Superhydrophobic Coatings on Cellulose-Based Materials with Alkyl Ketene Dimer Pickering Emulsion: Fabrication and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AKD Pickering Emulsion
- (1)
- 2.0 g of glacial acetic acid and 198.0 g of deionized water were mixed in a beaker to obtain 1.0 wt% acetic acid solution. Then, a certain amount of chitosan was added into the acetic acid solution. After mixing and stirring evenly, the chitosan powder was completely dissolved to prepare an acetic acid solution of chitosan.
- (2)
- 8.0 g of melted AKD was added to the heated chitosan acetic acid solution at 65 °C. This mixture was then emulsified at 10,000 rpm for 10 min using a high-speed homogenizer. During emulsification, a water bath insulated the AKD to prevent solidification, which could disrupt the process.
- (3)
- A 0.5 wt% dispersion solution of titanium dioxide (TiO2) was prepared using ultrasonic dispersion for 10 min. A designated amount of heated chitosan acetic acid solution and TiO2 dispersion was combined with 8.0 g of melted AKD, followed by emulsification at 10,000 rpm for 10 min. Once cooled to room temperature, an AKD emulsion with chitosan/TiO2 as emulsifiers was formed. The emulsification process of AKD by chitosan was the same as that of chitosan/TiO2 but without the addition TiO2 dispersion.
2.3. Preparation of Superhydrophobic AKD Coatings
2.4. Characterization
3. Results
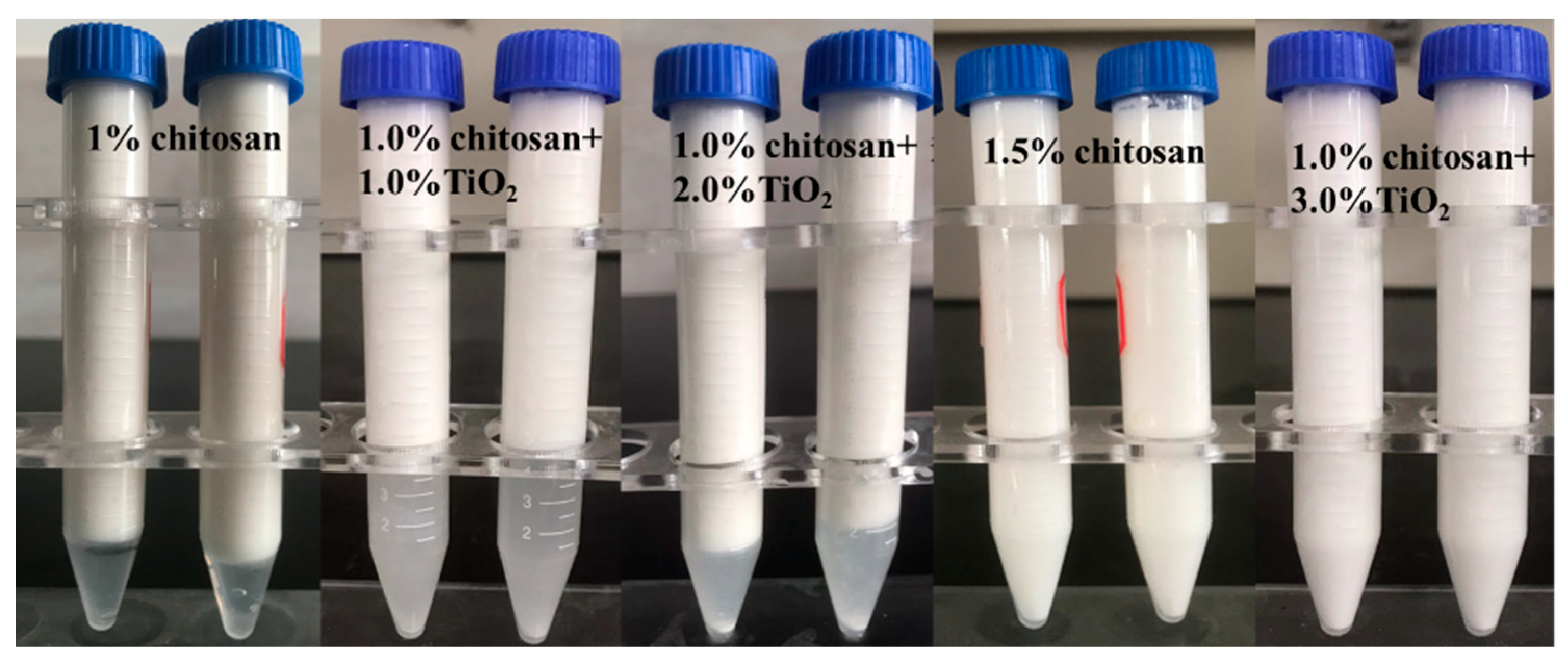
3.1. The Stability of AKD Pickering Emulsion
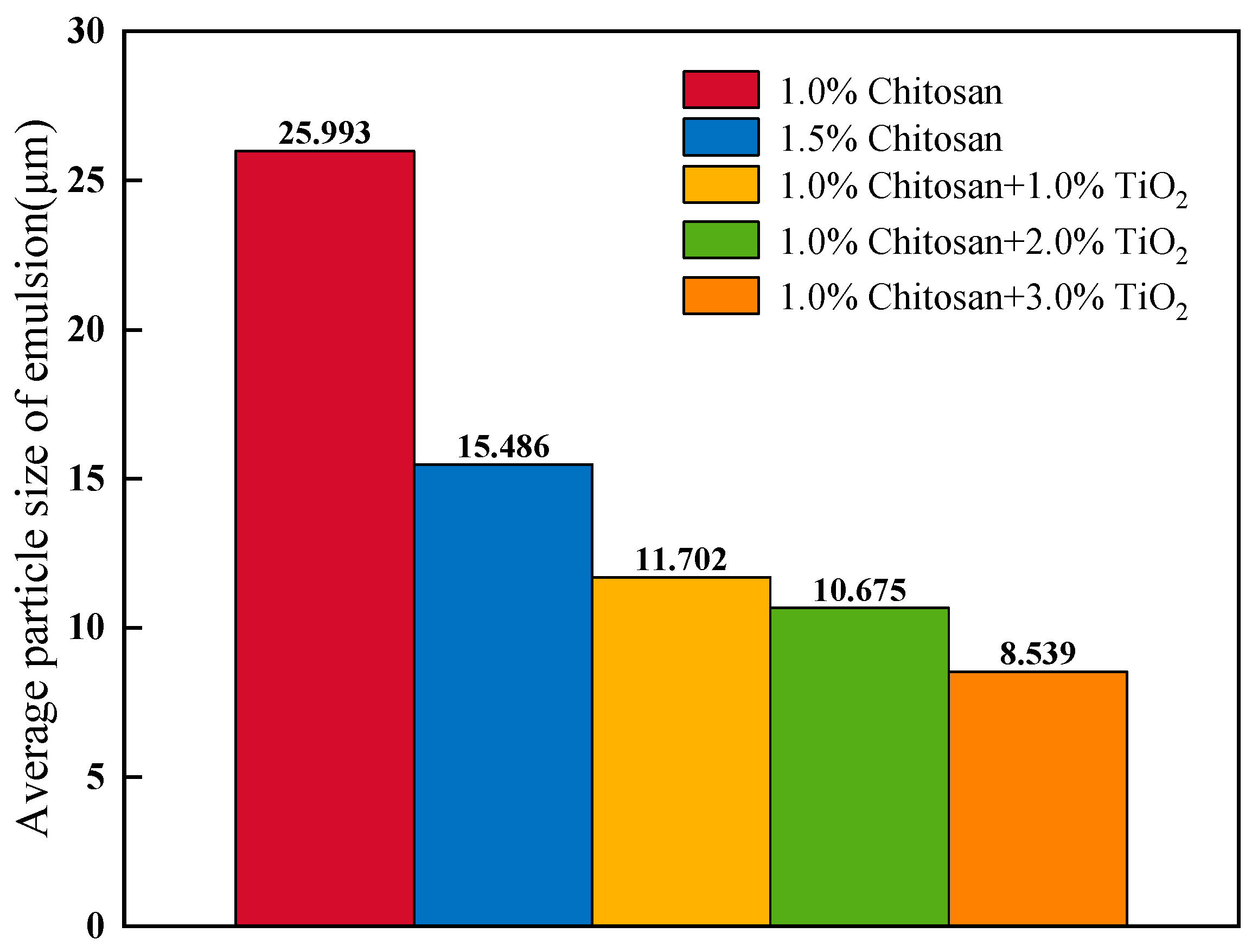
3.2. The Particle Size of AKD Emulsion
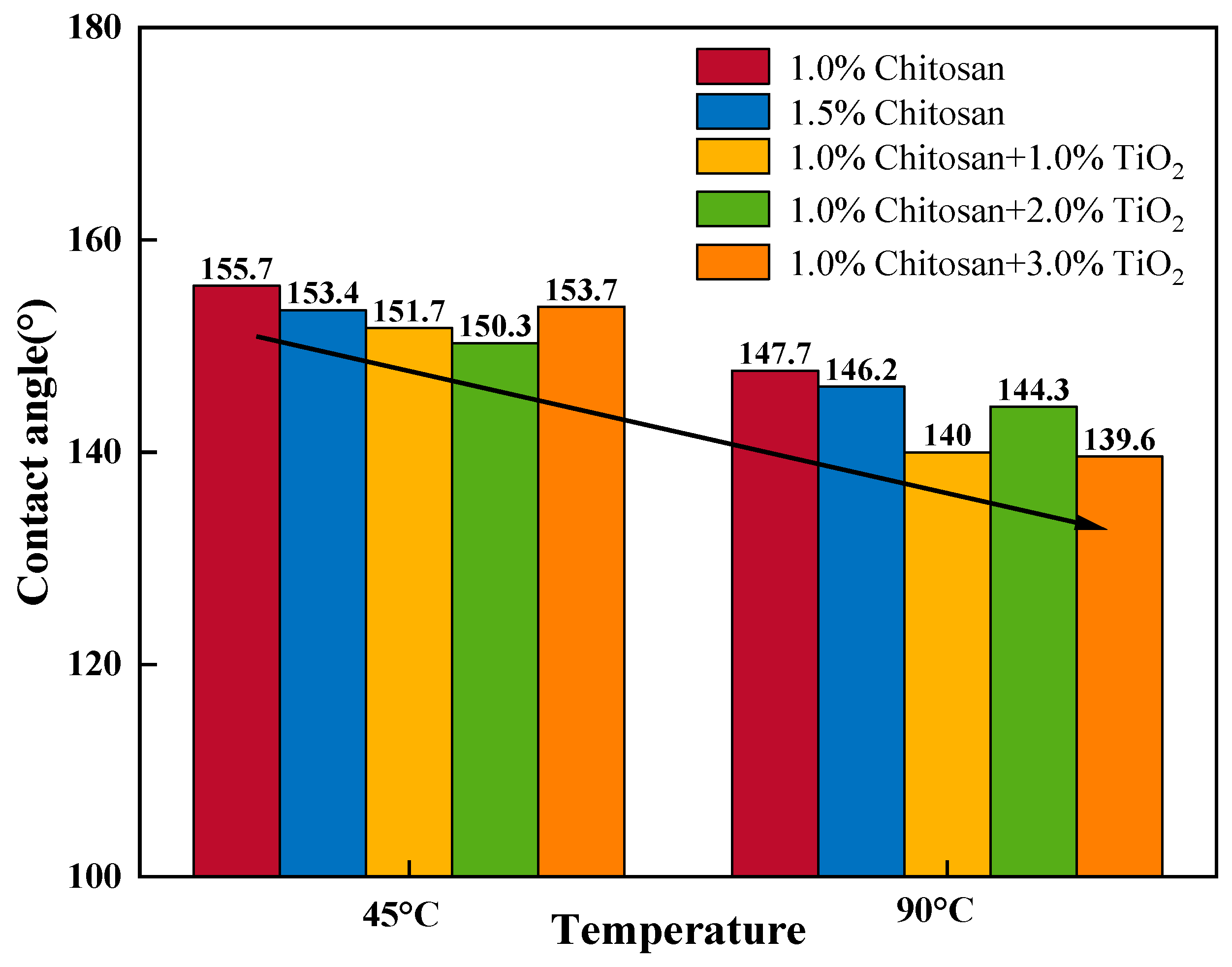
3.3. Hydrophobic Properties of AKD-Coated Filter Paper
