Adsorption of Fragrance Capsules onto Cellulose Nano- and Micro-Cellulose Fibers in Presence of Guar Biopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Mixing Protocol
2.4. Dynamic Light Scattering
2.5. ζ-Potential
2.6. Phase-Contrast Optical Microscopy
2.7. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.8. Scanning Electron Microscopy (SEM)
3. Results and Discussion
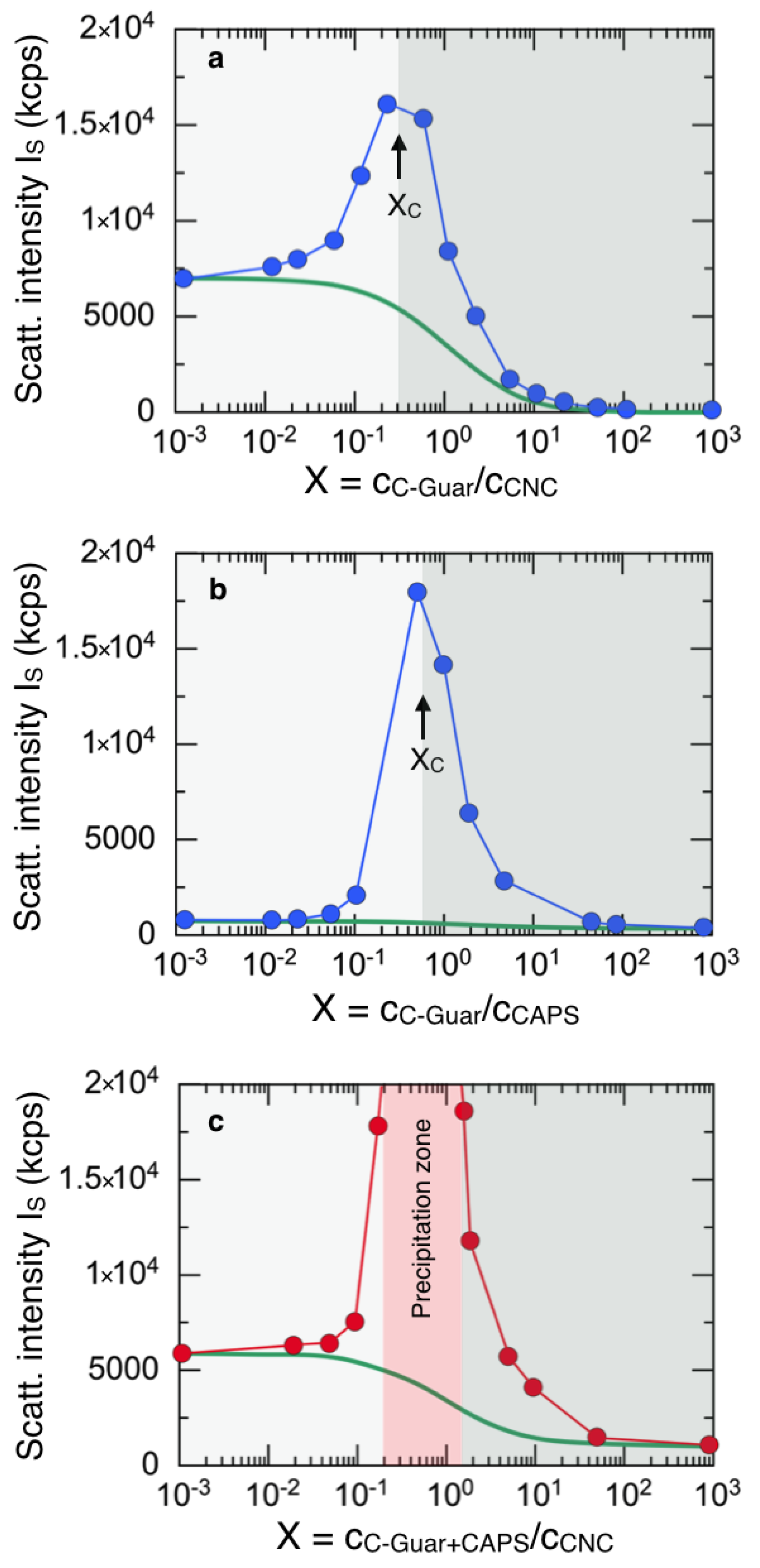
3.1. Interaction of Fragrance Capsules with Cationic Surfactants and C-Guar Polymers
3.2. Impact of Esterquat Surfactant on Fragrance Capsule/Nanocellulose Interaction
3.3. Impact of Cationic Guar Biopolymers on Fragrance Capsule/Nanocellulose Interaction
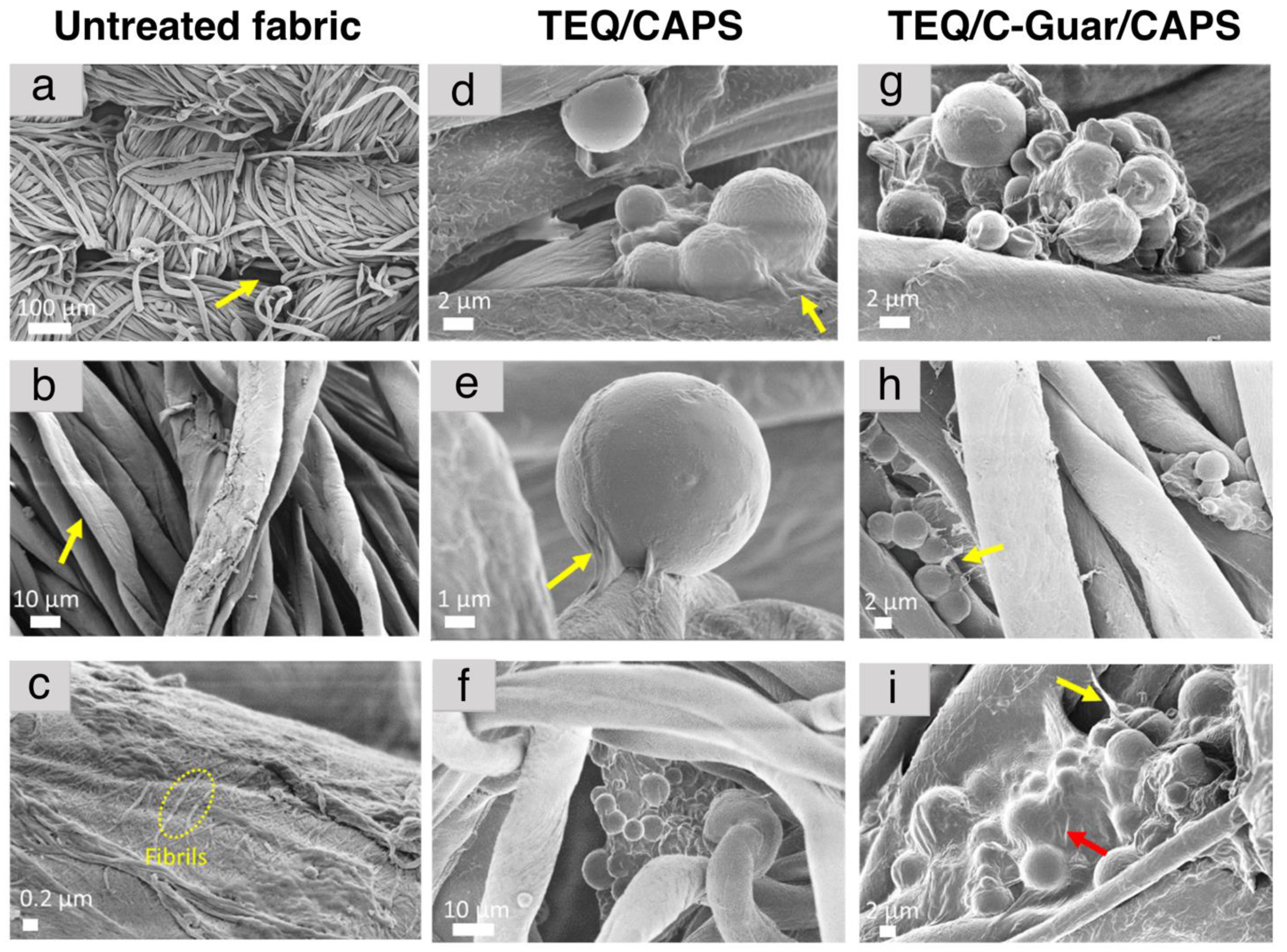
3.4. Adsorption of Fragrance Capsules on Cotton Microfibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crutzen, A.M. Study of the ditallowdimethylammonium chloride interaction with cellulose. J. Am. Oil Chem. Soc. 1995, 72, 137–143. [Google Scholar] [CrossRef]
- Murphy, D.S. Fabric Softener Technology: A Review. J. Surfact. Deterg. 2015, 18, 199–204. [Google Scholar] [CrossRef]
- Boh Podgornik, B.; Šandrić, S.; Kert, M. Microencapsulation for Functional Textile Coatings with Emphasis on Biodegradability—A Systematic Review. Coatings 2021, 11, 1371. [Google Scholar] [CrossRef]
- Basavaraj, M.G.; McFarlane, N.L.; Lynch, M.L.; Wagner, N.J. Nanovesicle formation and microstructure in aqueous ditallowethylesterdimethylammonium chloride (DEEDMAC) solutions. J. Colloid Interface Sci. 2014, 429, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Nakamura, K.; Hoshi, M.; Hara, T.; Kojima, H.; Itou, M.; Ikeda, R.; Okamoto, Y. Elucidation of Softening Mechanism in Rinse-Cycle Fabric Softeners. Part 2: Uneven Adsorption—The Key Phenomenon to the Effect of Fabric Softeners. J. Surfact. Deterg. 2016, 19, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dahl, V.; Kleinen, J.; Gambaryan-Roisman, T.; Venzmer, J. Influence of lipid bilayer phase behavior and substrate roughness on the pathways of intact vesicle deposition: A streaming potential study. Colloids Surf. A 2017, 521, 302–311. [Google Scholar] [CrossRef]
