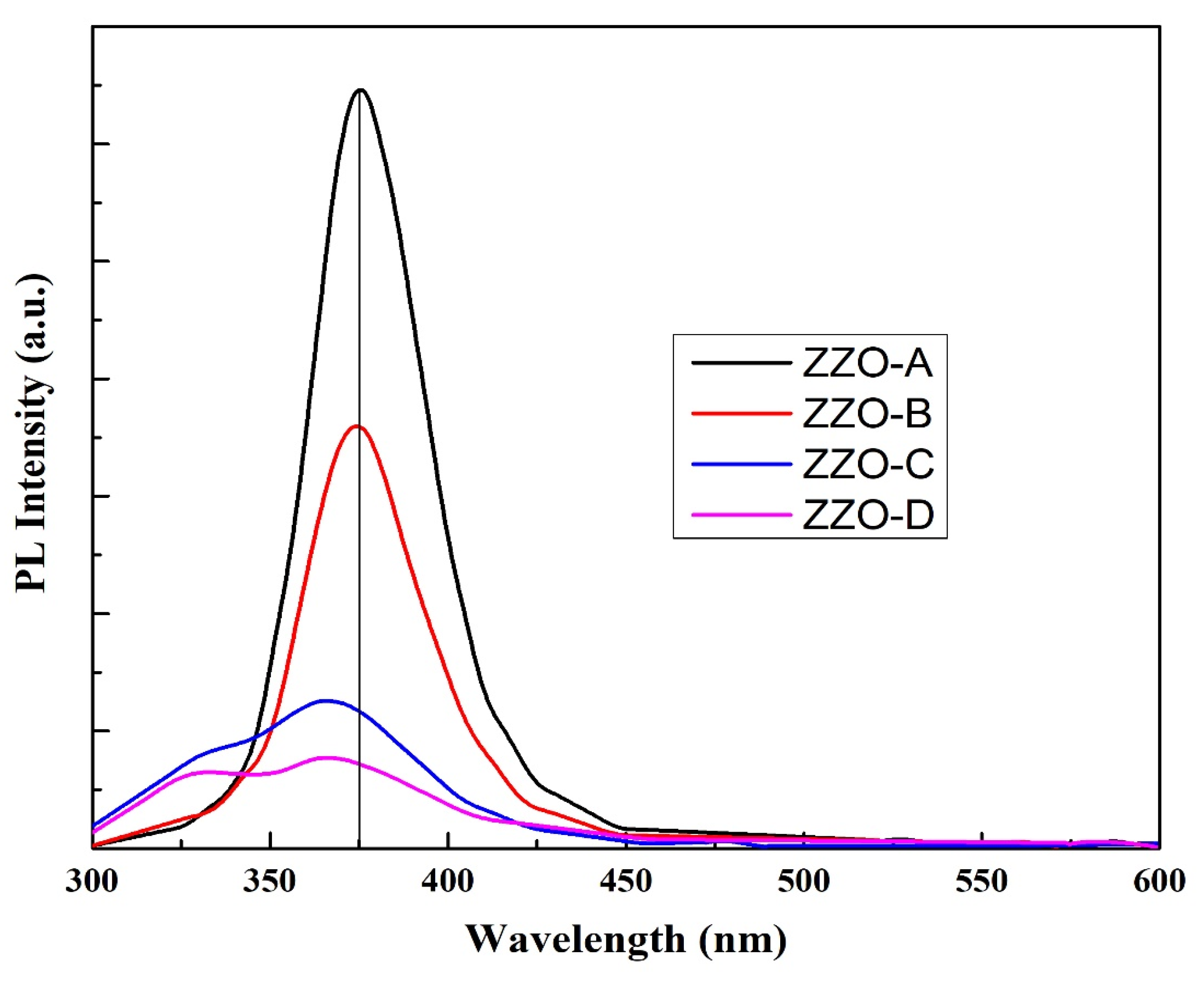
3.5.2. PL Analysis
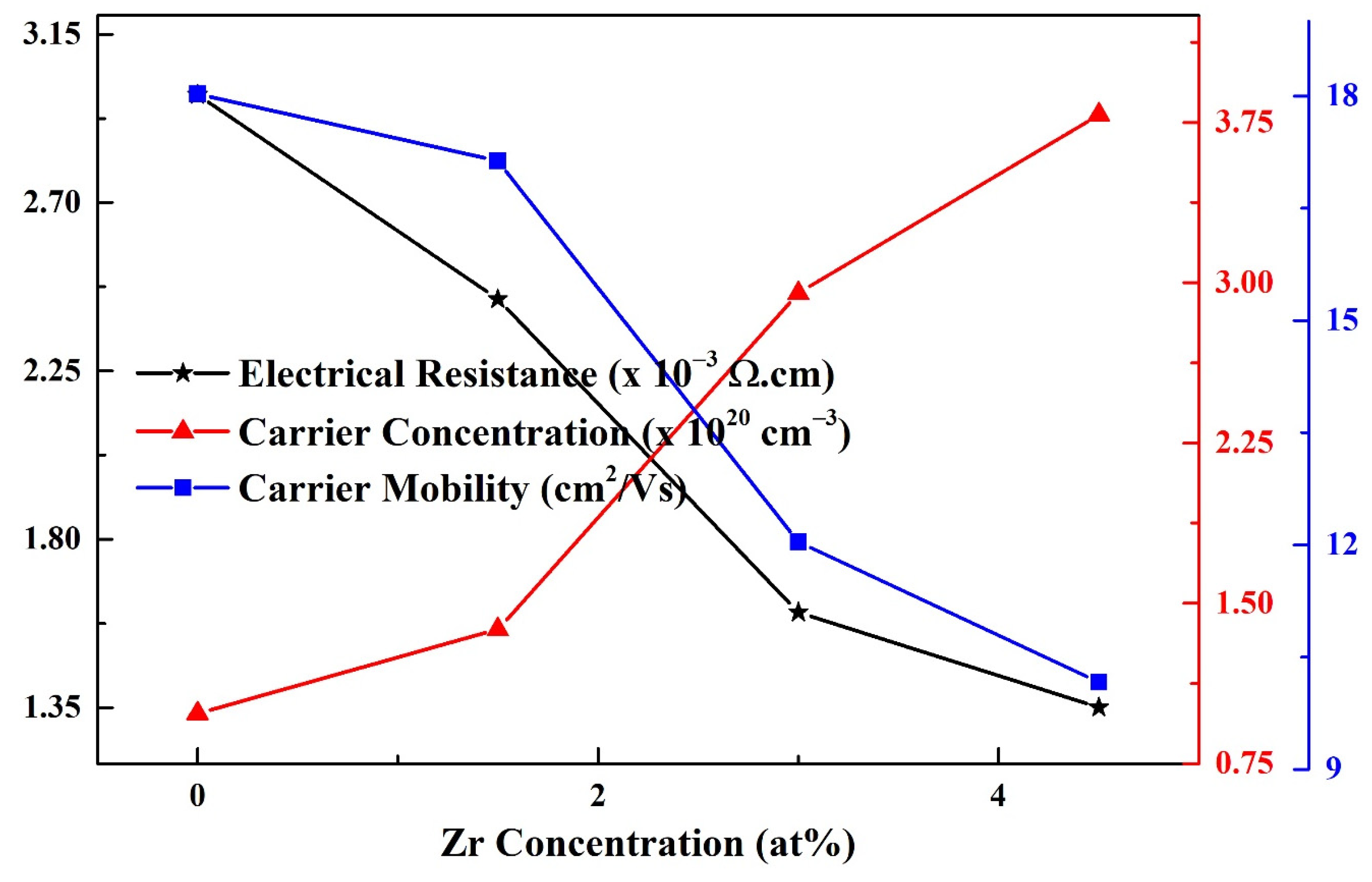
3.6. Electrical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, Z.; Jiang, Y. Surface modification and characterization of indium–tin oxide for organic light-emitting devices. J. Colloid Interface Sci. 2006, 302, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, J.; Mackenzie, A.; Phillips, W. Precise measurements of oxygen content: Oxygen vacancies in transparent conducting indium oxide films. Appl. Phys. Lett. 1991, 58, 2506–2508. [Google Scholar] [CrossRef]
- Valentini, A.; Quaranta, F.; Penza, M.; Rizzi, F.R. The stability of zinc oxide electrodes fabricated by dual ion beam sputtering. J. Appl. Phys. 1993, 73, 1143–1145. [Google Scholar] [CrossRef]
- Park, C.Y.; Lee, J.H.; Choi, B.H. Effects of surface treatment of ITO anode layer patterned with shadow mask technology on characteristics of organic light-emitting diodes. Org. Electron. 2013, 14, 3172–3179. [Google Scholar] [CrossRef]
- Kang, H.; Lu, Z.; Zhong, Z.; Gu, J. Structural, optical and electrical characterization of Ga-Mg co-doped ZnO transparent conductive films. Mater. Lett. 2018, 215, 102–105. [Google Scholar] [CrossRef]
- Wu, J.-L.; Lin, H.-Y.; Su, B.-Y.; Chen, Y.-C.; Chu, S.-Y.; Liu, S.-Y.; Chang, C.-C.; Wu, C.-J. Comparison of physical and electrical properties of GZO/ZnO buffer layer and GZO as source and drain electrodes of α-IGZO thin-film transistors. J. Alloys Compd. 2014, 592, 35–41. [Google Scholar] [CrossRef]
- Ozasa, K.; Ye, T.; Aoyagi, Y. Deposition of thin indium oxide film and its application to selective epitaxy for in situ processing. Thin Solid Films 1994, 246, 58–64. [Google Scholar] [CrossRef]
- Sawada, M.; Higuchi, M.; Kondo, S.; Saka, H. Characteristics of indium-tin-oxide/silver/indium-tin-oxide sandwich films and their application to simple-matrix liquid-crystal displays. Jpn. J. Appl. Phys. 2001, 40, 3332–3336. [Google Scholar] [CrossRef]
- Marikkannu, S.; Kashif, M.; Sethupathy, N.; Vidhya, V.; Piraman, S.; Ayeshamariam, A.; Bououdina, M.; Ahmed, N.M.; Jayachandran, M. Effect of substrate temperature on indium tin oxide (ITO) thin films deposited by jet nebulizer spray pyrolysis and solar cell application. Mater. Sci. Semicond. Process. 2014, 27, 562–568. [Google Scholar] [CrossRef]
