An Antimicrobial Marine Cage Surface Modified with Antibacterial Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
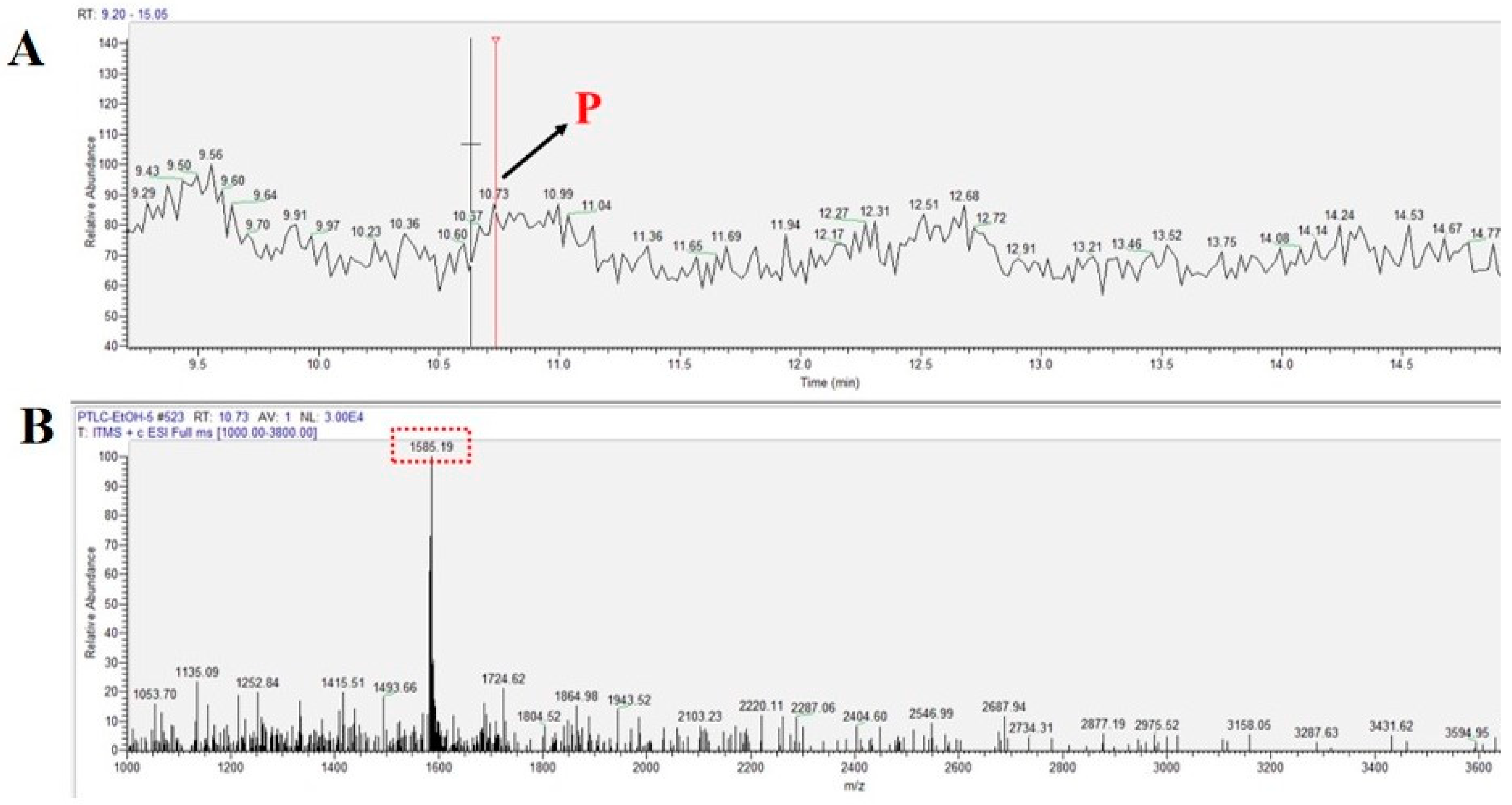
2.2. Extraction and Isolation of Peptide
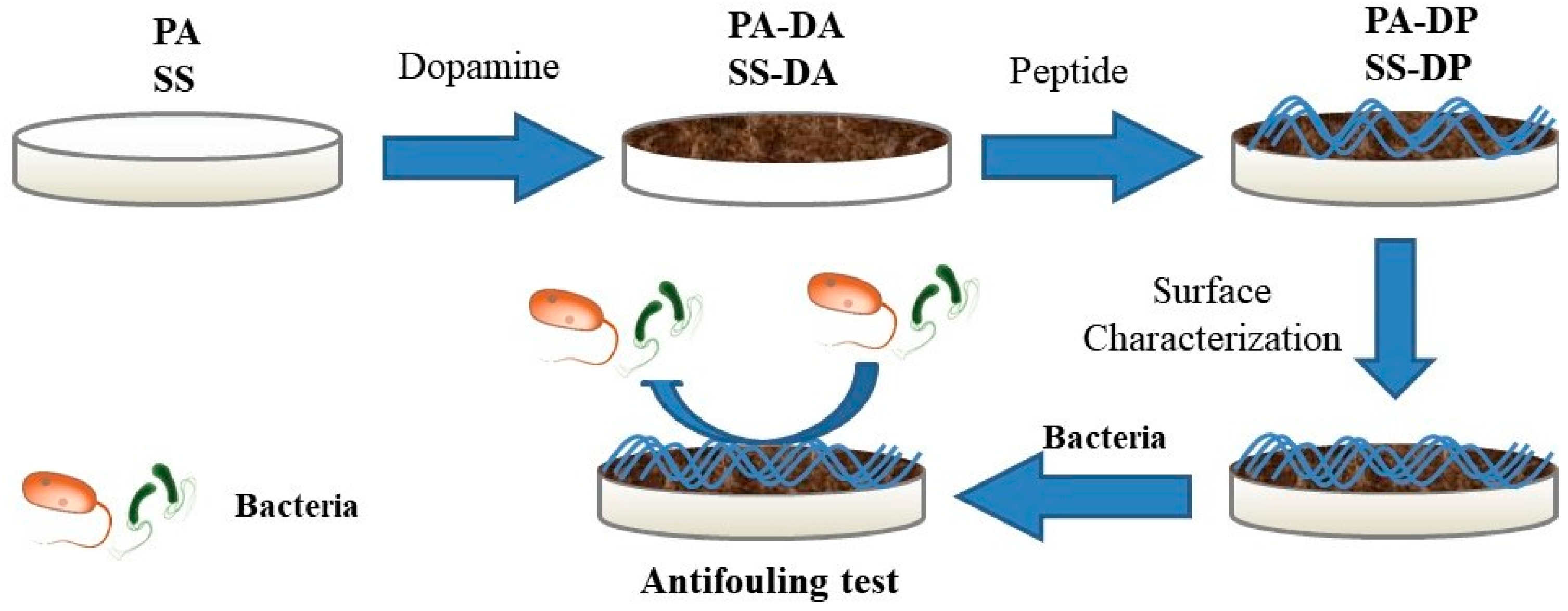
2.3. Fabrication of Peptide Modified Surface
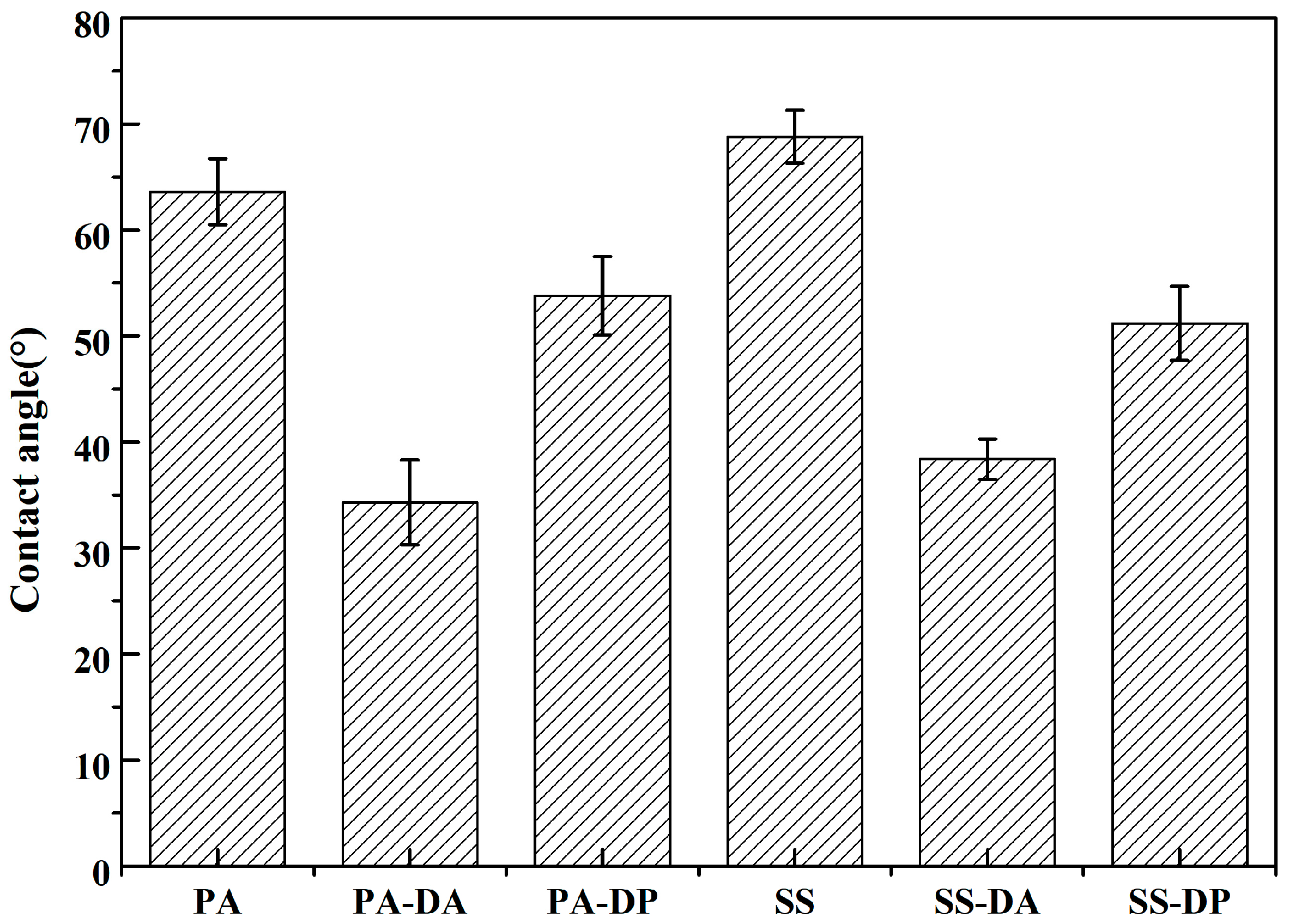
2.4. Surface Characterization
2.5. Antimicrobial Tests
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, C.; Shumway, S.; Whitlatch, R.; Getchis, T. Biofouling in marine molluscan shellfish aquaculture: A survey assessing the business and economic implications of mitigation. J. World Aquac. Soc. 2011, 42, 242–252. [Google Scholar] [CrossRef]
- Vladkova, T. Surface modification approach to control biofouling. In Marine and Industrial Biofouling; Springer Series on Biofilms; Springer: Berlin/Heidelberg, Germany, 2009; Volume 4, pp. 135–163. [Google Scholar]
- Abarzua, S.; Jacubowski, S. Biotechnological investigation for the prevention of biofouling 1. Biological and biochemical principles for the prevention of biofouling. Mar. Ecol. Prog. Ser. 1995, 123, 301–312. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.; Kane, R. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Tesler, A.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef] [PubMed]
- Sunny, S.; Vogel, N.; Howell, C.; Vu, T.; Aizenberg, J. Lubricant-infused nanoparticulate coatings assembled by layer-by-layer deposition. Adv. Funct. Mater. 2014, 24, 6658–6667. [Google Scholar] [CrossRef]
- Anderson, C.; Atlar, M.; Callow, M.; Candries, M.; Milne, A.; Townsin, R. The development of fouling-release coatings for seagoing vessels. J. Mar. Des. Oper. 2003, B4, 11–23. [Google Scholar]
