Enhanced Pickering Emulsion Stabilization of Cellulose Nanocrystals and Application for Reinforced and Hydrophobic Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Modified Cellulose Nanocrystals
2.3. BA-g-CNCS Stabilized for O/W Pickering Emulsions
2.4. Fabrication of Reinforcing and Hydrophobic Cotton Fabric
2.5. Characterization
3. Results and Discussion
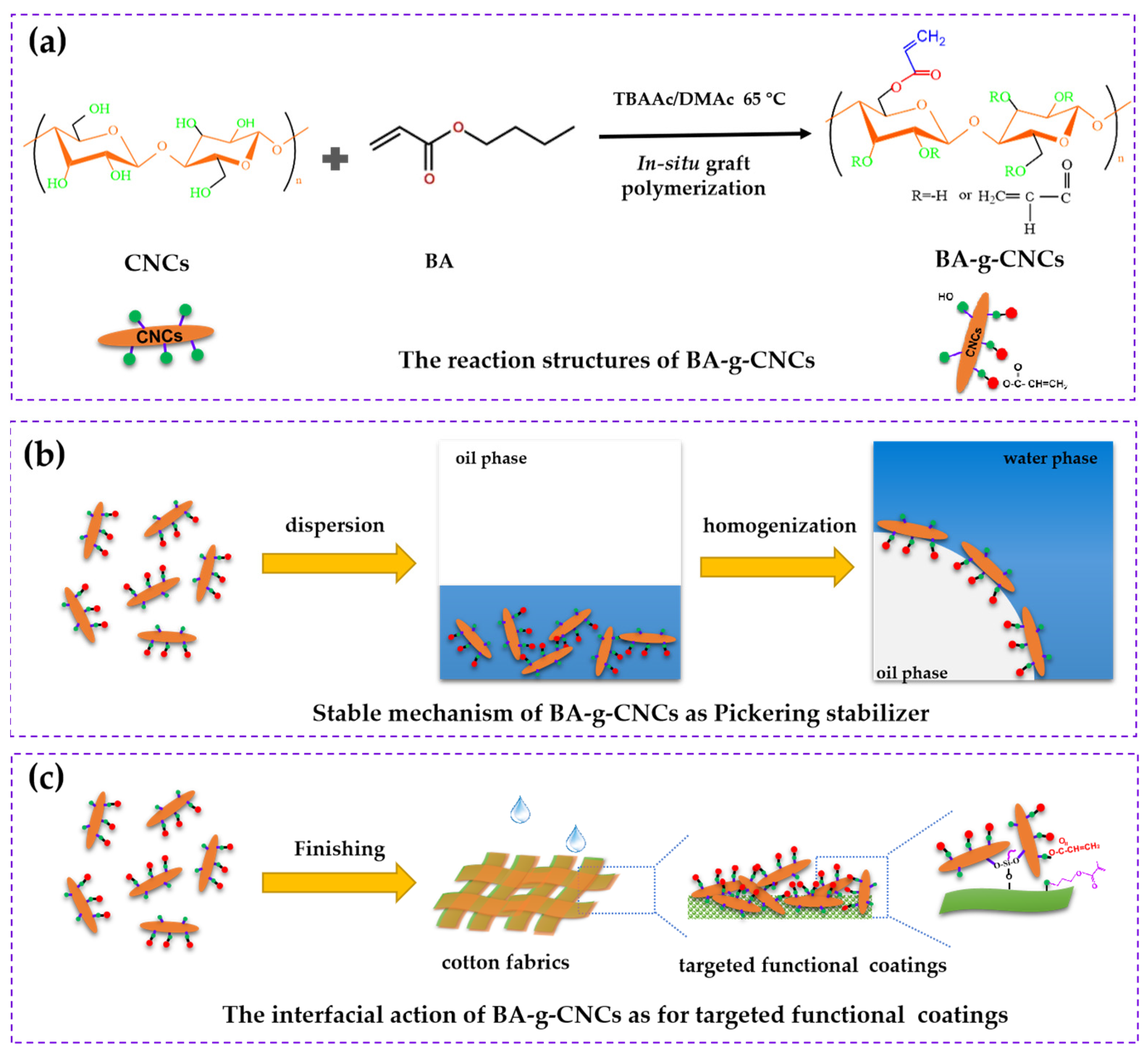
3.1. The Formation Mechanism of BA-g-CNCs
3.2. The Structures of BA-g-CNCs
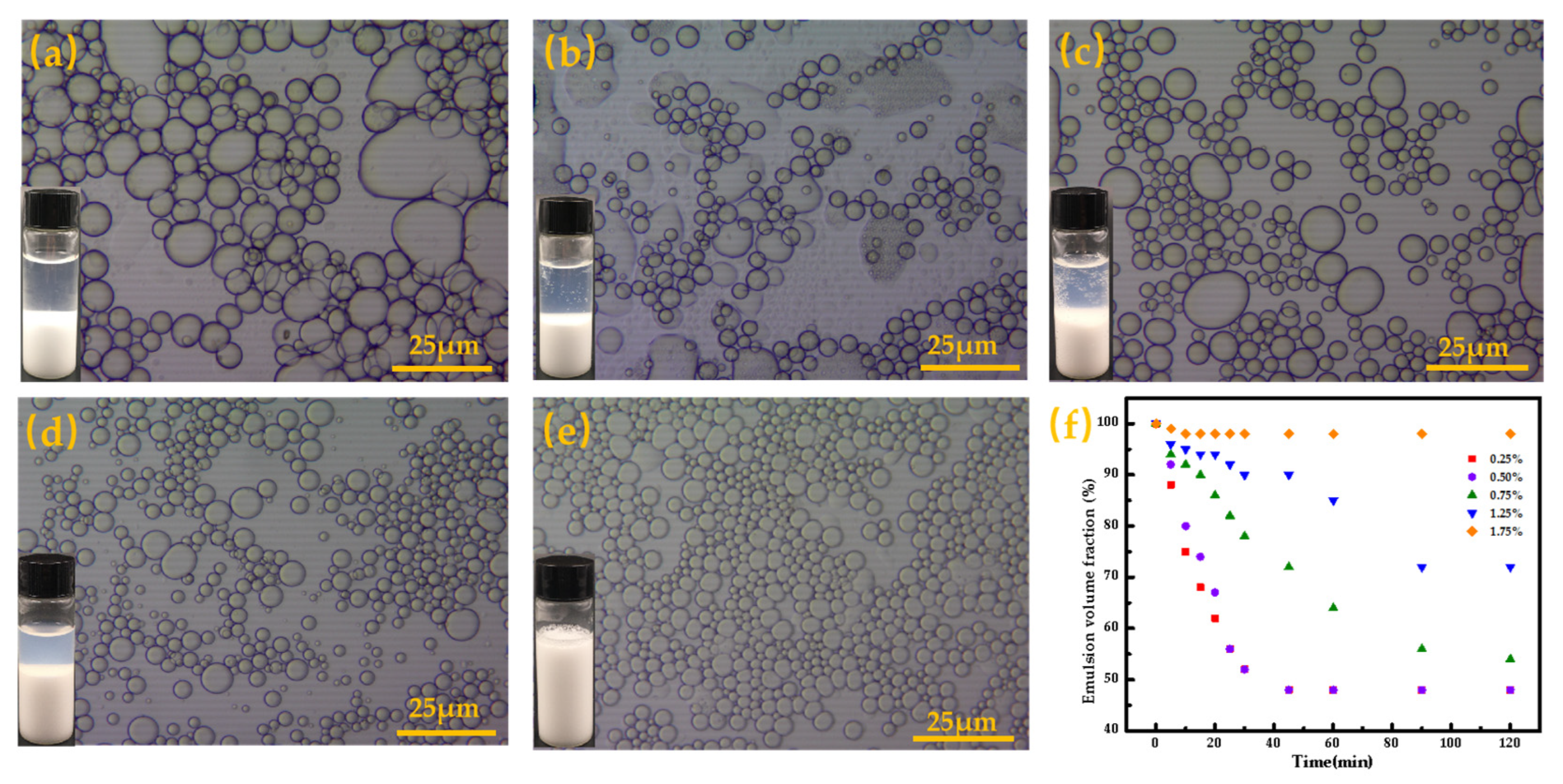
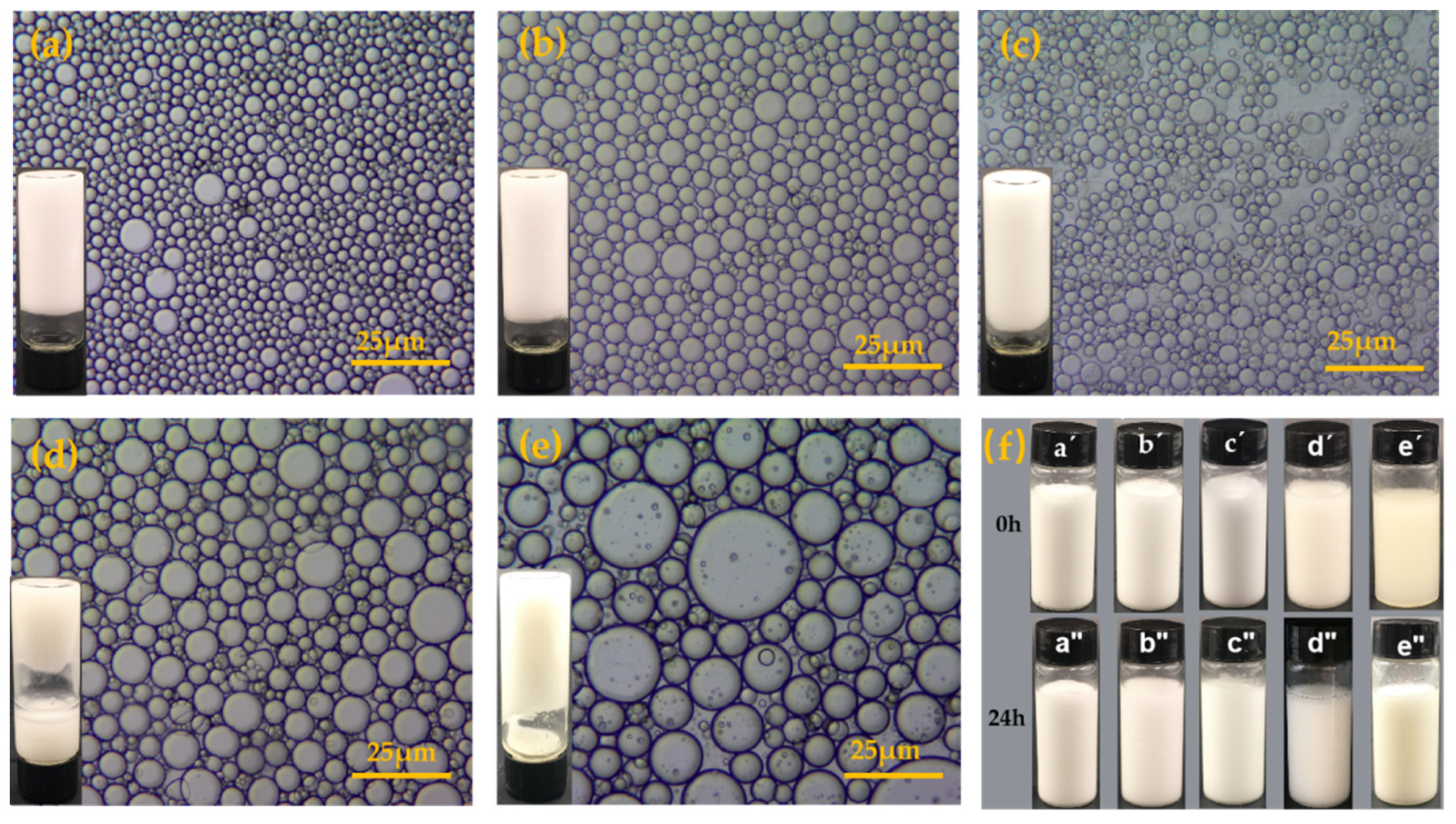
3.3. Stability and Morphology of O/W Pickering Emulsions of BA-g-CNCs as Pickering Stabilizers
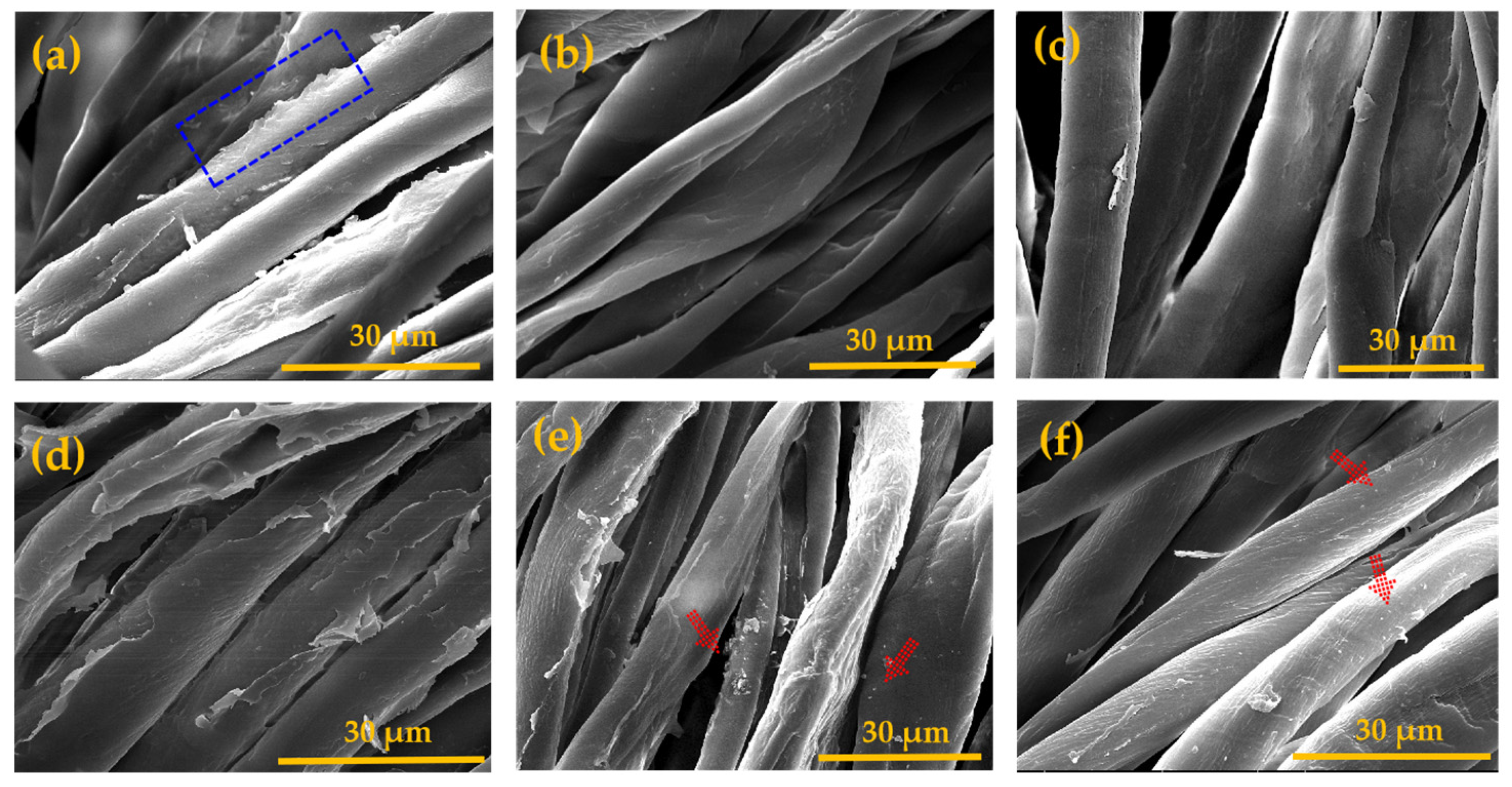
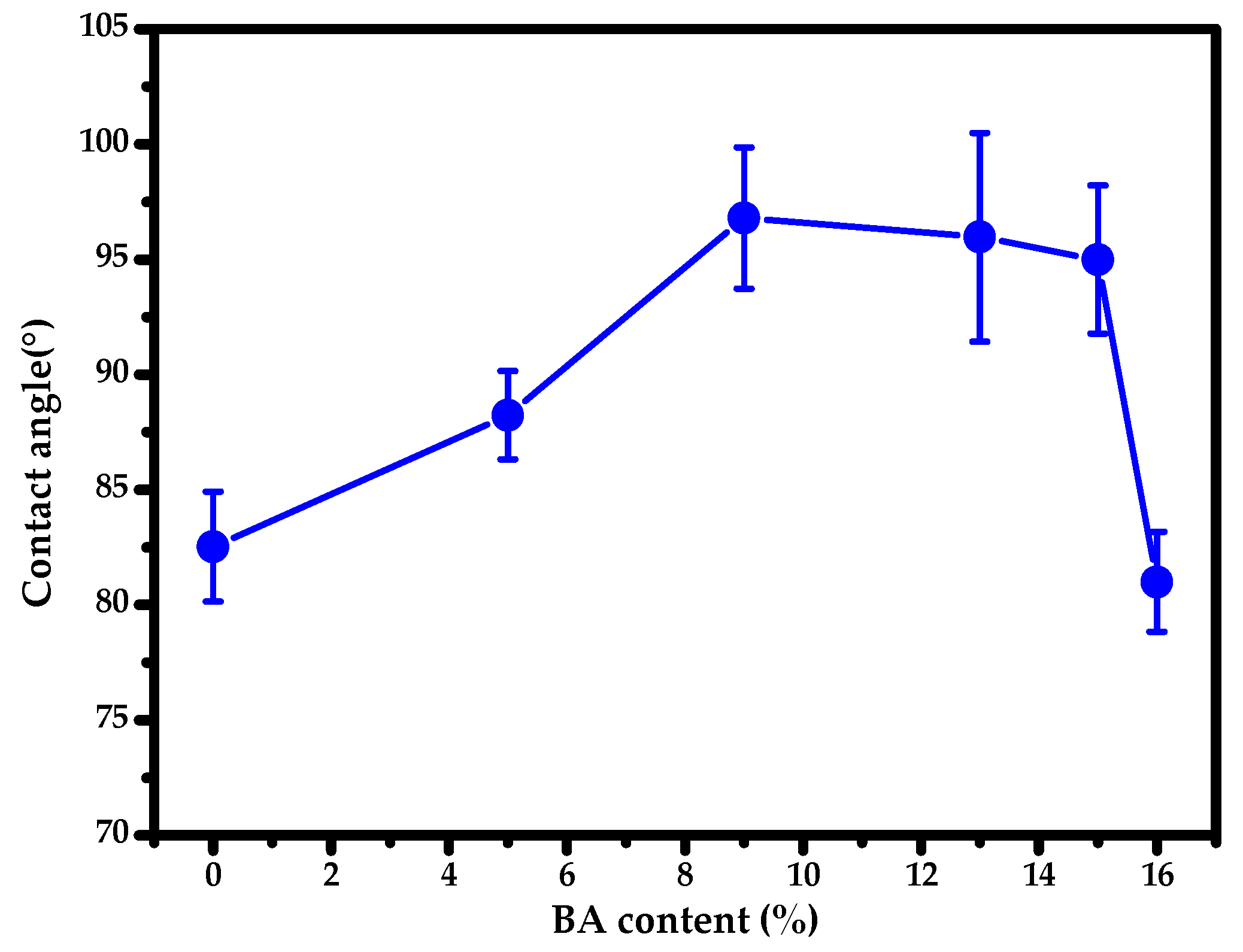
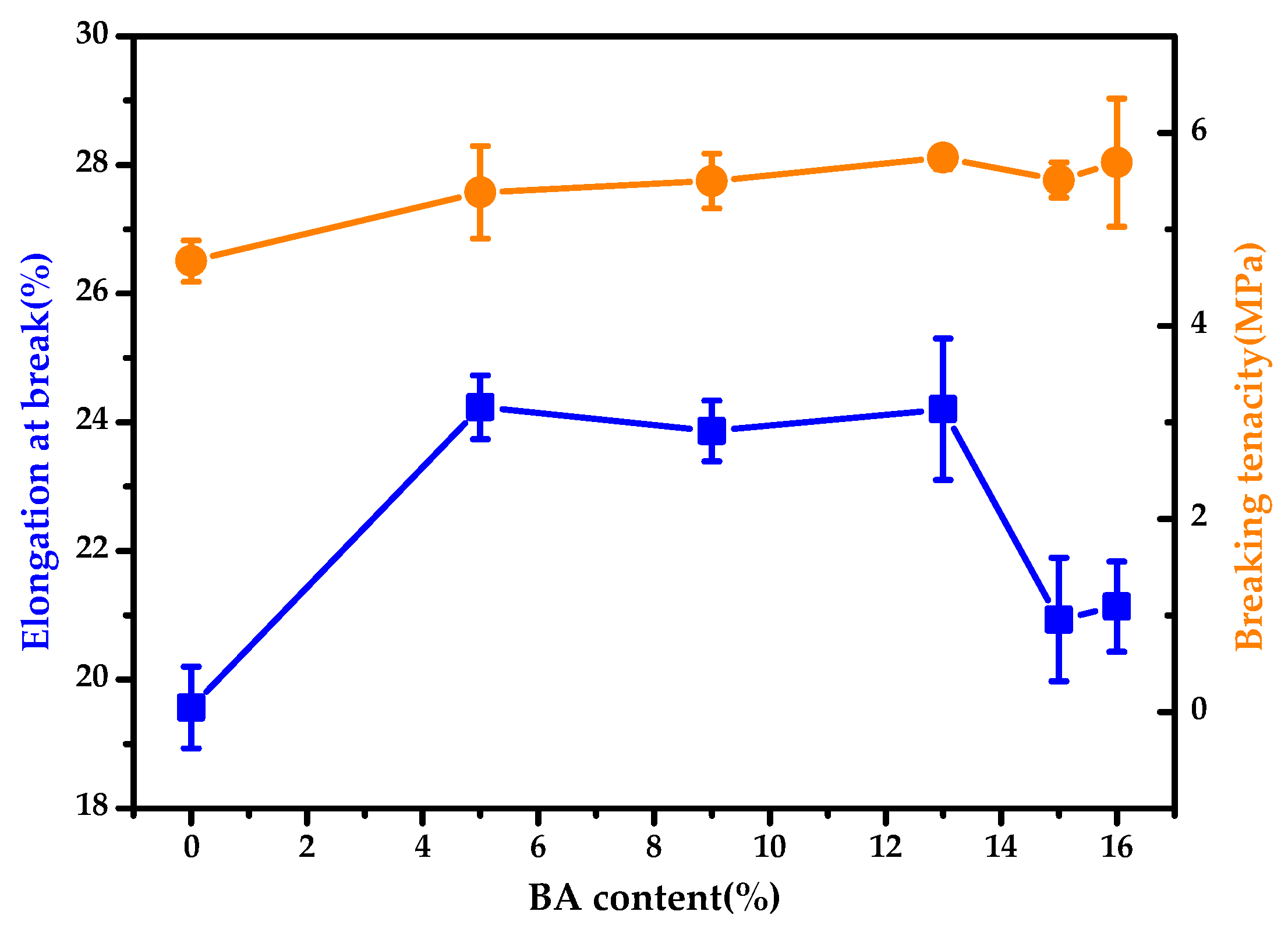
3.4. BA-g-CNCs as Reinforcing and Hydrophobic Cotton Fabric Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perrin, L.; Gillet, G.; Gressin, L.; Desobry, S. Interest of Pickering emulsions for sustainable micro/nanocellulose in food and cosmetic applications. Polymers 2020, 12, 2385. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Parajuli, S.; Alazzam, O.; Wang, M.; Mota, L.C.; Adhikari, S.; Wicks, D.; Ureña-Benavides, E. Surface properties of cellulose nanocrystal stabilized crude oil emulsions and their effect on petroleum biodegradation. Colloids Surf. A 2020, 596, 124705. [Google Scholar] [CrossRef]