- Huang, J.; Chu, S.; Kong, J.; Liu, J. ZnO pn homojunction random laser based on nitrogen doped p-type nanowires. In Proceedings of the 2013 IEEE Photonics Conference, Bellevue, WA, USA, 8–12 September 2013. [Google Scholar]
- Lu, Y.-J.; Shi, Z.-F.; Shan, C.-X.; Shen, D.-Z. ZnO-based deep-ultraviolet light-emitting devices. Chin. Phys. B 2017, 26, 047703. [Google Scholar] [CrossRef]
- Kim, W.-S.; Moon, Y.-K.; Kim, K.-T.; Shin, S.-Y.; Du Ahn, B.; Lee, J.-H.; Park, J.-W. The influence of hafnium doping on bias stability in zinc oxide thin film transistors. Thin Solid Films 2011, 519, 5161–5164. [Google Scholar] [CrossRef]
- Martinson, A.B.; Elam, J.W.; Hupp, J.T.; Pellin, M.J. ZnO Nanotube Based Dye-Sensitized Solar Cells. Nano Lett. 2007, 7, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Pauporté, T.; Le Bahers, T.; Viana, B.; Ciofini, I. Wavelength-Emission Tuning of ZnO Nanowire-Based Light-Emitting Diodes by Cu Doping: Experimental and Computational Insights. Adv. Funct. Mater. 2011, 21, 3564–3572. [Google Scholar] [CrossRef]
- Iwan, S.; Bambang, S.; Zhao, J.; Tan, S.; Fan, H.; Sun, L.; Zhang, S.; Ryu, H.; Sun, X. Green electroluminescence from an n-ZnO: Er/p-Si heterostructured light-emitting diode. Phys. B Condens. Matter 2012, 407, 2721–2724. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, J.; Ghorpade, U.; Shin, H.H.; Gang, M.G.; Park, S.D.; Kim, H.-J.; Lee, D.S.; Kim, J.H. Comparison study of ZnO-based quaternary TCO materials for photovoltaic application. J. Alloys Compd. 2019, 793, 499–504. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Seo, K.-M.; Kim, S.-H.; Ansari, S.A. Critical Aspects of Various Techniques for Synthesizing Metal Oxides and Fabricating Their Composite-Based Supercapacitor Electrodes: A Review. Nanomaterials 2022, 12, 1873. [Google Scholar] [CrossRef]
- Mwankemwa, B.S.; Malevu, T.D.; Sahini, M.G.; Vuai, S.A. Effects of vertically aligned ZnO nanorods surface morphology on the ambient-atmosphere fabricated organic solar cells. Results Mater. 2022, 14, 100271. [Google Scholar] [CrossRef]
- Rezaie, M.N.; Mohammadnejad, S.; Ahadzadeh, S. The impact of ZnO nanotube on the performance of hybrid inorganic/organic light-emitting diode as a single-mode ring-core UV waveguide. Surf. Interfaces 2022, 28, 101666. [Google Scholar] [CrossRef]
- Minami, T. Transparent conducting oxide semiconductors for transparent electrodes. Semicond. Sci. Technol. 2005, 20, S35–S44. [Google Scholar] [CrossRef]