- Zhao, Q.; Wang, S.; Müller-Steinhagen, H. Tailored surface free energy of membrane diffusers to minimize microbial adhesion. Appl. Surf. Sci. 2004, 230, 371–378. [Google Scholar] [CrossRef]
- Srinivasan, M.; Swain, G. Managing the use of copper-based antifouling paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef]
- Xie, C.; Guo, H.; Zhao, W.; Zhang, L. Environmentally friendly marine antifouling coating based on a synergistic strategy. Langmuir 2020, 36, 2396–2402. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.; Fuente-Nunez, C.; Phillips, K.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2021, 41, 94–120. [Google Scholar] [CrossRef]
- Boman, H.; Nilsson, I.; Rasmuson, B. Inducible antibacterial defense system in drosophila. Nature 1972, 237, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Andrea, A.; Molchanova, N.; Jenssen, H. Antibiofilm peptides and peptidomimetics with focus on surface immobilization. Biomolecules 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Segev-Zarko, L.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.; Shai, Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- Trepos, R.; Cervin, G.; Pile, C.; Pavia, H.; Hellio, C.; Svenson, J. Evaluation of cationic micropeptides derived from the innate immune system as inhibitors of marine biofouling. Biofouling 2015, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Li, D.; Irvin, R. A peptide-stainless steel reaction that yields a new bioorganic—Metal state of matter. Biomaterials 2011, 32, 5311–5319. [Google Scholar] [CrossRef]
- Cao, P.; He, X.; Xiao, J.; Yuan, C.; Bai, X. Peptide-modified stainless steel with resistance capacity of Staphylococcus aureus biofilm formation. Surf. Interface Anal. 2018, 50, 1362–1369. [Google Scholar] [CrossRef]