- Had, B.; Cfa, B.; Mad, C.; VS, A.; VH, B. Polymerization of cellulose nanocrystals-based Pickering HIPE towards green porous materials-Science Direct. Carbohydr. Polym. 2020, 243, 116411. [Google Scholar]
- Yi, J.; Gao, L.; Zhong, G.; Fan, Y. Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for β-carotene stabilization and delivery. Food Funct. 2020, 11, 768–778. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy protein nanoparticle aggregates as pickering stabilizers for oil-in-water emulsions. J. Agric. Food Chem. 2013, 61, 8888–8898. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy glycinin as food-grade Pickering stabilizers: PART. I. structural characteristics, emulsifying properties and adsorption/arrangement at interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar] [CrossRef]
- Liu, F.; Tang, C.H. Soy glycinin as food-grade Pickering stabilizers: PART. II. Improvement of emulsification and interfacial adsorption by electrostatic screening. Food Hydrocoll. 2016, 60, 620–630. [Google Scholar] [CrossRef]
- Ma, J.J.; Huang, X.N.; Yin, S.W. Bioavailability of quercetin in zein-based colloidal particles-stabilized Pickering emulsions investigated by the in vitro digestion coupled with Caco-2 cell monolayer model. Food Chem. 2021, 360, 130152. [Google Scholar] [CrossRef]
- Pei, X.; Zhai, K.; Wang, C.; Deng, Y.; Wang, P. Polymer brush graft-modified starch-based nanoparticles as Pickering emulsifiers. Langmuir 2019, 35, 7222–7230. [Google Scholar] [CrossRef]