- Klingshirn, C. ZnO: From basics towards applications. Phys. Status Solidi (b) 2007, 244, 3027–3073. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Lee, W.-C. Effect of dopants on the structural, optical and electrical properties of sol–gel derived ZnO semiconductor thin films. Curr. Appl. Phys. 2013, 13, 60–65. [Google Scholar] [CrossRef]
- Fay, S.; Steinhauser, J.; Oliveira, N.; Vallat-Sauvain, E.; Ballif, C. Opto-electronic properties of rough LP-CVD ZnO: B for use as TCO in thin-film silicon solar cells. Thin Solid Films 2007, 515, 8558–8561. [Google Scholar] [CrossRef]
- Faÿ, S.; Steinhauser, J.; Nicolay, S.; Ballif, C. Polycrystalline ZnO: B grown by LPCVD as TCO for thin film silicon solar cells. Thin Solid Films 2010, 518, 2961–2966. [Google Scholar] [CrossRef]
- Huang, C.; Wang, M.; Deng, Z.; Cao, Y.; Liu, Q.; Huang, Z.; Liu, Y.; Guo, W.; Huang, Q. Low content indium-doped zinc oxide films with tunable work function fabricated through magnetron sputtering. Semicond. Sci. Technol. 2010, 25, 045008. [Google Scholar] [CrossRef]
- Hafdallah, A.; Ynineb, F.; Aida, M.; Attaf, N. In doped ZnO thin films. J. Alloys Compd. 2011, 509, 7267–7270. [Google Scholar] [CrossRef]
- Wang, C.; Man, B.; Liu, M.; Chen, C.; Jiang, S.; Yang, S.; Xu, S.; Gao, X.; Hu, B. The intrinsic room-temperature ferromagnetism in ZnO: Co thin films deposited by PLD. Adv. Condens. Matter Phys. 2012, 2012, 363981. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.A.; King, P.J.; Dawson, K.; Romani, S.; Williams, P.A.; Ridealgh, J.; Rosseinsky, M.J. Atomic layer deposition of germanium-doped zinc oxide films with tuneable ultraviolet emission. J. Mater. Chem. 2012, 22, 12824–12829. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, J.H.; Cho, H.K. Tunable electrical and optical properties in composition controlled Hf: ZnO thin films grown by atomic layer deposition. J. Electrochem. Soc. 2012, 159, H384. [Google Scholar] [CrossRef]
- Gokulakrishnan, V.; Parthiban, S.; Jeganathan, K.; Ramamurthi, K. Investigation on the effect of Zr doping in ZnO thin films by spray pyrolysis. Appl. Surf. Sci. 2011, 257, 9068–9072. [Google Scholar] [CrossRef]
- Alam, M.W.; Ansari, M.Z.; Aamir, M.; Waheed-Ur-Rehman, M.; Parveen, N.; Ansari, S.A. Preparation and Characterization of Cu and Al Doped ZnO Thin Films for Solar Cell Applications. Crystals 2022, 12, 128. [Google Scholar] [CrossRef]