- Kumar, A.; Gilson, L.; Henrich, F.; Dahl, V.; Kleinen, J.; Gambaryan-Roisman, T.; Venzmer, J. Intact deposition of cationic vesicles on anionic cellulose fibers: Role of vesicle size, polydispersity, and substrate roughness studied via streaming potential measurements. J. Colloid Interface Sci. 2016, 473, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Berret, J.-F. Advanced Eco-Friendly Formulations of Guar Biopolymer-Based Textile Conditioners. Materials 2021, 14, 5749. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef]
- Mamusa, M.; Sofroniou, C.; Resta, C.; Murgia, S.; Fratini, E.; Smets, J.; Baglioni, P. Tuning the Encapsulation of Simple Fragrances with an Amphiphilic Graft Copolymer. ACS Appl. Mater. Interfaces 2020, 12, 28808–28818. [Google Scholar] [CrossRef]
- He, Y.; Bowen, J.; Andrews, J.W.; Liu, M.; Smets, J.; Zhang, Z. Adhesion of perfume-filled microcapsules to model fabric surfaces. J. Microencapsul. 2014, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Messina, G.M.L.; Heux, L.; Marletta, G.; Berret, J.-F. Adsorption of a fabric conditioner on cellulose nanocrystals: Synergistic effects of surfactant vesicles and polysaccharides on softness properties. Cellulose 2021, 28, 2551–2566. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Mousseau, F.; Christov, N.; Cristobal, G.; Vacher, A.; Airiau, M.; Bourgaux, C.; Heux, L.; Berret, J.-F. Fabric Softener–Cellulose Nanocrystal Interaction: A Model for Assessing Surfactant Deposition on Cotton. J. Phys. Chem. B 2017, 121, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Tekin, R.; Bac, N.; Erdogmus, H. Microencapsulation of Fragrance and Natural Volatile Oils for Application in Cosmetics, and Household Cleaning Products. Macromol. Symp. 2013, 333, 35–40. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mercadé-Prieto, R.; Pan, X.; Fernández-González, A.; Zhang, Z.; Bakalis, S. Quantification of Microcapsules Deposited in Cotton Fabrics before and after Abrasion Using Fluorescence Microscopy. Ind. Eng. Chem. Res. 2012, 51, 16741–16749. [Google Scholar] [CrossRef]