- Khan, M.; Wu, Z.; Mao, S.; Lin, J.; Shah, S.; Lin, J. Controlled grafted poly(quaternized-4-vinylpyridine-co-acrylic acid) brushes attract bacteria for effective antimicrobial surfaces. J. Mater. Chem. B 2018, 6, 3782–3791. [Google Scholar] [CrossRef]
- Cao, P.; Yuan, C.; Xiao, J.; He, X.; Bai, X. A biofilm resistance surface yielded by grafting of antimicrobial peptides on stainless steel surface. Surf. Interface Anal. 2018, 50, 516–521. [Google Scholar] [CrossRef]
- Shang, B.; Wang, Y.; Yang, P.; Peng, B.; Deng, Z. Synthesis of superhydrophobic polydopamine-Ag microbowl/nanoparticle array substrates for highly sensitive, durable and reproducible surface-enhanced Raman scattering detection. Sens. Actuators B Chem. 2018, 255, 995–1005. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.; Bow, H.; Tambyah, P.; Hadinoto, K.; Leong, S. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; McCloskey, B.; Choi, T.; Lee, C.; Kim, M.; Freeman, B.; Park, H. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.; Miller, W.; Messersmith, P. Mussel-inspired surface chemistry-for multifunctional coatings. Science 2007, 5849, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Thuy, L.; Ki, S.; Ko, S.; Kim, S.; Cho, W.; Choi, J.; Kang, S. Multipurpose antifouling coating of solid surfaces with the marine-derived polymer fucoidan. Macromol. Biosci. 2018, 10, 1800137. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.; Peddiraju, V.; Mishra, A. Effect of antimicrobial peptide (AMP) etethered stainless steel surfaces on the bacterial membrane. Mater. Today Chem. 2021, 21, 100541. [Google Scholar] [CrossRef]
- Cao, P.; Liu, D.; Zhang, Y.; Xiao, F.; Yuan, C.; Liang, F.; Liu, X.; Zhang, C. Dopamine-assisted sustainable antimicrobial peptide coating with antifouling and anticorrosion properties. Appl. Surf. Sci. 2022, 589, 153019. [Google Scholar] [CrossRef]