- Dhakal, T.; Vanhart, D.; Christian, R.; Nandur, A.; Sharma, A.; Westgate, C.R. Growth morphology and electrical/optical properties of Al-doped ZnO thin films grown by atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2012, 30, 021202. [Google Scholar] [CrossRef]
- Chalker, P.R.; Marshall, P.; Romani, S.; Roberts, J.; Irvine, S.; Lamb, D.; Clayton, A.; Williams, P. Atomic layer deposition of Ga-doped ZnO transparent conducting oxide substrates for CdTe-based photovoltaics. J. Vac. Sci. Technol. A Vac. Surf. Films 2013, 31, 01A120. [Google Scholar] [CrossRef]
- Saito, K.; Hiratsuka, Y.; Omata, A.; Makino, H.; Kishimoto, S.; Yamamoto, T.; Horiuchi, N.; Hirayama, H. Atomic layer deposition and characterization of Ga-doped ZnO thin films. Superlattices Microstruct. 2007, 42, 172–175. [Google Scholar] [CrossRef]
- Saadi, H.; Benzarti, Z.; Rhouma, F.I.H.; Sanguino, P.; Guermazi, S.; Khirouni, K.; Vieira, M.T. Enhancing the electrical and dielectric properties of ZnO nanoparticles through Fe doping for electric storage applications. J. Mater. Sci. Mater. Electron. 2021, 32, 1536–1556. [Google Scholar] [CrossRef]
- Saadi, H.; Benzarti, Z.; Sanguino, P.; Hadouch, Y.; Mezzane, D.; Khirouni, K.; Abdelmoula, N.; Khemakhem, H. Improving the optical, electrical and dielectric characteristics of ZnO nanoparticles through (Fe+ Al) addition for optoelectronic applications. Appl. Phys. A 2022, 128, 1–15. [Google Scholar] [CrossRef]
- Demirkol, U.; Pat, S.; Mohammadigharehbagh, R.; Musaoğlu, C.; Özgür, M.; Elmas, S.; Özen, S.; Korkmaz, Ş. Investigation of the substrate effect for Zr doped ZnO thin film deposition by thermionic vacuum arc technique. J. Mater. Sci. Mater. Electron. 2018, 29, 18098–18104. [Google Scholar] [CrossRef]
- Yang, L.-L.; Yang, J.-H.; Wang, D.-D.; Zhang, Y.-J.; Wang, Y.-X.; Liu, H.-L.; Fan, H.-G.; Lang, J.-H. Photoluminescence and Raman analysis of ZnO nanowires deposited on Si (1 0 0) via vapor–liquid–solid process. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 920–923. [Google Scholar] [CrossRef]
- Sathya, M.; Pushpanathan, K. Synthesis and Optical Properties of Pb Doped ZnO Nanoparticles. Appl. Surf. Sci. 2018, 449, 346–357. [Google Scholar] [CrossRef]
- Bian, H.; Ma, S.; Yang, G.; Zhu, H.; Xu, X.; Yan, S.; Gao, J.; Zhang, Z. The optical and electrical properties of ZnO: Zr films. J. Alloys Compd. 2016, 672, 20–26. [Google Scholar] [CrossRef]