- He, L.; Hu, J.; Deng, W. Preparation and application of flavor and fragrance capsules. Polym. Chem. 2018, 9, 4926–4946. [Google Scholar] [CrossRef]
- Ambrosi, M.; Fratini, E.; Baglioni, P.; Vannucci, C.; Bartolini, A.; Pintens, A.; Smets, J. Microcapsules for Confining Fluids: Prediction of Shell Stability from Advanced SAXS Investigations. J. Phys. Chem. C 2016, 120, 13514–13522. [Google Scholar] [CrossRef]
- Pretzl, M.; Neubauer, M.; Tekaat, M.; Kunert, C.; Kuttner, C.; Leon, G.; Berthier, D.; Erni, P.; Ouali, L.; Fery, A. Formation and Mechanical Characterization of Aminoplast Core/Shell Microcapsules. ACS Appl. Mater. Interfaces 2012, 4, 2940–2948. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.-Q.; Fan, H.-F.; Xia, Q. Heat-resistant sustained-release fragrance microcapsules. J. Appl. Polym. Sci. 2014, 131, 40053. [Google Scholar] [CrossRef]
- Jacquemond, M.; Jeckelmann, N.; Ouali, L.; Haefliger, O.P. Perfume-containing polyurea microcapsules with undetectable levels of free isocyanates. J. Appl. Polym. Sci. 2009, 114, 3074–3080. [Google Scholar] [CrossRef]
- León, G.; Paret, N.; Fankhauser, P.; Grenno, D.; Erni, P.; Ouali, L.; Berthier, D.L. Formaldehyde-free melamine microcapsules as core/shell delivery systems for encapsulation of volatile active ingredients. RSC Adv. 2017, 7, 18962–18975. [Google Scholar] [CrossRef]
- Neumann, C.; Bacher, L.; Musyanovych, A.; Tutus, M.; Latnikova, A. Formulation of Next-Generation Multicompartment Microcapsules by Reversible Electrostatic Attraction. Chem. Eur. J. 2021, 27, 9336–9341. [Google Scholar] [CrossRef]
- Golja, B.; Forte Tavčer, P. Patterned Printing of Fragrant Microcapsules to Cotton Fabric. Coatings 2022, 12, 593. [Google Scholar] [CrossRef]