- Wang, X.Y.; Heuzey, M.C. Chitosan-based conventional and Pickering emulsions with long term stability. Langmuir 2016, 32, 929–936. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Tey, B.T.; Chan, E.S. Effects of environmental factors on the physical stability of Pickering emulsions stabilized by chitosan particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Sun, G.; Nan, F.F.; Ngai, T.; Ma, G.H. Systematic studies of Pickering emulsions stabilized by uniform-sized PLGA particles: Preparation and stabilization mechanism. J. Mater. Chem. B 2014, 2, 7605–7611. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, C.; Du, M.; Lin, S.; Xu, X.; Yu, P. In-situ dispersion of casein to form nanoparticles for Pickering high internal phase emulsions. LWT 2021, 139, 110538. [Google Scholar] [CrossRef]
- Xu, Q.N.; Ma, J.Z.; Zhou, J.H.; Wang, Y.Y.; Zhang, J. Bio-based core-shell casein-based silica nano-composite latex by double-in situ polymerization: Synthesis, characterization and mechanism. Chem. Eng. J. 2013, 228, 281–289. [Google Scholar] [CrossRef]
- Hu, Z.; Marway, H.S.; Kasem, H.; Pelton, R.; Cranston, E.D. Dried and redispersible cellulose nanocrystal Pickering emulsions. ACS Macro Lett. 2016, 5, 185–189. [Google Scholar] [CrossRef]
- Buffiere, J.; Balogh-Michels, Z.; Borrega, M.; Geiger, T.; Zimmermann, T.; Sixta, H. The chemical-free production of nanocelluloses from microcrystalline cellulose and their use as Pickering emulsion stabilizer. Carbohydr. Polym. 2017, 178, 48–56. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for Pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Bertsch, B.; Fischer, P. Adsorption and interfacial structure of nanocelluloses at fluid intefaces. Adv. Colloid Interface Sci. 2020, 276, 102089. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Jin, L.Q. Research development on hydrophobic modification of cellulose nanofibrils. Adv. Mater. Res. 2013, 785–786, 440–443. [Google Scholar] [CrossRef]
- Parajuli, S.; Ureña-Benavides, E.E. Fundamental aspects of nanocellulose stabilized Pickering emulsions and foams. Adv. Colloid Interface Sci. 2022, 299, 102530. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface modification of cellulose nanocrystals. Nanoscale 2014, 6, 7764. [Google Scholar] [CrossRef]
- Peng, S.X.; Chang, H.; Kumar, S.; Moon, R.J.; Youngblood, J.P. A comparative guide to controlled hydrophobization of cellulose nanocrystals via surface esterification. Cellulose 2016, 23, 1825–1846. [Google Scholar] [CrossRef]
- Li, M.C.; Mei, C.; Xu, X.; Lee, S.; Wu, Q. Cationic surface modification of cellulose nanocrystals: Toward tailoring dispersion and interface in carboxymethyl cellulose films. Polymer 2016, 107, 200–210. [Google Scholar] [CrossRef]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Bacher, M.; Edler, M.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2018, 106, 1288–1296. [Google Scholar] [CrossRef]