- Machda, F.; Ogawa, T.; Okumura, H.; Ishihara, K.N. Damp-heat durability comparison of Al-doped ZnO transparent electrodes deposited at low temperatures on glass and PI-tape/PC substrates. Ceram. Int. 2020, 46, 16178–16184. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yuan, Y.-Z.; Li, C.-F.; Gao, X.-T.; Cao, X.-Z.; Li, J.-B. The structure and photoluminescence properties of RF-sputtered films of ZnO on Teflon substrate. Mater. Lett. 2008, 62, 2907–2909. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Vijaya, J.J.; Kennedy, L.J. Effects of Morphology and Zr Doping on Structural, Optical, and Photocatalytic Properties of ZnO Nanostructures. Ind. Eng. Chem. Res. 2012, 51, 16333–16345. [Google Scholar] [CrossRef]
- Smaali, A.; Abdelli-Messaci, S.; Lafane, S.; Mavlonov, A.; Lenzner, J.; Richter, S.; Kechouane, M.; Nemraoui, O.; Ellmer, K. Pulsed laser deposited transparent and conductive V-doped ZnO thin films. Thin Solid Films 2020, 700, 137892. [Google Scholar] [CrossRef]
- Tsay, C.-Y.; Fan, K.-S. Optimization of Zr-doped ZnO thin films prepared by sol-gel method. Mater. Trans. 2008, 49, 1900–1904. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Raifuku, I.; Ishikawa, Y.; Uraoka, Y.; Yanagi, H. Hierarchical core–shell heterostructure of H2O-oxidized ZnO nanorod@ Mg-doped ZnO nanoparticle for solar cell applications. Mater. Adv. 2020, 1, 1253–1261. [Google Scholar] [CrossRef]
- Hussain, S.; Jacob, J.; Usman, Z.; Mahmood, K.; Ali, A.; Arshad, M.; Khan, W.S.; Farooq, Z.; Farooq, M.; Ashfaaq, A.; et al. Length dependent performance of Cu2O/ZnO nanorods solar cells. Superlattices Microstruct. 2019, 126, 181–185. [Google Scholar] [CrossRef]
- Özgür, Ü.; Hofstetter, D.; Morkoç, H. ZnO devices and applications: A review of current status and future prospects. Proc. IEEE 2010, 98, 1255–1268. [Google Scholar] [CrossRef]
- Nithin, K.K.; Krishnappa, M.R. Synthesis and characterization of cobalt-doped cadmium oxide thin films prepared by sol–gel spin coating method. J. Physics: Conf. Ser. 2019, 1362, 012118. [Google Scholar]