- Murtaza, M.; Hussain, A.I.; Kamal, G.M.; Nazir, S.; Chatha, S.A.S.; Asmari, M.; Uddin, J.; Murtaza, S. Potential Applications of Microencapsulated Essential Oil Components in Mosquito Repellent Textile Finishes. Coatings 2023, 13, 1467. [Google Scholar] [CrossRef]
- Indrie, L.; Affandi, N.D.N.; Díaz-García, P.; Haji, A.; Ilies, D.C.; Zlatev, Z.; Taghiyari, H.R.; Grama, V.; Farima, D. Mechanical and Morphological Properties of Cellulosic Fabrics Treated with Microencapsulated Essential Oils. Coatings 2022, 12, 1958. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of nanoparticles by light-scattering techniques. Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Kert, M.; Forte Tavčer, P.; Hladnik, A.; Spasić, K.; Puač, N.; Petrović, Z.L.; Gorjanc, M. Application of Fragrance Microcapsules onto Cotton Fabric after Treatment with Oxygen and Nitrogen Plasma. Coatings 2021, 11, 1181. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, S.J.; Cheong, I.W.; Kim, J.H. Microencapsulation of fragrant oil via in situ polymerization: Effects of pH and melamine-formaldehyde molar ratio. J. Microencapsul. 2002, 19, 559–569. [Google Scholar] [CrossRef]
- Prata, A.S.; Grosso, C.R.F. Production of microparticles with gelatin and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Bruyninckx, K.; Dusselier, M. Sustainable Chemistry Considerations for the Encapsulation of Volatile Compounds in Laundry-Type Applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Obendorf, S.K.; Liu, H.; Tan, K.; Leonard, M.J.; Young, T.J.; Incorvia, M.J. Adsorption of Aroma Chemicals on Cotton Fabric in Different Aqueous Environments. J. Surfact. Deterg. 2009, 12, 43–58. [Google Scholar] [CrossRef]
- Madan, G.L.; Shrivastava, S.K. Electrokinetic studies of cotton. Part IV.: Role of water in influencing surface charge density of cotton in electrolyte solution. Colloid Polym. Sci. 1977, 255, 269–275. [Google Scholar] [CrossRef]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current characterization methods for cellulose nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Deutschman, C.P.; Han, L.; Pope, M.A.; Tam, K.C. Cellulose nanocrystals in smart and stimuli-responsive materials: A review. Mater. Today Adv. 2020, 5, 100055. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.-F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Grandisson, C.; Golemanov, K.; Ahuja, R.; Berret, J.-F. Silicone incorporation into an esterquat based fabric softener in presence of guar polymers. Colloids Surf. A 2021, 615, 126175. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Christov, N.; Jin, D.W. Composition Comprising a Quaternary Ammonium Compound, a Cationic Polysaccharide and a Nonionic Polysaccharide. France Patent WO 2015/192971 Al, 23 December 2015. [Google Scholar]
- Zhang, H.Z.; Jin, D.W.; Christov, N.; Cristobal, G. Compositions Comprising Quat and Polysaccharides. France Patent WO 2017/101798 Al, 22 June 2017. [Google Scholar]
- Revol, J.F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Bio. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Lodge, T.P.; Muthukumar, M. Physical Chemistry of Polymers: Entropy, Interactions, and Dynamics. J. Phys. Chem. 1996, 100, 13275–13292. [Google Scholar] [CrossRef]
- Johnson, W.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Galactomannans as Used in Cosmetics. Int. J. Toxicol. 2015, 34, 35S–65S. [Google Scholar] [CrossRef] [PubMed]
- Ungewiß, J.; Vietzke, J.-P.; Rapp, C.; Schmidt-Lewerkühne, H.; Wittern, K.-P.; Salzer, R. Quantitative determination of cationic modified polysaccharides on hair using LC–MS and LC–MS–MS. Anal. Bioanal. Chem. 2005, 381, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Fresnais, J.; Lavelle, C.; Berret, J.-F. Nanoparticle Aggregation Controlled by Desalting Kinetics. J. Phys. Chem. C 2009, 113, 16371–16379. [Google Scholar] [CrossRef]