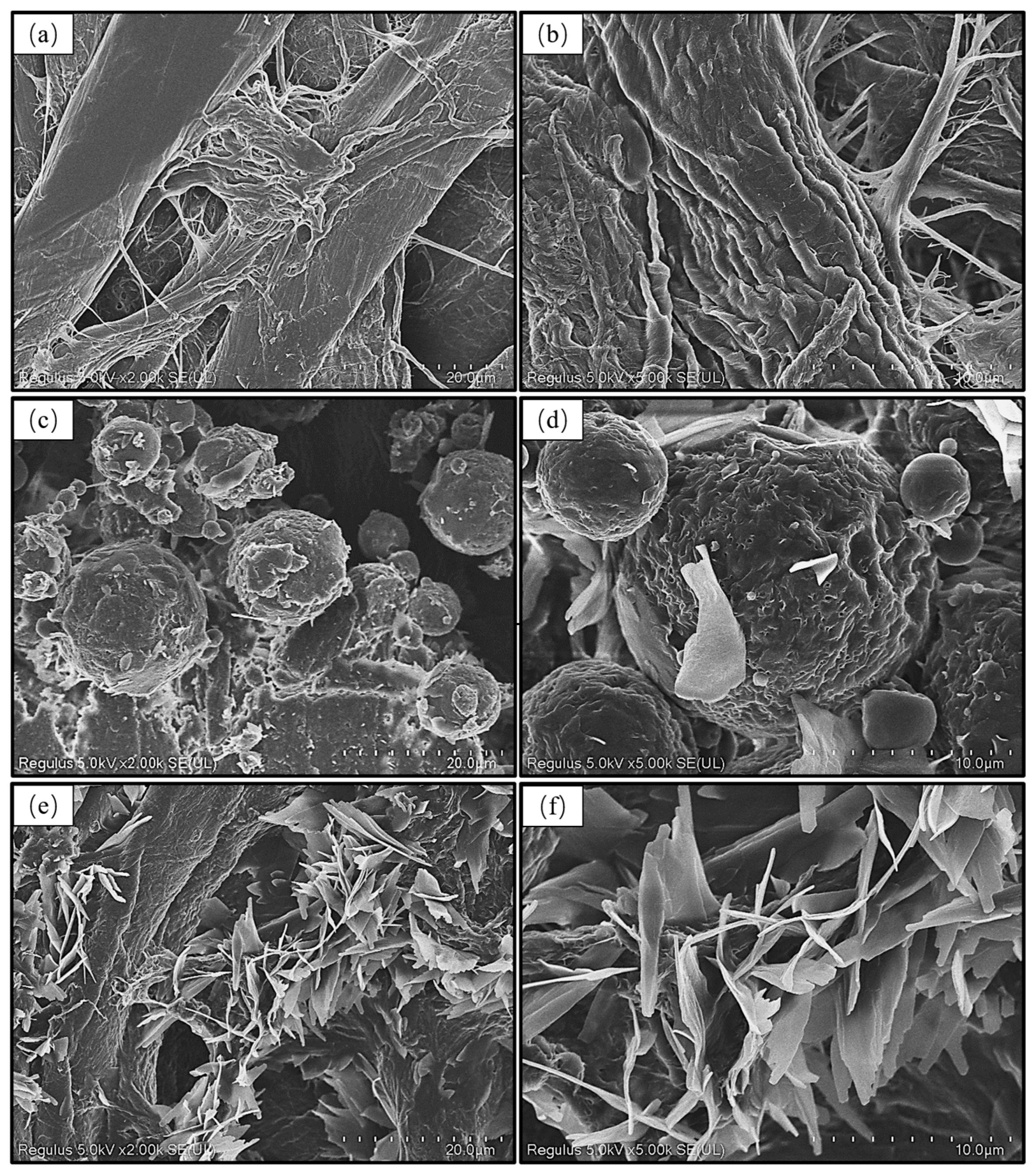
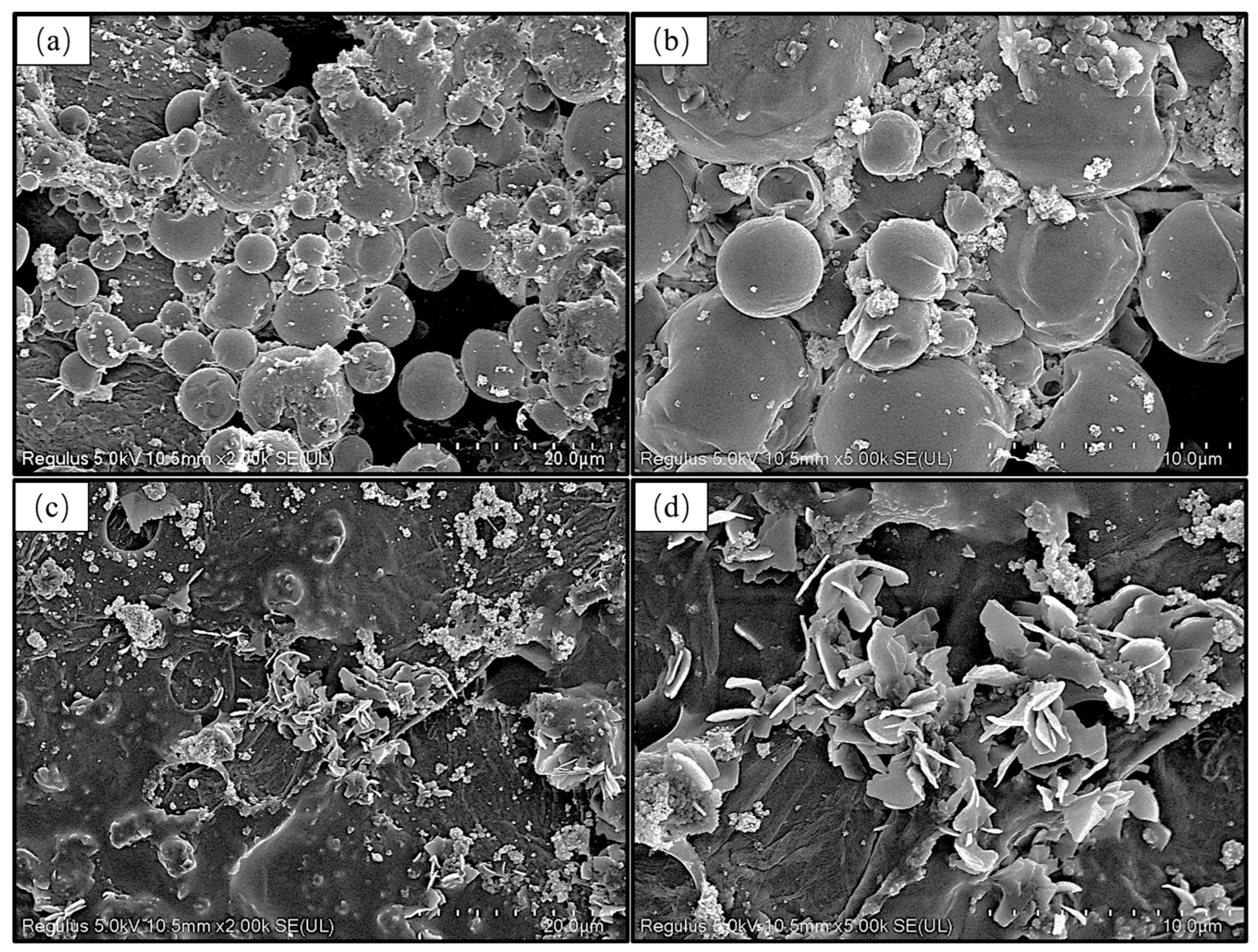
3.4. Surface Morphology of AKD Emulsion-Coated Filter Paper
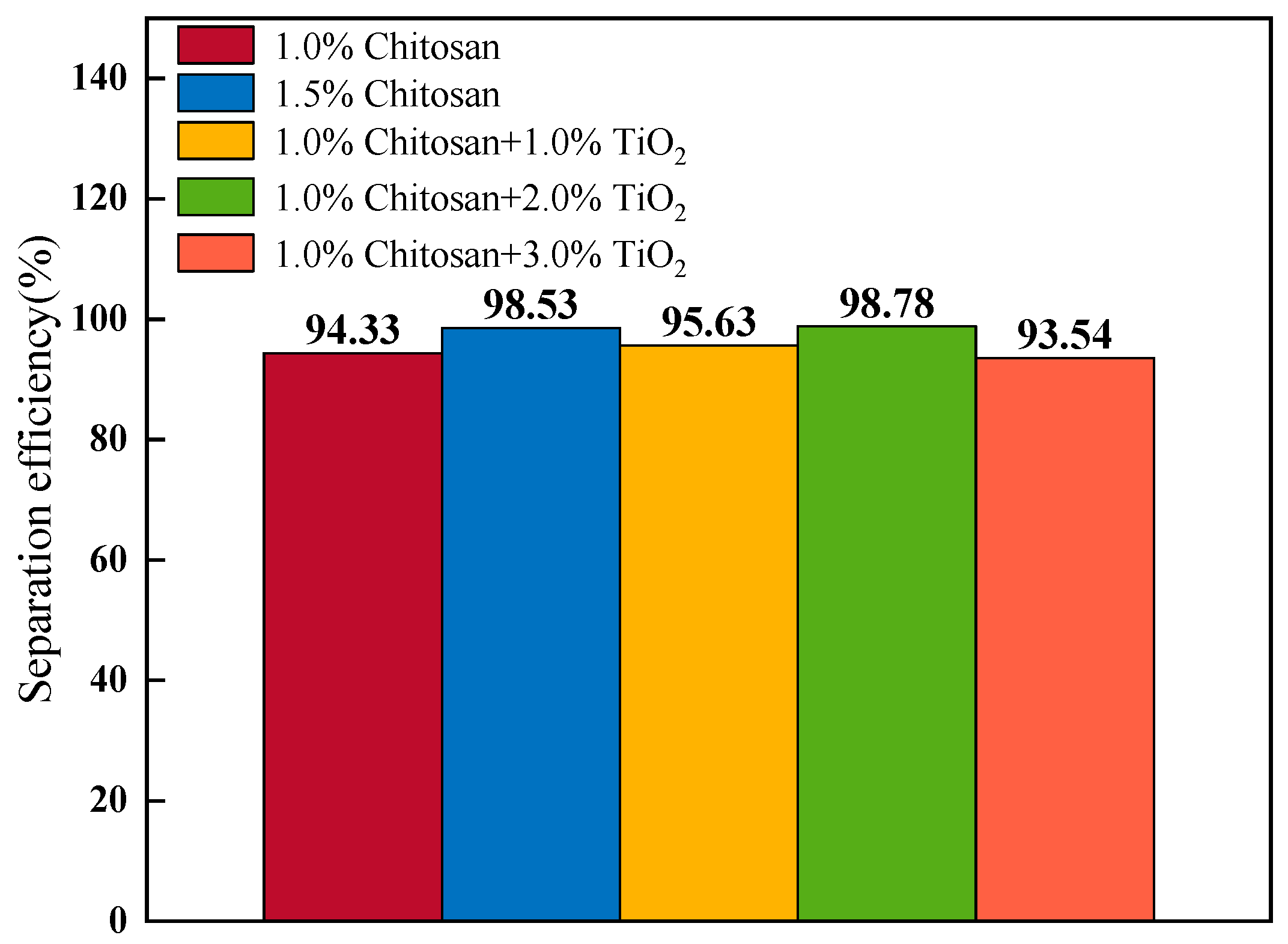
3.5. Oil–Water Separation Properties of AKD-Coated Filter Paper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.; Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef]
- Isimjan, T.T.; Wang, T.; Rohani, S. A novel method to prepare superhydrophobic, UV resistance and anti-corrosion steel surface. Chem. Eng. J. 2012, 210, 182–187. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, D.; Qiu, R.; Hou, B. Super-hydrophobic film prepared on zinc as corrosion barrier. Corros. Sci. 2011, 53, 2080–2086. [Google Scholar] [CrossRef]