- Paul, G.; Bandyopadhyay, S.; Sen, S.; Sen, S. Structural, optical and electrical studies on sol–gel deposited Zr doped ZnO films. Mater. Chem. Phys. 2003, 79, 71–75. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.B.; Viswanath, R.; Kirthan, B.; Prabhakara, M. Effect of zirconium doping on the structural, optical, electrochemical and antibacterial properties of ZnO nanoparticles prepared by sol-gel method. Chem. Data Collect. 2020, 29, 100505. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.; Viswanath, R.; Gowda, I.; Prabhakara, M. Bright red luminescence emission of macroporous honeycomb-like Eu3+ ion-doped ZnO nanoparticles developed by gel-combustion technique. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Supatutkul, C.; Pramchu, S.; Jaroenjittichai, A.; Laosiritaworn, Y. Electronic properties of two-dimensional zinc oxide in hexagonal, (4,4)-tetragonal, and (4,8)-tetragonal structures by using Hybrid Functional calculation. J. Physics: Conf. Ser. 2017, 901, 012172. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Saeed, S.; Wu, L.; Zhu, P.; Wang, D. Enhanced anharmonic phonon coupling and decay dominated by low-energy phonons in CdS nanowires. J. Raman Spectrosc. 2019, 50, 1492–1501. [Google Scholar] [CrossRef]
- Pradhan, A.; Zhang, K.; Loutts, G.; Roy, U.; Cui, Y.; Burger, A. Structural and spectroscopic characteristics of ZnO and ZnO: Er3+nanostructures. J. Physics: Condens. Matter 2004, 16, 7123–7129. [Google Scholar] [CrossRef]
- Khan, I.; Khan, S.; Nongjai, R.; Ahmed, H.; Khan, W. Structural and optical properties of gel-combustion synthesized Zr doped ZnO nanoparticles. Opt. Mater. 2013, 35, 1189–1193. [Google Scholar] [CrossRef]
- Artus, L.; Cusco, R.; Alarcon-Llado, E.; Gonzalez-Diaz, G.; Martil, I.; Jimenez, J.; Wang, B.; Callahan, M. Isotopic study of the nitrogen-related modes in N+- implanted ZnO. Appl. Phys. Lett. 2007, 90, 181911. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Yanagi, H. pH-controlled surface engineering of nanostructured ZnO films generated via a sustainable low-temperature H2O oxidation process. Appl. Surf. Sci. 2019, 467, 932–939. [Google Scholar] [CrossRef]
- Urbach, F. The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys. Rev. 1953, 92, 1324. [Google Scholar] [CrossRef]
- Rao, J.; Yu, A.; Shao, C.; Zhou, X. Construction of Hollow and Mesoporous ZnO Microsphere: A Facile Synthesis and Sensing Property. ACS Appl. Mater. Interfaces 2012, 4, 5346–5352. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmad, R.; Rashid, M.; Hassan, M.; Hussnain, A.; Khan, M.A.; ul Haq, M.E.; Shafique, M.; Riaz, S. Structural and magnetic studies on Zr doped ZnO diluted magnetic semiconductor. Curr. Appl. Phys. 2014, 14, 176–181. [Google Scholar] [CrossRef]