- Li, B.; Xu, W.; Kronlund, D.; Maattanen, A.; Liu, J.; Smatt, J.H.; Xu, C. Cellulose nanocrystals prepared via formic acid hydrolysis followed by TEMPO-mediated oxidation. Carbohydr. Polym. 2015, 133, 605–612. [Google Scholar] [CrossRef]
- Lin, W.S.; Hu, X.; You, X.; Sun, Y.; Wen, Y.; Yang, W.; Chen, H. Hydrophobic modification of nanocellulose via a two-step silanation method. Polymers 2018, 10, 1035. [Google Scholar] [CrossRef]
- Gan, L.; Liao, J.; Lin, N.; Hu, C.; Wang, H.; Huang, J. Focus on gradientwise control of the surface acetylation of cellulose nanocrystals to optimize mechanical reinforcement for hydrophobic polyester-based nanocomposites. ACS Omega 2017, 2, 4725–4736. [Google Scholar] [CrossRef]
- Peng, S.X.; Shrestha, S.; Yoo, Y.; Youngblood, J.P. Enhanced dispersion and propertiesof a two-component epoxy nanocomposite using surface modified cellulose nanocrystals. Polymer 2017, 112, 359–368. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable construction of temperaturesensitive supramolecular hydrogel based on cellulose and cyclodextrin. Polymers 2022, 14, 3801. [Google Scholar] [CrossRef]
- Dong, W.K.; Shin, J.; Choi, S.Q. Nano-dispersed cellulose nanofibrils-PMMA composite from Pickering emulsion with tunable interfacial tensions. Carbohydr. Polym. 2020, 247, 116762. [Google Scholar]
- Yao, H.; Zhou, J.; Li, H.; Zhao, J. Nanocrystalline cellulose/fluorinated polyacrylate latex via RAFT mediated surfactant- free emulsion polymerization and its application as waterborne textile finishing agent. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1305–1314. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, H.; Yao, H. Cellulose nanocrystals/fluorinated polyacrylate soap-free emulsion prepared via RAFT- assisted Pickering emulsion polymerization. Colloids Surf. B. 2019, 177, 321–328. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Graham, L.; Moorlag, C.; Dooley, B.M.; Cranston, E.D. Poly (methylmethacrylate)-grafted cellulose nanocrystals: One- step synthesis, nanocomposite preparation, and characterization. Can. J. Chem. Eng. 2016, 94, 811–822. [Google Scholar] [CrossRef]
- Wei, L. All acrylic based thermoplastic elastomers: Design and synthesis for improved mechanical performance. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2017. [Google Scholar]
- Hasa, E.; Tai, Y.L.; Guymon, C.A. Controlling phase separated domains in UV-curable formulations with H-functionalized prepolymers. Polym. Chem. 2022, 13, 3102–3115. [Google Scholar] [CrossRef]
- Michael, V.K.; Amir, S.P.; Richard, M.B.; Todd, H.; Marc, A.D.; Emily, D.C. Incorporation of polymer-grafted cellulose nanocrystals into latex-based pressure-sensitive adhesives. ACS Mater. Au 2022, 2, 176–189. [Google Scholar]
- Yu, Q.; Yang, W.; Wang, Q.; Dong, W.; Du, M.; Ma, P. Functionalization of cellulose nanocrystals with γ-MPS and its effect on the adhesive behavior of acrylic pressure sensitive adhesives. Carbohydr. Polym. 2019, 217, 168–177. [Google Scholar] [CrossRef]