- Mousseau, F.; Berret, J.-F. The Role of Surface Charge in the Interaction of Nanoparticles with Model Pulmonary Surfactants. Soft Matter 2018, 14, 5764–5774. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Studies on the Formation of Complex Minerals in Solution and on Their Stability. Annal. Chim. 1928, 9, 113–203. [Google Scholar]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. 2013, 52, 11998–12013. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Picco, A.S.; Galdino, F.E.; Albuquerque, L.J.C.; Berret, J.-F.; Cardoso, M.B. Nanoparticle–Protein Interaction: Demystifying the Correlation between Protein Corona and Aggregation Phenomena. ACS Appl. Mater. Interfaces 2022, 14, 28559–28569. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering; Wiley and Sons: New York, NY, USA, 1976. [Google Scholar]
- Leclercq, L.; Boustta, M.; Vert, M. A physico-chemical approach of polyanion-polycation interactions aimed at better understanding the in vivo behaviour of polyelectrolyte-based drug delivery and gene transfection. J. Drug. Target. 2003, 11, 129–138. [Google Scholar] [CrossRef]
- Mengarelli, V.; Auvray, L.; Pastre, D.; Zeghal, M. Charge inversion, condensation and decondensation of DNA and polystyrene sulfonate by polyethylenimine. EPJ E 2011, 34, 127. [Google Scholar] [CrossRef] [PubMed]
- Priftis, D.; Megley, K.; Laugel, N.; Tirrell, M. Complex coacervation of poly(ethylene-imine)/polypeptide aqueous solutions: Thermodynamic and rheological characterization. J. Colloid Interface Sci. 2013, 398, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, E.; Westphal, A.H.; Borst, J.W.; Stuart, M.A.C.; van der Gucht, J. Binodal Compositions of Polyeleetrolyte Complexes. Macromolecules 2010, 43, 6476–6484. [Google Scholar] [CrossRef]
- Mousseau, F.; Vitorazi, L.; Herrmann, L.; Mornet, S.; Berret, J.-F. Polyelectrolyte assisted charge titration spectrometry: Applications to latex and oxide nanoparticles. J. Colloid Interface Sci. 2016, 475, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, A.E.; De Hoe, G.X.; Tirrell, M.V. Expanding the structural diversity of polyelectrolyte complexes and polyzwitterions. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100897. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Obendorf, S.K.; Dixit, V.; Woo, D.J. Microscopy Study of Distribution of Laundry Fabric Softener on Cotton Fabric. J. Surfact. Deterg. 2009, 12, 225–230. [Google Scholar] [CrossRef]