- Norouzzadeh, P.; Mabhouti, K.; Golzan, M.; Naderali, R. Comparative study on dielectric and structural properties of undoped, Mn-doped, and Ni-doped ZnO nanoparticles by impedance spectroscopy analysis. J. Mater. Sci. Mater. Electron. 2019, 31, 7335–7347. [Google Scholar] [CrossRef]
- Sohail, A.; Faraz, M.; Arif, H.; Bhat, S.A.; Siddiqui, A.A.; Bano, B. Deciphering the interaction of bovine heart cystatin with ZnO nanoparticles: Spectroscopic and thermodynamic approach. Int. J. Biol. Macromol. 2017, 95, 1056–1063. [Google Scholar] [CrossRef]
- Bindemann, R.; Kluge, G.; Elliott, R.J.; Ipatova, I.P. (Eds.) Optical Properties of Mixed Crystals; Modern Problems in Condensed Matter Sciences; North-Holland Publ. Co.: Amsterdam, The Netherlands, 1988; Volume 23, ISBN 044870695. [Google Scholar]
- Devi, P.G.; Velu, A.S. Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J. Theor. Appl. Phys. 2016, 10, 233–240. [Google Scholar] [CrossRef]
- Philips’Gloeilampenfabrieken, O. A method of measuring specific resistivity and Hall effect of discs of arbitrary shape. Philips Res. Rep. 1958, 13, 1–9. [Google Scholar]
- Kumrueng, W.; Sawanthai, K.; Tubtimtae, A.; Ponhan, W. Effect of pH treatment on the structural and optical properties of Sn6Sb10S21 thin films facilely synthesized using a spin coating method. Opt. Mater. 2020, 105, 109917. [Google Scholar] [CrossRef]
- Joshi, G.; Saxena, N.; Mangal, R.; Mishra, A.; Sharma, T. Band gap determination of Ni-Zn ferrites. Bull. Mater. Sci. 2003, 26, 387–389. [Google Scholar] [CrossRef]
- Ramzan, M.; Imran, M.; Ullah, S.; Khan, M.A.; Naz, G.; Ghouri, M.; Iqbal, H.M. Fabrication and characterization of multifunctional thin multi-layer films for transparent conducting oxides. Prog. Org. Coat. 2020, 149, 105976. [Google Scholar] [CrossRef]
- Lee, D.J.; Kim, H.M.; Kwon, J.Y.; Choi, H.; Kim, S.H.; Kim, K.B. Structural and electrical properties of atomic layer deposited Al-doped ZnO films. Adv. Funct. Mater. 2011, 21, 448–455. [Google Scholar] [CrossRef]
- Kim, K.-T.; Kim, G.-H.; Woo, J.-C.; Kim, C.-I. Characteristics of Nickel-doped Zinc Oxide thin films prepared by sol–gel method. Surf. Coat. Technol. 2008, 202, 5650–5653. [Google Scholar] [CrossRef]