- Criscione, A.; Roisman, I.V.; Jakirlić, S.; Tropea, C. Towards modelling of initial and final stages of supercooled water solidification. Int. J. Therm. Sci. 2015, 92, 150–161. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Bressler, A.H.; Castano, C.E.; Fergusson, C.P.; Mohammadi, R. Rational strategy for the atmospheric icing prevention based on chemically functionalized carbon soot coatings. Appl. Surf. Sci. 2016, 390, 452–460. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Quéré, D. Rough ideas on wetting. Phys. A Stat. Mech. Appl. 2002, 313, 32–46. [Google Scholar] [CrossRef]
- Hamidon, N.N.; Hong, Y.; Salentijn, G.I.; Verpoorte, E. Water-based alkyl ketene dimer ink for user-friendly patterning in paper microfluidics. Anal. Chim. Acta 2018, 1000, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Q.; Song, X.M.; Cui, L.Q.; Gao, S.S.; Chen, F.S.; Wang, S.L.; Liu, J.L. Preparation and characterization of AKD sizing agent by “one-pot cooking”. Nord. Pulp Pap. Res. J. 2018, 33, 317–326. [Google Scholar] [CrossRef]
- Li, L.; Neivandt, D.J. The mechanism of alkyl ketene dimer (AKD) sizing on cellulose model films studied by sum frequency generation vibrational spectroscopy. Cellulose 2019, 26, 3415–3435. [Google Scholar] [CrossRef]
- Zhang, X.; Batchelor, W.; Shen, W. Building Dual-Scale Roughness Using Inorganic Pigments for Fabrication of Superhydrophobic Paper. Ind. Eng. Chem. Res. 2017, 56, 3618–3628. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Li, G.; Lucia, L.A.; Wang, H.; Yu, D. Unique alkyl ketene dimer Pickering-based dispersions: Preparation and application to paper sizing. J. Appl. Polym. Sci. 2018, 135, 45730. [Google Scholar] [CrossRef]