- Cao, P.; Yang, Y.; Uche, F.; Hart, S.; Li, W.; Yuan, C.Q. Coupling plant-derived cyclotides to metal surfaces: An antibacterial and antibiofilm study. Int. J. Mol. Sci. 2018, 19, 793. [Google Scholar] [CrossRef]
- Berisha, A.; Hazimeh, H.; Galtayries, A.; Decorse, P.; Kanoufi, F.; Combellas, C.; Pinson, J.; Podvorica, F. Grafting of an aluminium surface with organic layers. RSC Adv. 2016, 6, 78369–78377. [Google Scholar] [CrossRef]
- Lan, Y.; Hiebner, D.; Casey, E. Self-assembly and regeneration strategy for mitigation of membrane biofouling by the exploitation of enzymatic nanoparticles. Chem. Eng. J. 2021, 412, 128666. [Google Scholar] [CrossRef]
- Lou, T.; Bai, X.; He, X.; Liu, W.; Yang, Z.; Yang, Y.; Yuan, C. Enhanced antifouling properties of marine antimicrobial peptides by PEGylation. Front. Bioeng. Biotech. 2023, 11, 1124389. [Google Scholar] [CrossRef]
- Fevzioglu, M.; Ozturk, O.; Hamaker, B.; Campanella, O. Quantitative approach to study secondary structure of proteins by FT-IR spectroscopy, using a model wheat gluten system. Int. J. Biol. Macromol. 2020, 164, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Du, C.; He, X.; Zhang, C.; Yuan, C. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl. Surf. Sci. 2020, 510, 145512. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Guo, Q. An Antimicrobial Marine Cage Surface Modified with Antibacterial Peptides. Coatings 2023, 13, 1711. https://doi.org/10.3390/coatings13101711
Cao Z, Guo Q. An Antimicrobial Marine Cage Surface Modified with Antibacterial Peptides. Coatings. 2023; 13(10):1711. https://doi.org/10.3390/coatings13101711
Chicago/Turabian StyleCao, Zhimin, and Qian Guo. 2023. "An Antimicrobial Marine Cage Surface Modified with Antibacterial Peptides" Coatings 13, no. 10: 1711. https://doi.org/10.3390/coatings13101711