- Iqbal, M.F.; Ain, Q.U.; Yaqoob, M.M.; Zhu, P.; Wang, D. Temperature dependence of exciton–phonon coupling and phonon anharmonicity in ZnTe thin films. J. Raman Spectrosc. 2022, 53, 1265–1274. [Google Scholar] [CrossRef]

| Samples ID | ZZO-A | ZZO-B | ZZO-C | ZZO-D |
|---|---|---|---|---|
| Crystallite size (nm) (Scherrer formula) | 15.1 ± 0.1 | 17.2 ± 0.1 | 18.8 ± 0.1 | 20.3 ± 0.1 |
| Lattice constant a (Å) | 3.2447 | 3.2463 | 3.2521 | 3.2568 |
| Lattice constant c (Å) | 5.2188 | 5.2274 | 5.2364 | 5.2526 |
| c/a ratio | 1.6084 | 1.6102 | 1.6102 | 1.6128 |
| Micro strain ε (×10−3) (Scherrer formula) | 2.52 | 2.22 | 2.05 | 1.97 |
| Volume of unit cell V (Å3) | 14.6643 | 14.6957 | 14.7474 | 14.8144 |
| Bond Length L (Å) | 1.8865 | 1.8876 | 1.8908 | 1.894 |
| Crystallite size (nm) (W-H Plot) | 16.4 ± 0.1 | 17.6 ± 0.1 | 21.7 ± 0.1 | 21.9 ± 0.1 |
| Micro strain ε (×10−3) (W-H Plot) | 6.67 | 4.43 | 4.17 | 4.12 |
| Band gap (eV) | 3.32 | 3.36 | 3.39 | 3.51 |
| Samples ID | ZZO-A | ZZO-B | ZZO-C | ZZO-D |
|---|---|---|---|---|
| Resistivity (×10−3 Ω·cm) | 2.98 | 2.44 | 1.60 | 1.35 |
| Carrier concentration (×1020 cm−3) | 0.98 | 1.37 | 2.95 | 3.78 |
| Carrier mobility (cm2/Vs) | 18.0 | 17.1 | 12.0 | 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.u.R.; Ramzan, M.; Imran, M.; Zubair, M.; Shahab, S.; Ahmed, S.J.; Ferreira, F.; Iqbal, M.F. Tailoring the Structural, Optical and Electrical Properties of Zinc Oxide Nanostructures by Zirconium Doping. Coatings 2023, 13, 34. https://doi.org/10.3390/coatings13010034
Khan AuR, Ramzan M, Imran M, Zubair M, Shahab S, Ahmed SJ, Ferreira F, Iqbal MF. Tailoring the Structural, Optical and Electrical Properties of Zinc Oxide Nanostructures by Zirconium Doping. Coatings. 2023; 13(1):34. https://doi.org/10.3390/coatings13010034
Chicago/Turabian StyleKhan, Asad ur Rehman, Muhammad Ramzan, Muhammad Imran, Muhammad Zubair, Sana Shahab, Sara J. Ahmed, Fábio Ferreira, and Muhammad Faisal Iqbal. 2023. "Tailoring the Structural, Optical and Electrical Properties of Zinc Oxide Nanostructures by Zirconium Doping" Coatings 13, no. 1: 34. https://doi.org/10.3390/coatings13010034
APA StyleKhan, A. u. R., Ramzan, M., Imran, M., Zubair, M., Shahab, S., Ahmed, S. J., Ferreira, F., & Iqbal, M. F. (2023). Tailoring the Structural, Optical and Electrical Properties of Zinc Oxide Nanostructures by Zirconium Doping. Coatings, 13(1), 34. https://doi.org/10.3390/coatings13010034