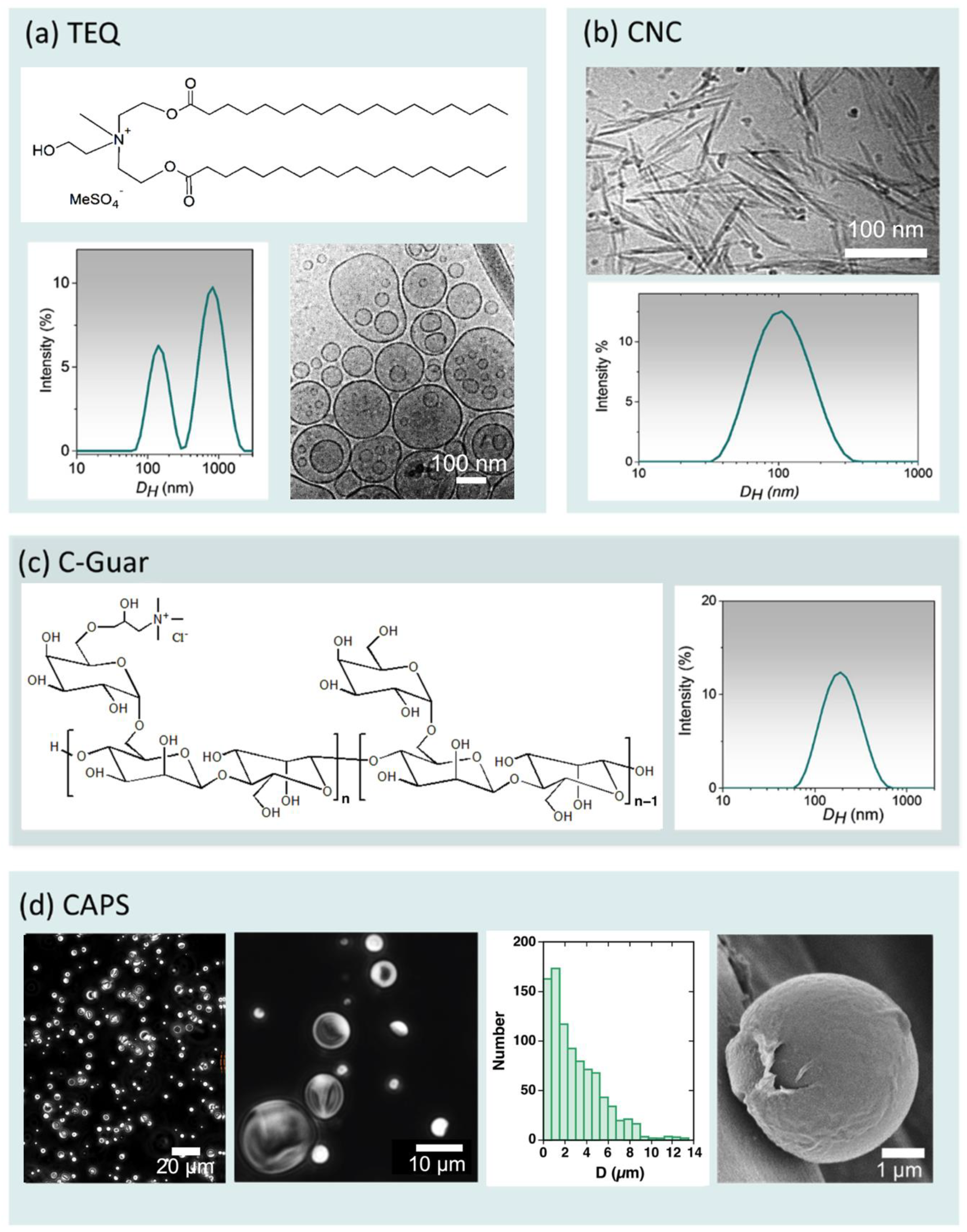


| Chemicals | Abbreviation | Concentration (wt. %) | Molecular or Colloidal State |
|---|---|---|---|
| Esterquat surfactant | TEQ | 4.0 | Multivesicular vesicles |
| Cationic guar | C-Guar | 0.2 | Branched polymers |
| Hydroxypropyl guar | HP-Guar | 0.4 | Branched polymers |
| Essential oil | Essential oil | 1.0 | Hydrophobic molecules |
| Fragrance capsules | CAPS | 1.0 | Microspheres |
| Additives (pigment, antimicrobial agents, salt) | Additives | <1.0 | Ions, molecules |
| Compounds | (nm) | pdi | -Potential (mV) |
|---|---|---|---|
| Surfactant (TEQ) | 220 | 0.45 | +47 |
| Cationic guar (C-Guar) | 250 | 0.80 | +30 |
| Fragrance capsules (CAPS) | 310 | 0.30 | −13 |
| Cellulose nanocrystal (CNC) | 120 | 0.21 | −38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikonomou, E.K.; Berret, J.-F. Adsorption of Fragrance Capsules onto Cellulose Nano- and Micro-Cellulose Fibers in Presence of Guar Biopolymers. Coatings 2023, 13, 1831. https://doi.org/10.3390/coatings13111831
Oikonomou EK, Berret J-F. Adsorption of Fragrance Capsules onto Cellulose Nano- and Micro-Cellulose Fibers in Presence of Guar Biopolymers. Coatings. 2023; 13(11):1831. https://doi.org/10.3390/coatings13111831
Chicago/Turabian StyleOikonomou, Evdokia K., and Jean-François Berret. 2023. "Adsorption of Fragrance Capsules onto Cellulose Nano- and Micro-Cellulose Fibers in Presence of Guar Biopolymers" Coatings 13, no. 11: 1831. https://doi.org/10.3390/coatings13111831
APA StyleOikonomou, E. K., & Berret, J.-F. (2023). Adsorption of Fragrance Capsules onto Cellulose Nano- and Micro-Cellulose Fibers in Presence of Guar Biopolymers. Coatings, 13(11), 1831. https://doi.org/10.3390/coatings13111831