- Che, K.M.; Zhang, M.Z.; He, J.L.; Ni, P.H. Polyphosphoester-modified cellulose nanocrystals for stabilizing Pickering emulsion polymerization of styrene. Chin. J. Polym. Sci. 2020, 38, 921–931. [Google Scholar] [CrossRef]
- Xu, P.; Cao, Y.; Wu, B.; Ma, P.; Dong, W.; Bai, H.; Zhang, H.; Zhu, H.; Chen, M. Effects of modified nanocrystalline cellulose on the hydrophilicity, crystallization and mechanical behaviors of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). New J. Chem. 2018, 42, 11972–11978. [Google Scholar] [CrossRef]
- Jie, Y.; Dai, Y.Y. Liquid crystal of nanocellulose whiskers’ grafted with acrylamide. Chin. Chem. Lett. 2012, 23, 367–370. [Google Scholar]
- Han, S.; Lyu, S.; Chen, Z.; Fu, F.; Wang, S. Combined stabilizers prepared from cellulose nanocrystals and styrene-maleic anhydride to microencapsulate phase change materials. Carbohydr. Polym. 2020, 234, 115923. [Google Scholar] [CrossRef] [PubMed]
- Kurukji, D.; Pichot, R.; Spyropoulos, F.; Norton, I.T. Interfacial behaviour of sodium stearoyllactylate (SSL) as an oil-in-water Pickering emulsion stabiliser. J. Colloid Interface Sci. 2013, 409, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.O.; Zhang, H.; Schilling, M.; Liu, X.; Wang, X.; Florian, R.; Zhang, K. High-internal-phase Pickering emulsions stabilized by polymeric dialdehyde cellulose-based nanoparticles. ACS Sustain. Chem. Eng. 2020, 8, 7371–7379. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Xu, H.; Wang, A. Preparation of carboxymethyl cellulose-based macroporous adsorbent by eco-friendly Pickering-MIPEs template for fast removal of Pb2+ and Cd2+. Front. Chem. 2019, 7, 603. [Google Scholar] [CrossRef]