- Quan, C.; Werner, O.; Wågberg, L.; Turner, C. Generation of superhydrophobic paper surfaces by a rapidly expanding supercritical carbon dioxide–alkyl ketene dimer solution. J. Supercrit. Fluids 2009, 49, 117–124. [Google Scholar] [CrossRef]
- Arbatan, T.; Zhang, L.; Fang, X.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Esmaeili, A.R.; Mir, N.; Mohammadi, R. A facile, fast, and low-cost method for fabrication of micro/nano-textured superhydrophobic surfaces. J. Colloid Interface Sci. 2020, 573, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H. Fabrication and characterization of pickering emulsions stabilized by octenyl succinic anhydride -modified gliadin nanoparticle. Food Hydrocolloid. 2019, 90, 19–27. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, Y.; Zhong, J.; Yu, C. Superhydrophobic Coatings on Cellulose-Based Materials with Alkyl Ketene Dimer Pickering Emulsion: Fabrication and Properties. Coatings 2023, 13, 1829. https://doi.org/10.3390/coatings13111829
Wang Y, Huang Y, Zhong J, Yu C. Superhydrophobic Coatings on Cellulose-Based Materials with Alkyl Ketene Dimer Pickering Emulsion: Fabrication and Properties. Coatings. 2023; 13(11):1829. https://doi.org/10.3390/coatings13111829
Chicago/Turabian StyleWang, Yating, Yuanfei Huang, Jing Zhong, and Chenghua Yu. 2023. "Superhydrophobic Coatings on Cellulose-Based Materials with Alkyl Ketene Dimer Pickering Emulsion: Fabrication and Properties" Coatings 13, no. 11: 1829. https://doi.org/10.3390/coatings13111829
APA StyleWang, Y., Huang, Y., Zhong, J., & Yu, C. (2023). Superhydrophobic Coatings on Cellulose-Based Materials with Alkyl Ketene Dimer Pickering Emulsion: Fabrication and Properties. Coatings, 13(11), 1829. https://doi.org/10.3390/coatings13111829