- He, Y.; Wu, F. Factors that affect Pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Mazela, B.; Tomkowiak, K.; Jones, D. Strength and moisture-related properties of filter paper coated with nanocellulose. Coatings 2022, 12, 1376. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Tao, H.; Li, Y.; Wang, Y.; Zhou, Y.; Xu, Q.; Ma, J. Enhanced Pickering Emulsion Stabilization of Cellulose Nanocrystals and Application for Reinforced and Hydrophobic Coatings. Coatings 2022, 12, 1594. https://doi.org/10.3390/coatings12101594
Zhang F, Tao H, Li Y, Wang Y, Zhou Y, Xu Q, Ma J. Enhanced Pickering Emulsion Stabilization of Cellulose Nanocrystals and Application for Reinforced and Hydrophobic Coatings. Coatings. 2022; 12(10):1594. https://doi.org/10.3390/coatings12101594
Chicago/Turabian StyleZhang, Fan, Haoran Tao, Yilin Li, Yanbing Wang, Yingying Zhou, Qunna Xu, and Jianzhong Ma. 2022. "Enhanced Pickering Emulsion Stabilization of Cellulose Nanocrystals and Application for Reinforced and Hydrophobic Coatings" Coatings 12, no. 10: 1594. https://doi.org/10.3390/coatings12101594
APA StyleZhang, F., Tao, H., Li, Y., Wang, Y., Zhou, Y., Xu, Q., & Ma, J. (2022). Enhanced Pickering Emulsion Stabilization of Cellulose Nanocrystals and Application for Reinforced and Hydrophobic Coatings. Coatings, 12(10), 1594. https://doi.org/10.3390/coatings12101594





